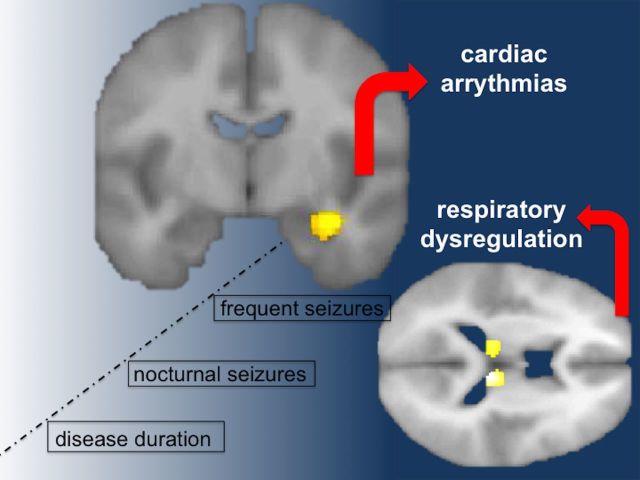
The mechanisms underlying sudden unexpected death in epilepsy (SUDEP) remain unclear. Wandschneider et al. reveal increased amygdalo-hippocampal volume in cases of SUDEP and in individuals at high risk, compared to individuals at low risk and people without epilepsy. Findings are consistent with histopathological reports in sudden infant death syndrome.
Keywords: sudden death, voxel based morphometry, hippocampus, autonomic, sudep risk
The mechanisms underlying sudden unexpected death in epilepsy (SUDEP) remain unclear. Wandschneider et al. reveal increased amygdalo-hippocampal volume in cases of SUDEP and in individuals at high risk, compared to individuals at low risk and people without epilepsy. Findings are consistent with histopathological reports in sudden infant death syndrome.
Abstract
Sudden unexpected death in epilepsy is a major cause of premature death in people with epilepsy. We aimed to assess whether structural changes potentially attributable to sudden death pathogenesis were present on magnetic resonance imaging in people who subsequently died of sudden unexpected death in epilepsy. In a retrospective, voxel-based analysis of T1 volume scans, we compared grey matter volumes in 12 cases of sudden unexpected death in epilepsy (two definite, 10 probable; eight males), acquired 2 years [median, interquartile range (IQR) 2.8] before death [median (IQR) age at scanning 33.5 (22) years], with 34 people at high risk [age 30.5 (12); 19 males], 19 at low risk [age 30 (7.5); 12 males] of sudden death, and 15 healthy controls [age 37 (16); seven males]. At-risk subjects were defined based on risk factors of sudden unexpected death in epilepsy identified in a recent combined risk factor analysis. We identified increased grey matter volume in the right anterior hippocampus/amygdala and parahippocampus in sudden death cases and people at high risk, when compared to those at low risk and controls. Compared to controls, posterior thalamic grey matter volume, an area mediating oxygen regulation, was reduced in cases of sudden unexpected death in epilepsy and subjects at high risk. The extent of reduction correlated with disease duration in all subjects with epilepsy. Increased amygdalo-hippocampal grey matter volume with right-sided changes is consistent with histo-pathological findings reported in sudden infant death syndrome. We speculate that the right-sided predominance reflects asymmetric central influences on autonomic outflow, contributing to cardiac arrhythmia. Pulvinar damage may impair hypoxia regulation. The imaging findings in sudden unexpected death in epilepsy and people at high risk may be useful as a biomarker for risk-stratification in future studies.
Introduction
The incidence of sudden death is 20-fold higher in people with epilepsy than in the general population; sudden unexpected death in epilepsy (SUDEP) is the most common cause of premature death in people with chronic epilepsy. There is currently little understanding of the underlying mechanisms of SUDEP, and post-mortem histopathology has shown no pathognomonic characteristics (Surges et al., 2009; Shorvon and Tomson, 2011; Surges and Sander, 2012). Meta-analyses of SUDEP risk factors (Hesdorffer et al., 2011; Ryvlin et al., 2013a) have identified frequent convulsive seizures (≥3/year) as a major risk factor and several studies indicate that unsupervised nocturnal seizures significantly contribute to SUDEP risk (Lamberts et al., 2012). The Mortality in Epilepsy Monitoring Unit Study (MORTEMUS) reported a consistent pattern in video-EEG monitored SUDEP cases, of a convulsive seizure, followed by early and fatal cardiorespiratory dysfunction (Ryvlin et al., 2013b). Some studies support a primary respiratory cause with central apnoea, which has been related to postictal generalized EEG suppression, indicating profound depression of CNS functions (Lhatoo et al., 2010). Other studies report primary peri-ictal cardiac arrhythmias and impaired heart rate variability accompanying SUDEP (Surges et al., 2010).
One recent imaging investigation (Mueller et al., 2014) reported severe volume loss in the dorsal mesencephalon in two SUDEP cases. A resting-state functional connectivity study identified reduced functional connectivity between the pons and right thalamus, the midbrain and right thalamus, the anterior cingulate cortex bilaterally and the thalamus, and the right and left thalamus in subjects at high risk compared to those at low risk of SUDEP (Tang et al., 2014). Imaging studies in other conditions with high risk of sudden death have also shown structural changes in brain regions bearing autonomic regulatory or respiratory functions, i.e. the dorsal and ventral medulla, putamen, and bilateral insular cortices in recent-onset obstructive sleep apnoea (Kumar et al., 2014), and the hypothalamus, posterior thalamus, caudal raphe, locus coeruleus, insular cortex and lateral medulla in congenital central hypoventilation syndrome. People suffering from the latter condition are especially at risk for sudden death (Patwari et al., 2010). Neuropathological studies in sudden infant death syndrome (SIDS) report brainstem abnormalities, i.e. brainstem gliosis and defects of neurotransmission in the medulla (Paine et al., 2014). Dentate gyrus abnormalities in the hippocampus were reported in a large subset of 153 sudden infant death syndrome cases, and may reflect defective neuronal migration and proliferation (Kinney et al., 2009, 2015).
Mice carrying mutations in the Kv1.1 potassium and SCN1A sodium channels have many phenotypes of human SUDEP, e.g. frequent convulsive seizures and premature death. A recent study in these mutant mice demonstrated that cortical EEG suppression coincided with spreading depolarization in the dorsal medulla, a region controlling cardiorespiratory pace making. Depolarizing blockade of these cells prevents normal autoresuscitation and produces cardiorespiratory arrest (Aiba and Noebels, 2015). To elucidate which brain regions may be implicated in SUDEP, we investigated whether regional abnormalities in grey matter volume appear in those who had SUDEP, compared to healthy controls. Due to the low incidence of SUDEP, exploring enriched risk groups has been suggested as a means to increase the yield of future studies (Ryvlin et al., 2013a). We explored whether regional imaging findings in people who died of SUDEP can be reproduced in a larger cohort of subjects at high risk for SUDEP. To assess whether imaging findings are common to SUDEP and those at high risk, independent from other epilepsy-related factors, we compared SUDEP cases and those at high risk to a population presumed to be at low risk of SUDEP. We also compared subjects at high risk and low risk of SUDEP to healthy controls.
Materials and methods
This retrospective study was conducted at a UK tertiary referral centre for epilepsy as part of database research on the ‘Prevention and Risk Identification of SUDEP’, approved by the National Research Ethics Committee (14/SW/0021).
The scans for SUDEP cases, those at low and high risk of SUDEP, and healthy controls were obtained from an overlapping period of case ascertainment, ensuring same imaging protocols were used for acquisition.
