Abstract
Adolescence is a time of increasing engagement in a variety of problem behaviors, including substance use and delinquency. Genetic risk for problem behavior increases over adolescence, is mediated partially by individual differences in sensation seeking, and is exacerbated by involvement with deviant peers. In this article, we describe how findings from behavioral genetic research on problem behavior intersect with research from developmental neuroscience. In particular, the incentive-processing system, including the ventral striatum, responds increasingly to rewards in adolescence, particularly in peer contexts. This developmental shift may be influenced by hormonal changes at puberty. Individual differences in the structure and function of reward-responsive brain regions may be intermediary phenotypes that mediate adolescents’ genetic risk for problem behavior. The study of problem behavior can be enriched by interdisciplinary research that integrates measures of brain structure and function into genetically informed studies.
Keywords: gene-environment interaction, behavior genetics, delinquency, substance use, problem behavior, risk-taking, externalizing, dual systems model, adolescence
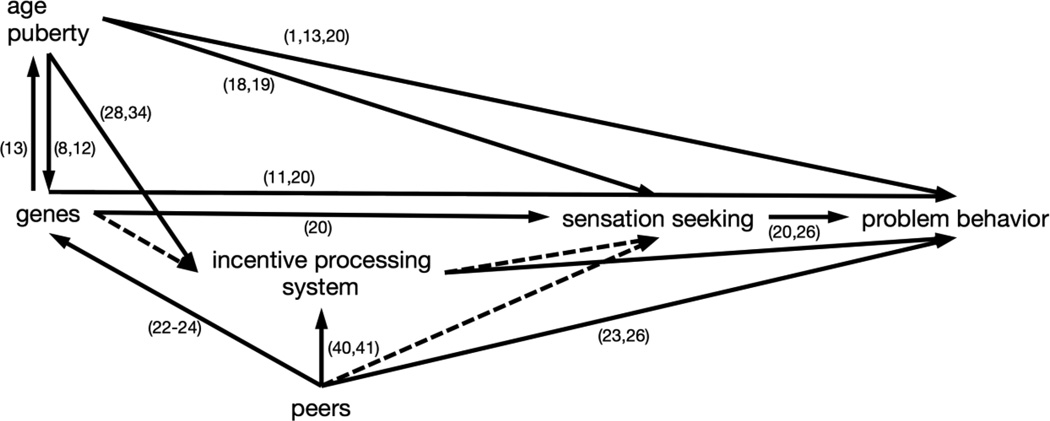
Adolescents engage disproportionately in problem behaviors, such as delinquency and substance use, that violate social norms and endanger their own and others’ well-being (1–3).1 In this article, we describe research from behavioral genetics and developmental neuroscience that advances understanding of biological risk for problem behavior. We scaffold our review around three findings from behavioral genetic research: Genetic influences on problem behavior increase with age and puberty, are mediated partially by genetic influences on sensation seeking, and are moderated by peer contexts. We integrate these findings with research on how neurobiological changes during adolescence contribute to teenagers’ propensity for problem behaviors. We focus on developmental changes in the incentive-processing system, including the ventral striatum (VS), the amygdala, and other subcortical areas that respond to rewards and threats. Like genetic risk for problem behavior, the incentive-processing system changes with age and puberty, is linked with sensation seeking, and is activated by peer contexts. These parallels suggest that findings from behavioral genetics and developmental neuroscience can be integrated to formulate hypotheses that can be addressed with interdisciplinary research (see Figure 1).
Figure 1. Overview of pathways from genes to problem behavior.
Solid arrows represent empirically supported pathways. Dashed arrows represent hypothesized pathways.
Note: Numbers in parentheses refer to studies cited in the References.
Three Findings from Behavior Genetic Research on Problem Behavior
Genetic Influences on Problem Behavior Increase with Age and Puberty
Average levels of problem behavior increase from childhood to adolescence (1, 3) and genetic influences on problem behavior also increase during this time. Genetic influences on rule-breaking behavior (e.g., property crime) increase from approximately 20% at age 10 to 80% at age 15 (8), a finding that has been replicated in four independent studies (9–10). In late adolescence, many forms of problem behavior are highly heritable, with genes accounting for more than 80% of the variance in a common latent factor linking antisocial behaviors, drug and alcohol use, and personality disinhibition (11).
