Abstract
Internal symmetry is commonly observed in the majority of fundamental protein folds. Meanwhile, sufficient evidence suggests that nascent polypeptide chains of proteins have the potential to start the co-translational folding process and this process allows mRNA to contain additional information on protein structure. In this paper, we study the relationship between gene sequences and protein structures from the viewpoint of symmetry to explore how gene sequences code for structural symmetry in proteins. We found that, for a set of two-fold symmetric proteins from left-handed beta-helix fold, intragenic symmetry always exists in their corresponding gene sequences. Meanwhile, codon usage bias and local mRNA structure might be involved in modulating translation speed for the formation of structural symmetry: a major decrease of local codon usage bias in the middle of the codon sequence can be identified as a common feature; and major or consecutive decreases in local mRNA folding energy near the boundaries of the symmetric substructures can also be observed. The results suggest that gene duplication and fusion may be an evolutionarily conserved process for this protein fold. In addition, the usage of rare codons and the formation of higher order of secondary structure near the boundaries of symmetric substructures might have coevolved as conserved mechanisms to slow down translation elongation and to facilitate effective folding of symmetric substructures. These findings provide valuable insights into our understanding of the mechanisms of translation and its evolution, as well as the design of proteins via symmetric modules.
Introduction
Symmetry is commonly observed in the majority of soluble and membrane-bound proteins in modern cells [1]. Most of these proteins are oligomeric complexes with two or more subunits assembled in a symmetric manner [1–3]. However, some researches also demonstrated that a large number of proteins consists of monomers that have an internal symmetric structure in the monomeric state. It has been reported that six out of ten fundamental protein superfolds exhibit internal structural symmetry [4]. The role of structural symmetry in protein function and evolution, and its utility in protein engineering and design has been the focus of a number of studies [5, 6]. Structural symmetry is thought to have arisen from intragenic duplication and fusion of smaller peptide modules [7–12]: 2-fold symmetry can be generated by single gene duplication and fusion; and higher order of symmetry requires subsequent duplication and fusion. The (αβ)8-barrel shows 2-fold internal structural symmetry and it is suggested that (βα)8-barrels are derived from ‘half-barrels’ [13]. The beta-trefoil is another common protein fold that exhibits three-fold structural symmetry [14]. Notably, a recent study on membrane proteins have also revealed a strong propensity toward internal symmetric architectures [15]. Generating large symmetric proteins from small structural units using preexisting genetic material may be one of nature’s major strategies to explore the existent protein folds [16]. This evolution process may confer several significant advantages to the functions of newly innovated proteins [17, 18]. For instance, tandemly duplicated superhelical structures exhibit an extensive accessible solvent surface that is capable of binding large substrates. Symmetry may also make certain proteins more compact and thermodynamically more stable, which confers great functional advantages: these proteins become more robust to mutations, or more resistant to destabilization induced by ligand binding.
In this paper, we focus on the relationship between mRNA sequence and protein structure from the viewpoint of symmetry. Although gene duplication and fusion have been proposed to be the origin of structural symmetry, it is agreed that sequence is much more divergent than structure. A number of methods have been developed to explore the internal similarity in protein sequences [19–21], which revealed that, for most of the structurally symmetric proteins, symmetry in sequence is typically of low level or undetectable. Previous researches have shown that when physiochemical properties of different amino acids are considered, symmetry in protein sequences can be more readily detected [22–25]. Here the intragenic symmetry for structural symmetric proteins is studied directly to explore the level of gene sequence conservation. While several other methods have been developed to detect large very similar DNA repeats [26], or tandem repeats [27], few methods have aimed at identifying small and divergent intragenic repeats that are related to protein structure. Here we shall use an improved recurrence quantification analysis to study the symmetry in both the nucleotide sequence and the triplet codon sequence [22, 28].
On the other hand, the studies on the mechanisms of protein translation have become a hotspot in recent years [29–34]. It is known that the rate of translation elongation for different regions within a single mRNAs is non-uniform [35, 36]; it may depend on several factors such as codon usage of the coding sequence, higher order of local mRNA structures, codon context and adjacent codons [37, 38]. The variations of translation rate can affect the co-translational folding process and thus have significant influence on the final conformation of the protein [39, 40]. For example, Kimchi-Sarfaty et al. reported that for MDR1 gene, frequent-to-rare codon substitutions appear to alter the shape of transport substrate binding site and substrate specificity of the gene product P-glycoprotein [41]. Replacement of rare codons by frequent ones for genes from Escherichia coli or Saccharomyces cerevisiae led to increased translation rate but with reduced activity of the cognate proteins [35, 42]. These evidences strongly support that the degeneracy of genetic code allows the mRNA sequences to contain an additional layer of information on protein structure.
Here we explore this issue for structural symmetric proteins. Since symmetry is a rule rather than an exception of nature, there should be some conserved mechanisms that genes adopt to modulate structural symmetry in proteins. Zhang et al. reported that discontinuous elongation of the peptide chain and selection of slow-translating clusters that locally slow down translation might be particularly important for multidomain proteins [40]. Tuller and colleagues reported a conserved pattern that the speed of translation is slow during the first 30–50 codons and this “ramp” should be significant for translation efficiency [43]. These results suggest that translational pauses or slow translated regions might be important during protein translation to coordinate the effective co-translational protein folding. In light of these works, we shall analyze different features of mRNAs to identify conserved mechanisms in genes that allow for an efficient folding of proteins into symmetric structure.
We carry out the proposed researches by using a set of representative proteins from the left-handed beta-helix (LβH) fold, taking advantage of our previous studies on the structural symmetry of this protein fold [44]. The LβH fold is interesting since it so far has been observed very infrequently [45]. It may represent the structure of a variety of amyloid fibrils associated with prion and Huntington’s diseases [46, 47]. The polypeptide chain coils into a left-handed helical structure formed by beta-strands and separated by loop regions. This fold generally associates with transferase activity and is presented in a broad range of taxons [48]. The structures of LβH fold have been reported to be two-fold symmetric [19, 44], which means that the structure of one single helix may be composed of two symmetric subhelices. Our results reveal that for all the proteins we studied, the same degree of two-fold intragenic symmetry can be detected, providing clear evidence of gene duplication and fusion in these proteins. Moreover, we observed a major decrease of local codon usage bias in the middle region of mRNA for all the proteins we studied, which might be a conserved feature of this protein fold. In some local segments connecting symmetric substructures, we also observed major or consecutive decreases of mRNA folding energy.
Materials and Methods
Data acquisition
There are four superfamilies of LβH fold, each containing a number of families and species. Because codon usage bias might be different across species, we chose one protein from every species of every family to construct a set of 18 LβH proteins. Here the only one protein from superfamily Guanosine diphospho-D-mannose pyrophosphorylase was discarded from study because of the very small size in helical structure. Among the 18 proteins, 15 were from bacteria, one from plant, and two from animals. The gene sequences of these proteins were obtained from ExPASy (http://web.expasy.org/blast/) and most of the genome data used in calculating codon usage frequency (i.e., occurrences of each codon) were obtained from genbank FTP (ftp://ftp.ncbi.nih.gov/). The calculation of codon usage frequency was based on the whole genome sequence of each species. Note that the codon usage data of the three non-bacteria species were taken from a codon usage table in http://www.kazusa.or.jp/codon/. The detailed information of each protein is presented in Table 1, including the superfamily, the protein name, the species, PDB ID, the protein segment being selected and its corresponding gene segment, and the source of the species' genome data. For the selected proteins, the α helices at N terminals or C terminals were excluded from analysis. Then, sequence alignment of the proteins was carried out to explore their evolutionary relationships (Table 2). Our results show that for the same protein from different species their sequence similarity can be very high. For example, the similarity between proteins 1LXA and 1J2Z is as high as 0.46. On the other hand, proteins of the same superfamily can have very low sequence similarity. For example, the similarity between proteins 1KK6 and 1QRE is as low as 0.15. The similarity values of protein 2F9C to most of the other proteins in the set are very high, probably because of the much longer length of this protein compared with others. Proteins 1M8N and 1LOS are different isoforms of the same protein; and thus they have very high sequence similarity. The data in this paper are available upon request to the authors.
