Abstract
Converging evidence points to a neural network that supports a range of abilities including remembering the past, thinking about the future, and introspecting about oneself and others. Neuroimaging studies find hippocampal activation during event construction tasks, and patients with hippocampal amnesia are impaired in their ability to (re)construct events of the past and the future. Neuroimaging studies of constructed experiences similarly implicate the medial prefrontal cortex (mPFC), but it remains unknown whether the mPFC is critical for such processes. The current study compares performance of five patients with bilateral mPFC damage, six patients with bilateral hippocampal damage, and demographically matched comparison participants on an event construction task. Participants were given a neutral cue word and asked to (re)construct events across four time conditions: real past, imagined past, imagined present, and future. These event narratives were analyzed for the number of internal and external details to quantify the extent of episodic (re)experiencing. Given the literature on the involvement of the mPFC in self-referential processing, we also analyzed the event narratives for self-references. The patients with mPFC damage did not differ from healthy comparison participants in their ability to construct highly detailed episodic events across time periods but displayed disruptions in their incorporation of the self. Patients with hippocampal damage showed the opposite pattern; they were impaired in their ability to construct highly detailed episodic events across time periods but not in their incorporation of the self. The results suggest differential contributions of hippocampus and medial prefrontal cortex to the distributed neural network for various forms of self-projection.
Keywords: mPFC, hippocampus, memory, self-projection, self-referential processing
1. Introduction
A core neural network has been proposed to underlie a range of abilities including remembering the past, thinking about the future, introspecting about oneself and others, and other complex cognitive and social behaviors (Buckner & Carroll, 2007; Schacter & Addis, 2007a). The neural substrates contributing to the proposed network include the medial temporal lobes, medial prefrontal cortex, lateral temporal cortices and lateral posterior parietal cortices (Addis, Wong, & Schacter, 2007; Buckner & Carroll, 2007; Spreng, Mar, & Kim, 2009). While the range of abilities supported by the network may seem disparate at first blush (e.g., imagining multiple futures, reconstructing the events of our past, considering the thoughts and feelings of ourselves and others) each contributes to the flexibility of human cognition and behavior and requires the flexible and creative (re)construction and use of mental representations for adaptive function (Buckner & Carroll, 2007). The collection of abilities supported by this network has been given various umbrella terms such as ‘self-projection’, ‘simulation’, and ‘prospection’ in an attempt to capture and describe the set of shared behavioral phenomena. Less is known, however, about the individual mechanisms and the unique contributions of each neural system to this complex set of abilities.
Given the role of hippocampus in representational flexibility (Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001) and in flexible cognition more broadly (Rubin, Watson, Duff, & Cohen, 2014) the hippocampus and medial temporal lobes have figured prominently in the work on the network for self-projection (we will use this umbrella term for the range of cognitive abilities from here on). Indeed, converging evidence reveals that in addition to the well established role of the hippocampus in recovering and reconstructing the past (Scoville & Milner, 1957; Cohen & Eichenbaum, 1993; Squire, 1992), the hippocampus also plays a critical role in constructing or imagining the future (e.g., Addis, Wong, & Schacter, 2007; Gaesser, Spreng, McLelland, Addis, & Schacter, 2013; Hassabis, Kumaran, Vann & Maguire, 2007; Okuda, Fujii, Ohtake, Tsukiura, Tanji, Suzuki, Kawashima, et. al., 2003; Race, Keane, & Verfaellie, 2011; Szpunar, Watson, & McDermott, 2007; Tulving, 1985; Zeman, Beschin, Dewar, & Della Sala, 2012), scene construction (e.g., Hassabis & Maguire, 2009; Mullallly & Maguire, 2014), creative thinking (Duff, Kurczek, Rubin, Cohen, & Tranel, 2013), and in the binding of relational information (co-occurrences of people, places, and things) across time and events (e.g., Davachi, 2006; Duff, Hengst, Tranel, & Cohen, 2007; Konkel, Warren, Duff, Tranel, & Cohen, 2008; Ranganath, 2010).
Methodologically, a common approach to investigating the role of the hippocampus in self-projection has been to employ modified versions of the Autobiographical Interview (AI) (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002) where participants are asked to (re)construct events that are real or imagined and that come from any time from the remote past to the distant future (e.g., Addis, Wong & Schacter, 2008). In those studies, the narratives are scored using the original AI scoring system to identify internal and external details (which map on to episodic and semantic memory respectively) for an index of “episodic-ness” of the narratives, i.e., how much they conveyed an impression of actually (re)experiencing the event (Irish, Addis, Hodges, & Piguet, 2012; Race, Keane, & Verfaellie, 2011). A consistent finding in patients with hippocampal amnesia using these methods is a deficit in the ability to project oneself into the past and into the future as measured by significantly fewer episodic details in their narratives relative to healthy participants (e.g., Hassabis et al., 2007; Kwan, Carson, Addis, & Rosenbaum, 2010; Race et al., 2011; although see Squire, van der Horst, McDuff, Frascino, Hopkins, & Mauldin, 2010). An open question is whether the hippocampus contributes to other forms of self-projection. Such data would be informative in answering questions about the specificity of individual neural systems to different aspects of self-projection and self-representation.
There is also an extensive literature on the role of the medial prefrontal cortex (mPFC) in various aspects of self-projection. Neuroimaging studies show involvement of the mPFC in self-referential processing and the neural representation of the self (e.g., Brunet, Sarfati, Hardy-Bayle, & Decety, 2000; Calarge, Andreasen, & O'Leary, 2003; Frith & Frith, 1999; D'Argembeau, Collette, Van der Linden, Laureys, Del Fiore, Degueldre, Luxen, A., et al., 2005; Fossati, Hevenor, S.J., Lepage, M. Graham, S.J. Grady, C., Keightley, M.L., Craik, F. et al., 2004; Gusnard, Akbudak, Schulman, & Raichle, 2001; Kelley, Macrae, Wyland, Caglar, Inati, & Heatherton, 2002), episodic memory (Addis, McIntosh, Moscovitch, Crawley, & McAndrews, 2004; Cabeza, Prince, Daselaar, Greenberg, Budde, Dolcos et al., 2004; Gilboa, 2004; Rogers, Kuiper, & Kirker, 1977; Symons & Johnson, 1997) and social functioning (Eisenberger & Lieberman, 2004). Research using neuropsychological patients with mPFC damage converges with many of the neuroimaging findings. Specifically, patients with mPFC damage show deficits in future thinking (e.g., Harlow, 1868; Benton, 1968; Bechara, Damasio, Damasio, & Anderson, 1994; Levine, Black, Cabeza, Sinden, McIntosh, Toth, Tulving, et al., 1998) and temporal discounting (e.g., Fellows & Farah, 2005); they also fail to show the self-reference effect (e.g., Philippi, Duff, Denburg, Tranel, & Rudrauf, 2012). While the mPFC has been implicated in various forms of self-projection across imaging and neuropsychological studies, a remaining gap in the literature is the study of self-projection using the same methods commonly employed in the memory literature. Furthermore, while other studies have examined different aspects of self-projection (e.g., past and future thinking) in patients whose damage includes the mPFC (Irish, Hodges, & Piguet, 2013) there are no studies to our knowledge that have examined different aspects of self-projection (e.g., into the past/future and into the self) within the same task in this population.
In the current study, we asked six patients with bilateral hippocampal damage and five patients with bilateral medial prefrontal cortex damage to complete a modified version of the AI where they (re)constructed events in four conditions: real past, imagined past, imagined present, and future. We then analyzed those narratives for internal and external details and the use of self-references. Given the previous literature on deficits in self-projection (i.e., the ability to remember the past and think about the future), we expected hippocampal amnesic patients to produce significantly fewer details across all time periods relative to demographically-matched healthy comparison participants, replicating previous findings. An open question, however, is if these deficits extend to other forms of self-projection or self-representation. Given the extensive literature on the use of personal pronouns as a measure of self-referential processing and self-representation (e.g., Carmody & Lewis, 2012; Davis & Brock, 1975; Esselen, Metzler, Pascual-Marqui & Jancke, 2008; Ingram, Cruet, Johnson, & Wisnicki, 1988; Kircher, Senior, Phillips, Benson, Bullmore, Brammer, et al., 2004; Lewis & Carmody, 2008; Rude, Gortner, & Pennebaker, 2004; Lombardo & Baron-Cohen, 2010; Lombardo, Chakrabarti, Lai, & Baron-Cohen, 2012), we also examined the narratives for the use of self-referential pronouns. In contrast, for the mPFC patients we expected to find deficits, consistent with the literature, in the ability to introspect about the self as measured by the use of self-referential pronouns in their narratives. Such an outcome would extend this literature by documenting deficits in self-referential processing in the narrative discourse of mPFC patients. While there is strong evidence from fMRI studies of mPFC involvement in episodic memory and future thinking, the necessity of the mPFC is unclear. The current study, with two groups of well-characterized neurological patients completing the same task, promises to further our understanding and characterization of the specificity of individual neural systems to different aspects of self-projection.