The age span during which genetic influences on problem behavior increase most rapidly (10 to 15 years) coincides with puberty, suggesting that genetic influences on problem behavior may be activated by pubertal development rather than (or in addition to) chronological age. Supporting this hypothesis, we recently found that genetic influences on rule-breaking forms of delinquency were moderated by pubertal status, even after controlling for age (12). In contrast, age did not moderate genetic influence on delinquency after controlling for pubertal status.
How might genetic risk for problem behavior be influenced by puberty? One possibility is that the relevant genes influence the timing or tempo of pubertal development itself. In a nationally representative study of adolescent twin girls (13), genetic predispositions toward earlier pubertal development contributed to higher risk for delinquency. Moreover, hormonal changes associated with puberty may activate genes that were not expressed in childhood. During puberty, average levels of gonadal (testosterone, progesterone, estradiol) and adrenal (DHEA and DHEA-S) hormones increase in males and females. These hormones bind to DNA-transcription factors that are distributed throughout the central nervous system and can thus affect gene expression directly (14). Finally, genetic influences on problem behavior may be amplified via transactions with environmental contexts (15). For example, regardless of age, adolescents who are more physically developed affiliate with older and more deviant peers (16). These peer influences, in turn, might influence problem behavior reciprocally, setting up a positive feedback loop that amplifies initial genetically influenced differences.
Genetic Influences on Problem Behavior Are Mediated by Sensation Seeking
Genetic influences on problem behavior increase during adolescence, but it is unclear which genes are involved or how these genetic differences translate into differences in problem behavior. One way to parse the causal chain between genotype and a complex phenotype, such as problem behavior, is to conduct a behavior genetic study that tests whether genetic influences on the complex phenotype are accounted for by genetic influences on a more basic, biologically proximate phenotype that is hypothesized to be intermediary. This study design can delineate complex pathways between genes and behavior by testing intermediary phenotypes at varying levels of analysis, from brain structure (e.g., white matter density) to brain function (e.g., ventral striatum activity) to component psychological process (e.g., selective attention) to personality trait (e.g., novelty seeking) and, finally, to behavior.
In studies using this type of behavioral genetic design, the personality trait of sensation seeking is a key psychological mediator of genetic influences on problem behavior. Sensation seeking—the tendency to prefer and seek novel and thrilling sensations and experiences—correlates phenotypically with many forms of problem behavior (17). At the population level, average levels of sensation seeking increase from childhood to middle adolescence (i.e., around 16 years old), a developmental trend that mirrors increases in problem behavior at this time (18). Given recent evidence that puberty might activate genes for problem behavior, in some studies, average levels of sensation seeking are associated more closely with pubertal development than with chronological age (19). Moreover, twin and family studies suggest that the same genes that influence sensation seeking also influence problem behavior. In a nationally representative study of adolescent twins and siblings ages 10 to 16, individual differences in the rate of increase in sensation seeking were due overwhelmingly to genetic differences (h2 ~ 80%). These genetic influences on sensation seeking accounted for nearly half (43%) of the genetic variance in change in adolescents’ delinquent behavior (20). Finally, research using measured DNA (21) provides convergent evidence that genes influencing problem behavior are also relevant for sensation seeking. In this study (21), which constructed a polygenic risk score from a genomewide association study of externalizing disorders in adults, this risk score predicted a small but significant percentage of variance in self-reported impulse control and sensation seeking in adolescents.