Table 1. Detailed information of the dataset.
| Superfamily | Protein name | Species | PDB ID | Protein segments | Gene segments | Source of genome data |
|---|---|---|---|---|---|---|
| Trimeric LpxA-like enzymes | UDP N-acetylglucosamine acyltransferase | Escherichia coli K12 | 1LXA | 1–198 | 1–594 | Ftp |
| Trimeric LpxA-like enzymes | UDP N-acetylglucosamine acyltransferase | Helicobacter pylori 26695 | 1J2Z | 2–193 | 4–579 | Ftp |
| Trimeric LpxA-like enzymes | Galactoside acetyltransferase | Escherichia coli K12 | 1KRR | 62–185 | 184–555 | Ftp |
| Trimeric LpxA-like enzymes | Maltose O-acetyltransferase | Escherichia coli K12 | 1OCX | 55–183 | 163–549 | Ftp |
| Trimeric LpxA-like enzymes | Xenobiotic acetyltransferase | Pseudomonas aeruginosa | 1XAT | 3–166 | 7–498 | Ftp |
| Trimeric LpxA-like enzymes | Xenobiotic acetyltransferase | Enterococcus faecium | 1KK6 | 1–180 | 1–540 | Ftp |
| Trimeric LpxA-like enzymes | N-acetylglucosamine 1-phosphate uridyltransferase GlmU | Escherichia coli K12 | 1HV9 | 252–438 | 754–1314 | Ftp |
| Trimeric LpxA-like enzymes | N-acetylglucosamine 1-phosphate uridyltransferase GlmU | Streptococcus pneumoniae | 1G97 | 252–447 | 754–1341 | Ftp |
| Trimeric LpxA-like enzymes | Glucose-1-phosphate adenylyltransferase small subunit | Solanum tuberosum | 1YP2 | 390–521 | 1168–1563 | Cdtable |
| Trimeric LpxA-like enzymes | gamma-carbonic anhydrase | Methanosarcina thermophila | 1QRE | 4–174 | 10–522 | Ftp |
| Trimeric LpxA-like enzymes | Ferripyochelin binding protein | Pyrococcus horikoshii | 1V3W | 1–144 | 1–432 | Ftp |
| Trimeric LpxA-like enzymes | Putative acetyltransferase | Bacillus cereus | 1XHD | 1–144 | 1–426 | Ftp |
| Trimeric LpxA-like enzymes | Serine acetyltransferase | Haemophilus influenzae | 1SSQ | 138–241 | 412–723 | Ftp |
| Trimeric LpxA-like enzymes | Hypothetical protein YdcK | Salmonella enterica | 2F9C | 3–322 | 7–966 | Ftp |
| Trimeric LpxA-like enzymes | Acetyltransferase PglD | Campylobacter jejuni | 3BSW | 72–195 | 214–588 | Ftp |
| An insect antifreeze protein | Thermal hysteresis protein | Choristoneura fumiferana (5-turn isoforms) | 1LOS | 3–90 | 7–270 | Cdtable |
| An insect antifreeze protein | Thermal hysteresis protein | Choristoneura fumiferana(7-turn isoforms) | 1M8N | 2–121 | 4–363 | Cdtable |
| Adhesin YadA, collagen-binding domain | Cell adhesion | Yersinia enterocolitica | 1P9H | 32–209 | 94–627 | Ftp |
Table 2. Similarity (S) of the sequence alignment.
| S | 1LXA | 1J2Z | 1KRR | 1OCX | 1XAT | 1KK6 | 1HV9 | 1G97 | 1YP2 | 1QRE | 1V3W | 1XHD | 1SSQ | 2F9C | 3BSW | 1LOS | 1M8N | 1P9H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1LXA | 1 | 0.46 | 0.4 | 0.36 | 0.27 | 0.21 | 0.22 | 0.23 | 0.32 | 0.27 | 0.33 | 0.33 | 0.36 | 0.38 | 0.39 | 0.44 | 0.32 | 0.25 |
| 1J2Z | 0.46 | 1 | 0.35 | 0.38 | 0.26 | 0.18 | 0.27 | 0.24 | 0.31 | 0.19 | 0.35 | 0.33 | 0.32 | 0.35 | 0.35 | 0.41 | 0.3 | 0.2 |
| 1KRR | 0.4 | 0.35 | 1 | 0.48 | 0.37 | 0.45 | 0.35 | 0.4 | 0.2 | 0.33 | 0.31 | 0.35 | 0.21 | 0.51 | 0.26 | 0.24 | 0.18 | 0.31 |
| 1OCX | 0.36 | 0.38 | 0.48 | 1 | 0.38 | 0.45 | 0.33 | 0.42 | 0.23 | 0.32 | 0.28 | 0.28 | 0.19 | 0.5 | 0.21 | 0.23 | 0.17 | 0.29 |
| 1XAT | 0.27 | 0.26 | 0.37 | 0.38 | 1 | 0.4 | 0.27 | 0.26 | 0.22 | 0.2 | 0.24 | 0.25 | 0.18 | 0.43 | 0.3 | 0.31 | 0.24 | 0.22 |
| 1KK6 | 0.21 | 0.18 | 0.45 | 0.45 | 0.4 | 1 | 0.21 | 0.19 | 0.3 | 0.15 | 0.34 | 0.27 | 0.31 | 0.36 | 0.3 | 0.4 | 0.27 | 0.16 |
| 1HV9 | 0.22 | 0.27 | 0.35 | 0.33 | 0.27 | 0.21 | 1 | 0.4 | 0.38 | 0.2 | 0.28 | 0.32 | 0.32 | 0.41 | 0.35 | 0.4 | 0.34 | 0.16 |
| 1G97 | 0.23 | 0.24 | 0.4 | 0.42 | 0.26 | 0.19 | 0.4 | 1 | 0.38 | 0.21 | 0.33 | 0.32 | 0.36 | 0.41 | 0.4 | 0.43 | 0.32 | 0.18 |
| 1YP2 | 0.32 | 0.31 | 0.2 | 0.23 | 0.22 | 0.3 | 0.38 | 0.38 | 1 | 0.27 | 0.2 | 0.21 | 0.23 | 0.45 | 0.19 | 0.28 | 0.12 | 0.26 |
| 1QRE | 0.27 | 0.19 | 0.33 | 0.32 | 0.2 | 0.15 | 0.2 | 0.21 | 0.27 | 1 | 0.34 | 0.3 | 0.35 | 0.43 | 0.31 | 0.4 | 0.27 | 0.18 |
| 1V3W | 0.33 | 0.35 | 0.31 | 0.28 | 0.24 | 0.34 | 0.28 | 0.33 | 0.2 | 0.34 | 1 | 0.4 | 0.21 | 0.48 | 0.34 | 0.34 | 0.21 | 0.26 |
| 1XHD | 0.33 | 0.33 | 0.35 | 0.28 | 0.25 | 0.27 | 0.32 | 0.32 | 0.21 | 0.3 | 0.4 | 1 | 0.31 | 0.49 | 0.19 | 0.32 | 0.21 | 0.23 |
| 1SSQ | 0.36 | 0.32 | 0.21 | 0.19 | 0.18 | 0.31 | 0.32 | 0.36 | 0.23 | 0.35 | 0.21 | 0.31 | 1 | 0.46 | 0.27 | 0.3 | 0.12 | 0.32 |
| 2F9C | 0.38 | 0.35 | 0.51 | 0.5 | 0.43 | 0.36 | 0.41 | 0.41 | 0.45 | 0.43 | 0.48 | 0.49 | 0.46 | 1 | 0.54 | 0.52 | 0.49 | 0.38 |
| 3BSW | 0.39 | 0.35 | 0.26 | 0.21 | 0.3 | 0.3 | 0.35 | 0.4 | 0.19 | 0.31 | 0.34 | 0.19 | 0.27 | 0.54 | 1 | 0.29 | 0.22 | 0.31 |
| 1LOS | 0.44 | 0.41 | 0.24 | 0.23 | 0.31 | 0.4 | 0.4 | 0.43 | 0.28 | 0.4 | 0.34 | 0.32 | 0.3 | 0.52 | 0.29 | 1 | 0.68 | 0.38 |
| 1M8N | 0.32 | 0.3 | 0.18 | 0.17 | 0.24 | 0.27 | 0.34 | 0.32 | 0.12 | 0.27 | 0.21 | 0.21 | 0.12 | 0.49 | 0.22 | 0.68 | 1 | 0.3 |
| 1P9H | 0.25 | 0.2 | 0.31 | 0.29 | 0.22 | 0.16 | 0.16 | 0.18 | 0.26 | 0.18 | 0.26 | 0.23 | 0.32 | 0.38 | 0.31 | 0.38 | 0.3 | 1 |
Methods for detecting symmetry in nucleotide sequences
We analyze symmetry in nucleotide sequences by using an improved recurrence quantification analysis [22, 28]. The method works as follows. Let S = x 1 x 2 x 3…x N denote a nucleotide sequence of length N, where x i represents one of the four nucleic acids. One constructs a set of all (N-d+1) possible segments of d (d < N) consecutive symbols:
