Abstract
Over the past decade, soy biodiesel (BD) has become a first alternative energy source that is economically viable and meets requirements of the Clean Air Act. Due to lower mass emissions and reduced hazardous compounds compared to diesel combustion emissions (CE), BD exposure is proposed to produce fewer adverse health effects. However, considering the broad use of BD and its blends in different industries, this assertion needs to be supported and validated by mechanistic and toxicological data. Here, adverse effects were compared in lungs and liver of BALB/cJ mice after inhalation exposure (0, 50, 150, or 500 μg/m3; 4 h/d, 5 d/wk, for 4 wk) to CE from 100% biodiesel (B100) and diesel (D100). Compared to D100, B100 CE produced a significant accumulation of oxidatively modified proteins (carbonyls), an increase in 4-hydroxynonenal (4-HNE), a reduction of protein thiols, a depletion of antioxidant gluthatione (GSH), a dose-related rise in the levels of biomarkers of tissue damage (lactate dehydrogenase, LDH) in lungs, and inflammation (myeloperoxidase, MPO) in both lungs and liver. Significant differences in the levels of inflammatory cytokines interleukin (IL)-6, IL-10, IL-12p70, monocyte chemoattractant protein (MCP)-1, interferon (IFN) γ, and tumor necrosis factor (TNF)-α were detected in lungs and liver upon B100 and D100 CE exposures. Overall, the tissue damage, oxidative stress, inflammation, and cytokine response were more pronounced in mice exposed to BD CE. Further studies are required to understand what combustion products in BD CE accelerate oxidative and inflammatory responses.
Epidemiologic and occupational studies demonstrated that ambient particular matter (PM) and diesel exhaust particles exert deleterious effects on human health, including exacerbation of preexisting lung disease, increased incidence of respiratory infections, decrement in lung capacity, and enhanced risk of lung cancer (Sawyer et al., 2010; LaGier et al., 2013). According to the U.S. Environmental Protection Agency (EPA), elevated levels of airborne PM, nitrogen oxides, and sulfur oxides contribute to serious health problems in the United States, producing increased acute respiratory symptoms, chronic bronchitis, and aggravation of asthma, cardiovascular diseases, and premature mortality (Ghio et al., 2012). The International Agency for Research on Cancer (IARC) recently identified diesel combustion emissions (CE) as a Group 1 human carcinogen after chronic exposure (Benbrahim-Tallaa et al., 2012). Diesel exhaust particles are mainly aggregates of spherical carbon particles coated with inorganic and organic substances. The inorganic fraction primarily consists of small solid carbon (or elemental carbon) PM ranging from 0.01 to 0.08 microns in diameter. The organic fraction is composed of organic compounds such as aldehydes, alkanes and alkenes, and high-molecular-weight polycyclic aromatic hydrocarbons (PAH) and PAH derivatives, such as nitro-PAH, which are extractable in organic solvents. Many of these PAH and PAH derivatives, especially nitro-PAH, were found to be potent mutagens and carcinogens (Garshick et al., 2004; Tokiwa et al., 1986). Nitro-PAH compounds are also formed during transport through the atmosphere by reactions of adsorbed PAH with nitric acid and by gas-phase radical-initiated reactions in the presence of oxides of nitrogen. A number of adverse, chronic, noncancer effects have been associated with exposure to diesel CE.
Occupational studies showed that there may be a greater incidence of cough, phlegm, and chronic bronchitis among those exposed to diesel CE than among those not exposed (Pronk et al., 2009). Reductions in pulmonary function have also been reported following occupational exposures in chronic studies (Rudell et al., 1996). In addition, diesel PM has been associated with changes in heart rate, increased incidence of arrhythmias, impairment of vasodilation, increase in blood pressure, and systemic inflammation (Hazari et al., 2011; Peretz et al., 2008; Tornqvist et al., 2007; Nemmar et al., 2007; 2009; Carll et al., 2013; Harkema et al., 2009; Knuckles et al., 2011). Human studies by Mills et al. (2005, 2007a, 2007b) demonstrated a dose-dependent increase in prothrombotic effects and acute myocardial ischemia upon exposure to diesel exhaust. Therefore, alternative fuels are becoming an emerging priority, as a renewable energy source that may exert reduced impacts on health (Lewtas, 2007).
Over the past decade, biodiesel (BD) became an alternative energy source that is economically viable and meets requirements of the Clean Air Act. BD is a form of diesel fuel manufactured from vegetable oils, animal fats, algae, recycled restaurant greases, and other sources. BD, produced from non-petroleum-based renewable resources, is believed to be safe and biodegradable and can be used in most diesel engines. As compared to traditional petroleum-based diesel (D), combustion of BD results in the reduction of some air pollutants (including PM and CO) and greenhouse gas emissions (e.g., B20 reduces CO by 15%) (McCormick, 2007; Rakopoulos et al., 2008; Ramadhas et al., 2004; U.S. EPA, 2002).
The potential broad use of BD in different operational areas, including transportation (on- and off-road vehicles), residential, and other manufacturing/production (mining, oil, and gas industries) facilities, may result in increased environmental and occupational exposures to BD exhaust particles. The effects of BD and BD blends on regulated and non-regulated emissions were studied and compared with those of for various on- and off-road diesel-powered applications (Boehman et al., 2005; McCormick 2005; Bugarski et al., 2006; Williams et al., 2006). Data indicate that the composition and concentration of the emissions depend on several factors, including operating conditions, type of engine, fuel composition, and additives used (Obert, 1973; Ullman, 1989), which in turn influence the amount of exposure in different occupations (Groves and Cain, 2000; Pronk et al., 2009). In general, the BD and its blends were found to reduce emissions of nonvolatile fractions (Boehman et al., 2005; McCormick, 2005; Bugarski et al., 2006) and to elevate particle-bound semivolatile organic fractions of BD exhaust particles (McDonald et al., 1995; McCormick, 2005; Purcell et al., 1996).
The speculative nature of reductions of adverse health effects produced by BD exposure is based primarily on the differences in chemical composition of BD and D combustion exhaust (Jung et al., 2006; Tsolakis 2006). The semivolatile derivatives from BD emission products were found to have higher toxicity than solid PM (McCormick, 2007). While several studies demonstrated the potential of neat BD (B100) and its blends to reduce exposure of underground miners to elemental carbon and PM (Bugarski et al., 2006), overall pulmonary toxicity of BD exposure has not been sufficiently addressed. In the present study, changes in oxidative stress, inflammatory biomarkers, and toxicity in the lung and liver of mice were investigated after inhalation exposure to B100 or D100 (50, 150, or 500 μg/m3; 4 h/d, 5 d/wk, for 4 wk).