Subjects with epilepsy were identified from a general clinical database at the tertiary referral centre. We identified 12 people who died with definite or probable SUDEP, and matched those with 53 living subjects with epilepsy identified from the same database according to the criteria below. All subjects had to have undergone a high-resolution T1 volume scan using the identical 3 T MRI scanner as part of their clinical care. Individuals with major brain lesions, such as those after partial temporal lobe resection, were not included to avoid problems with imaging normalization. Sufficient clinical data had to be available to subsequently identify subjects at low or high risk of SUDEP, as described below. All three groups were matched for gender, age, epilepsy syndrome, and epilepsy duration to control for duration-related structural changes. Groups were also matched for lesion pathology where possible.
Healthy controls were comparable to the epilepsy populations for gender and age.
Characteristics of subjects who died suddenly
Those deceased were classified as probable (n = 10) or definite (n = 2) SUDEP, according to a recent classification (Nashef et al., 2012). The median age at death was 35.5 [interquartile range (IQR) 2.8] years. Scans were acquired at a median of 2 (IQR 2.8) years antemortem. Videotelemetry data of seizures were available in five SUDEP cases. Further clinical information on the SUDEP cases are shown in Table 1. SUDEP, subjects at high or low risk, as well as control subjects, were comparable for gender and age at scan (Table 2).
Table 1.
Additional clinical characteristics of the SUDEP cohort
| Case | Epilepsy syndrome | SUDEP Category | Lesion on visual inspection of MRI by neuroradiologist | Duration tonic phase (s) | PGES | Duration PGES (s) |
|---|---|---|---|---|---|---|
| 1 | Juvenile myoclonic epilepsy | Probable | No | N/A | N/A | N/A |
| 2 | Focal, left temporal Primary generalized | Probable | Bulky left amygdala with mild FLAIR signal increase | N/A | no | N/A |
| 3 | Focal, bitemporal | Probable | no | 11 | yes | 30–43 |
| 4 | Focal, probably bitemporal | Probable | no | N/A | N/A | N/A |
| 5 | Multifocal, left mesial temporal and frontal | Probable | Left hippocampal sclerosis | 10 | yes | 33 |
| 6 | Focal, frontal | Probable | no | N/A | N/A | N/A |
| 7 | Focal, unclassified | Definite | Bilateral periventricular leucomalacia | N/A | N/A | N/A |
| 8 | Focal, frontal | Probable | Left hippocampal sclerosis | N/A | yes | 5 |
| 9 | Focal, left hemisphere neocortical | definite | Cavernoma left superior frontal gyrus | 6 - 23 | no | N/A |
| 10 | Unclassified | Probable | Cavernoma right inferior frontal, in white matter | N/A | N/A | N/A |
| 11 | Focal, probably bitemporal | Probable | Enlarged left amygdala > hippocampus | N/A | N/A | N/A |
| 12 | Focal, left hemisphere | Probable | Right superior temporal DNET | N/A | N/A | N/A |
DNET = dysembryoplastic neuroepithelial tumour; FLAIR = fluid-attenuated inversion recovery; N/A =not applicable; PGES = postictal generalized EEG suppression.
Table 2.
Demographic and clinical parameters
| SUDEP cases (n = 12) | At high risk (n = 34) | At low risk (n = 19) | Controls (n = 15) | df | X2 | P | |
|---|---|---|---|---|---|---|---|
| Age at scan (years) Median (IQR) | 33.5 (21.5) | 30.5 (12) | 30.0 (7.5) | 37 (16) | 3 | 2.85 | 0.241 |
| Age at onset (years) Median (IQR) | 16.5 (10) | 13.5 (7) | 14 (6) | N/A | 2 | 6.21 | 0.045 |
| Epilepsy duration (years) Median (IQR) | 11.5 (24.3) | 17 (11.25) | 15 (15) | N/A | 2 | 5.74 | 0.057 |
| Gender, male | 8 | 19 | 12 | 7 | 3 | 1.42 | 0.722* |
| >3 CSs/year | 8 | 24 | 0 | N/A | 2 | 26.09 | 0.000* |
| Nocturnal seizures | 8 | 27 | 0 | N/A | 2 | 31.9 | 0.000* |
| Polytherapy | 4 | 14 | 4 | N/A | 2 | 2.21 | 0.347* |
Pearson’s chi-square was used for dichotomous variables. As some cells had an expected count < 5, an exact significance test was selected. Kruskal-Wallis test was used for all other variables. CS = convulsive seizure.
P < 0.05; asterisk indicates exact P.
Characteristics of people at high or low risk for SUDEP
A risk score was created for each subject according to the most robust epilepsy-specific risk factors for SUDEP identified in recent combined-risk factor analyses (Hesdorffer et al., 2011) that were also implemented in a recent SUDEP imaging study (Tang et al., 2014). Odds ratios for individual SUDEP risk factors were therefore adjusted for different study groups. Those with either nocturnal seizures [odds ratio (OR) = 3.9], or frequent (≥3/year) convulsive seizures (OR = 15.46), were considered ‘high risk.’ Increased SUDEP risk is also associated with young age at disease onset (onset age < 16 years: OR = 1.72), and long disease duration (duration > 15 years: OR = 1.95) (Hesdorffer et al., 2011; Lamberts et al., 2012). For each subject, odds ratios for risk factors were added to define an individual overall risk score. In the SUDEP cohort, 11 of 12 SUDEP cases (91.7%) were correctly identified as high risk subjects if the summed risk score was at least 3.9 (median risk score 19.1, IQR 16.7). One subject with juvenile myoclonic epilepsy had died, probably from SUDEP, but was not known to have suffered from nocturnal seizures or frequent convulsive seizures. A cut-off of 3.9 was therefore used to stratify others into those with high (≥3.9) and low risk (<3.9) of SUDEP.
Individual risk scores and pathology identified on MRI of people at low and high risk for SUDEP are listed in Supplementary Table 1.
SUDEP cases, and those at low and high risk, were matched for epilepsy syndrome (SUDEP: 1/12 generalized genetic epilepsy; high risk: 0/34; low risk: 3/19), and as far as possible for type of pathology (Supplementary Table 2). We were primarily interested in identifying common structures and pathophysiological mechanisms underlying SUDEP and high risk for SUDEP, and the majority of those at low risk (10/19), and high risk (22/34), had no identifiable lesions on a high-resolution 3 T epilepsy protocol clinical MRI brain scan. Videotelemetry data of seizures were available in 30/34 of those at high risk, and 7/19 at low risk. Further information regarding epilepsy classification (as per videotelemetry and/or history) is shown in Supplementary Table 3.
Controls
Scans of 15 age- and gender-matched healthy control subjects were included from a previous study (Stretton et al., 2013). All controls had normal MRI scans.
MRI data acquisition
All participants had been previously scanned on the same 3 T GE Signa HDx scanner (General Electric), and were scanned with identical acquisition parameters. We used standard imaging gradients, with a maximum strength of 40 mT/m and slew rate of 150 T/m/s. As part of the clinical sequences, a coronal T1-weighted volumetric (3D) scan was acquired with 170 contiguous 1.1-mm thick slices (matrix 256 × 256, in-plane resolution 0.9375 × 0.9375 mm).
MRI data analysis
We used the Voxel Based Morphometry 8 toolbox (http://dbm.neuro.uni-jena.de/vbm), implemented in Statistical Parametric Mapping (SPM) 8 software (http://www.fil.ion.ucl.ac.uk/spm) for data analysis. Preprocessing included spatial normalization to the Montreal Neurological Institute (MNI) template, segmentation into the different tissue classes (grey matter, white matter, CSF), and modulation to correct for volume changes due to normalization. Intersubject registration was optimized by using the DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) algorithm. A quality check implemented in the VBM8 Toolbox did not identify any outliers, and grey matter images were then smoothed with a 10-mm full-width at half-maximum Gaussian kernel (Yasuda et al., 2010).