Genetic Influences on Problem Behavior Are Moderated by Peer Relationships
Genes account for approximately 80% of the variation in problem behavior by late adolescence. However, this heritability estimate is a population average that masks potential variability in the strength of genetic influences on problem behavior in different environmental contexts—a gene × environment interaction. One of the most consistently replicated gene × environment interaction effects is the moderating effect of peer contexts: Genetic influences on problem behavior are amplified among teenagers who have more deviant peers. This pattern is evident whether examining best friends or peer groups; whether using adolescents’ perceptions of their peers or peers’ own self-report behavior; and whether the key outcome is nonsubstance-related delinquent behavior, alcohol use, or other forms of substance use (22–24). Convergent evidence for the importance of gene × peer interactions has been found using diverse methods, including twin/family studies (e.g., 23), candidate genes (e.g., 24, 25), and polygene methods that aggregate measured genetic risk across the entire genome (e.g., 21). Consistent with the hypothesis that part of the genetic risk for problem behavior is mediated through sensation seeking, the correlation between peer deviance and one’s own delinquent behavior is amplified among adolescents who report high levels of sensation seeking (26).
Adolescent Neurodevelopment and the Emergence of Problem Behavior
As we have described, genetic influences on problem behavior increase with age and puberty, are partially mediated by sensation seeking, and are moderated by peer relationships. At the same time, neuroscience research has begun to elucidate how adolescent-typical patterns of neurodevelopment may heighten teenagers’ propensity for problem behavior. Behavioral genetic research and neuroscience research intersect at several points, particularly on the roles of puberty and peers.
The Dual Systems Model
According to the dual systems model of adolescent neurodevelopment (27), the escalation of risk-taking behavior in adolescence results from asynchronous development in two brain systems. First, the incentive-processing system, including the ventral striatum (VS), amygdala, and orbitofrontal cortex, undergirds processing of motivational and affective information, including information about potential rewards. Second, the cognitive control system, including the prefrontal cortex and anterior cingulate cortex, is involved in self-regulation, planning, and impulse control. The cognitive control system undergoes protracted development through adolescence and young adulthood, whereas the incentive-processing system becomes more responsive in early and middle adolescence. For example, white matter volume—which reflects increasing myelination, a process that allows signals to be conducted through the brain more rapidly and reliably—continues to increase throughout adolescence and young adulthood, particularly in tracts relevant for high-level cognitive control (28). At the same time, adolescents show stronger VS activation in a variety of reward paradigms than children or adults, and VS activation correlates positively with risk-taking and preference for immediate rewards in laboratory tasks, as well as with self-reported problem behavior (reviewed in 28, 29). This neurobiological maturity gap may underlie age-group differences in risky and socially deviant behavior, with adolescents more prone to problem behavior than either children (for whom the incentive-processing system is less active, so they are less sensitive to the potential rewards of problem behavior) or adults (for whom the cognitive control system is more mature, so they can more effectively anticipate the potential adverse consequences of problem behavior).
Puberty and the Incentive-Processing System
The hormonal changes of puberty may be particularly important for the development of the incentive-processing system. Among young adults, both naturally occurring individual differences in testosterone and experimental administrations of testosterone correlate with reward seeking on the Iowa Gambling Task (IGT; 30, 31); performance on the IGT, in turn, is associated with sensation seeking (32, 33). Higher testosterone levels are also associated with greater reward-related VS activation in both adolescent boys and girls (34), and testosterone administration causes higher VS activation in response to rewards among adults (35). Longitudinal increases in testosterone in early adolescence are also associated with increased activation in response to threat cues in both the amygdala and the striatum, which may contribute to adolescents finding ostensibly dangerous situations thrilling and rewarding (36).
Peers and the Incentive-Processing System
The incentive-processing system in adolescents is sensitive to peer environments. Adolescents prefer smaller, more immediate monetary rewards to larger, delayed rewards when they are with peers of the same age (37). The same brain regions (including the VS) that respond to monetary rewards also respond to social stimuli, such as attaining higher social status, receiving positive social feedback, or viewing attractive faces (38, 39). Among adolescents, the presence of peers increases responsiveness of the VS to reward (40, 41). This effect of peer presence is not observed among adults, consistent with the idea that adolescence is a distinct developmental period of elevated sensitivity to social stimuli (40). Moreover, this neural peer effect correlates with risk-taking on a simulated driving task and with self-reported resistance to peer influence (40). Neural responses to peers vary by age and sex, with older female adolescents showing pronounced activity in the VS when they think about being evaluated by socially desirable peers (42). Thus, peer contexts both exacerbate genetic risk for problem behavior and activate neurobiological systems implicated in the rise of problem behavior typical for this age group.