METHODS
Combustion Emissions (CE) Generation System and Conditions
Single 100-gal lots of pure 100% soy biofuel (B100) and pure 100% diesel fuel (D100) were purchased from Piedmont Biofuels, Pittsboro, NC, and Red Star Oil, Durham, NC, respectively. B100 and D100 CE for inhalation exposure experiments were generated using a 4.8-kW (6.4-hp) direct-injection, single-cylinder, 0.320-L displacement, Yanmar L70 V diesel generator operated at a constant 3600 rpm. Chamber temperatures, relative humidity, and noise were also monitored, and maintained within acceptable ranges (see Supplemental Material, Table S2). Mice were exposed to HEPA-filtered room air or B100/D100 CE emissions diluted to yield 50, 150, or 500 μg/m3 of fuel emission particulate 4 h/d, 5 d/wk, for a total period of 4 wk. Six animals per study group were utilized for all in vivo assays. The animals were weighed and sacrificed with intraperitoneal (ip) injection of sodium pentobarbital and exsanguinated, at 2 h following the last time point of exposure. A detailed description of the engine generation and emission monitoring system, as well as the procedures for generating diluted emissions, is provided in the Supplemental Material.
Animals
Pathogen-free BALB/c female mice, 10–12 wk old, weighing 17–20 g, were purchased from Charles River (Raleigh, NC). Once at the U.S. EPA animal care facilities in Research Triangle Park, NC (accredited by the Association for Assessment and Accreditation of Laboratory Animal Care), animals were housed in groups of 5 in polycarbonate cages with hardwood chip bedding (Beta Chip, Northeastern Products, Warrensburg, NY), provided a 12-h light (6:00) to dark (18:00) cycle, maintained at 22.3 ± 1.1 °C and 50 ± 10% humidity, and given access to both food (5P00 Prolab RMH 3000, PMI Nutrition International, Richmond, IN) and water ad libitum. Animals were acclimated for at least 10 d before the study began. Sentinel animals were housed in the same location and found to be free of common rodent pathogens. All procedures were approved by the U.S. EPA Animal Care and Welfare Committee. The animals were treated humanely and with regard for alleviation of suffering.
Study Design
Previous studies focusing on the mechanisms of the effects of diesel CE exposure were performed using the following concentrations: 500 μg/m3 and 300 μg/m3 in rats (Carll et al., 2013; Carll et al., 2012; Harkema et al., 2009), and 300 μg/m3 in mice (Knuckles et al., 2011). In particular, Finch et al. (2002) exposed F344 rats to biodiesel at concentrations of 40, 200, and 500 μg/m3. These exposure concentrations also reflect concentrations in coal mines and other occupations associated with use of diesel equipment (U.S. EPA, 2002). Thus, in order to elicit a measureable response, concentrations of 50, 150, and 500 μg/m3 were chosen for performing inhalation exposures described in this study. Further, these relatively high concentrations are necessary in order to reveal significant biological effects. The concentrations over the short 4-wk exposure, as investigated in this study, are further justified because humans may be exposed chronically for longer times.
BALB/cJ mice were selected as these rodents are one of the two mouse strains most commonly used as lab animal models, for performing B100 and D100 CE inhalation exposure studies. This was because of the following two reasons: (1) Several studies reported increased acute respiratory symptoms, chronic bronchitis, and aggravation of asthma upon exposure to PM (Pronk et al., 2009; Rudell et al., 1996), and (2) of the six mouse strains tested, BALB/cJ mice were reported to be the best inbred strain that closely mimics the phenotype of human occupational asthma (De Vooght et al., 2010).
Preparation of Lung/Liver Homogenates
The whole mouse lungs/liver were separated from other tissues and weighed before being homogenized with a tissue shredder (model 985-370, Biospec Products Inc., Racine, WI) in phosphate-buffered saline (PBS; pH 7.4) for 2 min. The homogenate suspension was frozen at −80°C until processed.
Total Protein and Lactate Dehydrogenase (LDH) Activity in the Tissue Homogenates
Measurement of total protein in the tissue homogenates was performed by a modified Bradford assay according to the manufacturer’s instructions (BioRad, Hercules, CA), with bovine serum albumin as a standard. The total protein values measured upon B100 and D100 CE exposure in lungs and liver are provided in Supplemental Table S5. The activity of lactate dehydrogenase (LDH) was assayed spectrophotometrically by monitoring the reduction of nicotinamide adenine dinucleotide at 340 nm in the presence of lactate using a Lactate Dehydrogenase Reagent Set (Pointe Scientific, Inc., Lincoln Park, MI).
Myeloperoxidase Levels in the Lung/Liver of Exposed Mice
Inflammatory response in the lung/liver of mice was assessed by measurement of myeloperoxidase (MPO) activity by enzyme-linked immunosorbent assay (ELISA). The concentration of MPO in tissue homogenates was measured using a commercially available ELISA immunoassay kit (Cell Sciences, Canton, MA) with detection limit ranging from 1.02 to 250 ng/ml. Each measurement of MPO activity in tissue homogenates was assayed in at least triplicate and normalized to total protein content in tissue samples.
Evaluation of Biomarkers of Oxidative Stress in the Lung/Liver
Oxidative damage to the lung/liver following exposure was evaluated by the presence of 4-hydroxynonenol (4-HNE) and protein carbonyl formation. 4-HNE, a by-product of lipid peroxidation, was measured in lung homogenates by ELISA using the OxiSelect HNE-His adduct kit (Cell Biolabs, Inc., San Diego, CA). The quantity of oxidatively modified proteins as assessed by measurement of protein carbonyls in lung homogenates was determined using the Biocell PC ELISA kit (Northwest Life Science Specialties). Sensitivity of the assay was <0.1 nmol/mg protein.
Fluorescence Assay for Low-Molecular-Weight Thiols
Low-molecular-weight thiol concentration in lung/liver homogenates was determined using ThioGloTM-1, a maleimide reagent, which produces highly fluorescent adducts upon its reaction with –SH groups. Low-molecular-weight thiols were estimated by an immediate fluorescence response registered upon addition of ThioGloTM-1 to the lung homogenate. A CytoFluor multiwell plate reader Series 4000 (Applied BioSystems, Foster City, CA) was employed for the assay of fluorescence using excitation at 360/40 nm and emission at 530/25 nm with a gain of 50. Data obtained were exported and analyzed using CytoFluor Software (Applied BioSystems, Foster City, CA).
Cytokine Analysis of Tissue Homogenates
Levels of cytokines were assayed in the supernatants of tissue homogenates. The concentrations of tumor necrosis factor (TNF)-α, (MCP)-1, interleukin (IL)-12p70, IL-6, IL-10, and interferon (IFN)-γ (sensitivity of assay is 5–7.3 pg/ml) were determined using the BDTM Cytometric Bead Array, Mouse Inflammation kit (BD Biosciences, San Diego, CA).
Statistical Analysis
Statistical analysis was performed using SigmaPlot 11.0 (San Jose, CA). Statistical significance of the observed outcomes, that is, treatment-related differences within each exposed group and their respective controls, was analyzed by Student’s t-test with Welch’s correction for unequal variances or one-way analysis of variance (ANOVA). One-way ANOVA with Bonferroni post hoc test was used for performing pairwise multiple comparisons between the different doses investigated within each group; p values of <.05 were considered to be statistically significant.
