Summary
Background
Dendrites often display remarkably complex and diverse morphologies that are influenced by developmental and environmental cues. Neuroplasticity in response to adverse environmental conditions entails both hypertrophy and resorption of dendrites. How dendrites rapidly alter morphology in response to unfavorable environmental conditions is unclear. The nematode Caenorhabditis elegans enters into a stress-resistant dauer larval stage in response to an adverse environment.
Results
Here we show that the IL2 bipolar sensory neurons undergo dendrite arborization and axon remodeling during dauer development. When dauer larvae are returned to favorable environmental conditions, animals resume reproductive development and IL2 dendritic branches retract, leaving behind remnant branches in post-dauer L4 and adult animals. The C. elegans furin homolog KPC-1 is required for dauer IL2 dendritic arborization and dauer specific nictation behavior. kpc-1 is also necessary for dendritic arborization of PVD and FLP sensory neurons. In mammals, furin is essential, ubiquitously expressed, and associated with numerous pathologies including neurodegenerative diseases. While broadly expressed in C. elegans neurons and epithelia, kpc-1 acts cell autonomously in IL2 neurons to regulate dauer-specific dendritic arborization and nictation.
Conclusions
Neuroplasticity of the C. elegans IL2 sensory neurons provides a paradigm to study stress-induced and reversible dendritic branching, and the role of environmental and developmental cues in this process. The newly discovered role of KPC-1 in dendrite morphogenesis provides insight into the function of proprotein convertases in nervous system development.
Introduction
Animals display numerous adaptations in response to stressful environmental conditions. Under ideal environmental conditions, the nematode C. elegans develops from an embryo through four larval stages before molting into the reproductive adult [1]. Under stressful conditions of high population density, starvation and high temperature, C. elegans develops into an alternative larval stage called dauer (Figure S1) [2]. Dauers are morphologically and behaviorally distinct from the non-dauer third larval L3 stage and adapted to survive stressful environmental conditions [3]. One adaptation is nictation behavior, mediated by the IL2 neurons, wherein the dauer larvae stand on their tail [3,4]. Upon return to a favorable environment, dauers reenter reproductive development (Figure S1) [2,5].
Differences in neuron morphology and gene expression are known to exist between dauers and non-dauers [6–8]. However, the nervous system of dauers is relatively unexplored compared to the adult hermaphrodite. Electron microscopy studies found differences between C. elegans dauers and non-dauers in the ciliated endings of neurons and surrounding glia [8,9].
Most neurons in C. elegans are morphologically simple [10]. Electron microscopy and reconstruction of the C. elegans nervous system suggested there were few, if any, branching dendrites [10]. However, recent studies using GFP revealed that the PVD and FLP polymodal sensory neurons undergo progressive dendritic arborization with increasing complexity as the animal develops from L2 to adult (reviewed in [11]).
Here we show that the IL2 neurons undergo dramatic remodeling during dauer development including dendrite arborization. Following an exit from dauer, IL2 processes retract and return to the non-dauer morphology. Using a candidate-gene approach, we identified the POU-homeodomain transcription factor UNC-86 and RFX transcription factor DAF-19 as regulators of dauer IL2 remodeling. From a forward genetic screen, we identified KPC-1, the C. elegans homolog of furin, as a cell autonomous regulator of dauer-specific IL2 arborization and function.
Results
Dauer IL2 neurons display dendrite arborization and multipolarity
In non-dauer larvae and adults, the six IL2 neurons (left-right pairs located in the subdorsal, subventral, and lateral hexaradiate zones) have single unbranched primary (1°) dendrites, ending in sensory cilia that are exposed to the external environment (Figure 1A) [10,19]. Although the six IL2 neurons have a similar gross structure during non-dauer stages and express many of the same genes, the lateral IL2s display different neuronal connectivity and morphology [10,19,20]. Based on these observations and the anatomical differences described herein, we refer to the dorsal and ventral IL2 neurons as IL2Qs (inner labial type 2 quadrants) while the left and right IL2s are referred to as IL2Ls (inner labial type 2 laterals). This nomenclature is currently used for a set of six sensory outer labial neurons [10].
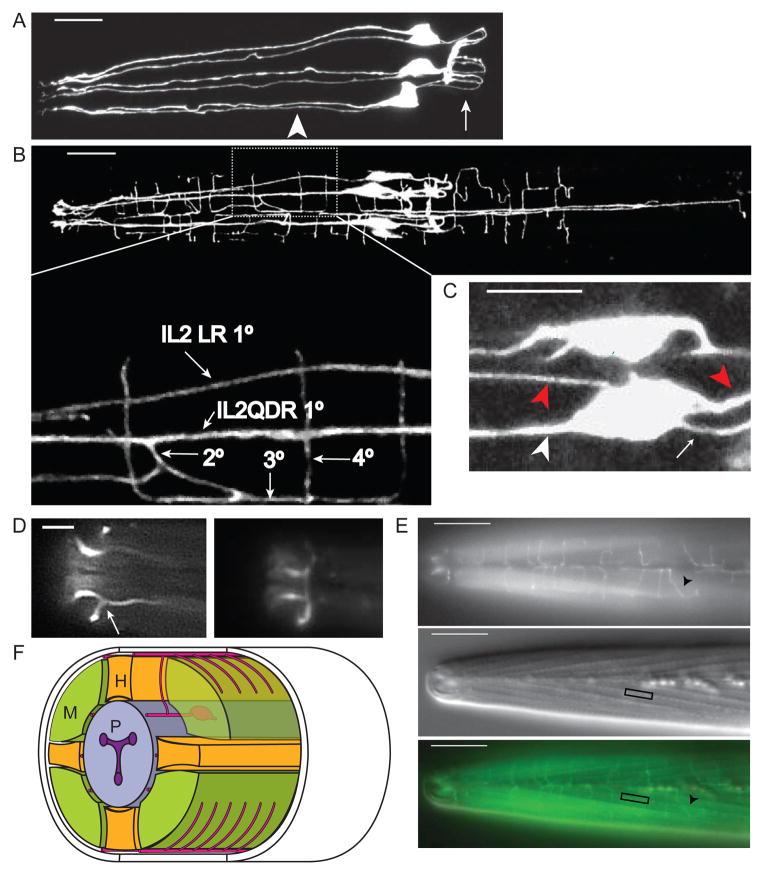
Figure 1. The IL2 neurons show extensive remodeling during dauer.