2. Methods
2.1. Participants
Participants were six (one female) patients with bilateral hippocampal damage, five patients (two female) with bilateral mPFC damage, and eleven healthy demographically-matched comparison participants (CP). The patients were recruited from the Patient Registry of the University of Iowa's Division of Behavioral Neurology and Cognitive Neuroscience and were characterized neuropsychologically and neuroanatomically according to established protocols in our laboratory (Frank, Damasio, & Grabowski, 1997; Tranel, 2007). The Institutional Review Board approved all procedures for Human Subjects Research at the University of Iowa.
At the time of data collection, the patients with hippocampal damage were in the chronic epoch of amnesia, with time-post-onset ranging from 8 to 18 years. Hippocampal patients were, on average, 53.2 years old (range 45 – 61 years), and had, on average, 15 years of education (range 12 – 16 years). Etiologies included anoxia/hypoxia (1606, 1846, 2363, 2563), resulting in bilateral hippocampal damage, as well as herpes simplex encephalitis (HSE; 1951, 2308), resulting in more extensive bilateral medial temporal lobe damage affecting the hippocampus, amygdala, and surrounding cortices (see Figure 1). For patients who were MRI compatible (excluding 2563) high-resolution volumetric MRI analyses revealed significant reduction to the hippocampus bilaterally with volumes reduced by more than 4.74 studentized residuals on average (SD = 2.35) compared to age matched comparisons. Visual inspection of a CT scan from patient 2563, who wears a pacemaker, confirmed damage limited to hippocampus. Performance on tests of neuropsychological functioning revealed a severe and selective impairment in declarative memory functioning (e.g., WMS-III GMI = 60.2 SD = 9.6) while performance across other cognitive domains was within normal limits (see Table 2). One participant with amnesia (1606) was unable to complete the entire protocol, completing one past, one imagined past, two present and two future constructions.
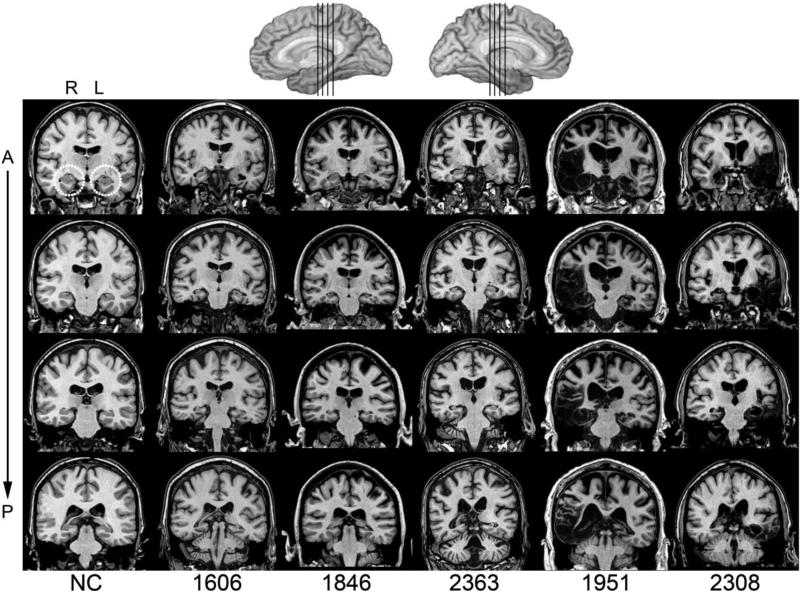
Figure 1.
Magnetic resonance scans of hippocampal patients. Images are coronal slices through four points along the hippocampus from T1-weighed scans. Volume changes can be noted in the region of the hippocampus bilaterally for patients 1606, 1846, and 2363 and significant bilateral damage to the MTL, including hippocampus, can be observed for 1951 and 2308. R = right; L = left; A = anterior; P = Posterior; NC = a healthy comparison brain.
Table 2.
Neuropsychological characteristics of hippocampal and mPFC participants.
| Group | Patient | WAIS-III FSIQ | WMS-III GMI | BN | TT | COWA | WCST | CFT | BDI | APP | SIP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hippocampus | 1606 | 91 | 66 | 32 | 44 | 43 | 6 | 34 | 9 | No | 1 |
| 1846 | 84 | 57 | 43 | 41 | 24 | 6 | 28 | 9 | No | 1 | |
| 1951 | 106 | 57 | 49 | 44 | 40 | 6 | 32 | 5 | No | 2 | |
| 2308 | 98 | 45 | 52 | 44 | 24 | N/A | 26 | 0 | No | 3 | |
| 2363 | 98 | 73 | 58 | 44 | 26 | 6 | 26 | N/A | No | 1 | |
| 2563 | 94 | 63 | 52 | 44 | 36 | 6 | 36 | 0 | No | 1 | |
| Mean (SD) | 95.2 (7.4) | 60.2 (9.6) | 47.7 (9.1) | 43.5 (1.2) | 32.2 (8.5) | 6 (0.0) | 30.3 (4.3) | 4.6 (4.5) | 0.0 (0.0) | 1.5 (0.8) | |
| mPFC | 318 | 143 | 109 | 60 | 44 | 54 | 6 | 36 | 0 | Yes (-3) | 3 |
| 2352 | 106 | 109 | 54 | 44 | 34 | 6 | 32 | 1 | Yes (-3) | 2 | |
| 2391 | 109 | 132 | 57 | 43 | 59 | 6 | 34 | 4 | Yes (-2) | 2 | |
| 2577 | 84 | 96 | 55 | 44 | 44 | 0 | 31 | 7 | Yes (-3) | 3 | |
| 3350 | 118 | 108 | 52 | N/A | 40 | 6 | 36 | 3 | Yes (-1) | 1 | |
| Mean (SD) | 112 (21.4) | 110.8 (13.1) | 55.6 (3.0) | 43.8 (0.5) | 46.2 (10.2) | 4.8 (2.7) | 33.8 (2.3) | 3.0 (2.7) | -2.4 (0.9) | 2.2 (0.8) |
Note. WAIS-III FSIQ = Wechsler Adult Intelligence Scale-III Full Scale Intelligence Quotient; WMS-III = Wechsler Memory Scale-III General Memory Index; BN = Boston Naming Test; TT = Token Test; COWA = Controlled Oral Word Association Test; WCST = Wisconsin Card Sort Test Number of Categories Achieved; CFT = Complex Figure Test Copy; BDI = Beck Depression Inventory; APP = Acquired Personality Problems; SIP = Social Conduct and Interpersonal Functioning; N/A = not available. APP = Acquired Personality Problems refer to whether or not the participant had acquired problems in personality functioning, as derived from data on the Iowa Scales of Personality Change. The numbers in parentheses denote the degree of severity, where 1 = mild, 2 = moderate, and 3 = severe; SIP = The extent of post-lesion change or impairment in aspects of social conduct and interpersonal functioning was rated on a three-point scale, with 1 = no change in impairment, 2 = moderate change or impairment, 3 = severe change or impairment. Characterizations of Acquired Personality Problems and Social and Interpersonal Functioning were rendered by a board-certified clinical neuropsychologist who was blind to the hypothesis of the current study at the time the ratings were performed.