RESULTS
Biodiesel and Diesel CE Analysis and Chamber Concentrations
Particle and various gas levels including humidity and temperature were closely monitored and regulated to ensure a constant exposure. Supplemental Material Table S2 presents a summary of the average concentrations and particle size data for the high (500 μg/m3) concentration exposures of B100 and D100. The concentrations of carbon monoxide (CO), nitric oxide (NO), and nitrogen dioxide (NO2) concentrations were all maintained below thresholds of concern. In addition, the sulfur dioxide (SO2) concentrations were below detection levels for all exposures. Time-integrated particle concentrations were determined by gravimetric analysis. Chamber particle concentrations determined independently by this method using integrated filter samples (one 4-h sample per exposure day) agreed with the tapered element oscillating microbalance (TEOM) measurements. Particle numbers were relatively high, and corresponded to particle size distributions (PSD) with well-established accumulation modes. Measurements for B100 and D100 median number and surface (assuming spherical particles) diameters in the inhalation chambers were 66 and 92 nm, and 77 and 134 nm, respectively. The final particle mass of the 500 μg/m3 target concentration for B100 was 521 ± 10 μg/m3 and for D100 was 490 ± 6 μg/m3. Assuming spherical particles with a density of 1.2 g/cm3, a mass median aerodynamic diameter of 113 nm and 168 nm for B100 and D100 was estimated based on scanning mobility particle sizer (SMPS) data.
Tissue Damage and Inflammatory Response
As a first step, the degree of cytotoxicity/tissue damage and inflammation was evaluated by assessing the LDH and MPO activity in the lungs and liver homogenates from BALB/cJ mice after 4 wk of inhalation exposures to B100 or D100 CE (Figure 1). A significant increase compared to control in the levels of LDH was observed upon exposure to both B100 and D100 CE. A dose-related rise of approximately 78 and 18% was observed in the lungs and liver of mice exposed to 500 μg/m3 of B100 CE (Figures 1A and 1B, black bars). Similar to B100, LDH levels in the D100 animals were elevated compared to control. A total increase of up to approximately 37 and 21% in LDH response occurred in the lungs and liver of mice exposed to 500 μg/m3 of D100 CE (Figures 1A and 1B, gray bars). While both D100 and B100 induced release of LDH in both lungs and liver, the extent of damage was greater in lung compared to liver.
FIGURE 1.
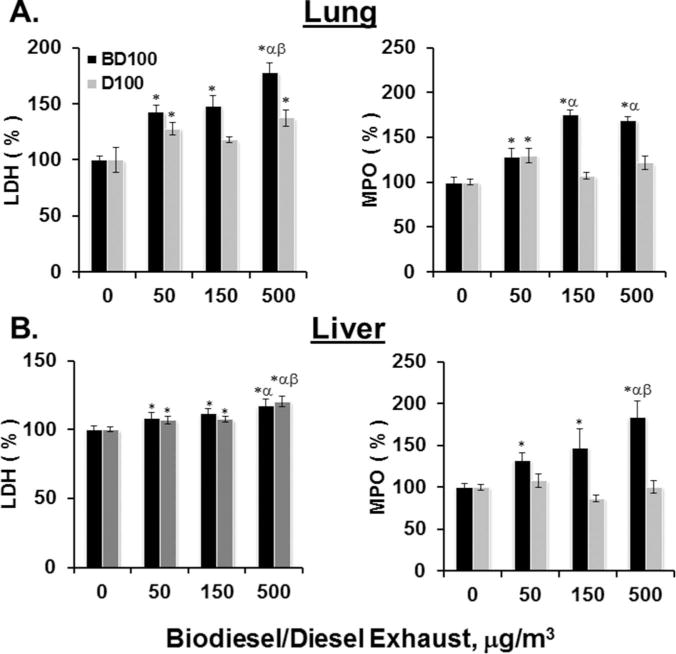
Tissue damage and inflammation as evaluated by changes in the activity of LDH and MPO. The changes in the activity of (i) LDH and (ii) MPO were assessed in the (A) lung tissue and (B) liver tissue of BALB/cJ mice in response to inhalation exposure of biodiesel (BD100, black bars) and diesel combustion exhaust particles (D100, gray bars) for a total of 4 wk at a rate of 4 h/d and 5 d/wk. Data are represented as percent compared to control in all cases. Average control values upon BD100 exposure for LDH and MPO in the lung were 47.5 ± 1.7 and 101.5 ± 5.3 U/mg protein, respectively and in the liver were 201.6 ± 5.1 and 22.1 ± 0.9 U/mg protein, respectively. Upon D100 exposure, average control values in the lungs and liver for LDH were 47. 2 ± 5.4 and 145.2 ± 2.8 U/mg protein, respectively and for MPO were 102.2 ± 2.9 and 23.8 ± 1.8 U/mg protein, respectively. Asterisk indicates significant at p < .05 vs. control (air) exposed mice. Means ± SD (n = 6 mice per group); α indicates significant at p < .05 vs. 50 μg/m3 exposed mice; β indicates significant at p < .05 vs. 150 μg/m3 exposed mice; β indicates significant at p < .05 vs. 500 μg/m3 exposed mice.
Dose-related MPO elevations of 29, 75, and 70% in lung and 32, 48, and 84% in liver were observed after inhalation exposures to 50, 150, and 500 μg/m3 of B100, respectively (Figure 1). Changes induced by D100 CE were less compared to B100 (Figure 1, MPO—black vs. gray bars). Only a rise of approximately 30% in lung and 8% in liver was observed in the MPO levels at the lower concentration of D100 CE (50 μg/m3), while at higher concentrations the levels were either not altered or decreased compared to controls (Figures 1A and 1B, gray bars). These results indicate that inhalation exposure of mice to B100 elicited significantly higher inflammatory responses than did D100 in both lung and liver.
Oxidative Stress Upon Exposure to B100 and D100 Combustion Exhaust Particles
Oxidative damage assessed by levels of 4-hydroxynonenol (4-HNE) and oxidatively modified proteins (protein carbonyls) in the lung and liver tissue of mice exposed to B100 and D100 (0, 50, 150, and 500 μg/m3) are presented in Figure 2. A dose-related tendency in the accumulation of 4-HNE and protein carbonyls and reductions in low-molecular-weight thiols (glutathione [GSH]) and oxidized protein thiols were detected upon exposure to B100 CE (Figure 2A, black bars). In all circumstances the magnitude of effect or evidence of a dose-related change was less with D100 than B100.
FIGURE 2.