The smoothed grey matter images were entered into a full-factorial design with group as factor to test for local differences in grey matter volume between groups. Voxels with grey matter values <0.2 (absolute threshold masking) were excluded to avoid edge effects between different tissue types. Age at scan was entered as a nuisance variable into the model.
The statistical threshold was set at P < 0.001, with a minimum cluster size of 30 contiguous voxels.
Statistical analysis of demographical and clinical data
Statistical analysis of demographical and clinical data was performed with SPSS Version 20.0 (SPSS Inc.). Pearson’s chi-square test with an exact significance test for cells with a count of less than five was used for dichotomous data. Kruskal-Wallis test was used for all other data (Table 2).
Results
Demographic and clinical data
All groups were comparable for gender and age at scan. Epilepsy groups were generally comparable for clinical parameters, except for factors included in the risk scoring, i.e. frequent convulsive seizures, nocturnal seizures, and onset of disease (Table 2). Of the epilepsy groups, 66.7% of SUDEP cases, 35.3% of high risk and 47.3% of low risk had a lesion on the scan (Supplementary Table 2).
Voxel-based morphometry
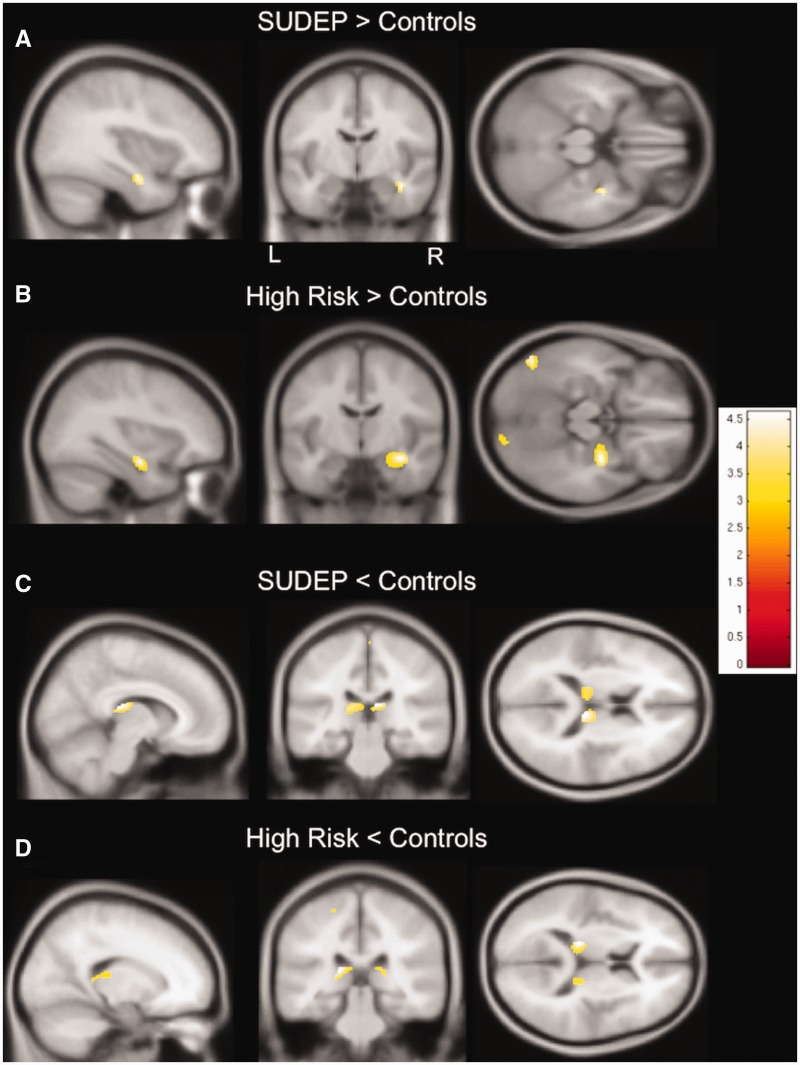
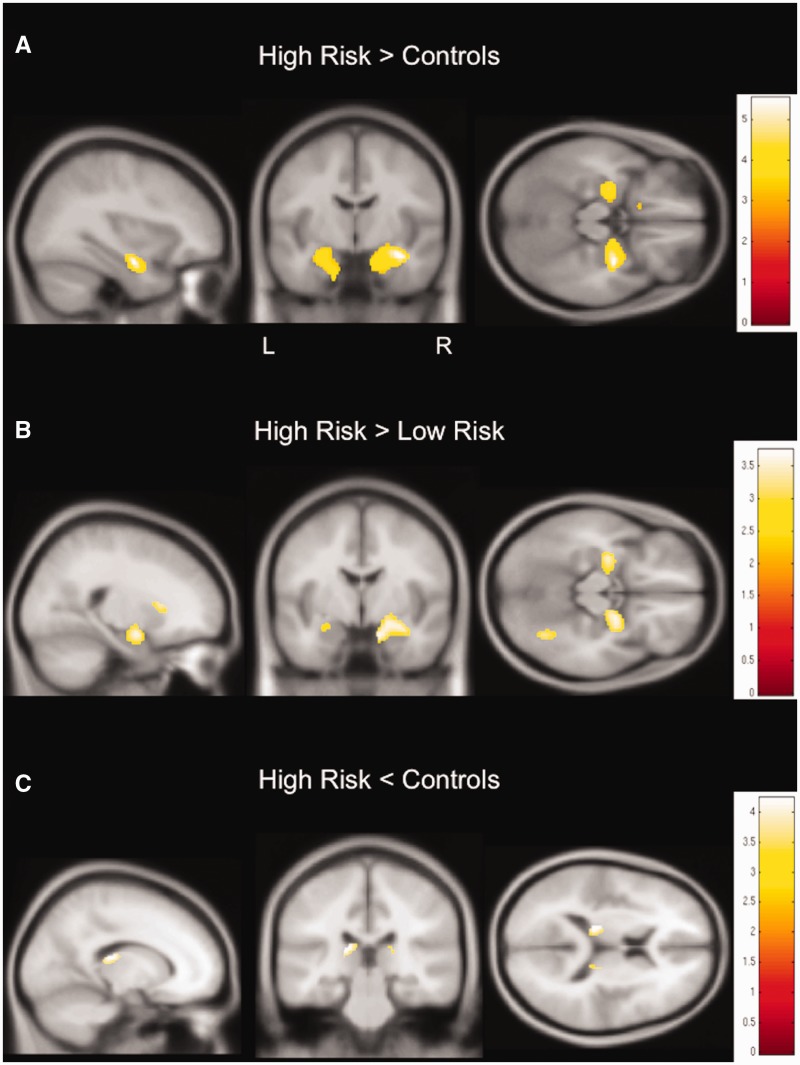
SUDEP cases showed increased grey matter volume within the right anterior hippocampus, and parahippocampal gyrus (Fig. 1A), and decreased grey matter volume in the pulvinar of the thalamus bilaterally (Fig. 1C), compared to controls. In those at high risk, we found similar changes within these regions, i.e. grey matter volume increase in the right hippocampus and parahippocampal gyrus (Fig. 1B), and decreased grey matter volume in the left pulvinar (Fig. 1C), when compared to controls.
Figure 1.