(A) Lateral Z-projection of a wild-type L3 expressing Pklp-6::GFP in IL2s. The six IL2s are arranged in a hexaradiate pattern with single 1° dendrites (arrowhead) anterior of the cell body. Axons (arrow) posterior from the cell body form a loop which innervate the nerve ring. Scale bar, 10 μm.
(B) Dorsal Z-projection of wild-type dauer expressing Pklp-6::GFP in IL2s. The IL2Q (for quadrant) dendrites in dauer are highly branched. Zoomed inset: The IL2QDR 1° dendrite extends a 2° dendrite towards the dorsal midline which branches, forming a 3° dendrite that travels along the dorsal midline. This 3° dendrite branches and extends 4° dendrites into the body-wall muscle quadrants. Similar branching patterns are seen on the ventral body-wall. Scale bar, 10 μm.
(C) Z-projection of IL2QDL dauer cell body expressing Pklp-6::GFP. The IL2Qs extend additional dauer-specific primary dendrites (1d°) (red arrowheads) in addition to the original non-dauer anteriorly directed 1° dendrite (white arrowhead), and posteriorly directed axon (white arrow). Scale bar, 5 μm.
(D) Dorsal view of dauer nose expressing Plag-2::GFP showing (left) the single branch (arrow) extending from the lateral IL2LL 1° dendrite and (right) a body wall view of the same animal showing the formation of the crown from branches emerging from the IL2L dendrites. Scale bar, 5 μm.
(E) Wild-type dauers extend fine dendritic processes along the body wall. Top: Dorsal/ventral view of body wall in wild-type dauer expressing Plag-2::GFP in IL2 neurons. Quaternary dendrites (arrowhead) extend from the dorsal/ventral midlines perpendicularly into the muscle quadrants. Middle: Same animal and focal plane with DIC Nomarski optics. Somatic muscle dense bodies (box), which serve as connections with the overlying hypodermis, are evident. Bottom: Merged image. Scale bars, 10 μm.
(F) Oblique transverse schematic of dauer IL2 branching. M:muscle, P:pharynx, H:hypodermis.
Using multiple fluorescent reporters expressed in the IL2s, we found that during dauer the IL2Qs undergo dendrite arborization resulting in a three-fold increase in total dendritic length (Figures 1 and S2A). Remodeling of IL2 axons was also observed, including branching and axonal thickening (Figure S2B).
The IL2Q dendritic arbors in dauers are distinct and more variable in structure from the well-characterized and highly stereotyped PVD dendritic arbors in adults [21]. During dauer, the IL2Q 1° dendrites extend secondary (2°) dendrites directly to the dorsal (for IL2QDs) and ventral (for IL2QVs) midlines (Figures 1B and 1F). This differs from PVD neurons where the 2° dendrites travel along the body wall dorsally and ventrally to the sub-lateral nerves (see Figure 2A in [21]). Upon reaching the dorsal or ventral midline, the IL2Q 2° dendrites branch and extend 3° dendrites in anterior and posterior directions along the midlines. As the IL2Q 3° dendrites travel along the midline, they extend 4° dendrites perpendicular from the midline into the body-wall muscle quadrants (Figure 1B and F). Each 4° dendrite is spaced and does not overlap with another, suggestive of self-avoidance. Occasionally, a given 4° dendrite branches again producing a 5° dendrite that extends the potential receptive field in the body-wall. Generally the body-wall 4° dendrites will remain in the ipsilateral body-wall quadrant. In rare instances, we observed wild-type (WT) dauers whose dendrites crossed the dorsal or ventral midlines and extended 4° dendrites to the contralateral side (Figure S2C).
In addition to dendritic branches emerging from the 1° dendrites, dauer IL2Qs extend additional 1° dendrites from the cell bodies, resulting in a shift from bipolar to multipolar neurons. We refer to these additional dendrites as dauer-specific primary dendrites (1d°) (Figure 1C). The 1d° dendrites extend to both the dorsal/ventral midlines as well as the lateral midlines, and remain within the ipsilateral body quadrant. For example, the IL2QDL may send 1d° dendrites to both the dorsal and lateral left midlines. Upon reaching the midlines the 1d° dendrites branch to form 2d° dendrites that extend in anterior and posterior directions. The 2d° dendrites then branch and extend 3d° dendrites perpendicular into the ipsilateral body-wall field, similar to the 4° dendrites that originate from the 1° dendrites.
The IL2Ls remodel during dauer in a pattern distinct from the IL2Qs. The dauer IL2Ls extend only a single 2° dendrite, at the distal end of the 1° dendrite, towards the lateral midline. Upon reaching the midline, the IL2L 2° dendrite extends 3° dendrites around the circumference of nose. Frequently (~50% in WT), the 3° dendrites from each lateral IL2 neuron meet to form a crown-like structure (Figure 1D). It is uncertain if the crown processes fuse as seen with the amphid sheath cells in dauers [8,9].
Following a return to favorable environmental conditions, dauers recover and reenter reproductive developmental [2] (Figure S1). During the 12–15 hour recovery period the IL2 dendritic arbors retract, leaving behind remnant branches. Remnant branches, typically short 2° or 1d° dendrites that approach but rarely meet the midline, are seen in 75.6% (N=41) of post-dauer L4 and adult animals (Figure 3A). This result illustrates the influence of the environment and early developmental events on the adult nervous system.