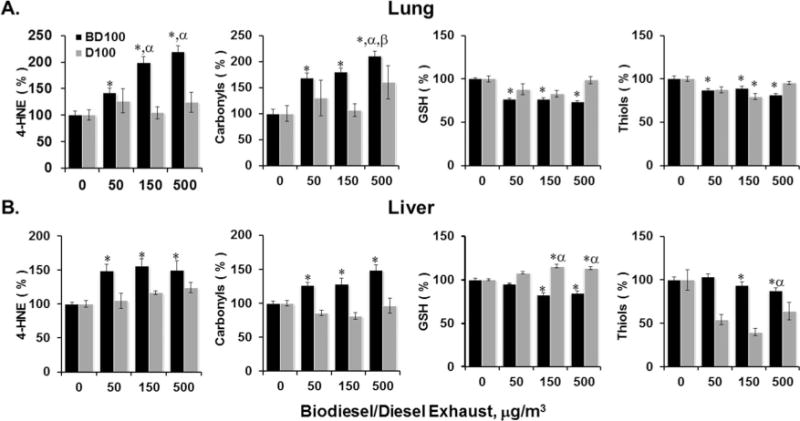
Biomarkers of oxidative stress in the lung and liver tissue of BALB/cJ mice following inhalation exposure of biodiesel and diesel combustion exhaust particles. The levels of 4-hydroxynonenal, protein carbonyls, glutathione, and protein thiols assessed in (A) lung and (B) liver without and at various concentrations (50, 150, or 500 μg/m3) of biodiesel/diesel combustion exhaust particles. Mice were exposed to exhaust particles via inhalation to the doses indicated. Black columns correspond to the exposure with BD100 CE; gray columns correspond to exposure with D100 CE. Animals were sacrificed 2 h post exposure. Means ± SE (n = 6 mice per group). Asterisk indicates significant at p < .05 vs. control (air) exposed mice. Means ± SD (n = 6 mice per group); α indicates significant at p < .05 vs. 50 μg/m3 exposed mice; β indicates significant at p < .05 vs. 150 μg/m3 exposed mice; β indicates significant at p < .05 vs. 500 μg/m3 exposed mice.
Similar to the lung, elevated levels of 4-HNE and protein carbonyls and decreased levels of GSH and protein thiols were found in liver following inhalation exposure to B100 CE. Again the increase in 4-HNE was less marked with D100 at 150 μg/m3 concentration, while the protein carbonyls were actually reduced with 50 and 150 μg/m3 concentrations of D100. In contrast to BD exposure, it was found that exposure to the D100 (50, 150, and 500 μg/m3) produced elevation in GSH (liver) but displayed greater reduction in oxidized thiols in liver compared to those observed in BD exposed mice (Figure 2). Overall, results indicate that the magnitude of oxidative damage found both in the lung and liver was relatively greater in animals exposed to B100 CE.
Cytokine Response
The cytokines IL-6, IL-10, IL-12p70, MCP-1, IFNγ, and TNF-α were used as markers of the proinflammatory response in the mouse lung and liver after inhalation exposure to B100 and D100 CE. Data (mean ± SD) for each dose investigated are presented in Table 1.
TABLE 1.
Accumulation of Cytokines in the Lung and Liver of BALB/cJ Mice Following Inhalation of B100 and D100 Combustion Exhaust Particles for 4 wk
| Exposure details | IFNγ | IL-10 | IL-12p70 | IL-6 | MCP-1 | TNF-α |
|---|---|---|---|---|---|---|
| B100 Lung | ||||||
| Control | 100 ± 13.25 | 100 ± 22.1 7 | 100 ± 7.89 | 100 ± 3.1 | 100 ± 9.85 | 100 ± 15.21 |
| 50 μg/m3 | 93.54 ± 6.48 | 130.37 ± 27.25 | 521.64 ± 157.45*,β | 223.39 ± 53.76* | 128.46 ± 9.79* | 102.45 ± 13.62 |
| 150 μg/m3 | 125.84 ± 14.14*α | 154.96 ± 20.73 | 245.08 ± 69.54* | 400.85 ± 114.07*α | 138.99 ± 13.66* | 118.65 ± 14.6 |
| 500 μg/m3 | 115.59 ± 21.94 | 253.47 ± 60.88*,α,β | 684.01 ±271.56*,β | 580.74 ± 77.57*,α,β | 158.28 ± 14.8*α | 168.71 ± 21.1*,α,β |
| B100 Liver | ||||||
| Control | 100 ± 5.98 | 100 ± 18.71 | 100 ± 11.98 | 100 ± 19.63 | 100 ± 6.11 | 100 ± 11.17 |
| 50 μg/m3 | 116.93 ± 4.34*,β,γ | 128.06 ± 12.42 | 143.01 ± 7.25*,γ | 277.68 ± 75.84* | 118.02 ± 6.48* | 161.97 ± 14.32* |
| 150 μg/m3 | 107.41 ± 5.66 | 128.34 ± 18.57 | 130.51 ± 9.02* | 229.36 ± 32.72* | 126.37 ± 11.38*,γ | 152.58 ± 14.55* |
| 500 μg/m3 | 106.35 ± 6.24 | 135.63 ± 33.15 | 119.2 ± 16.37 | 305.81 ± 69.42* | 108.09 ± 8.25 | 137.56 ± 24.55 |
| D100 Lung | ||||||
| Control | 100 ± 5.99 | 100 ± 12.42 | 100 ± 27.54 | 100 ± 6.02 | 100 ± 7.21 | 100 ± 11.44 |
| 50 μg/m3 | 147.41 ± 9.69* | 159.98 ± 16.82* | 129.73 ± 20.03 | 165.25 ± 33.39*,β | 121.12 ± 10.38* | 124.04 ± 12.08 |
| 150 μg/m3 | 174.26 ±45.26* | 136.48 ± 22.27 | 109.59 ± 24.19 | 109.22 ± 10.83 | 98.39 ± 5.81 | 133.29 ± 18.53* |
| 500 μg/m3 | 244.23 ± 79.8*,α,β | 277.89 ± 107.31*,β | 214.7 ± 77.91*,α,β | 211.74 ± 52.21*,β | 121.92 ± 3.97* | 208.37 ± 64.04*α |
| D100 Liver | ||||||
| Control | 100 ± 7.35 | 100 ± 27.34 | 100 ± 7.36 | 100 ± 28.3 | 100 ± 22.26 | 100 ± 9.38 |
| 50 μg/m3 | 104.49 ± 8.9 | 93.86 ± 15.96 | 131.35 ± 20.46*,β | 126.43 ± 27.13 | 76.99 ± 5.63* | 152.27 ± 21.32*,β |
| 150 μg/m3 | 93.2 ± 7.35 | 66.39 ± 6.54* | 89.3 ± 9.84 | 64.31 ± 7.42 | 78.4 ± 5.38* | 112.07 ± 13.58 |
| 500 μg/m3 | 101.55 ± 9.05 | 66.51 ± 9.51* | 123.36 ± 15.47*,β | 109.74 ± 25.53 | 78.4 ± 9.74* | 135.85 ± 15.48* |
Note. The data are presented as mean ± SD (n = 6 mice per group) and correspond to percent versus control (estimated based on pg/mg of total protein) in each case. Average control values (in pg/mg) for IFNγ, IL-10, IL-12p70, IL-6, MCP-1, and TNF-α in the lungs were 0.90 ± 0.12, 1.40 ± 0.28, 1.03 ± 0.14, 0.83 ± 0.03, 1.68 ± 0.15, and 0.75 ± 0.10, respectively, and in the liver were 0.15 ± 0.01, 0.67 ± 0.15, 0.64 ± 0.07, 0.20 ± 0.07, 0.39 ± 0.06, and 0.26 ± 0.03, respectively.