where i denotes the location of the first nucleic acid (or codon) of X i in the sequence. We find how many other segments are similar to each segment X i and the similarities between two segments is calculated based on Hamming distance:
| (1) |
A segment X j is defined similar to X i if the percentage of identical nucleic acids is larger than a threshold value S 0 (Table 3).
Table 3. Results and parameters.
| PDB id | Nt sym | S 0 | Cdbias sym | C 0 | rCAI |
|---|---|---|---|---|---|
| 1LXA | 2 | 0.35 | 2 | 0.01 | 1.1702 |
| 1J2Z | 2 | 0.35 | 2 | 0.02 | 1.0726 |
| 1KRR | 2 | 0.35 | 2 | 0.01 | 0.9798 |
| 1OCX | 2 | 0.35 | 0 | 0.01 | 1.0404 |
| 1XAT | 2 | 0.35 | 2 | 0.015 | 1.4127 |
| 1KK6 | 2 | 0.35 | 2 | 0.025 | 1.1597 |
| 1HV9 | 2 | 0.35 | 2 | 0.008 | 1.2223 |
| 1G97 | 2 | 0.35 | 2 | 0.01 | 1.1626 |
| 1YP2 | 2 | 0.30 | 2 | 0.025 | 1.0605 |
| 1QRE | 2 | 0.35 | 2 | 0.005 | 1.0621 |
| 1V3W | 2 | 0.35 | 2 | 0.03 | 1.0368 |
| 1XHD | 2 | 0.35 | 2 | 0.03 | 1.1514 |
| 1SSQ | 2 | 0.35 | 0 | 0.015 | 1.1794 |
| 2F9C | 2 | 0.30 | 2 | 0.005 | 1.0452 |
| 3BSW | 2 | 0.35 | 2 | 0.015 | 1.3315 |
| 1LOS | 2 | 0.30 | weak | 0.025 | 1.0293 |
| 1M8N | 2 | 0.30 | weak | 0.020 | 1.0348 |
| 1P9H | 2 | 0.35 | 2 | 0.005 | 1.0474 |
To assess the statistical significance of the pairwise alignment, P-value is calculated from the distribution of similarity scores in a large number of random sequences through randomly shuffling the original sequence. The similarity of the two aligned segments is considered statistically significant when P-value is lower than 0.01 (i.e., the probability of obtaining an alignment with at least the same similarity by self-alignment of shuffled sequences is lower than 0.01).
Methods for detecting symmetry in codon sequences
Because one amino acid is coded by a group of triplet codons and synonymous codons are not used in equal frequencies, we further analyze symmetry in the codon sequence. We use a revised Codon Adaption Index (rCAI) (defined in the next section) to measure the similarity between two aligned segments of codon sequences.
Let S = x 1 x 2 x 3…x N denote a codon sequence of length N, where x i represents one of the 61 codons. From the sequence S, one constructs a set of all (N−d + 1) possible segments of d (d < N) consecutive codons:
For each segment X i, we find how many other segments have similar codon usage feature to it. The rCAI value of each segment is calculated. Two segments are deemed similar if the difference between their rCAI values is smaller than a threshold value C 0 (Table 3).
Revised Codon Adaption Index
CAI is defined as the geometric mean of the relative synonymous codon usage (RSCU) values corresponding to each of the codons used in that sequence, divided by the maximum possible CAI for a gene sequence of the same amino acid composition [49, 50]. It is calculated as:
| (2) |
where RSCU k and RSCU max is the RSCU value for the kth codon and the maximum RSCU value among the synonymous codon group of kth codon, respectively. An RSCU value for a codon is the observed frequency of the codon divided by the frequency expected [49]:
| (3) |
where x ij is the number of occurrences of the j-th codon for i-th amino acid, and n i is the number of synonymous codons for i-th amino acid. For computational effectiveness, Eq (2) is computed as:
| (4) |
where w k is the relative adaptiveness of a codon and is denoted as x ij / x imax.
In this definition, the most frequently used codon from different groups of synonymous codons makes the same contribution to the result, i.e., there are up to 20 number of w k whose value is 1 thus make no difference to the CAI value. Our purpose is to compare the similarity of codon usage feature between any two segments, the most frequently used codons from different synonymous codon group is preferred to contribute differently. For this purpose, a little revision on the definition of CAI is made that the maximum possible CAI is substituted by the expected CAI:
| (5) |
The revised Codon Adaption Index (rCAI) is then used as the measurement of the similarity between codon sequences.
Local codon usage bias: the distribution of local CAI values over the codon sequence
Although encoding the same amino acid, synonymous codons are used with different frequencies. Synonymous codon choice can be influenced by mutation and selection sources such as Horizontal Gene Transfer [51, 52]. Selection on synonymous codons acts to increase the thermodynamic stability of DNA and RNA structures [53], as well as to assist co-translational protein folding [54]. Some codons are translationally favorable—they are selected for accurate and efficient translation in bacteria, yeast, worm, fly and even in mammals [55, 56]. Here the local codon bias is analyzed to investigate its potential correlation with structural symmetry. The distribution of local CAI values over the codon sequence is used as a measurement. In calculating the local CAI values, the length of the sliding window is set to be 20 consecutive codons, which is chosen based on the structure feature of LβH that the unit of one rung is 18 to 20 amino acids long [57]. The sliding window moves from the beginning to the end of the codon sequence, with one codon for each move. Then the local CAI of all d = 20 sliding windows are calculated and shown as the distribution over the codon sequence.