Patients with mPFC damage were, on average, 67.6 years of age (range: 61-73 years) and had, on average, 13.8 years of education (range: 11-18 years). Etiologies included meningioma resection (318, 2391, 3350) and sub-arachanoid hemorrhage and/or anterior communicating artery aneurysm (2352, 2577; see Table 1 for demographic information; see Figure 2 for lesion overlap map) with time post-onset ranging from 10 to 38 years. All mPFC patients had lesions that overlapped with the Montreal Neurological Institute (MNI) coordinates (x = 10, y =52, z = 2; putatively corresponding to BA 10 in the right hemisphere, a region that previous neuroimaging (Kelley, et al., 2002) and lesion (Phillipi, Duff, Denburg, Tranel & Rudrauf, 2012) work have implicated in self referential processing (this region has also been demonstrated to be differentially recruited during future simulation, e.g., Addis et al., 2007; Okuda et al., 2003). All mPFC patients have well-documented post-morbid changes in emotion processing, decision-making, personality, and/or social and interpersonal functioning (Croft, Duff, Kovach, Anderson, Adolphs, & Tranel, 2010; Koenigs & Tranel, 2008; Young, Bechara, Tranel, Damasio, Hauser, & Damasio, 2010). These deficits in various aspects of social and emotional processing are in stark contrast to relatively intact neuropsychological profiles, including performance within normal limits on neuropsychological tests of language, memory, intelligence and visual discrimination (see Table 2 for neuropsychological data).
Table 1.
Demographic characteristics of the participants with hippocampal and mPFC damage
| Group | Patient | Sex | Age | Hand | Ed | Chron | Etiology |
|---|---|---|---|---|---|---|---|
| Hippocampus | 1606 | M | 61 | R | 12 | 18 | Anoxia |
| 1846 | F | 45 | R | 14 | 15 | Anoxia | |
| 1951 | M | 56 | R | 16 | 28 | HSE | |
| 2308 | M | 52 | L | 16 | 9 | HSE | |
| 2363 | M | 52 | R | 18 | 10 | Anoxia | |
| 2563 | M | 53 | R | 16 | 8 | Anoxia | |
| Mean (SD) | 53.2 (5.3) | 15.0 (1.7) | 14.7 (7.6) | ||||
| mPFC | 318 | M | 73 | R | 14 | 38 | Meningioma Resection |
| 2352 | F | 64 | R | 14 | 15 | SaH; ACoA | |
| 2391 | F | 67 | R | 12 | 14 | Meningioma Resection | |
| 2577 | M | 73 | R | 11 | 15 | SaH; ACoA | |
| 3350 | M | 61 | R | 18 | 10 | Meningioma Resection | |
| Mean (SD) | 67.6 (5.4) | 13.8 (2.7) | 18.4 (11.1) |
Note: M = Male; F = Female; R = Right handed; Ed = years of education; Chron = chronicity, years between lesion onset and the current study; SaH = sub-arachnoid hemorrhage; ACoA = Anterior communicating artery aneurism; HSE = Herpes Simplex Encephalitis
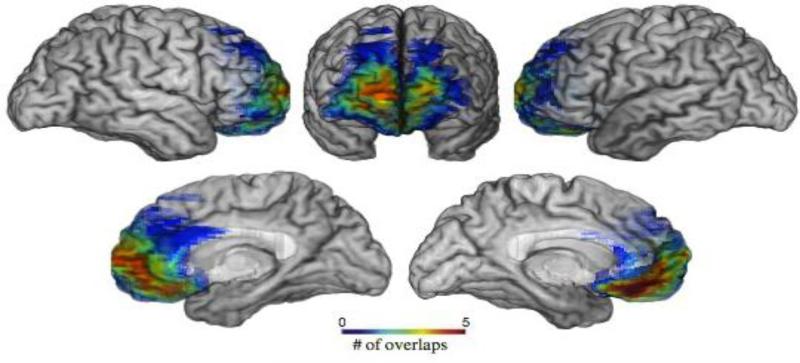
Figure 2.
Lesion overlap map of patients with damage to the medial prefrontal cortex (mPFC).
Note: Warmer colors indicate areas of greater overlap across participants.
Eleven healthy comparison participants were matched pair-wise on sex, age, and education separately to the each of the participants with brain damage in the hippocampus (n = 6; age – mean = 54.3; SD = 5.8; p = 0.29; education – mean =16.2; SD = 1.6; p = 0.43) and mPFC (n = 5; age – mean = 69.8; SD = 4.7; p = 0.51; education – mean = 16.8l; SD = 2.6; p = 0.11) groups.
2.2. Procedures
2.2.1. Dynamic Narrative Construction Elicitation Protocol
Participants were provided with a neutral cue word (e.g., clock, hotel, bird, restaurant) and asked to produce three unique narratives of autobiographical events in each of four distinct time periods (Real Past, Imagined Past, Imagined Present, and Future) for a total of twelve narratives. Neutral cue words were selected from the Affective Norms for Emotional Words database (Bradley & Lang, 1999). The cue words were well balanced across time period and did not differ significantly on measures of valence (p = 0.21), arousal (p = 0.26), frequency (p = 0.43), or imaginability (p = 0.53) (norms from Coltheart, 1981). For each event, participants were instructed that the event had to be autobiographical rather than an event that happened to someone else. Participants were also told that events should have actually occurred (Real Past) or be possible and reasonable to happen (Imagined Past, Present, Future) and that the events could have/should last between a few minutes and several hours but not more than a day. Participants were instructed to choose Real Past events and to construct Imagined Past events that happened to them only once and before they were 25 years old. For Present and Future events, participants were asked to construct events that had not happened to them before. For Future events, there was no limit for how far into the future the event could be set. These constraints were placed on the events to encourage participants to draw disproportionately from their episodic memory for which they had specific (but infrequent) autobiographical experiences rather than “a pool of generalized knowledge” (Cermak & O'Connor, 1983).
Once participants had selected an event that fit the criteria, participants were asked to provide a title, time, and location for the event. Participants then were asked to provide a one to two minute overview of what happened or might have happened during the event (i.e., the event that lasted a few minutes to a few hours). Participants were subsequently asked to select a specific moment from the event they described and were then given one to two minutes to produce a narrative of the setting and experience of that specific moment. All subsequent data analysis was focused on these one to two minute descriptions of the specific moment of the (re)constructed event. All instructions were given orally to participants and written instructions in bullet point format were also provided and left within view of participants to reduce memory demands. Participants were encouraged to use the full-allotted time. If a participant indicated they could not think of any thing else, the experimenter encouraged the participant to keep thinking and would remind the participant of the instructions. Sessions were video and audio taped.
2.2.2. Transcript Processing
All sessions were transcribed in their entirety using a two-stage consensus transcription procedure (e.g., Duff, Hengst, Tranel, & Cohen, 2008). During the first stage, the original transcriber recorded all utterances, audible sounds, and pause times of both the examiner and participant. In the second stage, the consensus transcriber and the original transcriber viewed the video together to generate the final version of the transcript – the consensus transcript. Any corrections or additions to the transcripts were rendered after discussion and consensus. Throughout the transcription process, words were broadly defined with little emphasis placed on morphological or syntactic form, consistent with our previous work. Each of the following were counted as words: false starts (e.g., getting the boat, actually getting the boat to go out = 10 words), fillers (e.g., uh, um = 2 words), and contractions (e.g., can't = 1 word).
2.2.3 Self-Projection Scoring
The narratives were scored for their episodic and semantic content following the scoring procedure protocol prescribed by Levine et al., (2002). We chose to examine self-projection using the AI procedures given their extensive use in the literature, facilitating comparison to other studies of narratives of event (re)construction (Irish et al., 2012; Race et al, 2011; Squire et al., 2010) and more novel uses of the procedures applied to narratives of pictures descriptions (e.g., Race et al, 2011; Race, Keane, & Verfaellie, 2013; Zeman et al., 2012). The coder isolated the main event, which was specific to a time and place, and then segmented it into details. Details were defined as unique occurrences, observations, or thoughts, which each independently conveyed information; these usually took the form of a grammatical clause, including a subject and predicate. At times, a single clause expressed more than one piece of information, and each piece was marked individually. For instance, “I drove to Dallas, Texas last month” includes an event (I drove), a place (Dallas, Texas), and a time (last month) detail.