Asterisk indicates significant at p < .05 vs. control (air) exposed mice. Means ± SD (n = 6 mice per group);
indicates significant at p < .05 vs. 50 μg/m3 exposed mice;
indicates significant at p < .05 vs. 150 μg/m3 exposed mice;
indicates significant at p < .05 vs. 500 μg/m3 exposed mice.
Responses in Lungs
The cytokine levels were found to be elevated in the lungs of mice exposed to both B100 and D100 CE. However, the extent of rise differed between each group and between different cytokines within the group. The inhalation exposure of B100 induced a dose-dependent increase in IL-6 and IL-12p70 (with the exception of 150 μg/m3), and a rise in the accumulation of MCP-1 and TNF-α in the lungs. Of all the cytokines probed, IL-6, IL-10, and IL-12p70 were the top three cytokines that showed significant elevation compared to control (Table 1, B100 Lung). The changes observed at the highest level of exposure to B100 (500 μg/m3) were as follows: IL-12p70 > IL-6 > IL-10 > TNF-α> MCP-1 > IFNγ. While IFNγ in the lung of mice exposed to B100 was found to be least perturbed, inhalation exposure to D100 produced a dose-trend increase in pulmonary IFNγ level compared to controls. The changes noted at the highest concentration of D100 CE exposure in the cytokine levels were as follows: IL-10 > IFNγ> IL-12p70 > IL-6 > TNF-α> MCP-1. A 2.5-fold increase in the level of IL-10 compared to control was found in lungs of mice exposed to either B100 or D100 CE at a dosage of 500 μg/m3 (Table 1). While IL-6 and IL-12p70 were approximately 2.7- and 3.2-fold higher upon exposure to B100 as compared to D100 CE, the changes in both MCP-1 and TNF-α levels were similar. Lastly, measured level of pulmonary IFNγ was significantly elevated only in lungs of mice exposed to D100 CE but not in pulmonary tissue of mice exposed to B100 (Table 1, B100 and D100 Lung data).
Responses in Liver
Similarly to the effects noted in lungs, exposure to B100 also induced a greater release of cytokines in liver. In particular, the release of IL-6 was significantly increased compared to control. The inhalation exposure to D100 exhaust resulted in a consistent decrease in MCP-1 and IL-10 (with the exception of 50 μg/m3) levels in liver (Table 1, D100 Liver). A significant elevation was found in the levels of IL-12p70, IFNγ, and TNF-α only at the lowest D100 inhalation exposure level (50 μg/m3).
To summarize, IL-6 and IL-12p70 significantly increased in both lung and liver upon exposure to B100 CE. The TNF-α levels especially in liver were also found to be elevated to similar extent. The IFNγ levels both in the liver and lung were not markedly changed upon B100 exposure. In contrast, the IFNγ levels in lungs of mice exposed to D100 CE showed a significant rise (Table 1). While IL-12p70 and TNF-α displayed similar effects both in lung and liver upon D100 exposure, IL-10 and MCP-1 exhibited opposite trends; that is, a rise in the levels of IL-10 and MCP-1 in lungs and a fall in liver was observed. Finally, no significant change in IFNγ levels was found in liver of mice exposed to D100 CE. Overall, the cytokine levels upon B100 and D100 CE exposures were more prominent in lungs as compared to liver.
DISCUSSION
Several studies showed that combustion products of BD produce less emissions of CO, PM, and PAH than D (McCormick et al., 2001; Rakopoulos et al., 2008; Ramadhas et al., 2004). To date, research on BD has mostly focused on the analysis of CE emission products of fuels derived from various production processes and manufacturing sources (Di et al., 2009; Tiyapongpattana et al., 2008). BD derived from soy-bean vegetable oil is largely composed of fatty acid derivatives (e.g., methyl linoleate; see Supplemental Material, Table S1), and its combustion products may include high levels of unsaturated aldehydes compared to neat D. The unsaturated sites, serving as major targets of lipid peroxidation, may result in formation of both oxidized and unsaturated aldehydes (Fullana et al., 2004; Da Silva and Pereira, 2008; Seaman et al., 2009). Such organic derivatives were found to exert higher toxicity than the solid PM fractions (McCormick, 2007). Recent studies reported that exposure to neat BD CE was more toxic than D, producing cardiovascular alterations as well as systemic and pulmonary inflammation in mice (Brito et al., 2010; Yanamala et al., 2013). Despite BD potential as a renewable and an economically viable fuel source, it is important to address whether and how inhalation exposure to BD CE might affect human health.
Previous studies on toxicity of BD emissions, other than mutagenicity studies, were performed either in rats over extended time periods (Finch et al., 2002) or in mice involving exposure to 550 μg/m3 of BD exhaust for only 1 h (Brito et al., 2010). In this study, oxidative stress and inflammatory responses were examined in lung and liver of mice upon inhalation exposure to 50, 150 or 500 μg/m3 of neat BD (B100) and D (D100) CE, 4 h/d, 5 d/wk, for a total period of 4 wk. Both B100 and D100 CE induced tissue damage, inflammation, and oxidative stress in mice after 4 wk of inhalation exposure, albeit to different extent. The MPO and LDH activity upon B100 exposure was significantly higher compared to D100, indicating greater tissue damage and inflammatory responses in mice exposed to B100 CE. Further, marked changes in the accumulation levels of oxidative stress markers 4-HNE, GSH, protein thiols, and carbonyls were found upon B100 CE exposures as compared to D100. This is in agreement with previous studies showing increased levels of organic and unsaturated aldehyde compounds in B100 combustion products (Graboski et al., 2003; McDonald et al., 1995; Purcell et al., 1996) and the potential of aldehydes to readily attack nucleophilic centers in protein and DNA to form carbonyl-retaining adducts (Uchida et al., 1998a, 1998b; Burcham and Fontaine, 2001). It is possible that the oxidative stress pathways elicited by B100 might be influenced by several factors, including but not limited to direct interaction of unsaturated aldehyde organic compounds released upon combustion, such as acrolein, 4-HNE, and others, reactive electrophiles, modified lipids/proteins, DNA adducts, and depletion/inactivation of antioxidants.