Local mRNA folding energy
Single-stranded mRNA sequences may form local secondary structures which is believed to have influences on protein translation. Secondary structure in the coding regions might generally be used to regulate the translation rate which might cause temporarily translation pause to facilitate protein folding. Here we study the local RNA structures alongside a codon sequence. Because the footprint length of a ribosome on mRNA is about 40 nucleotides, we calculate the local folding energy with a sliding window of 40 nucleotides downstream. The sliding window moves from the beginning to the end of the nucleotide sequence, with one nucleotide for each move. The folding energy of the segment framed by the sliding window is calculated by using Matlab function rnafold which predicts the minimun free energy of the RNA sequence using the thermodynamic nearest-neighbor approach (http://www.mathworks.com/help/bioinfo/ref/rnafold.html).
Randomization
To demonstrate that the features of the local codon usage and mRNA folding energy of LβH are specially selected by nature, we perform randomization on the gene sequences for comparison. For each native gene sequence, we randomly shuffle the set of codons among sites with identical amino acids, preserving the exact count of each codon and the precise order of encoded amino acids as in the native sequence [58]. We performe 10 times of the random procedure for each of the gene sequence and calculate the average profiles of both local codon usage bias and folding free energy. The averaged profiles for 10 random sequences are then compared with that of the natural gene sequences.
Results and Discussion
The gene sequence of protein 1LXA is two-fold symmetric
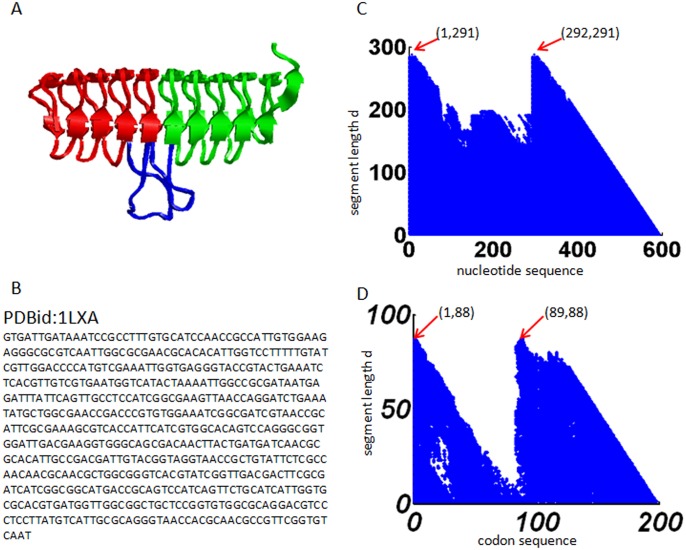
The protein 1LXA has a two-fold symmetric tertiary structure (Fig 1A): two symmetric substructures (shown in red and green, respectively) connected by an irregular extended loop in the middle (shown in blue). We apply the method described in Section 2.2 to analyze the nucleotide sequence of this protein, which has a length N = 594 (Fig 1B). Here we set S 0 = 0.35. The result for the nucleotide sequence (Fig 1C) shows two peaks at positions (1,291) and (292,291) which means that segments 1–291 and 292–582 are similar to each other; and thus the nucleotide sequence is two-fold symmetric. The correspondence between the symmetry in gene sequence and the symmetry in protein structure provide clear evidence of gene duplication and fusion event for this protein.
Fig 1. Results obtained from protein 1LXA.
(A) The gene sequence. (B) The tertiary protein structure. (C) The recurrence plot of the nucleotide sequence. (D) The recurrence plot of the codon sequence.
We also verify the two-fold symmetry of protein 1LXA from the perspective of codon usage bias. We apply the method described in Section 2.3 to analyze the codon sequence of this protein, which has a length N = 198. Result in codon usage bias (Fig 1D) also reveals a two-fold symmetry in the codon sequence, with the first segment containing codons 1–88 and the second segment containing codons 89–176. The codon sequence is thus two-fold symmetric. It suggests that the two symmetric subsegments of the gene sequence also share similar codon usage bias. Here the C 0 is set to be 0.01.
Two-fold symmetry is an evolutionarily conserved feature of LβH proteins
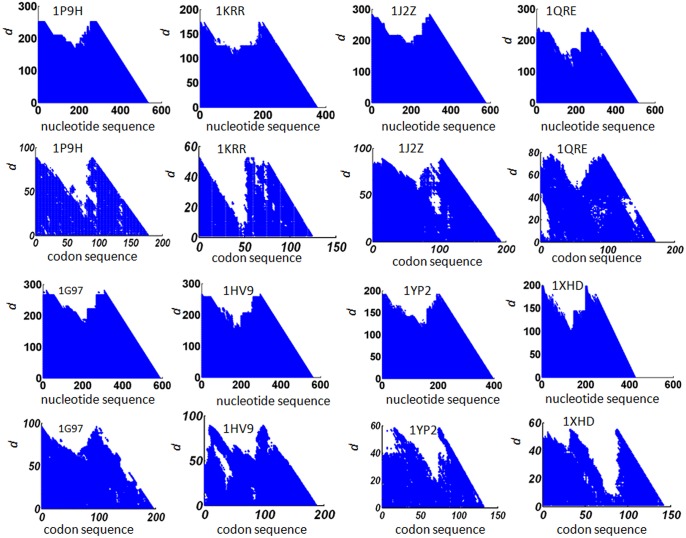
We apply the method described in Section 2.2 to analyze the nucleotide sequences of all the 18 LβH proteins listed in Table 1. The results of eight of them are presented in Fig 2; and the other results are presented in S1 Fig. For all the cases, two-fold symmetry is detected in the nucleotide sequence. The threshold for determining similarity S 0 was set in the range [0.30, 0.35], which is relatively high when compared with 0.25, the similarity for two protein sequences to have similar tertiary structures [59, 60]. This result suggests that one single gene duplication and fusion event to form a two-fold symmetric tertiary structure might be a common evolution process for this protein fold.
Fig 2. Recurrence plots for 8 proteins.
The first and third horizontal panel: the recurrence plot of the nucleotide sequence; the second and the fourth horizontal panel: the recurrence plot for codon usage bias in the codon sequence of the corresponding proteins. The PDB id of the protein is given in each of the plot.
We apply the method described in Section 2.3 to analyze the codon sequences of the 18 LβH proteins. The results of eight of them are presented in Fig 2. For 14 of the 18 cases, two-fold symmetry is detected, indicating that codon usage bias might be conserved during evolution. The threshold for determining similarity C 0 was set in the range [0.005, 0.03]. The wider range of similarity degree for codon usage bias may due to the divergence of individual revised CAI value for each of the gene sequence. To verify the effectiveness of our recurrence analysis, we compare it with the web server Swelf [61]. The software uses dynamic programming to identify internal repeats in DNA sequences, amino acid sequences, and three dimensional structures. It turns out that Swelf could not find repeats for most of the gene sequences in our dataset.