Two overarching categories were used to classify details: internal and external. Internal details pertained directly to the main event described by the participant and exhibit episodic re-experiencing. Internal details include reference to event, place, time, perceptual, and emotion/thought information. Details extraneous to the main event were considered external. Sub-categories for external details included those listed above as well as semantic, repetition, and other details. The number details were then tallied for each specific sub-category and summed to form internal and external composites, which were the main variables of interest. The proportion of internal-to-total details per memory reflected episodic re-experiencing irrespective of the total verbal output.
2.2.4. Self-Referential Processing Scoring
All narratives were coded for self references and references to other people. Coders marked all references to people and groups of people in both the subject (I went to Dallas) and object (The waiter handed the drinks to me) position of a sentence. References coded as self-reference included first person singular (I, me) and first person plural (we, us, our) pronouns. References to others included third person singular (he, she it, him, her, proper names, you) and third person plural (they, them) pronouns. Any repetitions of self and other references contained in a false start (I I went to Texas) were counted only once. To account for any differences in verbal output, amount or content, a proportion of self- to total-references was calculated.
We chose to examine self-referential processing through the use of personal pronouns for several reasons. First, there is an existing literature on self-referential processing and self-representation as indexed by the presence or absence of personal pronouns in healthy and disordered (e.g., autism) populations (e.g., Lombardo et al., 2012; Carmody & Lewis, 2012; Lewis & Ramsay, 2004; Mizuno, Liu, Williams, Keller, Minshew, & Just, 2011). In this way, tracking and coding pronouns as a tool to gain insight into self-referential processing does not differ from tracking and coding nouns, verbs, and adjectives (as internal and external details) to gain insight into episodic (re)construction abilities. Second, we wanted a measure of self-referential processing that could be applied to the participants’ narratives rather than in a second experimental task. This allowed us to mitigate against concerns that any observed differences between measures of self-referential processing and episodic (re)construction were the result of differences in task demands. Finally, we were interested in focusing on capturing self-referential processing ability (thinking about the self) rather than on a more general measure of theory of mind (thinking about the self and others).
2.2.5 Reliability of scoring
Raters blind to participant group membership rated approximately 20% of the dataset for all analyses. Inter- and intra-rater reliability for coding of internal and external details was assessed with intraclass correlation coefficients, 0.940 and 0.993, for internal and external details respectively. Inter- and intra-rater reliability for coding of self and other references was 0.990 and 0.944, respectively.
2.2.6. Data Analysis
Data analysis was performed on the verbal productions of the participants (not the experimenter). All measures (proportion of internal to overall details and proportion of self-references) were analyzed separately for each patient group (hippocampal and mPFC) and their respective comparison participants on the episodic moment with repeated measures ANOVAs with the between-subjects factor, group (patients vs. comparisons) and within-subject factor, time (Past, Imagined Past, Present, and Future). The patient groups (and their demographically matched comparison participants) were analyzed separately because previous research has demonstrated age related decline in the measures of interest including self-projection (Gaesser, Sacchetti, Addis, & Schacter, 2011) and self-referential processing (Mitchell, Raye, Ebner, Tubridy, Frankel, & Johnson, 2009; Rice & Pasupathi, 2010). An ANOVA indicated a significant group difference for age, F(3,18) – 15.96, p < 0.001 with follow-up tests indicating that both the hippocampal patients and their matched healthy comparison participants were significantly younger than the healthy comparison participants matched to the mPFC patients and the mPFC patients themselves.
Consistent with the literature (e.g., Cole, Morrison, & Conway, 2013; Murphy, Troyer, Levine, & Moscovitch, 2008; Steinworth, Levine, & Corkin, 2005), to account for differences in the length and content of the narratives for the event (re)constructions, proportions were calculated (e.g., proportion of internal details to the total number of details and proportion of self references to the total number of references). All variables were rank-ordered transformed prior to statistical analysis. Since multiple tests were run on the same elicitation data, in order to control for type I error, we applied Sidak corrections to all statistical tests separately for data and subsequently all p-values are reported from the Sidak correction (which, while reducing Type I error, may increase Type II error, indicating that results here are conservative).
3. Results
3.1 Word Count
All participants were able to provide unique autobiographical narratives for past and future, real and imagined conditions (3 Imagined Past, 3 Past, 3 Imagined Present, and 3 Future). We found that the patients with hippocampal damage produced significantly shorter narratives than their matched comparison participants, (F(1,10) = 6.56, p = 0.028, d = 0.40). Within the narratives of the hippocampal patients and their comparison participants, there was a significant effect of time, (F(3,30) = 5.28; p = 0.014, d = 0.35), but no group by time interaction, (F(3,30) = 1.14; p = 0.340, d = 0.10), indicating that hippocampal participants produced shorter narratives regardless of time condition.
For the mPFC patients and their matched healthy comparison participants, there was neither a significant effect of group, (F(1,8) = 0.05, p = 0.822, d = 0.001), time, (F(3,24) = 1.17; p = 0.342, d = 0.13), nor a group by time interaction, (F(3,24) = 2.17; p = 0.118, d = 0.21), indicating that mPFC and comparison participants produced narratives of similar length as measured by words for all time conditions.
Consistent with the literature, to account for differences in the length and content of the narratives for the event (re)constructions, proportions were calculated (e.g., proportion of internal details to the total number of details and proportion of self references to the total number of references).
3.2 Findings from the hippocampal patients
3.2.1 Self Projection
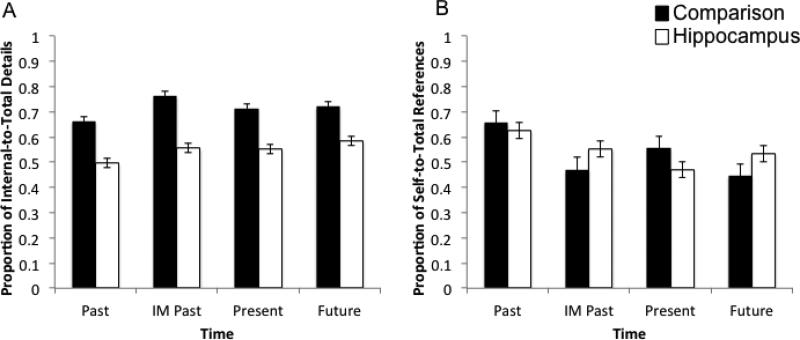
Across the entire data set and all participants, 2133 details were coded with 1370 of those coded as internal (HC = 448; comparison = 922) and 763 coded as external (HC = 385; comparison = 378). A 2 X 4 (group – HC, comparison; time – Past, IM Past, Present, Future) repeated measures ANOVA investigated the effect of time on the proportion of internal to total details that composed the narrative (re)constructions. Hippocampal patients produced a smaller proportion of internal to overall details, indicating less “episodicness” in the narratives of their event (re)constructions, (F(1,10) = 16.22, p = 0.002, d = 0.62). Furthermore, there was neither a main effect of time, (F(3,30) = 2.67, p = 0.065, d = 0.21), nor any group by time interaction, (F(3,30) = 0.07, p = 0.977, d = 0.01; Figure 3), indicating that amnesic participants produced fewer episodic details in their narratives irrespective of time period.
Figure 3.
Patients with hippocampal damage display significant disruptions in self-projection but not in self-referential processing.
Note: A) Proportion of internal to total details; B) Proportion of self to total references.
3.2.2. Self Referential Processing
Across the entire data set and all participants, 2140 references to people were coded with 1166 coded as self-references (HC=408; comparisons = 758) and 974 coded as references to others (HC = 375; comparison = 599). A 2 X 4 (group – HC, comparison; time – Past, IM Past, Present, Future) repeated measures ANOVA investigated the use of references to the self and to others in the narratives of the event (re)constructions. There was no effect of group on the proportion of self to total references (F(1,10) = 0.03, p = 0.859, d = 0.003), nor a group by time interaction (F(3,30) = 1.37, p = 0.271, d = 0.12; Figure 3) indicating that the hippocampal and comparison participants did not differ in the extent to which they incorporated themselves in the narratives of the event (re)constructions. There was a significant effect of time (F(3,30) = 3.45, p = 0.029, d = 0.26) with Past narratives having a higher proportion of self-to-total references than Imagined Past (0.043) and Present (0.025) narratives. Table 3 presents a representative example of a narrative for a hippocampal patient coded for word count, proportion of internal to total details, and proportion of self-references to total references.