Intersections Between Behavioral Genetic and Neuroscience Research
In summary, based on the behavior genetic and neuroscience findings we have reviewed thus far, average levels of problem behavior, sensation seeking, and reactivity of the incentive-processing system increase during adolescence and are linked with pubertal development. In addition, genetic influences on problem behavior increase with adolescents’ age and pubertal development, and are mediated by sensation seeking. Finally, peer relationships moderate the influence of genes and of sensation seeking on problem behavior, and activate the incentive-processing system. Considering these findings together, we propose a model (see Figure 1) in which genetically influenced individual differences in the structure and function of the incentive-processing system emerge at puberty and widen over adolescence. According to our model, these individual differences in neurodevelopment are also predicted to mediate genetic influences on sensation seeking and ultimately on problem behavior.
Although the studies we have described are consistent with our model, testing these hypotheses directly requires studies that simultaneously examine the links among genes, individual differences in brain structure and function, psychological functioning, and actual behavior. At least one candidate gene study has attempted to link measured genetic polymorphisms, imaging measures of brain activity, and problem behavior (43). In that study, adolescents who used illicit substances had significantly greater activity in the right insula and right anterior cingulate when successfully inhibiting responses on a stop-signal task; this right frontal network, in turn, was significantly associated with a polymorphism in SLC6A2, which codes for the norepinephrine transporter. However, the effect size for this single polymorphism was small (R2 ~ 1%) and has not yet been replicated. Additional interdisciplinary research that integrates measures of neurobiology into genetic studies (including both quantitative and molecular genetic designs) is needed.
Looking Ahead at Interdisciplinary Research
Researchers are increasingly interested in interdisciplinary studies that combine genetic and neuroimaging data (e.g., 44). Although quite demanding in terms of sample size, twin and family designs that incorporate measures of brain structure and function (e.g., 45) may be particularly valuable. In initial results from twin studies, some individual differences in brain structure and function are highly heritable. For example, grey matter density in the amygdala (which is involved in the incentive-processing system) is substantially heritable (h2 = .80) at the onset of puberty (46), and by adulthood, the heritability of frontal lobe volumes (which are involved in cognitive control) exceeds 90% (47). However, these studies have examined the heritability of phenotypes at a single level of analysis (e.g., brain structure); multivariate twin designs that measure phenotypes that traverse many levels of analysis (e.g., VS activity, sensation seeking, and problem behavior) are needed to test whether these phenotypes are influenced by the same underlying genetic polymorphisms.
Additionally, longitudinal genetically informative designs can answer critical questions about the direction of causation between neurobiological correlates and problem behaviors. Such questions are salient given that problem behavior often involves substance use, and cross-sectional designs provide little information about whether a structural or functional correlate represents an endogenous risk or is a consequence of exposure to substances. More generally, although ostensibly focused on understanding the development of problem behaviors in adolescence, much of the research we have described has been cross-sectional rather than longitudinal. As Loth and colleagues (48) commented, “adolescence-specific maturational growth curves, rather than adult norms, need to be considered when assessing individual differences” (p. 437).
Researchers should take a multivariate approach that considers both unity and diversity in the neurobiological and genetic underpinnings of problem behaviors. Illustrating the utility of a multivariate approach, a recent imaging study of 14- to 16-year-olds examined correlates of a general factor representing common variance across attention deficit hyperactivity disorder, conduct disorder, and symptoms of substance use, as well as unique correlates of behavioral subtypes. Sensation seeking and neural activity while anticipating a reward were associated uniquely with substance use, whereas the general factor was associated with impulsivity and neural activity during response inhibition (49). However, no study has yet investigated this topic using a longitudinal design that can test whether individual differences in neurobiological change are associated uniquely with some forms of problem behavior but not others.