Table 3 summarizes the results and parameters in this section, where the column“Nt sym”represents symmetry in nucleotide sequence. Because all the nucleotide sequences show two-fold symmetry, the column is labeled with 2 for all the rows. The column“Cdbias sym”represents symmetry in codon usage bias. Of the 18 sequences, 14 show two-fold symmetry, two show no symmetry, and two show weak symmetry. The column “rCAI”represents the rCAI value of the codon sequence.
Profiles of local codon usage bias and local mRNA folding free energy
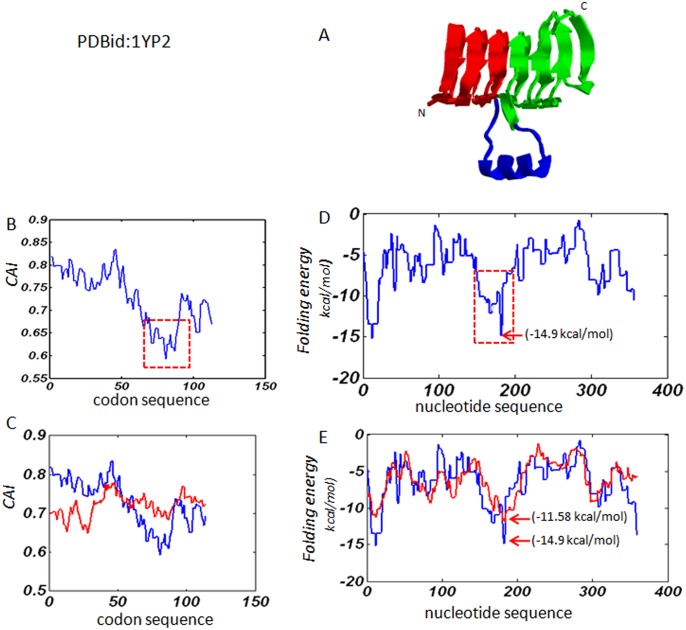
The protein 1YP2 has a two-fold symmetric tertiary structure (Fig 3A): six complete rungs (shown in red and green) connected by irregular extended structure in the middle (shown in blue). Fig 3B shows the profile of CAI values calculated along the sequence with a sliding window of length d = 20 codons. In the figure, a smaller CAI value indicates rarer codons are used nearby. The result suggests that rare or less frequently used codons are more likely to be used near the boundary between the two symmetric substructures. In Fig 3C, the CAI profile of 1YP2 (blue) is compared with the CAI profile (red) averaged from 10 random codon sequences of the same length. Clearly, the random profile is flat and lacks the nadir marking the boundary between two symmetric substructures. This demonstrates that the increased usage of rare codons near the connecting region is not random, but is specially selected by nature during evolution.
Fig 3. Results obtained from protein 1YP2.
(A). The cartoon structure: the two symmetric substructures are shown in red and green; the extended irregular structure in the middle of the helix is shown in blue. (B). The profile of local codon usage bias: the decreased region in the middle of the codon sequence is shown with dashed square lines. (C). The comparison between the profile of the natural gene sequence and the averaged profile of the same codon sequence randomized by 10 times. The blue line is for natural gene sequence and the red line is for the average of the random sequences. (D). The profile of local folding free energy, and the dashed square lines shows the region with decreased local folding free energy. (F). The comparison between the natural gene sequence and the random sequences. The blue line is for natural gene sequence and the red line is for the average of the random sequences.
Fig 3D illustrates the profile of local mRNA folding energy calculated along the sequence with a sliding window of length d = 40 nucleotides. In the figure, a lower energy implies the mRNA folding is locally more stable. The profile also shows a major decrease of local folding free energy in the middle of the nucleotide sequence, with the lowest energy of -14.9 kcal/mol, suggesting that higher order of secondary structure might be formed near the connecting region of symmetric substructures. In Fig 3E, the folding energy profile of 1YP2 (blue) is compared with a folding energy profile (red) averaged from 10 randomly generated sequences. Although the folding energy difference between the blue curve and the red curve is less dramatic than the CAI difference, the nadir of the major decreased region of the blue curve is significantly lower than that of the red curve: -14.9 kcal/mol versus -11.58 kcal/mol (Fig 3E). These results suggest that codons near the connecting region might be selected rather than randomly chosen for the formation of more stable structure.
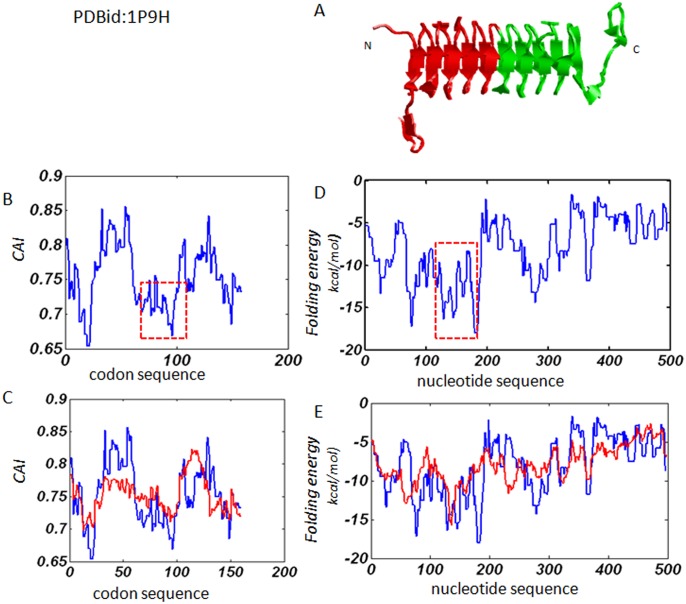
The protein 1P9H serves as another example. Unlike the structure of 1YP2, there is no extended irregular loop in the middle of the helix structure (Fig 4A). It yields results similar to 1YP2 in terms of both local codon usage bias and local mRNA folding free energy. Fig 4B shows a major decrease of local codon usage in the middle of the codon sequence. The comparison between the natural gene sequence and the average of 10 random codon sequences shows that the natural gene sequence has more biased codon usage than random sequences (Fig 4C). As such, a major decrease of local folding free energy in the middle of the nucleotide sequence is also detected (Fig 4D). Moreover, the natural gene sequence has more biased folding free energy distribution than random sequences (Fig 4E), indicating that the connecting region is more stable than the counterpart of a random sequence. Therefore, higher order of secondary structure is likely to form in the middle region.
Fig 4. Results obtained from protein 1P9H.
(A). The cartoon structure, with the two symmetric substructures shown in red and green respectively. (B). The profile of local codon usage bias: the region with major decreases in CAI value is shown with dashed square lines. (C). The comparison between the profile of the natural gene sequence and the averaged profile of the same codon sequence randomized by 10 times. The blue line is for natural gene sequence and the red line is for the average of the random sequences. (D) and (E). The profile of local folding free energy and the comparison between the natural gene sequence and the random sequences, respectively. The blue line is for natural gene sequence and the red line is for the average of the random sequences.