Table 3.
Example narratives for a healthy comparison, mPFC, and hippocampal participant
| Comparison | mPFC | Hippocampus | |
|---|---|---|---|
| Word Count | 135 | 204 | 163 |
| Self-Prospection | 0.82 | 0.82 | 0.23 |
| Self-Reference | 0.67 | 0.11 | 0.57 |
| Narrative | Okay I just came home from school and I was wearing a a little girl dress we didn't wear pants to school back then so I'm standing at the back door calling out and I can hear mom out in the garden and she answers that she's out there picking vegetables for for supper well there is a big long row of flowers called flocks back there that smelled really good they were light purple and there was an old shed back there where we kept the lawnmower and gardening stuff and it's just big and long row of pine trees that were kind of a wind break in the north side of the house and a huge garden I'm feeling relief when she answered. oh I suppose there were some farm smells the animals. | We were all we were all home from school it was Saturday and the sheriff sheriff and sheriff's deputy comes in the yard of course all the kids were looking through windows see what what's the sheriff in the yard for and he's out talking to dad so on then they get to dog and look at to dog looking under his chin and his around him and he was muddy and dirty and maybe some blood so it's a matter then of well what what's the deal my neighbor a mile or so across across the field the dog do that last night chasing hogs and killing a couple of hogs and they are out looking for the dogs and this dog was here that you have solid proof of who the dogs were whose dogs they were or maybe didn't see they probably couldn't describe him it's just there are more than one dog doing it out and so the deputy is checking our dog and he thinks he has enough evidence with the mud and so on on that he was involved so he was giv- he gave a choice we could dispose him ourselves or he could take him along. | I remember it being a big open space where you went in after you were done skiing and lots and lots of voices everybody was talking within the couple people that they came with and they were all talking about their skiing events for the day and feeling very very cold even though I was inside I still felt cold it took awhile to warm up going by the fire I remember going next to the fire after I had changed into my jeans into normal clothes and out of ski clothes and sitting by the fire to warm up talking about what we were going to eat supper that night if we were going to try and eat there at the ski lodge or if we were going to go out it was pretty loud in there in the lodge with people getting done skiing and everybody was in their own groups and everybody talking all at once and so that made it seem very loud |
3.3 Findings from the mPFC patients
3.3.1 Self Projection
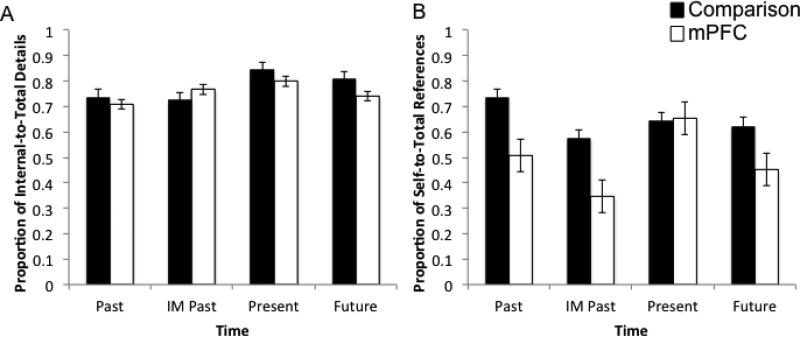
Across the entire data set and all participants, 2269 details were coded with 1716 details coded as internal (mPFC = 815; comparison = 901) and 533 coded as external (mPFC = 323; comparison = 230). A 2 X 4 (group – mPFC, comparison; time – Past, IM Past, Present, Future) repeated measures ANOVA investigated the effect of time on the proportion of internal to total details. There was not an effect of group, (F(1,8) = 1.35, p = 0.278, d = 0.14), time, (F(3,24) = 1.57, p = 0.216, d = 0.17) nor a group by time interaction, (F(3,24) = 0.28, p = 0.84, d = 0.03; Figure 4), as mPFC patients and comparison participants produced similar proportions of internal to total details, indicating highly similar levels of “episodiciness” in the narratives of the event (re)constructions.
Figure 4.
Patients with mPFC damage display significant disruptions in self-referential processing but not in self-projection.
Note: A) Proportion of internal to total details; B) Proportion of self to total references.
3.3.2 Self Referential Processing
Across the entire data set and all participants, 1453 references to people were coded with 793 references coded as referring to the self (mPFC = 368; comparison = 425) and 660 references coded as referring to others (mPFC = 380; comparison = 280). A 2 X 4 (group – mPFC, comparison; time – Past, IM Past, Present, Future) ANOVA investigated the use of self and other references in the narrative constructions. There was an effect of group on the proportion of self- to total-references (F(1,8) = 8.46, p = 0.020, d = 0.51), but not an effect of time (F(3,24) = 2.12, p = 0.124, d = 0.21) or a group by time interaction (F(3,24) = 1.33, p = 0.287, d = 0.14; Figure 4) indicating that patients with mPFC damage produced fewer self-references across all conditions. Table 3 presents a representative example of a narrative for a mPFC patient coded for word count, proportion of internal to total details, and proportion of self-references to total references.
4. Discussion
A core neural network comprising the MTL, the mPFC, and other structures, has been proposed to underlie the ability to remember the past, think about the future, and introspect about oneself and others (Buckner & Carroll, 2007; Schacter & Addis, 2007b; Addis, Wong, & Schacter, 2007; Spreng, Mar, & Kim, 2009). The current study asked five patients with bilateral mPFC damage, six patients with bilateral hippocampal damage, and demographically matched comparison participants to complete an event construction task, and the participants’ narrative productions of those event (re)constructions were then analyzed for internal and external details and the use of self-references to investigate whether distinct neural systems make specific and unique contributions to various aspects of self-projection. We found a double dissociation where patients with hippocampal damage, relative to healthy comparison participants, showed disruptions in their ability to construct highly detailed episodic events across all time periods, but this deficit did not extend to their ability to incorporate themselves in the narratives of those (re)constructions. The patients with mPFC damage showed the opposite pattern. They were able to construct highly detailed episodic events but incorporated themselves in the narratives of those (re)constructions less often than healthy participants. The results suggest differential contributions of the hippocampus and the mPFC to the core neural network for self-projection.
The findings here demonstrate that the patients with hippocampal damage were impaired in their ability to (re)construct events from the real (remote) past, the imagined past, the imagined present, and the future— results which are consistent with a growing body of work pointing to the role of the hippocampus in remembering the past and imaging the future in both neuroimaging studies (Addis et al., 2007; Addis & Schacter, 2008; Okuda et al., 2003; Schacter & Addis, 2007a; 2007b; Szpunar et al., 2007) and studies with patients with adult onset hippocampal amnesia (Hassabis et al., 2007; Kwan et al., 2010; Race et al., 2011, but see Squire et al, 2010). The deficit in (re)constructing events has been linked to the role of the hippocampus in scene construction (e.g., Hassabis et al., 2007; Hassabis & Maguire, 2009; Mullally & Maguire, 2014) and in the relational binding and subsequent retrieval of the temporal, spatial, and relational content that forms our mental representations of events, whether real or imagined, past or future (e.g., Buckner & Carroll, 2007; Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001; Schacter &Addis, 2007; Schacter, Addis, & Buckner, 2007; Race et al., 2011).
The novel finding here, in regard to the hippocampus, is that those deficits do not extend to the incorporation of the self in the narratives produced by the hippocampal patients. The patients with hippocampal damage produced shorter narratives overall, those narratives contained fewer details overall, and there were fewer references to characters in their narratives, i.e., fewer people (self or other) in the (re)constructed events. However, there was no difference in the proportion of self- to total- references relative to the healthy comparison participants, indicating that the patients with hippocampal damage have no deficit in self-referential processing.