Figure 3. Following recovery from the dauer stage, IL2Q arbors undergo incomplete retraction.
(A) Lateral Z-projection of post-dauer L4 expressing Pklp-6::GFP. Nematodes often will retain short remnant 2° (arrows) dendrites following recovery from dauer. Scale bar, 10 μm.
(B) Quantification of relative IL2Q dendrite length following the return of dauers to favorable conditions (plentiful food, low population density) fits a sigmoidal response curve (Y=1.24+[2.518÷(1+10(0.183x-0.616))], R2=0.803). A ratio of total/primary dendritic length was used to adjust for changes in total body length. Each data point represents a separate animal (N=35 animals).
IL2 dendritic arborization is observed in 100% of wild-type dauers from starved conditions or induced through exposure to dauer pheromone. Mutations in daf-2 and daf-7, which cause constitutive dauer formation under favorable environmental conditions, also result in dauer-specific IL2 arborization. Exposure to dauer-inducing conditions in non-dauer animals does not result in IL2 arborization (data not shown). We do not observe similar arborization during dauer in the following neurons: touch receptor neurons; PVDs; FLPs; CEPs; amphid and phasmid neurons. Together these data suggest that the IL2s possess the ability to dynamically remodel, and that arborization is an inherent component of the many morphological and behavioral adaptations seen during dauer.
Development of dauer-specific IL2Q morphology is a rapid and dynamic process
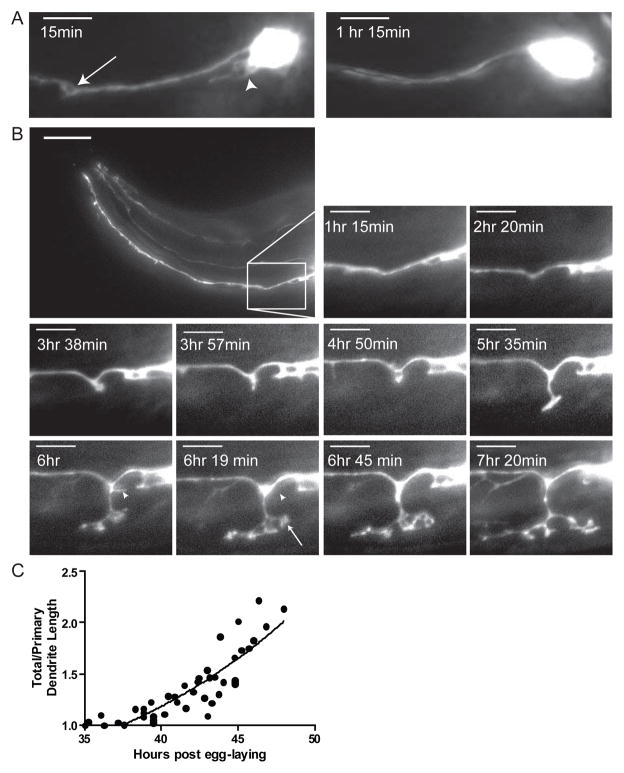
To follow the development of dauer-specific IL2Q arborization, we performed time-lapse imaging of the IL2Qs during the molt into dauer (Figure S1). At the beginning of this molt an initial 1d° dendrite forms on the IL2Q cell bodies (Figure 2A) and retracts soon after the closure of the stoma. After the original 1d° extension and retraction event, short puncta form and retract along the length of the original 1° dendrite over the next 3–4 hours (Figure 2B). Approximately 5–6 hours following molt onset, coinciding with the shrinking of the pharynx, several of the 2° dendritic sprouts extend towards the ventral or dorsal midlines. Approximately 6–8 hours following molt onset, the developing 2° dendrites rapidly grow in an exponential fashion, forming growth cones and 3° dendrites that expand and collapse (Figure 2B, compare 6hr to 6hr 19 min). Once the 2° dendrites reach the dorsal and ventral midline they branch to form both anteriorly and posteriorly directed 3° dendrites. Continued observation beyond approximately 8 hours following molt onset was technically impossible due to radial shrinkage and subsequent movement within old cuticles. In summary, dendritic arborization begins slowly and rapidly increases leading to an exponential increase in total dendritic length (Figure 2C).
Figure 2. Time-lapse imaging of IL2Qs during dauer formation reveals rapid dendrite arborization.
(A) Z-projection time-lapse images of a single animal expressing Pklp-6::tdTomato following the onset of the L2d molt into dauer. 15 minutes following the L2d molt, 2° dendritic sprouts (arrow) and 1d° dendrites (arrowhead) appear on an IL2QDL neuron. 75 minutes after the onset of the L2d molt, these sprouts have retracted. Scale bar, 10 μm.
(B) Lateral Z-projection time-lapse images of single animal expressing Pklp-6::tdTomato during the L2d molt into dauer. The formation of putative growth cones (arrow at 6 hr 19 min) as well as the retraction of branches (arrowheads at 6 hr – 6 hr 19 min) of IL2Qs is seen. Inset scale bar, 5 μm.
(C)Quantification of relative IL2Q dendritic length during dauer formation following the onset of the L2d molt fits an exponential curve (Y= 0.849*e(0.066x), R2=0.7436). A ratio of total/primary dendritic length was used to adjust for changes in total body length. Each animal examined is represented by multiple time-points (N=9 animals, 5–25 time points per animal).