Similarly to biomarkers of oxidative stress, inflammation, and tissue damage found in mice after inhalation exposures, B100 and D100 differentially affected cytokine responses in lung and liver. While the response in the anti-inflammatory cytokine IL-10 was almost similar between D100 and B100, the accumulation of proinflammatory cytokines, in particular IL-12p70, IFNγ, and the chemokine MCP-1, differed between BD and D exposures. B100 exposure resulted in a dose-trend rise in MCP-1 (monocyte chemotactic protein or CCL2) of up to 60%, compared to only 22% in lungs upon D100 exposure. The elevated levels of MCP-1 upon B100 exposure, suggesting an increase in the monocyte/macrophage recruitment to the lung (Figure 3A), are further paralleled by enhanced MPO activity in lung homogenates compared to D100 (Figure 1A). An infiltration of macrophages in pulmonary tissue during a subchronic exposure of soybean BD CE was also observed previously (Finch et al., 2002; Brito et al., 2010). Further, exposure to organic fractions of diesel exhaust particles was found to elicit a proinflammatory response in human airway epithelial (Bonvallot et al., 2001) and other cells (Fahy et al., 1999). Data also demonstrated that compared to D100, B100 CE exposure induced increased levels of IL-12p70, a critical cytokine that promotes the differentiation of T cells into Th1 cells. This further induces the release of IFNγ, triggering cell-mediated immunity. Despite the elevated levels of IL-12p70 release upon B100 exposure, no change in IFNγ levels was noted in this study (Table 1 and Figure 3B). While it is difficult to pinpoint the mechanism that is responsible for the observed levels of IFNγ production upon B100 CE exposure as compared to D100 (Figure 2), it is evident that increased levels of oxidative stress, MPO activity, and MCP-1 and lower levels of GSH compared to D100 CE, all favoring the Th2 type of response, may be the contributing factors that lead to inhibition of IFNγ release upon B100 CE exposure (Figures 1 and 2, and Table 1). As IFNγ/IFNγR interaction is a prerequisite for macrophage activation via the classical pathway (Mosser, 2003; Gordon, 2003), the unaltered/slightly reduced levels of IFNγ found in the present study upon B100 exposure point toward involvement of nonclassical pathways of macrophage activation, such as signaling via toll-like receptors (TLR) and other receptors (Figure 3A). Some of these putative signaling pathways that might be involved in B100 and D100 CE-induced macrophage activation are presented in Figure 3A. A relative balance between Th1 and Th2 responses is crucial for maintaining optimal health. While D100 exposure resulted in inducing both Th1 (e.g., IFNγ,TNF-α) and Th2 (e.g., IL-6, IL-10) types of immune responses to a similar extent, B100 CE favored the Th2 type of response (Figure 3B). This is further corroborated by studies where elevated levels of oxidative stress, as seen upon B100 exposure, were associated with the release of IL-4/IL-10 cytokines (Ma et al., 2002), which suppress Th1 response (IFNγ, TNF-α) and trigger Th2 responses (IL-6, IL-10). Thus, the increased post combustion organic component of BD (Graboski et al., 2003), as well as the elevated oxidative stress responses upon B100 CE exposure, may serve as the basis for these differential immune effects exhibited by BD and D (Figure 3). However, more detailed studies are needed to support this hypothesis.
FIGURE 3.
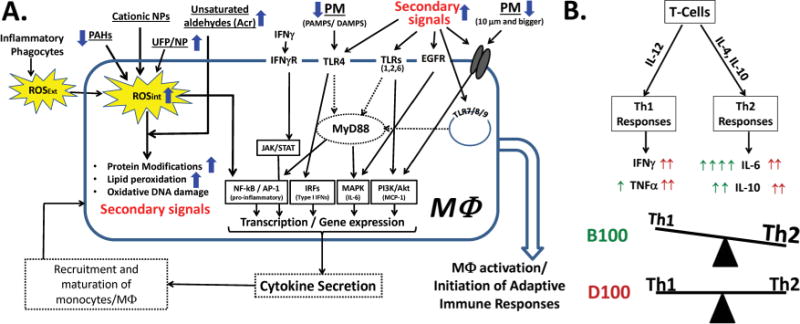
Differential immune responses triggered by B100 and D100 CEs in the lungs. Schematic representation of (A) putative signaling mechanisms involved in macrophage (MΦ) activation, and (B) Th1/Th2 responses induced by B100 and D100 CE. The blue arrows in (A) correspond to the relative changes observed in B100 CE compared to D100. In (B) the red and green arrows indicate the levels of each cytokine upon B100 and D100 CE exposures in the lungs, respectively. The accumulation of ROS generated internally (ROSint) due to the direct interaction of soluble organic emissions (like aldehydes, UFP, etc.) with biomolecules, acting as secondary signals, might play a dominant role in triggering differential immune responses upon BD exposure compared to D. The simultaneous signaling cascade driven by these secondary signals preferentially through nonclassical pathways upon BD exposure may promote MФ maturation leading to the production of IL-12. The increased organic component as well as the secondary intermediates/signals formed due to increased oxidative stress upon BD exposure may shift the immune response toward Th2 in comparison to D as shown in B (color figure available online).
Compared to lung, the overall responses in the liver were less pronounced upon B100 and D100 CE exposures. While the accumulation of IL-6 was significantly increased, cytokines MCP-1, IL-10, IL-12p70, and IFNγ response were only numerically elevated compared to control after B100 exposure (Table 1, B100 Liver). Interestingly, upon D100 exposure the levels of MCP-1 and IL-10 were found to be decreased compared to their responses in lung. The fall in IL-10 release corresponded to a rise in GSH levels observed in the liver after D100 exposure. This is further supported by the fact that excessive oxidative stress leads to depletion of GSH and/or induction of heme-oxygenase-1 (HO-1), which in turn may regulate the production of IL-10 in alveolar macrophages (Miyata and van Eeden 2011). Thus, an increase in antioxidant enzymes (e.g., HO-1) and its related pathways might lead to reduced levels of IL-10 as seen in the case of liver upon D100 exposure (Figure 2, Liver).
CONCLUSIONS
To summarize, the studies performed here suggest that B100 CE, despite its decreased levels of PM and PAH emissions, might on an equal mass basis produce adverse effects compared to petroleum D exposure. Based on the studies presented here, it is postulated that increased organic matter including unsaturated aldehyde components from B100 combustion may result in enhanced oxidative stress in lungs and liver. Thus, evidence suggests that the increased oxidative stress found after exposure to BD was due to either direct interactions of the latter organic compounds with critical biomolecules in lung and liver or to increasing the levels of intracellular reactive oxygen species (ROS) formed via depletion of essential antioxidants. Further studies focusing on deciphering the details of BD emissions and chemical characterization of the different effluents upon BD combustion are important not only for toxicity assessments, but also for hazard identification and safety assessment of BD and BD blends.