These results from patients with hippocampal damage provide further support for the proposed contribution of the hippocampus to the core neural network in support of remembering the past and thinking about the future. The lack of disruption in self-referential processing in these patients, as measured by the use of self-referential pronouns, is consistent with previous work demonstrating an intact self-reference effect in a single case study of an individual with hippocampal amnesia (Sui & Humphrey, 2013). However, while the hippocampus does not appear to be critical for self-referential processing, future studies should investigate the possibility that the hippocampus does support other aspects of thinking about the self and others. Indeed, previous investigations have found connections between hippocampal functioning, declarative memory, and empathy – the ability to put your self in another person's shoes (Beadle, Tranel, Cohen, & Duff, 2013), prosocial behavior (Gaesser & Schacter, 2014) and social cognition (Rubin, Watson, Duff, & Cohen, 2014; Spreng, 2013). However, the hippocampus and declarative memory do not appear to be necessary for engaging all aspects of theory of mind (Rabin, Braverman, Gilboa, Stuss & Rosenbaum, 2012; Rosenbaum, Stuss, Levine, & Tulving, 2007).
The lack of impairment in self-referential processing in patients with hippocampal damage is in contrast to the deficit in the patients with mPFC damage. The current findings with patients with mPFC damage are consistent with the literature indicating the critical contributions of the medial frontal lobes to self-referential processing (Craik, Moroz, Moscovitch, Stuss, Winocur, Tulving, & Kapur, 1999; D'Argembeau et al., 2005; Fossati et al., 2004; Gusnard, et al., 2001; Kelley et al., 2002; Philippi et al., 2012). While the literature on self-referential processing generally focuses on how information is encoded, here all information was self-generated by the participants, with the deficit in self-referential processing in individuals with mPFC damage evident in their discourse and narratives. This result is also particularly striking given that a requirement of our study was that the events had to personally involve the participant. That is, while the patients with mPFC damage were successful at producing highly detailed narratives of autobiographical events they do not reference themselves to the same degree as they do other characters in the (re)constructed events.
The patients with mPFC damage were not impaired in their (re)construction of events: the patients with mPFC damage produced as many internal details as healthy comparison participants for all time periods. This result is somewhat surprising given the literature describing the role of mPFC in future thinking (Addis et al., 2004; Buckner & Carroll, 2007; Cabeza et al., 2004; Gilbert & Wilson, 2007; Gilboa, 2004; Philippi, Tranel, Duff & Rudrauf, 2014; Schacter & Addis, 2007a; Schacter et al., 2007; Schacter, Addis, & Buckner, 2008) and autobiographical memory more broadly (Addis et al., 2004; Cabeza et al., 2004; Gilboa, 2004; Levine, 2004; Maguire, Mummery, & Buchel, 2000). In fact, in a recent study of patients with diffuse damage caused by various forms of dementia, voxel-based morphometry revealed divergent neural correlates related to performance on past and future thinking within each patient group, with frontal atrophy in bvFTD patients associated with deficits in future thinking (Irish, et al., 2013). We speculate that one possibility for the lack of impairment may be task demands. For example, Saver and Damasio (1991) asked patient EVR to describe what he would do in a number of future situations and scenarios and EVR was able to give the correct response to what he could or should do, despite his inability to actually perform in the correct/appropriate manner in real-life circumstances. We asked patients to construct narratives from different time periods, both real and imagined, in a laboratory setting but did not ask them to make a plan or representation that would be the basis for a future act. This distinction between successfully generating an appropriate response and the ability to act on that response is reminiscent of the dissociation noted in the literature in patients with mPFC damage between their normal intellectual abilities and performance on standardized tests and their real-life application of those abilities (Anderson, Bechara, Damasio, Tranel & Damasio, 1999; Bechara, Tranel, Damasio & Damasio, 1996; Bechara, Tranel, & Damasio, 2000; Burgess, Veitch, Costello, & Shallice, 2000; Damasio, Tranel, & Damasio, 1991; Shallice & Burgess, 1991).
While the event construction task used here has not, to our knowledge, been used with other patients with mPFC damage, previous work has looked at performance using similar (although not identical) methods in patients with damage to other frontal regions (dorsomedial and ventrolateral PFC; regions outside the “core network” proposed by Schacter et al., 2007) as well as damage to posterior parietal cortices (PPC). Berryhill and colleagues (2010) reported that the patients with damage to dorsomedial and ventrolateral PFC were impaired in their ability to construct imagined experiences while the PPC patients were unable to construct autobiographical or imagined experiences. The findings from Berryhill et al (2010) appear consistent with the proposal by Stuss and Levine (2002) suggesting that more dorsal portions of the frontal lobe contribute to functions that are more cognitive while more ventral portions contribute to abilities that are more affective in nature. We wonder if we might have observed a different outcome in the mPFC group if the event (re)construction cue words had been socially or emotionally salient (e.g., family vs tree). Indeed, in addition to the proposal by Stuss and Levine (2002), the mPFC has been implicated in the more emotional aspects of autobiographical memory (Addis et al., 2004; Cabeza et al., 2004; Gilboa, 2004; Levine, 2004; Maguire et al., 2000) and theory of mind (Brunet, et al., 2000; Calarge, et al., 2003; Frith & Frith, 1999; Gallagher, 2000).
Recent writings have emphasized the interactions of the hippocampus and the medial prefrontal cortex in supporting various cognitive abilities (e.g., Preston & Eichenbaum, 2013; Rubin, Watson, Duff, & Cohen, 2014; Simons & Spiers, 2003). Here, we find differential contributions of the hippocampus and medial prefrontal cortex to the various forms of self-projection within the proposed network. We speculate that these two systems interact along the following lines: The hippocampus makes an early contribution to the ability to flexibly create or re-instantiate the relational information (i.e., co-occurrences of people, places, and things, along with the spatial, temporal, and interactional relations among them) that makes up an episode (past or future, real or imagined). Once those representations are made available by the hippocampus, the mPFC may perform additional social and emotional processes and update those representations, perhaps in an iterative fashion (see Wang, Cohen, & Voss, 2015). The idea that hippocampus and mPFC differ in the nature and timing of their contributions to complex behavior is supported by other work from our lab in which we have shown distinct patterns of performance in patients with hippocampal and mPFC damage across a variety of domains including decision-making (Bechara et al., 1994; Gupta, Duff, Denburg, Cohen, Bechara, & Tranel, 2009), language use and processing (Duff & Brown-Schmidt, 2012), and moral updating (Croft et al., 2010) (see Rubin et al., 2014 for review).
One further aspect of the current findings that may be noteworthy, although may be qualified since no direct comparison was made, is that the magnitude of the deficit in the hippocampal patients in the ability to (re)construct narratives of episodic events appears larger than the deficit in mPFC patients in self-referential processing, although both findings are statistically significant. This pattern of behavioral results could be predicted from functional connectivity research. Andrews-Hanna and colleagues have demonstrated distinct functional contributions of default network components including the hippocampus as a part of a MTL subsystem that increases activity during episodic decisions and the default network hub, the amPFC, with activity during decisions of personal significance, introspection about one's mental states, and evoked emotion (Andrews-Hanna, Reidler, Huang, & Buckner, 2010; Andrews-Hanna, Reidler, Sepulrcre, Poulin, & Buckner, 2010). Since the amPFC is identified as one of two hubs (along with the posterior cingulate cortex - PCC) in the default network while the hippocampus was a component of one of two subsystems, the smaller deficit in self-referential processing for patients with mPFC damage may be due to intact contributions from the PCC where the hippocampus has no secondary neural contributions. While we find this potential connection between our behavioral data and the neuroimaging literature to be intriguing and a future direction worth pursing, such connections are speculative, as we did not directly compare the groups and the groups differ in age.