We examined the morphology of IL2 neurons following a transfer of dauers to favorable environmental conditions (plentiful food, low population density) (Figure 3). While commitment to dauer recovery is made within the first hour upon return to favorable conditions [5], no obvious changes in IL2 arbors are seen in dauers during the first hour following transfer. Within three hours in favorable conditions, loss of the 4° body wall dendrites first occurs. Almost all 4° body-wall dendrites are retracted by the onset of pharyngeal pumping (3–4 hours following transfer to food); however, 2° dendrites and 1d° dendrites are still present. Most branches are retracted, albeit often incompletely, at the molt into L4 (12–14 hours post transfer) (Figure 3).
IL2Q and IL2L dendritic morphology are regulated by independent transcription factors
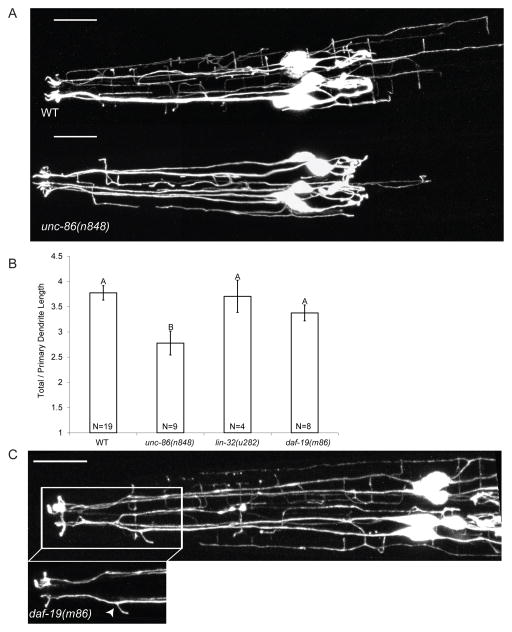
To begin to understand the mechanisms regulating dauer-specific IL2 branching, we examined several candidate genes. The POU homeodomain transcription factor UNC-86 is required for cell identity of several neurons, including PVD and IL2 [22]. UNC-86 also controls dendritic outgrowth in PVD [23]. As expected, null alleles of unc-86 result in a complete lack of IL2 reporter GFP expression, presumably due to cell fate defects (data not shown). However, the unc-86(n848) reduction-of-function mutant [24] possesses IL2 neurons with normal cell somas, axons, and 1° dendrites. Additionally, unc-86(n848) mutants show normal expression of several IL2-specific reporters, suggesting retention of IL2 cell fate. Sequencing of unc-86(n848) revealed a point mutation at a splice donor site within the DNA-binding homeodomain (Figure S3C). Interestingly, unc-86(n848) causes a significant decrease in the number of 4° body-wall dendrites, resulting in a total decrease in dendritic length, number of branch points and a redistribution of the arbor (Figure 4A and B and S3A and B). These results indicate that unc-86 is required for IL2Q dendritic arborization.
Figure 4. The transcription factors UNC-86 and DAF-19 regulate distinct components of dauer-specific IL2 remodeling.
(A) Z-projections of WT (top) and unc-86(n848) (bottom)dauers expressing P F28A12.3::GFP. Scale bars, 10 μm.
(B) unc-86(n848) dauers show significantly smaller IL2Q arbors than WT. Dendritic length was measured as a ratio of total/primary dendritic length to compensate for differences in body length between genotypes. See also Figure S3. Data are mean ± SEM. Genotypes with different letters above bars are statistically different (α=0.01) as determined by ANOVA followed by Tukey’s post-hoc test for comparison of multiple genotypes.
(C) Dorsal Z-projection of daf-19(m86) dauer expressing PF28A12.3::GFP with inset of IL2LL dendrite showing supernumerary branching (arrowhead). Scale bar, 10 μm.
The helix-loop-helix transcription factor, LIN-32, acts in parallel with UNC-86 to determine IL2 cell identity [25]. Indeed, a hypomorphic lin-32 mutant fails to express Pklp-6:: GFP in the full complement of six IL2 neurons (x̄ =1.71±0.97 neurons/animal, N=34 animals). However, typical dauer arborization is seen in IL2Q neurons that express Pklp-6::GFP (Figure 4B and S3D). The LIM homeodomain transcription factor MEC-3 is also required for PVD dendritic branching and acts in concert with UNC-86 for PVD and touch receptor neuron development [26,27]. In mec-3 mutants, IL2 dauer-specific arborization appears normal (data not shown).
The RFX transcription factor DAF-19 is a master regulator of ciliogenesis, with daf-19(m86) null mutants lacking all cilia [28,29]. Interestingly, IL2Q branching is normal in daf-19(m86) mutants suggesting that external signals sensed by the IL2Q cilia do not directly initiate IL2Q branching (Figure 4B). In WT dauers, the IL2Ls extend only a single 2° process which forms a crown-like structure (Figure 1D). However, daf-19(m86) dauers often display an additional 2° processes on one or both IL2Ls (19/34 daf-19(m86) with extra IL2L processes, 1/21 WT with extra IL2L processes, p<0.001 Fisher’s Exact Test) (Figure 4C). We next examined the IL2s in daf-19(n4132), an allele that disrupts a DAF-19 isoform necessary for the function but not development of IL2 cilia [20]. Interestingly, daf-19(n4132) dauers do not display additional IL2L processes (N=24 animals). These results indicate that either DAF-19 is acting outside the IL2Ls to inhibit additional processes in dauers or that the presence of IL2 cilia has an inhibitory action on IL2L process formation. Combined, these data are consistent with the IL2Qs and IL2Ls being two separate neuronal classes.