Supplementary Material
Acknowledgments
The authors are grateful to Bill Linak (U.S. EPA) for assistance in inhalation engineering and to Mary Daniels and Liz Boykin (U.S. EPA) for laboratory work. They also thank Dr. Vince Castranova and Dr. Teh-hsun B. Chen (CDC/NIOSH/HELD) and Dr. Mark Higuchi (U.S. EPA) for their discussion, comments, and feedback. This work was supported by NIOSH, 2927ZKCY.
This article has been reviewed by the U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health or U.S. EPA policy. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
This article is not subject to U.S. copyright.
References
- Benbrahim-Tallaa L, Baan RA, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Guha N, Loomis D, Straif K, International Agency for Research on Cancer Monograph Working Group Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012;13:663–664. doi: 10.1016/s1470-2045(12)70280-2. [DOI] [PubMed] [Google Scholar]
- Boehman AL, Song J, Alam M. Impact of biodiesel blending on diesel soot and the regeneration of particulate filters. Energy Fuels. 2005;19:1857–1864. [Google Scholar]
- Bonvallot V, Baeza-Squiban A, Baulig A, Brulant S, Boland S, Muzeau F, Barouki R, Marano F. Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression. Am J Respir Cell Mol Biol. 2001;25:515–521. doi: 10.1165/ajrcmb.25.4.4515. [DOI] [PubMed] [Google Scholar]
- Brito JM, Belotti L, Toledo AC, Antonangelo L, Silva FS, Alvim DS, Andre PA, Saldiva PH, Rivero DH. Acute cardiovascular and inflammatory toxicity induced by inhalation of diesel and biodiesel exhaust particles. Toxicol Sci. 2010;116:67–78. doi: 10.1093/toxsci/kfq107. [DOI] [PubMed] [Google Scholar]
- Bugarski AD, Schnakenberg GH, Mischler SE, Noll JD, Patts LD, Hummer JA. Effectiveness of selected diesel particulate matter control technologies for underground mining applications: Isolated zone study, 2004. Washington, DC: U.S. Department of Health and Human Services; 2006. (DHHS (NIOSH) pub. no. 2006-138, Report of investigations 9668). [Google Scholar]
- Burcham PC, Fontaine F. Extensive protein carbonylation precedes acrolein-mediated cell death in mouse hepatocytes. J Biochem Mol Toxicol. 2001;15:309–316. doi: 10.1002/jbt.10007. [DOI] [PubMed] [Google Scholar]
- Carll AP, Hazari MS, Perez CM, Krantz QT, King CJ, Winsett DW, Costa DL, Farraj AK. Whole and particle-free diesel exhausts differentially affect cardiac electrophysiology, blood pressure, and autonomic balance in heart failure-prone rats. Toxicol Sci. 2012;128:490–499. doi: 10.1093/toxsci/kfs162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carll AP, Lust RM, Hazari MS, Perez CM, Krantz QT, King CJ, Winsett DW, Cascio WE, Costa DL, Farraj AK. Diesel exhaust inhalation increases cardiac output, bradyarrhythmias, and parasympathetic tone in aged heart failure-prone rats. Toxicol Sci. 2013;131:583–595. doi: 10.1093/toxsci/kfs295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva TO, Pereira PAD. Influence of time, surface-to-volume ratio, and heating process (continuous or intermittent) on the emission rates of selected carbonyl compounds during thermal oxidation of palm and soybean oils. J Agric Food Chem. 2008;56:3129–3135. doi: 10.1021/jf0734525. [DOI] [PubMed] [Google Scholar]
- De Vooght V, Vanoirbeek JA, Luyts K, Haenen S, Nemery B, Hoet PH. Choice of mouse strain influences the outcome in a mouse model of chemical-induced asthma. PloS One. 2010;5:e12581. doi: 10.1371/journal.pone.0012581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Y, Cheung CS, Huang Z. Experimental investigation on regulated and unregulated emissions of a diesel engine fueled with ultra-low sulfur diesel fuel blended with biodiesel from waste cooking oil. Sci Total Environ. 2009;407:835–846. doi: 10.1016/j.scitotenv.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Fahy O, Tsicopoulos A, Hammad H, Pestel J, Tonnel AB, Wallaert B. Effects of diesel organic extracts on chemokine production by peripheral blood mononuclear cells. J Allergy Clin Immunol. 1999;103:1115–1124. doi: 10.1016/s0091-6749(99)70187-9. [DOI] [PubMed] [Google Scholar]
- Finch GL, Hobbs CH, Blair LF, Barr EB, Hahn FF, Jaramillo RJ, Kubatko JE, March TH, White RK, Krone JR, Menache MG, Nikula KJ, Mauderly JL, Van Gerpen J, Merceica MD, Zielinska B, Stankowski L, Burling K, Howell S. Effects of subchronic inhalation exposure of rats to emissions from a diesel engine burning soybean oil-derived biodiesel fuel. Inhal Toxicol. 2002;14:1017–1048. doi: 10.1080/08958370290084764. [DOI] [PubMed] [Google Scholar]
- Fullana A, Carbonell-Barrachina AA, Sidhu S. Comparison of volatile aldehydes present in the cooking fumes of extra virgin olive, olive, and canola oils. J Agric Food Chem. 2004;52:5207–5214. doi: 10.1021/jf035241f. [DOI] [PubMed] [Google Scholar]
- Garshick E, Laden F, Hart JE, Rosner B, Smith TJ, Dockery DW, Speizer FE. Lung cancer in railroad workers exposed to diesel exhaust. Environ Health Perspect. 2004;112:1539–1543. doi: 10.1289/ehp.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Graboski MS, McCormick RL, Alleman TL, Herring AM. (Final report to NREL/SR-510-31461).The effect of biodiesel composition on engine emissions from a DDC Series 60 diesel engine. 2003 http://www.biodiesel.org/reports/20030201_gen-361.pdf.