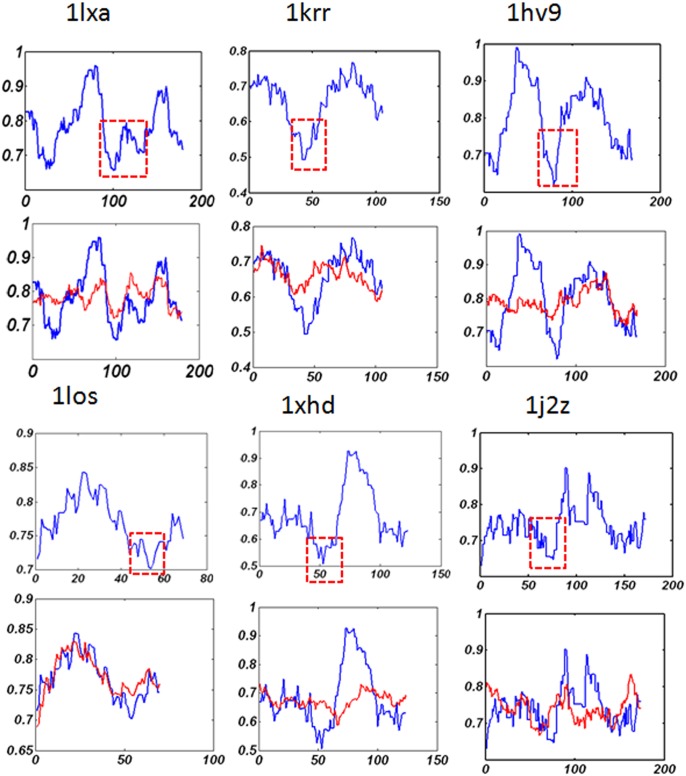
According to the tertiary structure, some LβH proteins such as 1LXA and 1YP2 contain extended loop regions in the middle of the helix structure; while some proteins such as protein 1P9H and protein 1HV9 (whose tertiary structure can be obtained from protein data bank) do not contain irregular loop region. In order to further verify that the above discovery involves a conserved feature of the entire LβH protein folds, we extend the study to all the representative sets of LβH proteins. Fig 5 gives the results for 6 LβH proteins and S2 Fig gives the results for the other 10 proteins.
Fig 5. The profile for local codon usage bias and the comparison of natural gene sequences to the random sequences.
The x-axis is the codon sequence and the y-axis is the local CAI value. The major decrease of local codon usage bias is shown with dashed square lines. The blue line is for natural gene sequence and the red line is for the average of the random sequences.
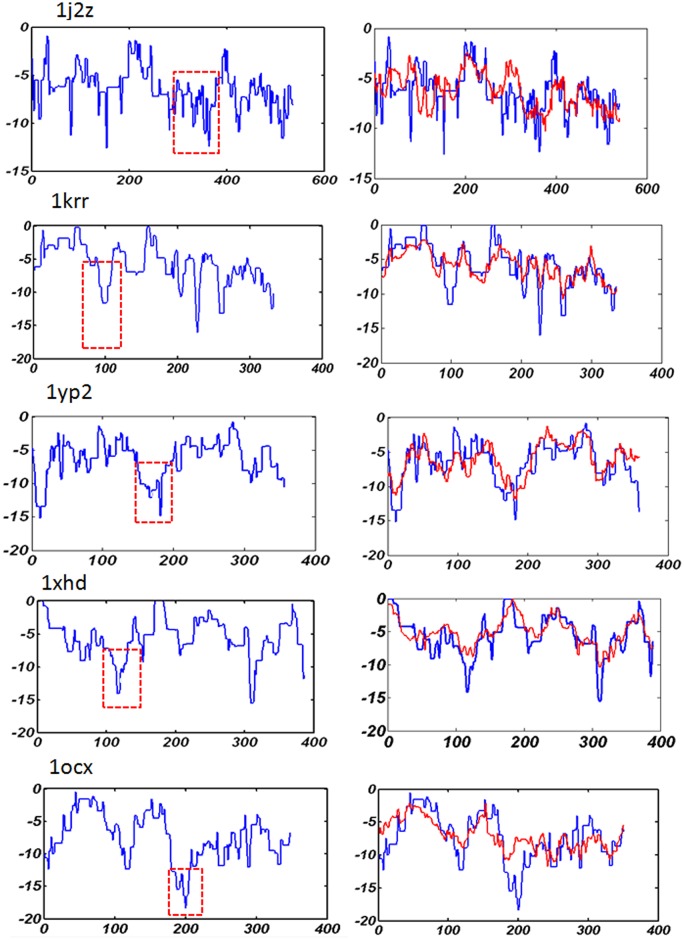
The study reveals that, for nearly all the representative proteins in the dataset, there is a major decrease of local codon usage bias in the middle of the codon sequence. It implies that the usage of less frequently used codons near the boundaries of the two symmetric substructures of LβH fold might be a conserved mechanism during the evolution process. Previous studies reported that the location of rare codon clusters often indicates domain termini and/or boundaries in multidomain proteins [35], and it may temporally separate the protein synthesis for hierarchical cotranslational folding of multidomains [40]. Our results on local codon usage bias suggest that these findings might also be true for single domain protein with internal structural symmetry. The study on the local folding free energy also shows that there are major or consecutive decreases of local folding free energy near the connecting region of symmetric substructures (Fig 6, and S3 and S4 Figs). Comparisons with random sequences show that natural gene sequences have more biased local folding free energy than random gene sequences. A recent study on mRNA folding suggested that mRNA secondary structures serve as elongation brakes to control the speed and hence the fidelity of protein translation [62]. Here the results in our study indicate that the tuning of translation elongation by local mRNA stability might relate to structural symmetry in proteins. Since both clusters of rare codons and formation of higher order of local mRNA secondary structure may lead to translation pause or slow-down regions, our results may suggest a sequential folding pathway of nascent peptide chain of symmetric structures.
Fig 6. The profile for local mRNA folding free energy and the comparison of natural gene sequences to the random sequences.
The x-axis is the nucleotide sequence and the y-axis is the local folding free energy. The region with major decrease of local mRNA folding free energy is shown with dashed square lines. The blue line is for natural gene sequence and the red line is for the average of the random sequences.
Conclusion
In this paper, we studied the relationship between the structural symmetry of proteins and the nucleotide sequence, codon bias, and mRNA folding energy in LβH fold. The results show that a similar degree of symmetry can be readily detected in both the nucleotide sequence and the triplet codon sequence. In addition, a major decrease of local codon usage bias in the middle of the codon sequence can be identified as a common feature. Major or consecutive decreases in local mRNA folding energy near the boundaries of the symmetric substructures can also be observed. Taken together, our results suggest that gene duplication and fusion event to form two-fold symmetric tertiary structure may be a common evolution process of LβH fold. Meanwhile, the selection over local codon usage and local mRNA secondary structure near the connecting regions of symmetric substructures might have coevolved and become a conserved mechanism for tuning translation of this protein fold. These findings provide valuable insights into the evolution and mechanisms of translation, and may also shed light on the design and engineering of symmetric protein folds.
Supporting Information
The first, third and fifth horizontal panel: the recurrence plot of the nucleotide sequence; the second, fourth and sixth horizontal panel: the recurrence plot for codon usage bias in the codon sequence of the corresponding proteins. The PDB id of the proteins are given in each of the plot.
(RAR)
The x-axis is the codon sequence and the y-axis is the local CAI value. The major decrease of local codon usage bias is shown with dashed square lines. The blue line is for natural gene sequence and the red line is for the average of the random sequences.
(RAR)
The x-axis is the nucleotide sequence and the y-axis is the local folding free energy. The region with major decrease of local mRNA folding free energy is shown with dashed square lines. The blue line is for natural gene sequence and the red line is for the average of the random sequences.
(RAR)
The x-axis is the nucleotide sequence and the y-axis is the local folding free energy. The region with major decrease of local mRNA folding free energy is shown with dashed square lines. The blue line is for natural gene sequence and the red line is for the average of the random sequences.