KPC-1, a furin proprotein convertase (PC) homolog, is necessary for proper dendrite arbor organization
To identify novel genes involved in dauer-specific branching, we performed a mutagenesis screen. We examined approximately 1500 haploid genomes for mutants with defects in dauer-specific IL2 remodeling. From this screen we isolated an allele of the PC-encoding gene, kpc-1 (Kex2/Proprotein Convertase). kpc-1(my24) mutants have highly disorganized and truncated dauer-specific IL2Q branching with 100% penetrance (Figure 5A and Supplemental Experimental Procedures). kpc-1(my24) dauers show resistance to 1% SDS and no obvious defects in dauer formation or recovery or in IL2 retraction following dauer recovery. Non-dauer kpc-1 mutants show no obvious defects in dendrite morphology (Figure S5A).
Figure 5. KPC-1 is required for multidendritic neuron arborization.
(A) Z-projections of wild-type (top) and kpc-1(my24) (bottom)dauers expressing P klp-6::GFP. Disruption of kpc-1 results in disorganized IL2 dendritic arbors in 100% of animals examined (N>100). See also Figure S5. Scale bar, 10 μm.
(B) Exon/Intron diagram of kpc-1. The kpc-1(my24) allele introduces a c→t missense mutation resulting in a P440L amino acid change at a highly conserved residue (highlighted yellow in alignment) four amino acids C-terminal from the catalytic serine (highlighted red in alignment). The kpc-1(gk8) deletion allele removes the majority of the catalytic domain.
(C) Lateral Z-projections of PVD neurons in wild-type (left) and kpc-1(gk8) (right) adults expressing PF49H12.4::GFP. Similar to the IL2 neurons, disruption of kpc-1 leads to disorganized and truncated arbors in PVDs. Scale bar, 10 μm. See also Figure S5C.
PCs are highly conserved serine proteases, which cleave numerous proproteins into their active forms [12]. PCs contain an N-terminal signal peptide and prodomain that is autocatalytically cleaved (Figure 5B). The catalytic domain contains conserved aspartate, histidine, and serine residues which serve as the catalytic triad necessary for nucleophilic attack of cleavage sites [13]. The conserved “P domain” is essential for PC function and is thought to stabilize the PC [30]. The C-terminus is variable among PCs and may include transmembrane and cysteine-rich domains [13,31].
kpc-1(my24) introduces a missense mutation resulting in a P440L amino acid change at a highly conserved proline four residues C-terminal to the catalytic serine (Figure 5B). Examination of the crystal structure of mouse furin [32] shows the homologous proline is located within the catalytic pocket and a change to a leucine residue may obstruct cleavage of substrates (Figure S4). kpc-1(my24) fails to complement kpc-1(gk8), a previously isolated deletion allele which removes most of the catalytic domain (Figure 5B).Furthermore, kpc-1(my24) and kpc-1(gk8) display a similar severity of IL2 branching defects, suggesting that kpc-1(my24) is a loss of function allele (Figure S5B). There are four PCs encoded in the C. elegans genome [17]. However, mutations in the other three PC genes bli-4, aex-5 and egl-3 do not produce obvious defects in IL2 dauer-specific dendritic branching, indicating a unique role for KPC-1 among the PCs in IL2 remodeling during dauer (data not shown).
We next explored the role of KPC-1 in the multidendritic sensory neurons PVD and FLP. We found that, similar to the IL2 dauer arbors, the PVD and FLP multidendritic arbors are highly disorganized and truncated in adult kpc-1 mutants (Figure 5C and S5C). Interestingly, previous microarray data identified kpc-1 as upregulated in the PVDs during arborization [23]. kpc-1 mutants are wild-type for amphid, phasmid and IL2 dye-filling and showed no obvious defects in touch-receptor neuron morphology (data not shown) or function [33]. These data suggest that KPC-1 is necessary for proper organization of multidendritic arbors in C. elegans.
KPC-1 acts cell-autonomously to regulate dauer-specific IL2 arborization
Using a transcriptional reporter consisting of the 3kb 5′ region upstream of kpc-1 fused to GFP (Pkpc-1::GFP), we found that kpc-1 is expressed in numerous neuronal and epithelial cells during dauer (Figure 6A). Processes adjacent to the body wall in dauers resemble those of IL2Q 4° dendrites (Figure 6B). To determine whether kpc-1 is expressed in the IL2s, we coinjected the Pkpc-1::GFP reporter with a Pklp-6::tdTomato reporter that is expressed exclusively in the IL2s. During non-dauer stages, kpc-1 is not expressed in the IL2 neurons (data not shown). In dauers Pkpc-1::GFP and Pklp-6::tdTomato are coexpressed, indicating that kpc-1 is upregulated in the IL2s during dauer (Fig. 6C). Non-dauer expression was consistently observed in the ventral nerve cord and pharynx with strong expression in the g2 pharyngeal gland cells and vpi pharyngeal intestinal valve cells (Figures S6).
Figure 6. kpc-1 is expressed broadly but acts cell autonomously to regulate IL2Q dauer-specific remodeling.
(A) Ventral Z-projection of dauer expressing Pkpc-1::GFP with expression in numerous neuronal and non-neuronal cells throughout the head. Scale bar, 10 μm.
(B) Ventral body-wall plane of same animal as in (A) showing GFP expression in IL2Q dauer-specific branches (arrow) as well as several additional neuronal commissures (arrowhead, amphid commisure). Scale bar, 10 μm.
(C) Dauer expressing both the IL2-specific reporter Pklp-6::tdTomato (left) and Pkpc-1::GFP (middle). An overlay image (right) demonstrates that Pkpc-1::GFP is expressed in the IL2s. Scale bar, 5 μm.
(D) kpc-1(gk8) defects in dauer-specific IL2Q arborization are rescued by constructs of either an IL2-specific promoter driving full length wild-type kpc-1 (Pklp-6::KPC-1) or full length kpc-1 tagged with dsRed and driven by the kpc-1 endogenous promoter (KPC-1::dsRed). Rescue was assessed by examining dauer-specific IL2Q arborization in kpc-1(gk8) dauers expressing Pklp-6::GFP in three independent transgenic lines (N=22–25 dauers per line). A mean ± SEM of the three lines is given. Statistical tests comparing the rescued lines with WT and kpc-1(gk8) cannot be performed due to a lack of variation in controls, however Pklp-6::KPC-1 and KPC-1::dsRed are not statistically different (Fisher’s Exact Test, p=0.9303).