Regional grey matter volume differences between SUDEP and people at high risk and controls. (A) SUDEP cases show increased grey matter volume in the right hippocampus and parahippocampal gyrus compared to healthy subjects. (B) Similarly to SUDEP cases, subjects at high risk show increased grey matter volume in the right hippocampus and parahippocampal gyrus compared to healthy controls. (C) Compared to controls, grey matter volume is decreased in SUDEP cases in the pulvinar bilaterally. (D) Likewise, grey matter volume is decreased in those at high risk in the left pulvinar, compared to healthy controls. T-values are represented in the coloured bars. P < 0.001, 30 voxel threshold extent; L = left; R = right.
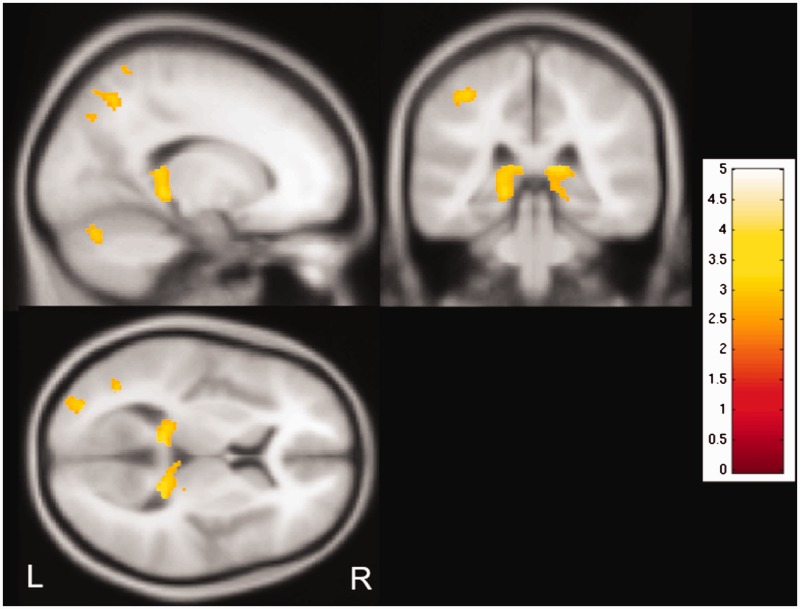
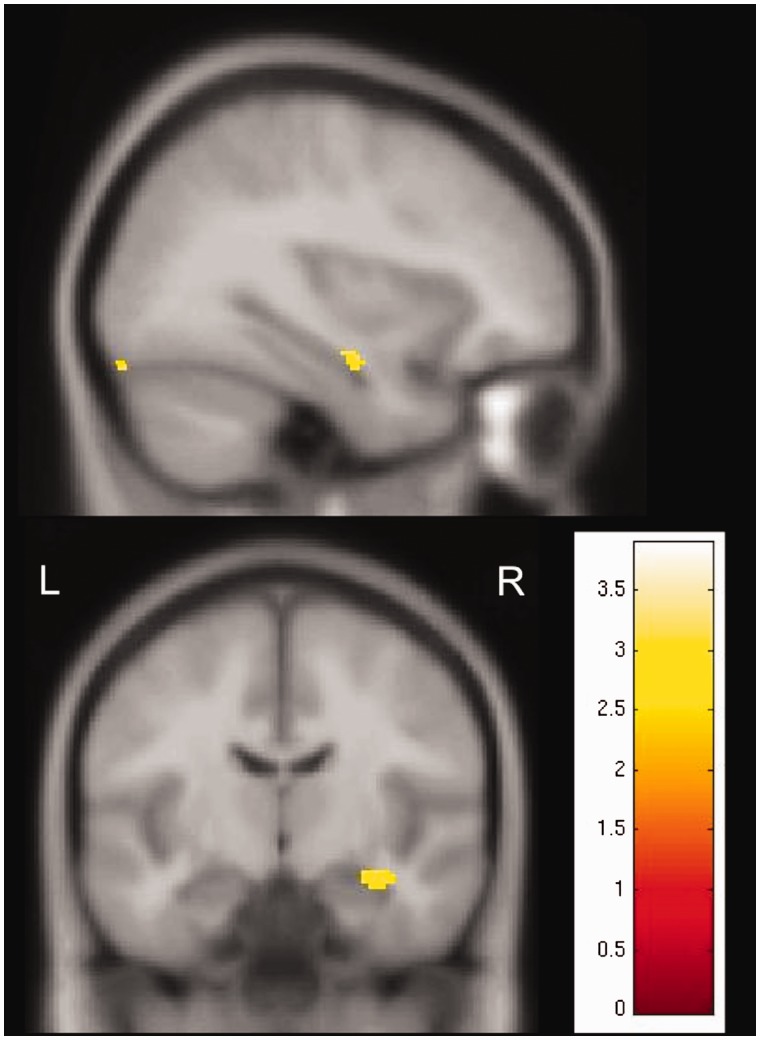
A post hoc analysis across all cases suggested a negative correlation of grey matter volume within the pulvinar bilaterally with disease duration (Fig. 2; P < 0.005, 30 voxel threshold extent).
Figure 2.
Correlation of grey matter volume with disease duration. Regional grey matter volume in bilateral thalamic pulvinar shows a negative correlation with epilepsy duration, i.e. grey matter volume decreases with longer duration (P < 0.005, 30 voxel threshold extent). T-values are represented in the coloured bar. L = left; R = right.
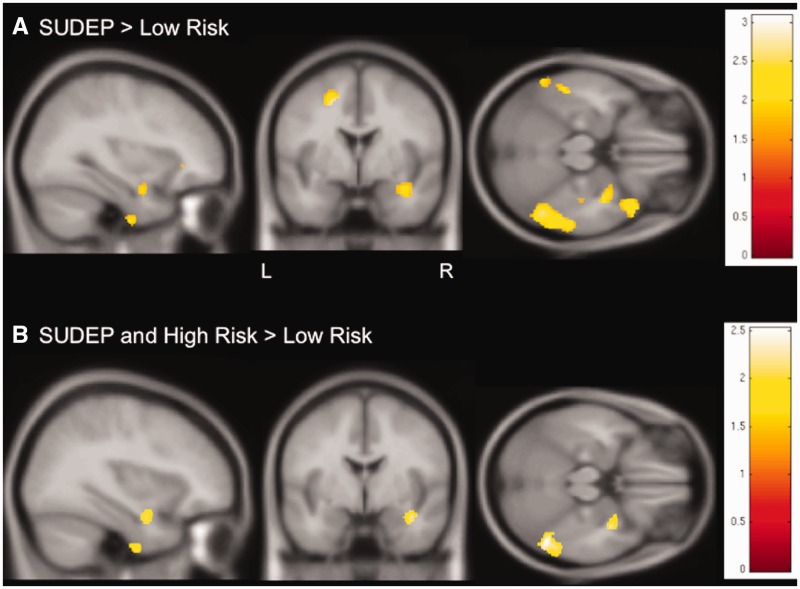
Both SUDEP cases and those at high risk showed areas of increased grey matter volume in the right hippocampus and parahippocampal gyrus, compared to those at low risk (threshold of significance P < 0.05, 30 voxels threshold extent; Fig. 3).
Figure 3.
Regional grey matter volume differences between SUDEP cases and those at high risk in comparison to people at low risk. (A) Similar to findings in comparison to controls (Fig. 1A), but at a lower threshold level (P < 0.05, 30 voxels threshold extent), SUDEP cases show increased grey matter volume in the right hippocampus and parahippocampal gyrus in comparison to those at low risk. (B) People at high risk and SUDEP cases share common areas of increased grey matter volume within the right hippocampus and parahippocampal gyrus when compared to those at low risk (conjunction, P < 0.05, 30 voxels threshold extent). T-values are represented in the coloured bars. L = left; R = right.