Finally, even rats show heritable individual differences in risk-taking behavior (50). This cross-species consistency suggests that the biological changes characteristic of adolescence have evolved to serve an adaptive purpose—to motivate exploration and separation from caregivers. Adolescents with genetic or neurobiological characteristics associated with problem behavior could engage in forms of risk-taking that are socially sanctioned or even prosocial. Yet the positive trajectories of biologically “at risk” teenagers, as well as the environmental opportunities and individual characteristics that foster prosocial risk-taking, are poorly understood. Addressing this question will stimulate a broader consideration of how the unique characteristics of teenagers can be leveraged to maximize healthy outcomes for themselves and for society as a whole.
Acknowledgments
Thank you to Elliot M. Tucker-Drob and members of the Harden and Tucker-Drob labs for helpful comments on earlier drafts of this article. This work was supported by grants 1-R21-AA020588 and 1-R21-AA023322 from the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Following Jessor and Jessor’s (4) formulation, we use the term problem behavior here, but other research traditions use different nomenclatures. Clinical psychologists have typically emphasized behaviors that are symptoms of DSM-defined externalizing disorders, including oppositional defiant disorder, conduct disorder, and substance use disorder. Epidemiologists and neuroscientists are often more interested in risk-taking behavior, such as cigarette smoking, sex without condoms, and reckless driving, which may be dangerous without necessarily indicating clinical pathology. The tradition of developmental psychopathology frequently operationalizes externalizing to include normal-range behaviors such as lying to parents, which are neither clinical-level symptoms nor necessarily risky. Finally, criminologists have focused on delinquent or criminal behaviors that violate laws. The term problem behavior is intended to imply a broad conceptualization of the focal construct, including health-risk behaviors (e.g., sex without condoms), delinquent behaviors (e.g., shoplifting, drinking alcohol), and clinical symptoms. Certainly, each individual problem behavior has unique causes; for example, adolescent cigarette smoking has decreased in recent historical periods (5), whereas marijuana use has increased (6). Yet these behaviors are united by the fact that they are, in fact, problems (for the adolescent or for society); they show similar developmental trends, increasing in prevalence in adolescence and early adulthood; they are highly intercorrelated; and they share common underlying causes (7).
References
- 1.Loeber R, Farrington DP. Age-crime curve. In: Bruinsma G, Weisburg D, editors. Encyclopedia of criminology and criminal justice. New York, NY: Springer; 2014. pp. 12–18. [Google Scholar]
- 2.McGue M, Iacono WG. The association of early adolescent problem behavior with adult psychopathology. American Journal of Psychiatry. 2005;162:1118–1124. doi: 10.1176/appi.ajp.162.6.1118. [DOI] [PubMed] [Google Scholar]
- 3.Swendsen J, Burstein M, Case B, Conway KP, Dierker L, He J, Merikangas KR. Use and abuse of alcohol and illicit drugs in U.S. adolescents: Results of the National Comorbidity Survey–Adolescent Supplement. Archives of General Psychiatry. 2012;69:390–398. doi: 10.1001/archgenpsychiatry.2011.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessor R, Jessor SL. Problem behavior and psychosocial development: A longitudinal study of youth. New York, NY: Academic Press; 1977. [Google Scholar]
- 5.Miech R, Koester S. Trends in U.S., past-year marijuana use from 1985 to 2009: An age-period-cohort analysis. Drug and Alcohol Dependence. 2012;124:259–267. doi: 10.1016/j.drugalcdep.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Midlöv P, Calling S, Sundquist J, Sundquist K, Johansson SE. The longitudinal age and birth cohort trends of smoking in Sweden: A 24-year follow-up study. International Journal of Public Health. 2014;59:243–250. doi: 10.1007/s00038-013-0535-5. [DOI] [PubMed] [Google Scholar]