(E) Z-projection of kpc-1(gk8) dauer head expressing KPC-1::dsRed. In most neurons KPC-1::dsRed is localized exclusively within the cell bodies and is not observed in neuronal processes. However in the ventral nerve cord KPC-1::dsRed is found in both cell bodies and neuronal processes (See Figure S6B). Scale bar, 10 μm.
We next asked if KPC-1 acts cell autonomously in the IL2s to control dauer-specific branching. We expressed KPC-1(+) under the control of the IL2-specific klp-6 promoter (Pklp-6::KPC-1) in a kpc-1(gk8) mutant background. The Pklp-6::KPC-1 transgene is sufficient to rescue the kpc-1 IL2 branching phenotype, indicating that KPC-1 acts cell autonomously to control dauer-specific IL2 arborization (Figure 6D).
To determine the subcellular localization of KPC-1, we created a translational reporter consisting of the 3kb 5′ region and the entire kpc-1 genomic sequence fused to dsRed. KPC-1::dsRed rescues the kpc-1(gk8) branching phenotype indicating that the transgene is functional in IL2 neurons (Figure 6D). The KPC-1 subcellular localization differs among neuronal types. In the majority of neurons, including the IL2s, KPC-1::dsRed is non-nuclear and excluded from all but the most proximal segments of the 1° dendrite and axon (Figure 6E). In ventral cord neurons, KPC-1 localizes to cell bodies and processes (Figure S6B).
KPC-1 regulates movement in both dauers and non-dauers
The kpc-1(gk8) is described as having a slight uncoordinated phenotype [17]. We find that adult kpc-1 mutants show fewer body bends than WT animals (Figure S6C). Adult motility is not rescued by the IL2-specific Pklp-6::KPC-1 transgene (data not shown). As kpc-1 is expressed in the ventral nerve cord (Figure S6B), which regulates movement, it seems likely that kpc-1 affects the function of these neurons.
Unlike non-dauer larval and adult stages, dauers tend to remain motionless [2]. Dauers are uniquely capable of performing a behavior termed nictation in which the animal elevates the majority of its body into the air for extended periods of time [3]. In nature, this behavior is thought to facilitate transport of dauers to new nutrient rich environments by passing arthropods. The IL2 neurons are required for nictation in dauers [4]. We found that kpc-1 and unc-86(n848) dauers are defective in nictation (Figure 7). As both unc-86 and kpc-1 mutants display motility defects, one possibility is that simply being uncoordinated (Unc) results in nictation defects. We previously examined the nictation behavior of several Unc or neurotransmitter/neuropeptide mutants including the proprotein convertase mutant egl-3 and GABA mutants unc-26 and unc-29, and showed that these mutants are capable of nictation [4]. To determine whether kpc-1 acts cell autonomously to regulate nictation, we expressed the IL2-specific Pklp-6::KPC-1 transgene in dauers and observed rescue of nictation defects. We conclude that kpc-1 acts cell autonomously in IL2 neurons to regulate dauer-specific nictation behavior (Figure 7) and dendritic arborization (Figure 6D).
Figure 7. kpc-1 and unc-86 regulate nictation behavior.
(A) Quantification of percent time spent nictating versus non-nictating [(Tnic/T)×100] in actively moving dauers as previously described [4]. kpc-1 and unc-86(n848) mutant dauers are defective in nictation ratio while IL2-specific rescue of kpc-1 restores nictation ratio to WT levels.
(B) Quantification of nictation initiation index [N/(Tnic-T)×100] as previously described [4]. kpc-1 and unc-86(n848) mutants are defective while IL2-specific rescue of kpc-1 restores initiation index to WT levels.
Data are means ± SEM. Genotypes with different letters above bars are statistically different (α=0.01) as determined by Kruskall-Wallis followed by Dunn’s multiple comparison. N=13–50 animals/genotype.
Discussion
Our finding of rapid and reversible dendritic arborization during dauer presents a new system for studying the molecular pathways leading from environmental stress to neuronal remodeling. Neuron morphology is affected by developmental and environmental conditions and the dendritic processes of neurons appear acutely sensitive to changing environments [34]. For example, following exposure to stress the mammalian brain undergoes contrasting patterns of dendrite resorption in the hippocampus and increased dendrite arborization in the amygdala [35]. In humans, alterations seen in dendrite morphology following extreme stress may serve a causative role in anxiety-related pathologies such as Post-Traumatic Stress Disorder (PTSD) [36].
Shared and distinct transcriptional mechanisms regulate PVD, FLP and IL2Q arborization. For example, the POU-homeodomain transcription factor, UNC-86, regulates arborization in both PVD neurons [23] and IL2Q dauer neurons (Figure 4A,B), while the LIM homeodomain transcription factor MEC-3 acts in concert with UNC-86 to regulate PVD dendritic branching, but not IL2Q arborization. Our study does not directly separate developmental morphogenesis from stress-induced plasticity per se. As both unc-86 and kpc-1 are necessary for both PVD and dauer-specific arborization, it is likely that these genes are acting on developmental morphogenesis rather than directly through a stress-induction pathway. One of the most striking phenotypic differences between the PVD/FLP and IL2 arbors is the ability of the IL2 arbor to retract upon a change in environmental conditions. Future examination of the loss of IL2Q arbors during dauer recovery may provide molecular insight into mechanisms regulating dendritic retraction.