Subgroup analyses
To ensure that the findings were not driven by gross brain pathologies, we conducted a subgroup analysis in those at risk who had non-lesional MRI scans (low risk n = 10; high risk n = 22). In the majority of SUDEP cases (66.7%), lesions were evident on clinical scans; hence, due to small sample size, the same subgroup analysis could not be conducted. Age at scan was entered as a nuisance variable. Compared to controls, those at high risk and without lesions still showed increases in anterior hippocampal grey matter volume, as well as in the amygdala, albeit this time bilaterally (Fig. 4A). Similarly, grey matter volume in both hippocampi and amygdalae was increased in people at high risk compared to those at low risk, but more prominent in the right than left amygdala and hippocampus (Fig. 4B).
Figure 4.
Regional grey matter volume differences between those at low and high risk with non-lesional epilepsy and controls. Findings appear similar to previous findings in the whole sample (Fig. 1), but are more bilateral: subjects at high risk without identifiable pathology on clinical structural scans show an increase of grey matter volume in both anterior hippocampi and amygdalae when compared to controls (A; P < 0.001, 30 voxels threshold extent) and when compared to people at low risk (B; P < 0.005, 30 voxels threshold extent).
Grey matter volume is decreased in the bilateral posterior thalamus in those at high risk when compared to controls (C; P < 0.005, 30 voxels threshold extent). T-values are represented in the coloured bars.
To explore whether findings in the right medial temporal lobe are only related to frequent convulsive seizures, we compared those with more than three convulsive seizures per year to those with fewer convulsive seizures per year in the high risk and SUDEP groups. Fourteen subjects had fewer convulsive seizures (four SUDEP, 10 high risk) and 32 had frequent convulsive seizures (eight SUDEP, 24 high risk). Age at scan and gender were entered as nuisance variables. There were no differences within the medial temporal region between both groups. Compared to controls, both groups showed common areas of increased grey matter volume in the right hippocampus (conjunction, P < 0.005; Fig. 5). We also compared total right hippocampal volumes in both groups using an automated segmentation tool (Winston et al., 2013). There were no significant differences in right hippocampal volumes between subjects with frequent and less frequent convulsive seizures (right hippocampal volume in cm3 in subjects with less than three convulsive seizures per year: median 3.036, IQR 0.65; in subjects with three or more convulsive seizures per year: median 2.90, IQR 0.52 cm3; Mann-Whitney U = 130.000, P = 0.646).
Figure 5.
Common areas of increased grey matter volume in subjects with frequent and less frequent convulsive seizures compared to controls. Amongst SUDEP cases and people at high risk of SUDEP, subjects with frequent convulsive seizures (i.e. ≥3/year) and less frequent convulsive seizures (<3/year) share common areas of increased grey matter volume in the right hippocampus when compared to healthy controls. Conjunction, P < 0.005. T-values are represented in the coloured bars. L = left; R = right.
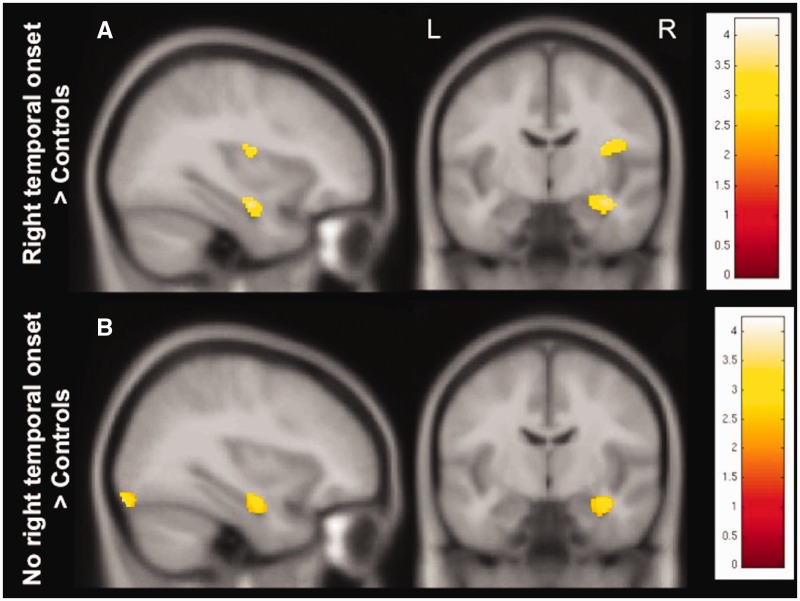
To relate seizure onset site to right medial temporal findings, subjects with right temporal seizure onset were compared to those with a different, right extratemporal or left hemisphere onset. Ictal EEG data were available in nine SUDEP, 30 high risk and four low risk individuals. In six high risk and one SUDEP case, seizure onset could not be localized and these cases were therefore excluded. There were no differences between these two groups in volumetric findings. In comparison to controls, both groups showed an increase in grey matter volume in the right hippocampus (P < 0.005, 30 voxels threshold extent; Fig. 6).
Figure 6.
Grey matter volume changes in subjects with and without right temporal seizure onset. In comparison to healthy controls, subjects with right temporal seizure onset (Fig. 6A), as well as those without (Fig. 6B) showed an increase of grey matter volume in the right hippocampus (P < 0.005, 30 voxels threshold extent). T-values are represented in the coloured bars. L = left; R = right.
Discussion
Anatomical differences between subjects with SUDEP and high risk versus those at low risk
We identified increased grey matter volume within the right hippocampus and parahippocampal gyrus in SUDEP cases and in subjects at high risk for SUDEP, compared to those at low risk and controls. There was increased grey matter volume in both hippocampi, extending to the amygdala when comparing non-lesional high and low risk subjects.
The posterior thalamus (pulvinar) showed disease duration-dependent grey matter volume reduction in all patient groups.
MRIs of all cases and controls were subsequently reviewed again by an experienced neuroradiologist (C.M.), specifically looking for the presence or absence of hippocampal pathology (Table 1 and Supplementary Table 1). No new lesions within these regions were identified on visual inspection of individual cases, suggesting that the findings are at a group level.
Neuropathological studies in sudden unexplained death in childhood and in sudden infant death syndrome have found abnormalities in the same region; dentate gyrus abnormalities in a large subset of 153 sudden infant death syndrome cases (Kinney et al., 2015) were interpreted as a developmental vulnerability, potentially leading to respiratory/autonomic instability, or even autonomic seizures and death during sleep when challenged by homeostatic stressors. Hippocampal and temporal lobe anomalies were also described in 62% of sudden unexplained death in childhood cases (Kinney et al., 2009). Microdysgenetic features of the hippocampal formation included dentate gyrus and subicular anomalies, granular nodular heterotopia, subventricular neuroblasts and hamartia, all indicative of aberrant neurodevelopment. Similar to SUDEP cases, those sudden unexplained death in childhood individuals with structural anomalies were found dead during sleep and in the prone position, and more commonly had an individual or family history of febrile seizures, creating a potential link between hippocampal/temporal lobe maldevelopment, susceptibility to seizures, and sudden death.
Increase in grey matter volume, which has appeared in several epilepsy syndromes in previous voxel-based morphometry studies (Yasuda et al., 2010), has been suggested as indicative of dystopic neurons and diminished grey-white matter demarcation (Yasuda et al., 2010), and findings in the current study may therefore reflect abnormal neurodevelopmental processes. Neuropathological studies in SUDEP show that pathology can be present in the hippocampus (e.g. gliosis) (Zhuo et al., 2012). There are so far, however, no quantitative neuropathological studies of the hippocampus in SUDEP, which would be needed to confirm any subtle abnormalities such as microdysgenesis.