(RAR)
Acknowledgments
The authors would like to thank the referees for their valuable suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
GLL received funding from the National Natural Science Foundation of China (61135004): http://www.nsfc.gov.cn/. He conceived and designed the experiments, and contributed to the writing of the manuscript. GYW received funding from the National Natural Science Foundation of China (61471186): http://www.nsfc.gov.cn/. He conceived and designed the experiments, and contributed to the writing of the manuscript. TCH received funding from the Hunan Provincial Natural Science Foundation of China (14JJ7075): http://www.hnst.gov.cn/zxgz/zkjj/. He contributed to the writing of the manuscript. TCH received funding from the key Scientific Research fund of Hunan Provincial Education Department (13A091): http://gov.hnedu.cn/zwgk.jsp. TCH received funding from the key Scientific Research fund of Provincial Key Laboratory of Informational Service for Rural Area of Southwestern Hunan: http://www.hnst.gov.cn/zxgz/zkjj/. He contributed to the writing of the manuscript. TCH received funding from the Aid program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province: http: http://gov.hnedu.cn/zwgk.jsp. He contributed to the writing of the manuscript.
References
- 1. Goodsell DS, Olson AJ. Structural symmetry and protein function. Annual review of biophysics and biomolecular structure. 2000;29(1):105–53. [DOI] [PubMed] [Google Scholar]
- 2. Venkatakrishnan A, Levy E, Teichmann S. Homomeric protein complexes: evolution and assembly. Biochemical Society Transactions. 2010;38(4):879 10.1042/BST0380879 [DOI] [PubMed] [Google Scholar]
- 3. Marsh JA, Teichmann SA. Structure, dynamics, assembly, and evolution of protein complexes. Annual review of biochemistry. 10.1146/annurev-biochem-060614-034142 [DOI] [PubMed] [Google Scholar]
- 4. Salem GM, Hutchinson EG, Orengo CA, Thornton JM. Correlation of observed fold frequency with the occurrence of local structural motifs. Journal of molecular biology. 1999;287(5):969–81. [DOI] [PubMed] [Google Scholar]
- 5. Broom A, Doxey AC, Lobsanov YD, Berthin LG, Rose DR, Howell PL, et al. Modular Evolution and the Origins of Symmetry: Reconstruction of a Three-Fold Symmetric Globular Protein. Structure. 2012;20(1):161–71. 10.1016/j.str.2011.10.021 [DOI] [PubMed] [Google Scholar]
- 6. Blaber M, Lee J. Designing proteins from simple motifs: opportunities in Top-Down Symmetric Deconstruction. Current Opinion in Structural Biology. 2012. [DOI] [PubMed] [Google Scholar]
- 7. McLachlan A. Repeating sequences and gene duplication in proteins. Journal of molecular biology. 1972;64(2):417–37. [DOI] [PubMed] [Google Scholar]
- 8. McLachlan A. Repeated helical pattern in apolipoprotein-AI. Nature. 1977;267: 465–66. [DOI] [PubMed] [Google Scholar]
- 9. Lang D, Thoma R, Henn-Sax M, Sterner R, Wilmanns M. Structural evidence for evolution of the β/α barrel scaffold by gene duplication and fusion. Science. 2000;289(5484):1546–50. [DOI] [PubMed] [Google Scholar]
- 10. Huang Y, Xiao Y. Detection of gene duplication signals of Ig folds from their amino acid sequences. PROTEINS: Structure, Function, and Bioinformatics. 2007;68(1):267–72. [DOI] [PubMed] [Google Scholar]
- 11. Vogel C, Morea V. Duplication, divergence and formation of novel protein topologies. Bioessays. 2006;28(10):973–8. [DOI] [PubMed] [Google Scholar]
- 12. Apic G, Gough J, Teichmann SA. Domain combinations in archaeal, eubacterial and eukaryotic proteomes. Journal of molecular biology. 2001;310(2):311–25. [DOI] [PubMed] [Google Scholar]
- 13. Henn-Sax M, Höcker B, Wilmanns M, Sterner R. Divergent Evolution of (betaalpha) 8-Barrel Enzymes. Biological chemistry. 2001;382(9):1315–20. [DOI] [PubMed] [Google Scholar]
- 14. Blaber M, Lee J, Longo L. Emergence of symmetric protein architecture from a simple peptide motif: evolutionary models. Cellular and Molecular Life Sciences. 2012:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forrest L. Structural Symmetry in Membrane Proteins. Annual Review of Biophysics. 2015;44(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolynes PG. Symmetry and the energy landscapes of biomolecules. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrade MA, Perez-Iratxeta C, Ponting CP. Protein repeats: structures, functions, and evolution. Journal of structural biology. 2001;134(2–3):117–31. [DOI] [PubMed] [Google Scholar]
- 18. Kertesz M, Wan Y, Mazor E, Rinn JL, Nutter RC, Chang HY, et al. Genome-wide measurement of RNA secondary structure in yeast. Nature. 2010;467(7311):103–7. 10.1038/nature09322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor WR, Heringa J, Baud F, Flores TP. A Fourier analysis of symmetry in protein structure. Protein engineering. 2002;15(2):79–89. [DOI] [PubMed] [Google Scholar]
- 20. Heger A, Holm L. Rapid automatic detection and alignment of repeats in protein sequences. PROTEINS: Structure, Function, and Bioinformatics. 2000;41(2):224–37. [DOI] [PubMed] [Google Scholar]
- 21. Szklarczyk R, Heringa J. Tracking repeats using significance and transitivity. Bioinformatics. 2004;20(suppl 1):i311–i7. [DOI] [PubMed] [Google Scholar]
- 22. Xu R, Xiao Y. A common sequence-associated physicochemical feature for proteins of beta-trefoil family. Computational biology and chemistry. 2005;29(1):79–82. [DOI] [PubMed] [Google Scholar]
- 23. Ji X, Chen H, Xiao Y. Hidden symmetries in the primary sequences of beta-barrel family. Computational biology and chemistry. 2007;31(1):61–3. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Huang Y, Xiao Y. Structural-symmetry-related sequence patterns of the proteins of beta-propeller family. Journal of molecular graphics & modelling. 2008;26(5):829. [DOI] [PubMed] [Google Scholar]
- 25. Li M, Huang Y, Xu R, Xiao Y. Nonlinear analysis of sequence symmetry of beta-trefoil family proteins. Chaos, Solitons & Fractals. 2005;25(2):491–7. [Google Scholar]
- 26. Achaz G, Boyer F, Rocha EPC, Viari A, Coissac E. Repseek, a tool to retrieve approximate repeats from large DNA sequences. Bioinformatics. 2007;23(1):119–21. [DOI] [PubMed] [Google Scholar]
- 27. Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic acids research. 1999;27(2):573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eckmann JP, Kamphorst SO, Ruelle D. Recurrence plots of dynamical systems. EPL (Europhysics Letters). 1987;4(9):973. [Google Scholar]
- 29. Fredrick K, Ibba M. How the sequence of a gene can tune its translation. Cell. 2010;141(2):227–9. Epub 2010/04/21. 10.1016/j.cell.2010.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cannarozzi G, Schraudolph NN, Faty M, von Rohr P, Friberg MT, Roth AC, et al. A role for codon order in translation dynamics. Cell. 2010;141(2):355–67. 10.1016/j.cell.2010.02.036 [DOI] [PubMed] [Google Scholar]