The current study differed in some ways from previous work that are worth mentioning. First, the bulk of work on episodic (re)construction has asked participants to narrative an entire event (e.g., a trip to a museum, wedding day). Reflecting on these previous studies, we wondered it in asking participants to narrate an entire event (which can last a few minutes or a several hours) the product is some aggregate measure of multiple moments that occurred across the event (moments that could differ considerably with respect to time, place, people and other relational details but that still make up the event). More specifically, we hypothesized that such tasks possibly dilute the rich representational information in the specific moments. Given our interest in investigating the role of the hippocampus in (re)construction processes we believed focusing on a specific moment was a robust measure of such capabilities. We also believed that focusing on a specific moment, rather than the knitting together of moments across an extended event, would give the hippocampal patients the best chance of success. We take the striking deficit in (re)constructing an individual moment across all time conditions as further evidence of the critical role of the hippocampus in (re)construction processes more generally. While the nature of the deficit in patients with hippocampal damage observed here using this modified elicitation technique is remarkably similar to previous work using the traditional event method, future work comparing the two methods is warranted. Second, we did not control for temporal distance, which has been shown to influence retrieval and simulation processes in healthy individuals (e.g., Anderson, Dewhurst, & Nash, 2012). How temporal distance may interact with moment (re)construction is unknown and is an interesting topic for future research. Certain limitations of this study should also be noted, principally the relatively small sample size. However, our sample size is nearly identically to other investigations on this topic (e.g., Race, et al., 2011, Race, et al., 2013; Squire et al., 2010; Zeman, et al., 2012) and our effect sizes are in the moderate to large range.
In summary, we report differential contributions of the hippocampus and medial prefrontal cortex to the core neural network for self-projection. It appears that the hippocampus is required to (re)construct the mental representation of a specific event as patients with hippocampal damage, but not mPFC damage, are impaired relative to healthy comparison participants in the (re)creation of richly detailed narratives of specific autobiographical events across time. The mPFC appears to integrate and highlight relevant social information, measured here as the number of self-references, into these mental representations as patients with mPFC damage, but not hippocampal damage, had a small proportion of self-references in their narratives. Future studies might be aim to further delineate the nature and time-course of interaction between hippocampus, mPFC, and the rest of the neural network for self-projection in order to provide a finer-grained understanding of the roles of and interactions between these neural systems in service of remembering the past, thinking about the future, introspecting about oneself and others, and other complex cognitive and social behaviors.
Highlights.
-We examined the role of the MTL and mPFC in self-projection and self-referential processing
--MTL patients were impaired in self-projection but not self-referential processing
-mPFC patients were impaired in self-referential processing but not self-projection
-MTL and mPFC make differential contributions to the neural network supporting the ability to remember the past, think about the future, and introspect about oneself Differential contributions of hippocampus and medial prefrontal cortex to self-projection and self-referential processing
Acknowledgements
We thank the Duff Communication and Memory Laboratory for assistance with transcribing and coding the sessions. We thank Joel Bruss for creating the figures of the patient lesions. The study was supported by NIDCD F32 DC008825 to MCD; NIMH RO1 MH062500 to NJC; and NINDS P01 NS19632 to DT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: A partial least squares approach. NeuroImage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. Constructive episodic simulation: temporal distance and detail of past and future events modulate hippocampal engagement. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychological Science. 2008;19:33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in the human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Dewhurst SA, Nash RA. Shared cognitive processes underlying past and future thinking: The impact of imagery and concurrent task demands on event specificity. Journal of Experimental Psychology: Learning Memory & Cognition. 2012;38:356–365. doi: 10.1037/a0025451. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Hunang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. Journal of Neurophysiology. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle J, Tranel D, Cohen NC, Duff MC. Empathy in hippocampal amnesia. Frontiers in Psychology. 2013;4:1–12. doi: 10.3389/fpsyg.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1-3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Benton A. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Berryhill ME, Picasso L, Arnold R, Drowos D, Olson IR. Similarities and differences between parietal and frontal patients in autobiographical and constructed experience tasks. Neuropsychologia. 2010;48(5):1385–1393. doi: 10.1016/j.neuropsychologia.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Technical Report C-1. The Center for Research in Psychophysiology, University of Florida; 1999. Affective norms for English words (ANEW): Instruction manual and affective ratings. [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. NeuroImage. 2000;11(2):157–166. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Science. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, et al. Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. Journal of Cognitive Neuroscience. 2004;16(9):1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Calarge C, Andreassen NC, O'Leary DS. Visualizing how one brain understands another: A PET study of theory of mind. American Journal of Psychiatry. 2003;160(11):1954–1964. doi: 10.1176/appi.ajp.160.11.1954. [DOI] [PubMed] [Google Scholar]
- Carmody DP, Lewis M. Self representation in children with and without autism spectrum disorder. Child Psychiatry & Human Development. 2012;43:227–237. doi: 10.1007/s10578-011-0261-2. [DOI] [PubMed] [Google Scholar]
- Cermak LS, O'Connor M. The anterograde and retrograde retrieval ability of a patient with amnesia due to encephalitis. Neuropsychologia. 1983;21(3):213–234. doi: 10.1016/0028-3932(83)90039-8. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, Amnesia, and The Hippocampal System. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Cole SN, Morrison CM, Conway MA. Episodic future thinking: Linking neuropsychological performance with episodic detail in young and old adults. The Quarterly Journal of Experimental Psychology. 2013;66(9):1687–1706. doi: 10.1080/17470218.2012.758157. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Quarterly Journal of Experimental Psychology. 1981;33A:497–505. [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S. In search of the self: A positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- Croft KE, Duff MC, Kovach C, Anderson SW, Adolphs R, Tranel D. Detestable or marvelous? Neuroanatomical correlates of character judgments. Neuropsychologia. 2010;48:1789–1801. doi: 10.1016/j.neuropsychologia.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Somatic markers and the guidance of behavior: theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and dysfunction. Oxford University Press; New York: 1991. pp. 217–229. [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, et al. Self-referential reflective activity and its relationship with rest: A PET study. NeuroImage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinions in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davis D, Brock TC. Use of first person pronouns as a function of increased objective self-awareness and prior feedback. Journal of Experimental Social Psychology. 1975;11:381–388. [Google Scholar]
- Duff MC, Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Frontiers in Human Neuroscience. 2012;6:69. doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Collaborative discourse facilitates efficient communication and new semantic learning in amnesia. Brain and Language. 2008;106(1):41–54. doi: 10.1016/j.bandl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Talking across time: Using reported speech as a communicative resource in amnesia. Aphasiology. 2007;21(6, 7, 8):702–716. doi: 10.1080/02687030701192265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Kurczek J, Rubin R, Cohen NJ, Tranel D. Hippocampal amnesia impairs creative thinking. Hippocampus. 2013;23(12):1143–1149. doi: 10.1002/hipo.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford University Press; New York: 2001. [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural system for physical and social pain. Trends in Cognitive Science. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Esslen M, Metzler S, Pascual-Marqui R, Jancke L. Pre-reflective and reflective self-reference: A spatiotemporal EEG analysis. NeuroImage. 2008;42:437–449. doi: 10.1016/j.neuroimage.2008.01.060. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, Craik F, et al. Distributed self in episodic memory: Neural correlates of successful retrieval of self-encoded positive and negative personality traits. NeuroImage. 2004;22:1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: An interactive, multimodal visualization and analysis system for neuroanatomical imaging. NeuroImage. 1997;5(1):13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds – A biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gaesser D, Sacchetti DC, Addis DR, Schacter DL. Characterizing age-related changes in remembering the past and imagining the future. Psychology and Aging. 2011;26:80–84. doi: 10.1037/a0021054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser D, Schacter DL. Episodic simulation and episodic memory can increase intention to help others. Proceedings of the National Academy of Sciences U.S.A. 2014;111(12):4415–4420. doi: 10.1073/pnas.1402461111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, McLelland VC, Addis DR, Schacter DL. Imagining the future: evidence for a hippocampal contribution to constructive processing. Hippocampus. 2013;23:1150–1161. doi: 10.1002/hipo.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. Philosophical conceptions of the self: Implications for cognitive science. Trends in Cognitive Science. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gilbert DT, Wilson TD. Prospection: Experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory - one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42(10):1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47(7):1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences, U.S.A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society. 1868;2:327–347. [Google Scholar]