Increased grey matter volumes in the hippocampus may also represent gliosis. Gliosis is a response to injury, and includes neuronal and synaptic functional alterations (Sofroniew, 2009) that have been associated with hyperexcitability in epilepsy (Binder and Steinhäuser, 2006). Gliosis within the hippocampus may therefore alter neuronal activity facilitating the risk of SUDEP, e.g. through hyperexcitability and/or limbic network dysfunction implicating also autonomic function.
A recent study evaluating structural imaging prediction patterns for seizure freedom after surgery in temporal lobe epilepsy found unilateral or bilateral atrophy of the hippocampus, amygdala and entorhinal cortex in most subjects, although one subgroup showed bilaterally increased hippocampal and amygdala volumes (Bernhardt et al., 2015). Subjects in this group were more likely to have unsuccessful epilepsy surgery, supporting the concept that gliosis may facilitate processes of treatment-resistant disease. Histopathology confirmed hippocampal gliosis in almost all subjects of this subgroup. Astrogliosis and cellular hypertrophy have been described in neuropathological studies in hippocampal tissue of subjects with refractory temporal lobe epilepsy (Das et al., 2012). Mild gliosis is also present in cases of amygdala enlargement (Minami et al., 2015).
Longitudinal voxel-based morphometry studies report a decrease of grey matter volume in mesiotemporal structures and the thalamus with longer disease duration and more active disease, i.e. frequent seizures (Bernhardt et al., 2009, 2013; Coan et al., 2009). Similarly, changes in the posterior thalamus correlate with disease duration in our cohort and this suggests a dynamic origin of grey matter volume alterations in our study. A potential mechanism for gliosis in epilepsy could be repeated hypoxic insults, particularly through convulsive seizures (Macey et al., 2009).
That increased grey matter volume may represent gliosis and plasticity following neural injury (Yasuda et al., 2010) is corroborated by data in other sudden death entities: increased grey matter volume in the putamen appears in people with newly-diagnosed obstructive sleep apnoea, who are subjected to repeated hypoxic episodes, with the increased volumes usually attributed to transitional processes in glial death accompanying the neural injury in the syndrome (Kumar et al., 2014).
Association with autonomic dysfunction and significance of laterality of findings
Several functional imaging studies in humans (Shoemaker et al., 2012, 2015), and stimulation studies in animals (Terreberry and Neafsey, 1987) have identified the hippocampus as an essential component of limbic circuitry modulating autonomic function, with substantial influences on blood pressure regulation (Harper et al., 2000).
Major hippocampal influence on autonomic activity through efferent projections can also be assumed from intracerebral stimulation studies in subjects with refractory epilepsy (Catenoix et al., 2011). These influences are corroborated by reports of people with mesial temporal lobe epilepsy who show decreased heart rate variability in relation to seizures and interictal epileptic discharges, which were more pronounced during sleep, when most cases of SUDEP occur (Moseley et al., 2011). Of interest, heart rate variability normalizes in some after successful temporal lobe epilepsy surgery (Hilz et al., 2002).
Hippocampal grey matter volume increases in our cohort may partially underlie seizure generation and ictal and peri-ictal autonomic dysfunction. However, increased right hippocampal grey matter volume was even present in those individuals with known right medial temporal epilepsy when compared to healthy controls (no cases of hippocampal sclerosis in either group). The increased grey matter volume was surprising, as longitudinal voxel-based morphometry data describe progressive atrophy of the ipsilateral hippocampus in the medial temporal lobe, especially in those subjects with higher seizure frequency and longer epilepsy duration, i.e. higher SUDEP risk (Bernhardt et al., 2009, 2013; Coan et al., 2009). This may suggest that our findings are not associated with a primarily seizure-related autonomic dysfunction; but they may be associated with an interictal autonomic dysregulation. At this time this is a speculative suggestion, and needs investigation of autonomic function in similar cohorts.
Asymmetry of grey matter volume increases
A significant aspect of the grey matter volume hippocampal increase in SUDEP cases and subjects at high risk was the asymmetry, with the volume changes on the right side. The lateralization of tissue change in an autonomic regulatory area poses a serious concern for sympathetic and parasympathetic outflow. If laterality on sympathetic influences is preserved to medullary output nuclei, the consequences to cardiac arrhythmia generation are severe, as asymmetric sympathetic outflow leads to such phenomena as potentially fatal long Q-T syndrome (Schwartz, 1998). The right insula plays a more prominent role with sympathetic regulation, while parasympathetic regulation is primarily mediated on the left insula (Oppenheimer, 2006), as determined by a series of stimulation, lesion, stroke, and imaging studies, including human epilepsy surgical studies (Oppenheimer et al., 1992). Both injury to the right limbic system and direct insular stimulation have been associated with sympathetic over-regulation (Oppenheimer, 2006). Sudden death after acute right-sided insular strokes and increased complex arrhythmias appears more often than in any other lesion localization (Soros and Hachinski, 2012). Right insular injury in obstructive sleep apnoea shows distorted blood pressure recovery patterns to a challenge (Harper et al., 2003; Henderson et al., 2003) and right hemisphere strokes, particularly when involving the insula, are accompanied by increased nocturnal blood pressure, higher noradrenaline levels and QTc prolongations (Oppenheimer, 2006). The insular effects appear to be mediated by projections to the ventral medial frontal cortex, hypothalamus, and hippocampus through integrated circuitry (Shoemaker et al., 2015). The lateralized (right) increased mesiotemporal grey matter volume in our cohort may contribute to chronic, asymmetric hyper-sympathetic activation, or a sympathetic system lacking in appropriate responsiveness, which would contribute to mechanisms that pose a risk for sudden death.
Similar scenarios develop for obstructive sleep apnoea and for heart failure, which induce severe injury preferentially in the right insula, and consequential very high resting, and unresponsive, sympathetic tone (Macey et al., 2002; Woo et al., 2005). An imbalance between parasympathetic and sympathetic drive places an individual at risk, resulting in a tendency to postictal bradycardia/asystole as noted in the MORTEMUS study (Ryvlin et al., 2013b).
Decreased grey matter volume in the posterior thalamus
A second major finding was that grey matter volume was reduced in the posterior thalamus, and correlated with disease duration. The finding was not unique to SUDEP. A decrease of grey matter volume in the posterior thalamus correlated with disease duration in all subjects with epilepsy (Fig. 2), and one may speculate that those changes may develop in low risk subjects, given sufficient duration of seizures. However, the finding of posterior thalamic grey matter volume should be taken in the context of roles for that structure in respiratory regulation. Substantial evidence, ranging from lesion and stimulation studies in the foetal lamb (Koos et al., 1998, 2004), to functional MRI studies in adolescents and children with congenital central hypoventilation syndrome (Macey et al., 2005), show the significant role of the posterior thalamus in mediating breathing responses following manipulation of oxygen levels, with special participation in the inhibition of breathing following hypoxic exposure (Koos et al., 1998, 2004). We speculate that injury to the posterior thalamus is common in people with epilepsy, that the evidence suggests that disease duration potentiates that injury, and that such injury poses particular risk to the hypoxia normally accompanying ictal episodes, causing thalamic structures to fail to adequately recover from low oxygen. A thalamic role must, however, be viewed in the context that in people who succumbed to SUDEP or who were at high risk also were burdened with right-sided grey matter volume increases in the hippocampal region, which would compromise appropriate blood pressure responses that accompany apnoea. Thus, the combination of injury, diminished posterior thalamic and altered right-sided hippocampal grey matter volume may impose a set of circumstances leading to vital failure.