IL2 neurons play roles in dauer maintenance and dauer-specific nictation behavior [4,37,38]. unc-86 and kpc-1 mutant dauers are defective in both IL2Q arborization and nictation. Furthermore, cell-specific rescue of kpc-1 restored nictation behavior to wild-type levels. While this evidence is indirect, it is tempting to hypothesize that IL2Q branches have a function in nictation. In the adult, the PVD and FLP neurons are polymodal sensory neurons, serving as both nociceptors and proprioceptors [21,39,40]. The morphological similarity between dauer IL2 arbors and PVD/FLP adult arbors may indicate a shared function as polymodal sensory neurons.
We found that kpc-1 is required for the organization of multidendritic arbors in C. elegans. KPC-1 is homologous to furin, an essential mammalian PC [17,41]. Furin activates semaphorins in vitro and is involved in axon guidance in the developing chick tectum [15,16] while Drosophila Fur1/furin mutants have synaptic target recognition defects [42]. In addition to our discovery of dendrite arborization, we found gross anatomical changes to IL2 axon morphology (Figure S2B); it will therefore be of great interest to examine IL2 synapses with TEM for likely changes in synaptic connectivity in dauer animals and the role of kpc-1 in these processes.
The substrate(s) of KPC-1 remain elusive. Furin is known to cleave numerous substrates [12]. Based on its similarity with furin, KPC-1 may be necessary for processing TGF-β ligands [17]. However, single mutants of the four TGF-β ligands with predicted furin cleavage sites (daf-7, unc-129, dbl-1, tig-2) did not phenocopy the kpc-1 mutant IL2 arborization defect (data not shown). The identification of individual substrates responsible for IL2Q arborization is an important but challenging goal. An expansion of our genetic screen, combined with the use of a proteomics approach may facilitate identification of KPC-1 substrates and understanding of PC biology.
Experimental Procedures
All nematodes were grown under standard conditions [1]. The following transgenic strains were used to image the IL2 neurons and considered wild-type in an N2 Bristol background: PT2519 myIs13[Pklp-6::GFP + pBX] III; JK2868 qIs56[Plag-2::GFP] V [37,43]; PT2038 pha-1; myEx632[Pklp-6::tdTOMATO + pBX]; PT2506 ofEx731[PF28A12.3::GFP] [44]. Additional methods are provided in the Supplemental Experimental Procedures.
Supplementary Material
Highlights.
C. elegans IL2 neurons develop complex dendritic arbors during dauer.
Dauer-specific IL2 arborization is rapid and reversible.
KPC-1/Furin regulates arborization of three classes of multidendritic neurons.
KPC-1 acts cell autonomously to regulate dauer IL2 arborization and nictation.
Acknowledgments
We thank the CGC, funded by the NIH Office of Research Infrastructure Programs (P40 OD010440), S. Shaham, T. Lamitina, M. Chalfie and P. Swoboda for providing strains; Noriko Goldsmith for assistance with confocal microscopy; Natalia Morsci and members of the Barr lab for unpublished data and critical reading of the manuscript; C. Britt Carlson and Michael Klaszky for technical assistance; and David Hall for valuable advice. This work was funded by the USDA (2010-65106-20587) and the New Jersey Commission on Spinal Cord Research (CSCR12FEL004) to NES and the NIH (5R01DK59418) to MMB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;1:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riddle DL, Albert PS. In: Genetic and environmental regulation of dauer larvae developmentC. elegans. Riddle DL II, Blumenthal T, Meyer BJ, Priess JR, editors. Plainview, NY: Cold Spring Harbor Laboratory Press; 1998. pp. 739–768. [PubMed] [Google Scholar]
- 3.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;2:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 4.Lee H, Choi MK, Lee D, Kim HS, Hwang H, Kim H, Park S, Paik YK, Lee J. Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat Neurosci. 2011;1:107–112. doi: 10.1038/nn.2975. [DOI] [PubMed] [Google Scholar]
- 5.Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984;2:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 6.Kim K, Li C. Expression and regulation of an FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. J Comp Neurol. 2004;4:540–550. doi: 10.1002/cne.20189. [DOI] [PubMed] [Google Scholar]
- 7.Peckol EL, Troemel ER, Bargmann CI. Sensory experience and sensory activity regulate chemosensory receptor gene expression in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2001;20:11032–11038. doi: 10.1073/pnas.191352498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albert PS, Riddle DL. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J Comp Neurol. 1983;4:461–481. doi: 10.1002/cne.902190407. [DOI] [PubMed] [Google Scholar]
- 9.Procko C, Lu Y, Shaham S. Glia delimit shape changes of sensory neuron receptive endings in C. elegans. Development. 2011;7:1371–1381. doi: 10.1242/dev.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond Ser B-Biol Sci. 1986;1165:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 11.Hall DH, Treinin M. How does morphology relate to function in sensory arbors? Trends Neurosci. 2011;9:443–451. doi: 10.1016/j.tins.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artenstein AW, Opal SM. Proprotein convertases in health and disease. N Engl J Med. 2011;26:2507–2518. doi: 10.1056/NEJMra1106700. [DOI] [PubMed] [Google Scholar]
- 13.Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;5:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 14.Scamuffa N, Calvo F, Chretien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. 2006;12:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- 15.Tassew NG, Charish J, Seidah NG, Monnier PP. SKI-1 and furin generate multiple RGMa fragments that regulate axonal growth. Dev Cell. 2012;2:391–402. doi: 10.1016/j.devcel.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Adams RH, Lohrum M, Klostermann A, Betz H, Puschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 1997;20:6077–6086. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thacker C, Rose AM. A look at the Caenorhabditis elegans Kex2/Subtilisin-like proprotein convertase family. Bioessays. 2000;6:545–553. doi: 10.1002/(SICI)1521-1878(200006)22:6<545::AID-BIES7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 18.Husson SJ, Clynen E, Baggerman G, Janssen T, Schoofs L. Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): mutant analysis by mass spectrometry. J Neurochem. 2006;6:1999–2012. doi: 10.1111/j.1471-4159.2006.04014.x. [DOI] [PubMed] [Google Scholar]