- 31. Kudla G, Murray AW, Tollervey D, Plotkin JB. Coding-sequence determinants of gene expression in Escherichia coli. Science. 2009;324(5924):255–8. Epub 2009/04/11. 10.1126/science.1170160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pechmann S, Chartron JW, Frydman J. Local slowdown of translation by nonoptimal codons promotes nascent-chain recognition by SRP in vivo. Nature structural & molecular biology. 2014;21(12):1100–5. Epub 2014/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou M, Guo J, Cha J, Chae M, Chen S, Barral JM, et al. Non-optimal codon usage affects expression, structure and function of clock protein FRQ. Nature. 2013;495(7439):111–5. Epub 2013/02/19. 10.1038/nature11833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tuller T, Zur H. Multiple roles of the coding sequence 5' end in gene expression regulation. Nucleic acids research. 2015;43(1):13–28. Epub 2014/12/17. 10.1093/nar/gku1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Komar AA. A pause for thought along the co-translational folding pathway. Trends in biochemical sciences. 2009;34(1):16–24. 10.1016/j.tibs.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 36. Varenne S, Buc J, Lloubes R, Lazdunski C. Translation is a non-uniform process: effect of tRNA availability on the rate of elongation of nascent polypeptide chains. Journal of molecular biology. 1984;180(3):549–76. [DOI] [PubMed] [Google Scholar]
- 37. Thanaraj T, Argos P. Ribosome‐mediated translational pause and protein domain organization. Protein Science. 1996;5(8):1594–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gershenson A, Gierasch LM. Protein folding in the cell: challenges and progress. Current opinion in structural biology. 2011;21(1):32–41. 10.1016/j.sbi.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsai CJ, Sauna ZE, Kimchi-Sarfaty C, Ambudkar SV, Gottesman MM, Nussinov R. Synonymous mutations and ribosome stalling can lead to altered folding pathways and distinct minima. Journal of molecular biology. 2008;383(2):281–91. 10.1016/j.jmb.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang G, Hubalewska M, Ignatova Z. Transient ribosomal attenuation coordinates protein synthesis and co-translational folding. Nature structural & molecular biology. 2009;16(3):274–80. [DOI] [PubMed] [Google Scholar]
- 41. Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, et al. A" silent" polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315(5811):525–8. [DOI] [PubMed] [Google Scholar]
- 42. Crombie T, Swaffield JC, Brown AJ. Protein folding within the cell is influenced by controlled rates of polypeptide elongation. Journal of molecular biology. 1992;228(1):7–12. [DOI] [PubMed] [Google Scholar]
- 43. Tuller T, Carmi A, Vestsigian K, Navon S, Dorfan Y, Zaborske J, et al. An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell. 2010;141(2):344–54. Epub 2010/04/21. 10.1016/j.cell.2010.03.031 [DOI] [PubMed] [Google Scholar]
- 44. Shen X. Conformation and sequence evidence for two-fold symmetry in left-handed beta-helix fold. Journal of Theoretical Biology. 2011;285(1):77–83. 10.1016/j.jtbi.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 45. De Winter R, Moss T, Roufa D, Kelly E, DeRose M, Draper G. A Left-Handed Parallel beta Helix in the Structure of UDP-N-Acetylglucosamine Acyltransferase. Cell Biol. 1995;15:3023. [DOI] [PubMed] [Google Scholar]
- 46. Govaerts C, Wille H., Prusiner S., and Cohen F. Evidence for assembly of prions with left-handed β-helices into trimers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(8342). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ji H, and Zhang H.,. [beta]-sheet constitution of prion proteins. Trends in biochemical sciences. 2010;35(3):129–34. 10.1016/j.tibs.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 48. Parisi G, Fornasari M., and Echave J. Evolutionary analysis of gamma-carbonic anhydrase and structurally related proteins. Mol Phylogenet Evol. 2000;14(323–34). [DOI] [PubMed] [Google Scholar]
- 49. Sharp PM, Li WH. The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic acids research. 1987;15(3):1281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharp PM, Tuohy TM, Mosurski KR. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic acids research. 1986;14(13):5125–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Médigue C, Rouxel T, Vigier P, Hénaut A, Danchin A. Evidence for horizontal gene transfer in Escherichia coli speciation. Journal of molecular biology. 1991;222(4):851–6. [DOI] [PubMed] [Google Scholar]
- 52. Médigue C, Viari A, Hénaut A, Danchin A. Escherichia coli molecular genetic map (1500 kbp): update II. Molecular microbiology. 1991;5(11):2629–40. [DOI] [PubMed] [Google Scholar]
- 53. Gu W, Zhou T, Wilke CO. A universal trend of reduced mRNA stability near the translation-initiation site in prokaryotes and eukaryotes. PLoS computational biology. 2010;6(2):e1000664 10.1371/journal.pcbi.1000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nature structural & molecular biology. 2009;16(6):574–81. [DOI] [PubMed] [Google Scholar]
- 55. Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Molecular biology and evolution. 1985;2(1):13–34. [DOI] [PubMed] [Google Scholar]
- 56. Chamary J, Parmley JL, Hurst LD. Hearing silence: non-neutral evolution at synonymous sites in mammals. Nature Reviews Genetics. 2006;7(2):98–108. [DOI] [PubMed] [Google Scholar]
- 57. Iengar P, Joshi N., and Balaram P.,. Conformational and sequence signatures in β helix proteins. Structure. 2006;14(3): p. 529–542. [DOI] [PubMed] [Google Scholar]
- 58. Katz L, Burge CB. Widespread selection for local RNA secondary structure in coding regions of bacterial genes. Genome Research. 2003;13(9):2042–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sweet RM, Eisenberg D. Correlation of sequence hydrophobicities measures similarity in three-dimensional protein structure. Journal of molecular biology. 1983;171(4):479–88. [DOI] [PubMed] [Google Scholar]
- 60. Mount DW. Sequence and genome analysis Bioinformatics: Cold Spring Harbour Laboratory Press: Cold Spring Harbour; 2004;2. [Google Scholar]
- 61. Abraham AL, Rocha EP, Pothier J. Swelfe: a detector of internal repeats in sequences and structures. Bioinformatics. 2008;24(13):1536–7. Epub 2008/05/20. 10.1093/bioinformatics/btn234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang JR, Chen X, Zhang J. Codon-by-codon modulation of translational speed and accuracy via mRNA folding. PLoS Biol. 2014;12(7):e1001910 Epub 2014/07/23. 10.1371/journal.pbio.1001910 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The first, third and fifth horizontal panel: the recurrence plot of the nucleotide sequence; the second, fourth and sixth horizontal panel: the recurrence plot for codon usage bias in the codon sequence of the corresponding proteins. The PDB id of the proteins are given in each of the plot.
(RAR)
The x-axis is the codon sequence and the y-axis is the local CAI value. The major decrease of local codon usage bias is shown with dashed square lines. The blue line is for natural gene sequence and the red line is for the average of the random sequences.
(RAR)
The x-axis is the nucleotide sequence and the y-axis is the local folding free energy. The region with major decrease of local mRNA folding free energy is shown with dashed square lines. The blue line is for natural gene sequence and the red line is for the average of the random sequences.
(RAR)
The x-axis is the nucleotide sequence and the y-axis is the local folding free energy. The region with major decrease of local mRNA folding free energy is shown with dashed square lines. The blue line is for natural gene sequence and the red line is for the average of the random sequences.
(RAR)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.