The mechanisms underlying decreased thalamic grey matter volume should be considered; the decline emerges in several epilepsy syndromes (Yasuda et al., 2010), and appears to be, in part, independent of epilepsy severity, presence of MRI lesions, and duration (Keller et al., 2002). Strong relationships of disease duration and declines in grey matter volume and changes in white matter tract microstructure, i.e. mean fractional anisotropy declines, have been described, and may underlie progressive brain changes in response to active disease, i.e. recurrent seizures (Keller et al., 2012).
High risk for SUDEP has been reported in those who fail epilepsy surgery (Sperling et al., 2005). Persistent seizures in subjects who had undergone amygdalo-hippocampectomy for unilateral temporal lobe epilepsy were associated with preoperative atrophy of bilateral dorsomedial and pulvinar thalamic regions (Keller et al., 2015), with the investigators arguing that these regions are important hubs of seizure modulation and spread. We suggest that the risk may stem from failure of a combined interaction of breathing and blood pressure control.
Limitations
The criteria used to define our risk groups, and the cut-off between high and low risk, were arbitrary. The finding that SUDEP and those at high risk show similar patterns is consistent with our definition of risk groups. Eleven of 12 SUDEP cases were classified as high risk with our criteria.
A major limitation of our study is to disentangle whether our finding of right hippocampal grey matter volume increase is a specific SUDEP risk factor or rather a marker of severe epilepsy.
As there was only one low risk case in our SUDEP group, we could not establish whether increased right hippocampal grey matter volume is present in SUDEP cases despite being labelled low risk. This would have marked our finding as more SUDEP-specific. In vivo imaging biomarkers of SUDEP risk should be present in both subjects who later on died from, and those at high risk of, SUDEP. We argue that the smaller the difference we observe between those two groups, the better our classification and definition of high risk criteria. Similarly, main risk factors for SUDEP—like frequent, uncontrolled convulsive seizures—will have to be present in both SUDEP and high risk groups (Hesdorffer et al., 2011), and hence, are also the major distinguishing factor of high risk versus low risk subjects in our study. By the nature of SUDEP and our study, it is therefore impossible to fully disentangle the effect of severe epilepsy from a specific SUDEP biomarker itself.
Due to methodological challenges (Ashburner and Ridgeway, 2013), there are only few longitudinal voxel-based morphometry studies in people with mesial temporal lobe epilepsy. All of them show grey matter atrophy within mesial temporal structures and beyond (e.g. thalamus) over time, which are more progressive with longer disease duration and higher seizure frequency (Bernhardt et al., 2009, 2013; Coan et al., 2009). Evaluation of subregional mesiotemporal disease progression revealed that progressive atrophy particularly involves the anterior part of the hippocampus (Bernhardt et al., 2013). These reports are in clear contrast with our findings of increased grey matter volume particularly in the anterior hippocampus, and suggest that these are not only caused by frequent seizures. There are poor data on exact seizure counts in our groups, but when subjects in the high risk and SUDEP groups where dichotomized into those with frequent (i.e. more than three convulsive seizures per year) and those with less frequent convulsive seizures, there were no significant group differences within the right hippocampus, but both groups showed common areas of increased right hippocampal grey matter volume when compared to healthy controls (Fig. 5). In addition, total right hippocampal volume measures did not differ between groups. This underscores our argument that the findings represent more specific SUDEP markers than just markers of severe epilepsy.
In keeping with the longitudinal data, posterior thalamic grey matter atrophy correlates with disease duration in our cohort and we can therefore confirm that this finding is not a specific SUDEP biomarker.
We appreciate that epilepsy groups in this study combine various different epilepsy subtypes, and include subjects with lesional and non-lesional MRI scans (Supplementary Table 2). Right hippocampal sclerosis was, however, not present in either epilepsy group, and therefore does not explain differences in right hippocampal grey matter volume. Structural abnormalities were common among our SUDEP population (66.7% of cases), and we acknowledge that our SUDEP group may therefore not be representative of all SUDEP cases.
A previous study (Mueller et al., 2014) described decreased midbrain volume using graph analysis methodology in two SUDEP cases compared to controls. We did not aim to examine brainstem volumes, although our whole-brain analysis included the brainstem; we found no abnormal changes in the brainstem within any group. Voxel-based morphometry has substantial limitations in evaluating brainstem segmentation, due to the difficulty in resolving internal brainstem architecture reliably and consistently (Lambert et al., 2013). Disturbances in brainstem attributes may be better evaluated with newer procedures for examining tissue changes, such as diffusion MRI.
Conclusion
Increased right hippocampal and parahippocampal grey matter volume and grey matter volume decline in the posterior thalamus appear to be related to SUDEP risk. In the case of grey matter volume increases, the relationship is independent of markers of severe epilepsy, such as frequent convulsive seizures. The volume increases are potentially of dynamic origin, representing gliosis in response to repetitive injury from severe epilepsy, while the thalamic volume declines may result from excitotoxic or other injury sources. The thalamic injury may lead to an inability to recover breathing to a hypoxic challenge from apnoea, while the hippocampal/parahippocampal pathology may contribute to asymmetric influences on autonomic outflow, establishing circumstances for cardiac arrhythmia and hypotension. The structural changes may be useful biomarkers to assist determination of pathophysiology of SUDEP.
Funding
This work was undertaken at UCLH/UCL who receives a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. It was partially funded by the NIH –National Institute of Neurological Disorders and Stroke (U01-NS090405-01 and U01-NS090407-01. The Center for SUDEP Research) and Wellcome Trust (project grant 083148). S.B.V. is funded by the National Institute for Health Research University College London Hospitals Biomedical Research Centre (NIHR BRC UCLH/UCL High Impact Initiative). J.W.S. is supported by the Marvin Weil Epilepsy Research Fund.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviation
- SUDEP
sudden unexpected death in epilepsy
References
- Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med 2015; 7; 282ra46 10.1126/scitranslmed.aaa4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Ridgway GR. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci 2013; 6: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Kim H, Bernasconi N. Patterns of subregional mesiotemporal disease progression in temporal lobe epilepsy. Neurology 2013; 81: 1840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Hong SJ, Bernasconi N, Bernasconi A. Magnetic resonance imaging pattern learning in temporal lobe epilepsy: classification and prognostics. Ann Neurol 2015; 77: 436–46. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology 2009; 72: 1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Steinhäuser C. Functional changes in astroglial cells in epilepsy [Review]. Glia 2006; 54: 358–68. [DOI] [PubMed] [Google Scholar]
- Catenoix H, Magnin M, Mauguiere F, Ryvlin P. Evoked potential study of hippocampal efferent projections in the human brain. Clin Neurophysiol 2011; 122: 2488–97. [DOI] [PubMed] [Google Scholar]
- Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology 2009; 73: 834–42. [DOI] [PubMed] [Google Scholar]
- Das A, Wallace GC, Holmes C, McDowell ML, Smith JA, Marshall JD, et al. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience 2012; 220: 237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Bandler R, Spriggs D, Alger JR. Lateralized and widespread brain activation during transient blood pressure elevation revealed by magnetic resonance imaging. J Comp Neurol 2000; 417: 195–204. [DOI] [PubMed] [Google Scholar]