- 19.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975:313–338. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Schwartz HT, Barr MM. Functional specialization of sensory cilia by an RFX transcription factor isoform. Genetics. 2010;4:1295–1307. doi: 10.1534/genetics.110.122879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albeg A, Smith CJ, Chatzigeorgiou M, Feitelson DG, Hall DH, Schafer WR, Miller DM, 3rd, Treinin M. C. elegans multi-dendritic sensory neurons: morphology and function. Mol Cell Neurosci. 2011;1:308–317. doi: 10.1016/j.mcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finney M, Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell. 1990;5:895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- 23.Smith CJ, Watson JD, Spencer WC, O’Brien T, Cha B, Albeg A, Treinin M, Miller DM., 3rd Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Dev Biol. 2010;1:18–33. doi: 10.1016/j.ydbio.2010.05.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamelin M, Scott IM, Way JC, Culotti JG. The mec-7 β-tubulin gene of Caenorhabditis elegans is expressed primarily in the touch receptor neurons. EMBO J. 1992;8:2885–2893. doi: 10.1002/j.1460-2075.1992.tb05357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaham S, Bargmann CI. Control of neuronal subtype identity by the C. elegans ARID protein CFI-1. Genes Dev. 2002;8:972–983. doi: 10.1101/gad.976002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol. 2003;1:81–102. doi: 10.1016/s0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Way JC, Chalfie M. mec-3, a homeobox-containing gene that specifies differentiation of the touch receptor neurons in C. elegans. Cell. 1988;1:5–16. doi: 10.1016/0092-8674(88)90174-2. [DOI] [PubMed] [Google Scholar]
- 28.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;2:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 29.Swoboda P, Adler HT, Thomas JH. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol Cell. 2000;3:411–421. doi: 10.1016/s1097-2765(00)80436-0. [DOI] [PubMed] [Google Scholar]
- 30.Zhou A, Martin S, Lipkind G, LaMendola J, Steiner DF. Regulatory roles of the P domain of the subtilisin-like prohormone convertases. J Biol Chem. 1998;18:11107–11114. doi: 10.1074/jbc.273.18.11107. [DOI] [PubMed] [Google Scholar]
- 31.Seidah NG. The proprotein convertases, 20 years later. Methods Mol Biol. 2011:23–57. doi: 10.1007/978-1-61779-204-5_3. [DOI] [PubMed] [Google Scholar]
- 32.Henrich S, Cameron A, Bourenkov GP, Kiefersauer R, Huber R, Lindberg I, Bode W, Than ME. The crystal structure of the proprotein processing proteinase furin explains its stringent specificity. Nat Struct Biol. 2003;7:520–526. doi: 10.1038/nsb941. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Kim K, Li C, Barr MM. FMRFamide-like neuropeptides and mechanosensory touch receptor neurons regulate male sexual turning behavior in Caenorhabditis elegans. J Neurosci. 2007;27:7174–7182. doi: 10.1523/JNEUROSCI.1405-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni VA, Firestein BL. The dendritic tree and brain disorders. Mol Cell Neurosci. 2012;1:10–20. doi: 10.1016/j.mcn.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;15:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller MM, McEwen BS. Establishing an agenda for translational research on PTSD. Ann N Y Acad Sci. 2006:294–312. doi: 10.1196/annals.1364.023. [DOI] [PubMed] [Google Scholar]
- 37.Ouellet J, Li S, Roy R. Notch signalling is required for both dauer maintenance and recovery in C. elegans. Development. 2008;15:2583–2592. doi: 10.1242/dev.012435. [DOI] [PubMed] [Google Scholar]
- 38.Lewis JA, Hodgkin JA. Specific neuroanatomical changes in chemosensory mutants of the nematode Caenorhabditis elegans. J Comp Neurol. 1977;3:489–510. doi: 10.1002/cne.901720306. [DOI] [PubMed] [Google Scholar]
- 39.Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, Kindt KS, Hwang SW, Miller DM, 3rd, Treinin M, Driscoll M, et al. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci. 2010;7:861–868. doi: 10.1038/nn.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Schulze E, Baumeister R. Temperature- and touch-sensitive neurons couple CNG and TRPV channel activities to control heat avoidance in Caenorhabditis elegans. PLoS One. 2012;3:e32360. doi: 10.1371/journal.pone.0032360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roebroek AJ, Umans L, Pauli IG, Robertson EJ, van Leuven F, Van de Ven WJ, Constam DB. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development. 1998;24:4863–4876. doi: 10.1242/dev.125.24.4863. [DOI] [PubMed] [Google Scholar]
- 42.Kurusu M, Cording A, Taniguchi M, Menon K, Suzuki E, Zinn K. A screen of cell-surface molecules identifies leucine-rich repeat proteins as key mediators of synaptic target selection. Neuron. 2008;6:972–985. doi: 10.1016/j.neuron.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blelloch R, Anna-Arriola SS, Gao D, Li Y, Hodgkin J, Kimble J. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev Biol. 1999;1:382–393. doi: 10.1006/dbio.1999.9491. [DOI] [PubMed] [Google Scholar]
- 44.Phirke P, Efimenko E, Mohan S, Burghoorn J, Crona F, Bakhoum MW, Trieb M, Schuske K, Jorgensen EM, Piasecki BP, et al. Transcriptional profiling of C. elegans DAF-19 uncovers a ciliary base-associated protein and a CDK/CCRK/LF2p-related kinase required for intraflagellar transport. Dev Biol. 2011;1:235–247. doi: 10.1016/j.ydbio.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.