- Groves J, Cain JR. A survey of exposure to diesel engine exhaust emissions in the workplace. Ann Occup Hyg. 2000;44:435–447. [PubMed] [Google Scholar]
- Harkema JR, Wagner JG, Kaminski NE, Morishita M, Keeler GJ, McDonald JD, Barrett EG, HEI Health Review Committee Effects of concentrated ambient particles and diesel engine exhaust on allergic airway disease in Brown Norway rats. Res Rep Health Effects Inst. 2009;145:5–55. [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011;119:951–957. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Kittelson DB, Zachariah MR. Characteristics of SME biodiesel-fueled diesel particle emissions and the kinetics of oxidation. Environ Sci Technol. 2006;40:4949–4955. doi: 10.1021/es0515452. [DOI] [PubMed] [Google Scholar]
- Knuckles TL, Buntz JG, Paffett M, Channell M, Harmon M, Cherng T, Lucas SN, McDonald JD, Kanagy NL, Campen MJ. Formation of vascular S-nitrosothiols and plasma nitrates/nitrites following inhalation of diesel emissions. J Toxicol Environ Health A. 2011;74:828–837. doi: 10.1080/15287394.2011.570225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGier AJ, Manzo ND, Dye JA. Diesel exhaust particles induce aberrant alveolar epithelial directed cell movement by disruption of polarity mechanisms. J Toxicol Environ Health A. 2013;76:71–85. doi: 10.1080/15287394.2013.738169. [DOI] [PubMed] [Google Scholar]
- Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat Res. 2007;636:95–133. doi: 10.1016/j.mrrev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Ma JY, Ma JK. The dual effect of the particulate and organic components of diesel exhaust particles on the alteration of pulmonary immune/inflammatory responses and metabolic enzymes. J Environ Sci Health C Environ Carcinogen Ecotoxicol Rev. 2002;20:117–147. doi: 10.1081/GNC-120016202. [DOI] [PubMed] [Google Scholar]
- McCormick RL. Effects of biodiesel on NOX emissions. Presented at Air Resources Board Biodiesel Workshop; Golden, CO: National Renewable Energy Laboratory; 2005. [Google Scholar]
- McCormick RL. The impact of biodiesel on pollutant emissions and public health. Inhal Toxicol. 2007;19:1033–1039. doi: 10.1080/08958370701533509. [DOI] [PubMed] [Google Scholar]
- McCormick RL, Graboski MS, Alleman TL, Herring AM, Tyson KS. Impact of biodiesel source material and chemical structure on emissions of criteria pollutants from a heavy-duty engine. Environ Sci Technol. 2001;35:1742–1747. doi: 10.1021/es001636t. [DOI] [PubMed] [Google Scholar]
- McDonald JF, Purcell DL, McClure BT, Kittelson DB. (SAE Tech Paper no. 950400).Emissions characteristics of soy methyl ester fuels in an IDI compressions ignition engine. 1995 http://papers.sae.org/950400.
- Mills NL, Lucking AJ, Beveridge J, Flint L, Paterson F, Boon NA, Fokkens P, Blomberg A, Sandstrom T, Donaldson K, Cassee FR, Newby DE. Inhalation of particulate air pollution impairs vascular function in man. J Am Coll Cardiol. 2007a;49:380a. [Google Scholar]
- Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007b;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukocyte Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Al-Maskari S, Ali BH, Al-Amri IS. Cardiovascular and lung inflammatory effects induced by systemically administered diesel exhaust particles in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L664–L670. doi: 10.1152/ajplung.00240.2006. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Dhanasekaran S, Yasin J, Ba-Omar H, Fahim MA, Kazzam EE, Ali BH. Evaluation of the direct systemic and cardiopulmonary effects of diesel particles in spontaneously hypertensive rats. Toxicology. 2009;262:50–56. doi: 10.1016/j.tox.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Obert EF. Internal combustion engines and air pollution. 3rd. New York, NY: Harper and Row; 1973. [Google Scholar]
- Peretz A, Kaufman JD, Trenga CA, Allen J, Carlsten C, Aulet MR, Adar SD, Sullivan JH. Effects of diesel exhaust inhalation on heart rate variability in human volunteers. Environ Res. 2008;107:178–184. doi: 10.1016/j.envres.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk A, Coble J, Stewart PA. Occupational exposure to diesel engine exhaust: A literature review. J Expos Sci Environ Epidemiol. 2009;19:443–457. doi: 10.1038/jes.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell DL, McClure BT, McDonald J, Basu HN. Transient testing of soy methyl ester fuels in an indirect injection, compression ignition engine. J Am Oil Chem Soc. 1996;73:381–388. [Google Scholar]
- Rakopoulos CD, Rakopoulos DC, Hountalas DT, Giakoumis EG, Andritsakis EC. Performance and emissions of bus engine using blends of diesel fuel with bio-diesel of sunflower or cottonseed oils derived from Greek feedstock. Fuel. 2008;87:147–157. [Google Scholar]
- Ramadhas AS, Jayaraj S, Muraleedharan C. Use of vegetable oils as IC engine fuels—A review. Renew Energy. 2004;29:727–742. [Google Scholar]
- Rudell B, Ledin MC, Hammarstrom U, Stjernberg N, Lundback B, Sandstrom T. Effects on symptoms and lung function in humans experimentally exposed to diesel exhaust. Occup Environ Med. 1996;53:658–662. doi: 10.1136/oem.53.10.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer K, Mundandhara S, Ghio AJ, Madden MC. The effects of ambient particulate matter on human alveolar macrophage oxidative and inflammatory responses. J Toxicol Environ Health A. 2010;73:41–57. doi: 10.1080/15287390903248901. [DOI] [PubMed] [Google Scholar]
- Seaman VY, Bennett DH, Cahill TM. Indoor acrolein emission and decay rates resulting from domestic cooking events. Atmos Environ. 2009;43:6199–6204. [Google Scholar]
- Tiyapongpattana W, Wilairat P, Marriott PJ. Characterization of biodiesel and biodiesel blends using comprehensive two-dimensional gas chromatography. J Separation Sci. 2008;31:2640–2649. doi: 10.1002/jssc.200800234. [DOI] [PubMed] [Google Scholar]
- Tokiwa H, Ohnishi Y. Mutagenicity and carcinogenicity of nitroarenes and their sources in the environment. Crit Rev Toxicol. 1986;17:23–60. doi: 10.3109/10408448609037070. [DOI] [PubMed] [Google Scholar]
- Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T, Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- Tsolakis A. Effects on particle size distribution from the diesel engine operating on RME-biodiesel with EGR. Energy Fuels. 2006;20:1418–1424. [Google Scholar]
- Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998a;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: Potential markers for oxidative stress. Proc Natl Acad Sci USA. 1998b;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman TL. Investigation of the effects of fuel composition on heavy duty diesel engine emissions. Society of Automotive Engineers; 1989. (SAE Technical Paper no. 892072). http://papers.sae.org/892072. [Google Scholar]
- U.S. Environmental Protection Agency. A comprehensive analysis of biodiesel impacts on exhaust emissions. Washington, DC: U.S. Environmental Protection Agency; 2002. Oct, [Google Scholar]
- Williams A, McCormick RL, Hayes R, Ireland J. Biodiesel effects on diesel particle filter performance. National Renewable Energy Laboratory; 2006. (Milestone Report NREL/TP-540-39606). http://www.nrel.gov/docs/fy06osti/39606.pdf. [Google Scholar]
- Yanamala N, Hatfield MK, Farcas MT, Schwegler-Berry D, Hummer JA, Shurin MR, Birch ME, Gutkin DW, Kisin E, Kagan VE, Bugarski AD, Shvedova AA. Biodiesel versus diesel exposure: Enhanced pulmonary inflammation, oxidative stress, and differential morphological changes in the mouse lung. Toxicol Appl Pharmacol. 2013 doi: 10.1016/j.taap.2013.07.006. Available online 22 July 2013, ISSN 0041-008X, http://dx.doi.org/10.1016/j.taap.2013.07.006. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