- Hassabis D, Kumaran D, Vann S, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences. 2007;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. The construction system of the brain. Philosophical Transactions Royal Society London B Biological Science. 2009;64:1263–1271. doi: 10.1098/rstb.2008.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RE, Cruet D, Johnson BR, Wisnicki KS. Self-focused attention, gender, gender role, and vulnerability to negative affect. Journal of Personality & Social Psychology. 1988;55:967–978. doi: 10.1037//0022-3514.55.6.967. [DOI] [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges J, Piguet O. Exploring the content and quality of episodic future simulations in semantic dementia. Neuropsychologia. 2012;50:3488–3495. doi: 10.1016/j.neuropsychologia.2012.09.012. 2012. [DOI] [PubMed] [Google Scholar]
- Irish M, Hodges JR, Piguet O. Episodic future thinking is impaired in the behavioural variant of frontotemporal dementia. Cortex. 2013;49(9):2377–2388. doi: 10.1016/j.cortex.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, et al. Towards a functional neuroanatomy of self processing: Effects of faces and words. Brain Research: Cognitive Brain Research. 2000;10:133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Prefrontal cortex damage abolishes brand-cued changes in cola preference. Social, Cognitive, and Affective Neuroscience. 2008;3(1):1–6. doi: 10.1093/scan/nsm032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Frontiers in Human Neuroscience. 2008;2 doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan D, Carson N, Addis DR, Rosenbaum RS. Deficits in past remembering extent to future imagining in a case of developmental amnesia. Neuropsychologia. 2010;48:3179–3186. doi: 10.1016/j.neuropsychologia.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Levine B. Autobiographical memory and the self in time: brain lesion effects, functional neuroanatomy, and life-span development. Brain and Cognition. 2004;55:54–68. doi: 10.1016/S0278-2626(03)00280-X. [DOI] [PubMed] [Google Scholar]
- Levine B, Black SE, Cabeza R, Sinden M, McIntosh AR, Toth JP, Tulving E, et al. Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121:1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay J, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- Lewis M, Carmody DP. Self-representation and brain development. Developmental Psychology. 2008;44:1329–1334. doi: 10.1037/a0012681. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay D. Development of self-recognition, personal pronoun use, and pretend play during the 2nd year. Child Development. 2004;75(6):1821–1831. doi: 10.1111/j.1467-8624.2004.00819.x. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Baron-Cohen S. Unravelling the paradox of the autistic self. WIREs: Cognitive Science. 2010;1(3):393–403. doi: 10.1002/wcs.45. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Lai M, Baron-Cohen S. Self-referential and social cognition in a case of autism and agenesis of the corpus callosum. Molecular Autism. 2012;3(14):1–15. doi: 10.1186/2040-2392-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ, Buchel C. Patterns of hippocampal-cortical interaction dissociate temporal lobe memory subsystems. Hippocampus. 2000;10:475–482. doi: 10.1002/1098-1063(2000)10:4<475::AID-HIPO14>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Ebner NC, Tubridy SM, Frankel H, Johnson MK. Age-group differences in medial cortex activity associated with thinking about self-relevant agendas. Psychological Aging. 2009;24(2):438–449. doi: 10.1037/a0015181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Liu Y, Williams DL, Keller TA, Minshew NJ, Just MA. The neural basis of deictic shifting in linguistic perspective-taking in high functioning autism. Brain. 2011;134:2422–2435. doi: 10.1093/brain/awr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. Memory, Imagination, and Predicting the Future: A Common Brain Mechanism? Neuroscientist. 2014;20(3):220–234. doi: 10.1177/1073858413495091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, Troyer AK, Levine B, Moscovitch M. Episodic, but not semantic, autobiographical memory is reduced in amnestic mild cognitive impairment. Neuropsychologia. 2008;46(13):3116–3123. doi: 10.1016/j.neuropsychologia.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R. Thinking of the future and the past: The roles of the frontal pole and the medial temporal lobes. NeuroImage. 2003;19(4):1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Philippi C, Duff MC, Denburg N, Tranel D, Rudrauf D. Medial prefrontal cortex damage abolishes the self-reference effect. Journal of Cognitive Neuroscience. 2012;24(2):475–481. doi: 10.1162/jocn_a_00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi C, Tranel D, Duff MC, Rudrauf D. Damage to the Default mode network disrupts autobiographical memory retrieval. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu070. doi: 10.1093/scan/nsu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefontal cortex in memory. Current Biology. 2013;23:R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin JS, Braverman A, Gilboa A, Stuss DT, Rosenbaum RS. Theory of mind development can withstand compromised episodic memory development. Neuropsychologia. 2012;50:3781–3785. doi: 10.1016/j.neuropsychologia.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Race E, Keane M, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. Journal of Neuroscience. 2011;31(28):10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, Keane M, Verfaellie M. Living in the moment: Patients with MTL amnesia can richly describe the present despite deficits in past and future thought. Cortex. 2013;49(6):1764–1766. doi: 10.1016/j.cortex.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. Binding items and contexts: the cognitive neuroscience of episodic memory. Current Directions in Psychological Science. 2010;19:131–137. [Google Scholar]
- Rice C, Pasupathi M. Reflecting on self-relevant experiences: Adult age differences. Developmental Psychology. 2010;46(2):479–490. doi: 10.1037/a0018098. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. Journal of Personality and Social Psychology. 1977;35(9):677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Stuss DT, Levine B, Tulving E. Theory of mind is independent of episodic memory. Science. 2007;318:1257. doi: 10.1126/science.1148763. [DOI] [PubMed] [Google Scholar]
- Rubin RD, Watson PD, Duff MC, Cohen NC. The role of the hippocampus in flexible cognition and social behavior. Frontiers in Human Neuroscience. 2014;8:742. doi: 10.3389/fnhum.2014.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rude SS, Gortner E, Pennebaker JW. Language use of depressed and depression-vulnerable college students. Cognition & Emotion. 2004;18:1121–1133. [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial prefrontal damage. Neuropsychologia. 1991;29(12):1241–1249. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. On the constructive episodic simulation of past and future events. Behavioral & Brain Sciences. 2007a;30:299–351. [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society (B) 2007b;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic Simulation of Future Events: Concepts, Data, and Application. Annals of the New York Academy of Sciences, Special Issue: The Year in Cognitive Neuroscience. 2008;1124:39–60. doi: 10.1196/annals.1440.001. 2008. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. The Journal of Neurology, Neurosurgery and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Higher-order cognitive impairments and frontal lobe lesions in man. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal Lobe Function and Dysfunction. Oxford University Press; New York: 1991. pp. 125–138. [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interaction in long-term memory. Nature Reviews Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Spreng N. Examining the role of memory in social cognition. Frontiers in Psychology. 2013;4:437. doi: 10.3389/fpsyg.2013.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:485–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Squire L. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, van der Horst A, McDuff SGR, Frascino JC, Hopkins RO, Mauldin KN. Role of the hippocampus in remembering the past and imagining the future. Proceedings of the National Academy of Sciences. 2010;107:19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinworth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: Evidence from H.M. and W.R. Neuropsychologia. 2005;43:479–496. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Reviews of Psychology. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Sui J, Humphreys GW. Self-referential processing is distinct from semantic elaboration: Evidence from long-term memory effects in a patient with amnesia and semantic impairments. Neuropsychologia. 2013;51(13):2663–2673. doi: 10.1016/j.neuropsychologia.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: A meta-analysis. Psychological Bulletin. 1997;121(3):371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Neural substrates of envisioning the future. Proceedings of the National Academy of Sciences USA. 2007;104(2):642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D. Theories of clinical neuropsychology and brain-behavior relationships: Luria and beyond. In: Morgan JE, Ricker JH, editors. Textbook of clinical neuropsychology. Taylor and Francis; New York: 2007. pp. 27–39. [Google Scholar]
- Tulving E. How many memory systems are there? American Psychologist. 1985;40(4):385–398. [Google Scholar]
- Wang JX, Cohen NJ, Voss JL. Covert rapid action-memory simulation (CRAMS): A hypothesis of hippocampal-prefrontal interactions for adaptive behavior. Neurobiology of Learning and Memory. 2015;117:22–33. doi: 10.1016/j.nlm.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Bechara A, Tranel D, Damasio H, Hauser M, Damasio A. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron. 2010;65:845–51. doi: 10.1016/j.neuron.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman A, Beschin N, Dewar M, Della Sala S. Imagining the present: Amnesia may impair descriptions of the present as well as of the future and the past. Cortex. 2012;49(3):637–645. doi: 10.1016/j.cortex.2012.03.008. [DOI] [PubMed] [Google Scholar]