- 7.Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- 8.Burt SA, Neiderhiser JM. Aggressive versus nonaggressive antisocial behavior: Distinctive etiological moderation by age. Developmental Psychology. 2009;45:1164–1176. doi: 10.1037/a0016130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burt SA, Klump KL. The etiological moderation of aggressive and nonaggressive antisocial behavior by age. Twin Research and Human Genetics. 2009;12:343–350. doi: 10.1375/twin.12.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burt SA. Evidence for meaningful etiological distinctions within the broader construct of antisocial behavior. In: Rhee SH, Ronald A, editors. Behavior genetics of psychopathology. New York, NY: Springer; 2014. [Google Scholar]
- 11.Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- 12.Harden KP, Patterson MW, Briley DA, Engelhardt LE, Kretsch N, Mann FD, Tackett JL, Tucker-Drob EM. Developmental changes in genetic and environmental influences on rule-breaking and aggression: Age and pubertal development. Journal of Child Psychology and Psychiatry. 2015 doi: 10.1111/jcpp.12419. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harden KP, Mendle J. Gene-environment interplay in the association between pubertal timing and delinquency in adolescent girls. Journal of Abnormal Psychology. 2012;121:73–87. doi: 10.1037/a0024160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicology and Teratology. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Tucker-Drob EM, Briley D, Harden KP. Genetic and environmental influences on cognition across development and context. Current Directions in Psychological Science. 2013;22:349–355. doi: 10.1177/0963721413485087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge X, Brody GH, Conger RD, Simons RL, Murry VM. Contextual amplification of pubertal transition effects on deviant peer affiliation and externalizing behavior among African American children. Developmental Psychology. 2002;38:42–54. doi: 10.1037//0012-1649.38.1.42. [DOI] [PubMed] [Google Scholar]
- 17.Newcomb MD, McGee L. Influence of sensation seeking on general deviance and specific problem behaviors from adolescence to young adulthood. Journal of Personality and Social Psychology. 1991;61:614–628. doi: 10.1037//0022-3514.61.4.614. [DOI] [PubMed] [Google Scholar]
- 18.Harden KP, Tucker-Drob EM. Individual differences in the development of sensation seeking and impulsivity during adolescence: Further evidence for a dual systems model. Developmental Psychology. 2011;47:739–746. doi: 10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- 19.Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Harden KP, Quinn PD, Tucker-Drob EM. Genetically influenced change in sensation seeking drives the rise of delinquent behavior during adolescence. Developmental Science. 2012;15:150–163. doi: 10.1111/j.1467-7687.2011.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvatore JE, Aliev F, Bucholz K, Agrawal A, Hesselbrock V, Bessselbrock M, Dick DM. Polygenic risk for externalizing disorders: Gene-by-development and gene-by-environment effects in adolescents and young adults. Clinical Psychological Science. 2014:1–13. doi: 10.1177/2167702614534211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brendgen M. Genetics and peer relations: A review. Journal of Research on Adolescence. 2012;22:419–437. [Google Scholar]
- 23.Harden KP, Hill JE, Turkheimer E, Emery RE. Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behavior Genetics. 2008;38:339–347. doi: 10.1007/s10519-008-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latendresse SJ, Bates JE, Goodnight JA, Lansford JE, Budde JP, Goate A, Dodge K, Pettit GS, Dick DM. Differential susceptibility to adolescent externalizing trajectories: Examining the interplay between CHRM2 and peer group antisocial behavior. Child Development. 2011;82:1797–1814. doi: 10.1111/j.1467-8624.2011.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kretschmer T, Vitaro F, Barker ED. The association between peer and own aggression is moderated by the BDNF val-met polymorphism. Journal of Research on Adolescence. 2014;24:177–185. doi: 10.1111/jora.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann FD, Kretsch N, Tackett JL, Tucker-Drob EM, Harden KP. Person × environment interactions on adolescent delinquency: Sensation seeking, peer deviance and parental monitoring. Personality and Individual Differences. 2015;76:129–134. doi: 10.1016/j.paid.2014.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geier CF. Adolescent cognitive control and reward processing: Implications for risk taking and substance use. Hormones and Behavior. 2013;64:333–342. doi: 10.1016/j.yhbeh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:1–9. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanton SJ, Liening SH, Schultheiss OC. Testosterone is positively associated with risk taking in the Iowa Gambling Task. Hormones and Behavior. 2011;59:252–256. doi: 10.1016/j.yhbeh.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Van Honk J, Schutter DJ, Hermans EJ, Putman P, Tuiten A, Koppeschaar H. Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology. 2004;29:937–943. doi: 10.1016/j.psyneuen.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Crone EA, Vendel I, van der Molen MW. Decision-making in disinhibited adolescents and adults: Insensitivity to future consequences or driven by immediate reward? Personality and Individual Differences. 2003;35:1625–1641. [Google Scholar]
- 33.Suhr JA, Tsanadis J. Affect and personality correlates of the Iowa Gambling Task. Personality and Individual Differences. 2007;43:27–36. [Google Scholar]
- 34.de Macks ZA, Moor BG, Overgaauw S, Güroğlu B, Dahl RE, Crone EA. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Developmental Cognitive Neuroscience. 2011;1:506–516. doi: 10.1016/j.dcn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermans EJ, Bos PA, Ossewaarde L, Ramsey NF, Fernández G, Van Honk J. Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. Neuroimage. 2010;52:277–283. doi: 10.1016/j.neuroimage.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Spielberg JM, Olino TM, Forbes EE, Dahl RE. Exciting fear in adolescence: Does pubertal development alter threat processing? Developmental Cognitive Neuroscience. 2014;8:86–95. doi: 10.1016/j.dcn.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien L, Albert D, Chein J, Steinberg L. Adolescents prefer more immediate rewards when in the presence of their peers. Journal of Research on Adolescence. 2011;21:747–753. [Google Scholar]
- 38.Kampe KK, Frith CD, Dolan RJ, Frith U. Psychology: Reward value of attractiveness and gaze. Nature. 2001;413:589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- 39.Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith AR, Steinberg L, Strang N, Chein J. Age differences in the impact of peers on adolescents’ and adults’ neural response to reward. Developmental Cognitive Neuroscience. 2015;11:75–82. doi: 10.1016/j.dcn.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guyer AE, McClure EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whelan R, Conrod PJ, Poline J, Lourdusamy A, Banaschewski T, Barker GJ, Garavan H the IMAGEN Consortium. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience. 2012;15:920–927. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- 44.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, Struve M the IMAGEN Consortium. The IMAGEN study: Reinforcement-related behaviour in normal brain function and psychopathology. Molecular Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 45.van Soelen ILC, Brouwer RM, Peper JS, van Leeuwen M, Koenis MMG, van Beijsterveldt TCEM, Swagerman SC, Kahn RS, Pol HEH, Boomsma DI. Brain SCALE: Brain structure and cognition: An adolescent longitudinal twin study into the genetic etiology of individual differences. Twin Research and Human Genetics. 2012;15:453–467. doi: 10.1017/thg.2012.4. [DOI] [PubMed] [Google Scholar]
- 46.Peper JS, Schnack HG, Brouwer RM, Van Baal GCM, Pjetri E, Szekely E, van Leeuwen M, van den Berg SM, Collins DL, Evans AC, Boomsma DI, Kahn RS, Pol HEH. Heritability of regional and global brain structure at the onset of puberty: A magnetic resonance imaging study in 9-year-old twin pairs. Human Brain Mapping. 2009;30:2184–2196. doi: 10.1002/hbm.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Pol H, Hilleke E. Genetic influences on human brain structure: A review of brain imaging studies in twins. Human Brain Mapping. 2007;28:464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loth E, Carvalho F, Schumann G. The contribution of imaging genetics to the development of predictive markers for addictions. Trends in Cognitive Sciences. 2011;15:436–446. doi: 10.1016/j.tics.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde A, Conrod PJ. Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. American Journal of Psychiatry. 2014;171:1310–1319. doi: 10.1176/appi.ajp.2014.13111499. [DOI] [PubMed] [Google Scholar]
- 50.Ashenhurst JR, Seaman M, Jentsch D. Responding in a test of decision-making under risk is under moderate genetic control in the rat. Alcoholism: Clinical and Experimental Research. 2012;36:941–949. doi: 10.1111/j.1530-0277.2011.01701.x. [DOI] [PubMed] [Google Scholar]