- Harper RM, Macey PM, Henderson LA, Woo MA, Macey KE, Frysinger RC, et al. FMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J Appl Physiol 2003; 94: 1583–95. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Woo MA, Macey PM, Macey KE, Frysinger RC, Alger JR, et al. Neural responses during Valsalva maneuvers in Obstructive Sleep Apnea Syndrome. J Appl Physiol 2003; 94: 1063–74. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, et al. Combined analysis of risk factors for SUDEP. Epilepsia 2011; 52: 1150–9. [DOI] [PubMed] [Google Scholar]
- Hilz MJ, Devinsky O, Doyle W, Mauerer A, Dutsch M. Decrease of sympathetic cardiovascular modulation after temporal lobe epilepsy surgery. Brain 2002; 125: 985–95. [DOI] [PubMed] [Google Scholar]
- Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage 2002; 16: 23–31. [DOI] [PubMed] [Google Scholar]
- Keller SS, Schoene-Bake JC, Gerdes JS, Weber B, Deppe M. Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: cross-sectional evidence for progressive neurologic injury. PloS One 2012; 7: e46791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Richardson MP, Schoene-Bake JC, O Muircheartaigh J, Elkommos S, Kreilkamp B, et al. Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Ann Neurol 2015; 77: 760–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Chadwick AE, Crandall LA, Grafe M, Armstrong DL, Kupsky WJ, et al. Sudden death, febrile seizures, and hippocampal and temporal lobe maldevelopment in toddlers: a new entity. Pediatr Dev Pathol 2009; 12: 455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Cryan JB, Haynes RL, Paterson DS, Haas EA, Mena OJ, et al. Dentate gyrus abnormalities in sudden unexplained death in infants: morphological marker of underlying brain vulnerability. Acta Neuropathol 2015; 129: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos BJ, Chau A, Matsuura M, Punla O, Kruger L. Thalamic locus mediates hypoxic inhibition of breathing in fetal sheep. J Neurophysiol 1998; 79: 2383–93. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Kawasaki Y, Hari A, Bohorquez F, Jan C, Roostaeian J, et al. Electrical stimulation of the posteromedial thalamus modulates breathing in unanesthetized fetal sheep. J Appl Physiol 2004; 96: 115–23. [DOI] [PubMed] [Google Scholar]
- Kumar R, Farahvar S, Ogren JA, Macey PM, Thompson PM, Woo MA, et al. Brain putamen volume changes in newly-diagnosed patients with obstructive sleep apnea. Neuroimage Clin 2014; 4: 383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Lutti A, Helms G, Frackowiak R, Ashburner J. Multiparametric brainstem segmentation using a modified multivariate mixture of Gaussians. Neuroimage Clin 2013; 2: 684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia 2012; 53: 253–7. [DOI] [PubMed] [Google Scholar]
- Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol 2010; 68: 787–96. [DOI] [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 2002; 166: 1382–7. [DOI] [PubMed] [Google Scholar]
- Macey PM, Woo MA, Macey KE, Keens TG, Saeed MM, Alger JR, et al. Hypoxia reveals posterior thalamic, cerebellar, midbrain and limbic deficits in Congenital Central Hypoventilation Syndrome. J Appl Physiol 2005; 98: 958–69. [DOI] [PubMed] [Google Scholar]
- Macey PM, Richard CA, Kumar R, Woo MA, Ogren JA, Avedissian C, et al. Hippocampal volume reduction in congenital central hypoventilation syndrome. PloS One 2009; 4: e6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami N, Morino M, Uda T, Komori T, Nakata Y, Arai N, et al. Surgery for amygdala enlargement with mesial temporal lobe epilepsy: pathological findings and seizure outcome. J Neurol Neurosurg Psychiatry 2015; 86: 887–94. [DOI] [PubMed] [Google Scholar]
- Moseley BD, Wirrell EC, Nickels K, Johnson JN, Ackerman MJ, Britton J. Electrocardiographic and oximetric changes during partial complex and generalized seizures. Epilepsy Res 2011; 95: 237–45. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Bateman LM, Laxer KD. Evidence for brainstem network disruption in temporal lobe epilepsy and sudden unexplained death in epilepsy. Neuroimage Clin 2014; 5: 208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012; 53: 227–33. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S. Cerebrogenic cardiac arrhythmias: cortical lateralization and clinical significance [Review]. Clin Auton Res 2006; 16: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb AW, Girvin JP, Hachinski VC. Cardiovascular effects of human insular stimulation. Neurology 1992; 42: 1727–32. [DOI] [PubMed] [Google Scholar]
- Paine SML, Jacques TS, Sebire NJ. Neuropathological features of unexplained sudden unexpected death in infancy: current evidence and controversies [Review]. Neuropathol Appl Neurobiol 2014; 40: 364–84. [DOI] [PubMed] [Google Scholar]
- Patwari PP, Carroll MS, Rand CM, Kumar R, Harper R, Weese-Mayer DE. Congenital central hypoventilation syndrome and the PHOX2B gene: a model of respiratory and autonomic dysregulation. Respir Physiol Neurobiol 2010; 173: 322–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Tomson T. Prevention of sudden unexpected death in epilepsy: a realistic goal?. Epilepsia 2013a; 54 (Suppl 2): 23–8. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013b; 12: 966–77. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ. The autonomic nervous system and sudden death [Review]. Eur Heart J 1998; (Suppl F): F72–80. [PubMed] [Google Scholar]
- Shoemaker JK, Wong SW, Cechetto DF. Cortical circuitry associated with reflex cardiovascular control in humans: does the cortical autonomic network “speak” or “listen” during cardiovascular arousal. Anat Rec (Hoboken) 2012; 295: 1375–84. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Norton KN, Baker J, Luchyshyn T. Forebrain organization for autonomic cardiovascular control. Auton Neurosci 2015; 188: 5–9. [DOI] [PubMed] [Google Scholar]
- Shorvon S, Tomson T. Sudden unexpected death in epilepsy [Review]. Lancet 2011; 378: 2028–38. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation [Review]. Trends Neurosci 2009; 32: 638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soros P, Hachinski V. Cardiovascular and neurological causes of sudden death after ischaemic stroke [Review]. Lancet Neurol 2012; 11: 179–88. [DOI] [PubMed] [Google Scholar]
- Sperling MR, Harris A, Nei M, Liporice JD, O’Connor MJ. Mortality after epilepsy surgery. Epilepsia 2005; 46: 49–53. [DOI] [PubMed] [Google Scholar]
- Stretton J, Winston GP, Sidhu M, Bonelli S, Centeno M, Vollmar C, et al. Disrupted segregation of working memory networks in temporal lobe epilepsy. Neuroimage Clin 2013; 2: 273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surges R, Sander JW. Sudden unexpected death in epilepsy: mechanisms, prevalence, and prevention [Review]. Curr Opin Neurol 2012; 25: 201–7. [DOI] [PubMed] [Google Scholar]
- Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms [Review]. Nat Rev Neurol 2009; 5: 492–504. [DOI] [PubMed] [Google Scholar]
- Surges R, Taggart P, Sander JW, Walker MC. Too long or too short? New insights into abnormal cardiac repolarization in people with chronic epilepsy and its potential role in sudden unexpected death [Review]. Epilepsia 2010; 51: 738–44. [DOI] [PubMed] [Google Scholar]
- Tang Y, Chen Q, Yu X, Xia W, Luo C, Huang X, et al. A resting-state functional connectivity study in patients at high risk for sudden unexpected death in epilesy. Epilepsy Behav 2014; 41: 33–8. [DOI] [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res Bull 1987; 19: 639–49. [DOI] [PubMed] [Google Scholar]
- Winston GP, Cardoso MJ, Williams EJ, Burdett JL, Bartlett PA, Espak M, et al. Automated hippocampal segmentation in patients with epilepsy: available free online. Epilepsia 2013; 54: 2166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, et al. Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail 2005; 11: 437–46. [DOI] [PubMed] [Google Scholar]
- Yasuda CL, Betting LE, Cendes F. Voxel-based morphometry and epilepsy [Review]. Expert Rev Neurother 2010; 10: 975–84. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Zhang Y, Zielke HR, Levine B, Zhang X, Chang L, et al. Sudden unexpected death in epilepsy: Evaluation of forensic autopsy cases. Forensic Sci Int 2012; 223: 171–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.