Abstract
Sesamin is a furofuran lignan biosynthesized from the precursor lignan pinoresinol specifically in sesame seeds. This lignan is shown to exhibit anti-hypertensive activity, protect the liver from damages by ethanol and lipid oxidation, and reduce lung tumor growth. Despite rapidly elevating demand, plant sources of lignans are frequently limited because of the high cost of locating and collecting plants. Indeed, the acquisition of sesamin exclusively depends on the conventional extraction of particular Sesamum seeds. In this study, we have created the efficient, stable and sustainable sesamin production system using triple-transgenic Forsythia koreana cell suspension cultures, U18i-CPi-Fk. These transgenic cell cultures were generated by stably introducing an RNAi sequence against the pinoresinol-glucosylating enzyme, UGT71A18, into existing CPi-Fk cells, which had been created by introducing Sesamum indicum sesamin synthase (CYP81Q1) and an RNA interference (RNAi) sequence against pinoresinol/lariciresinol reductase (PLR) into F. koreanna cells. Compared to its transgenic prototype, U18i-CPi-Fk displayed 5-fold higher production of pinoresinol aglycone and 1.4-fold higher production of sesamin, respectively, while the wildtype cannot produce sesamin due to a lack of any intrinsic sesamin synthase. Moreover, red LED irradiation of U18i-CPi-Fk specifically resulted in 3.0-fold greater production in both pinoresinol aglycone and sesamin than production of these lignans under the dark condition, whereas pinoresinol production was decreased in the wildtype under red LED. Moreover, we developed a procedure for sodium alginate-based long-term storage of U18i-CPi-Fk in liquid nitrogen. Production of sesamin in U18i-CPi-Fk re-thawed after six-month cryopreservation was equivalent to that of non-cryopreserved U18i-CPi-Fk. These data warrant on-demand production of sesamin anytime and anywhere. Collectively, the present study provides evidence that U18i-CP-Fk is an unprecedented platform for efficient, stable, and sustainable production of sesamin, and shows that a transgenic and specific light-regulated Forsythia cell-based metabolic engineering is a promising strategy for the acquisition of rare and beneficial lignans.
Introduction
The consistent and appropriate intake of low-cost healthy diets and clinical drugs are the most promising and effective ways to improve the quality of life including a healthy life expectancy and to prevent lifestyle-related diseases. Particularly, the recent escalation in the number of elderly individuals has increased the importance of efficient dietary supplement and drug development. Over the past few decades, plant specialized metabolites (formerly termed secondary metabolites), including alkaloids, flavonoids, isoflavonoids, and lignans, have attracted attention as dietary supplements and medicines. Lignans are naturally occurring phenylpropanoid dimers (C6-C3 units; e.g., coniferyl alcohol), in which the phenylpropane units are linked by the central carbons of the side chains [1–7]. Lignans have been characterized from Podophyllum, Linum, Sesamum, Forsythia and various other plant families [1–7]. Sesamin is classified as a furofuran lignan and is the most abundant water-insoluble lignan in Sesamum indicum (sesame) seeds [1–7]. This Sesamum lignan is biosynthesized by the sesame cytochrome P450, CYP81Q1, through the formation of two methylenedioxy bridges in a precursor lignan, pinoresinol (Fig 1) [5–8]. Sesamin was also shown to exert diverse beneficial effects on mammals including human [1–7], including an anti-hypertensive effect [9], the reduction of breast tumor growth [10], and recovery of liver damage caused by ethanol and lipid oxidation [11, 12]. These findings indicate that the demand for sesamin will rapidly increase in the near future. However, sesamin is acquired via extraction from sesame seed oil, and although sesame plants produce more sesamin than any other plant, the oil contains a maximum of 0.4–0.6% (w/w) sesamin [1–7]. Furthermore, sesame seeds are cultivated only once per year, thereby limiting opportunities to obtain large amounts of this compound. These shortcomings, combined with elevating demands on sesamin, indicate that a novel strategy for systematic sesamin production is clearly required.
Fig 1. Biosynthesis pathways of major lignans in Forsythia and Sesamum.
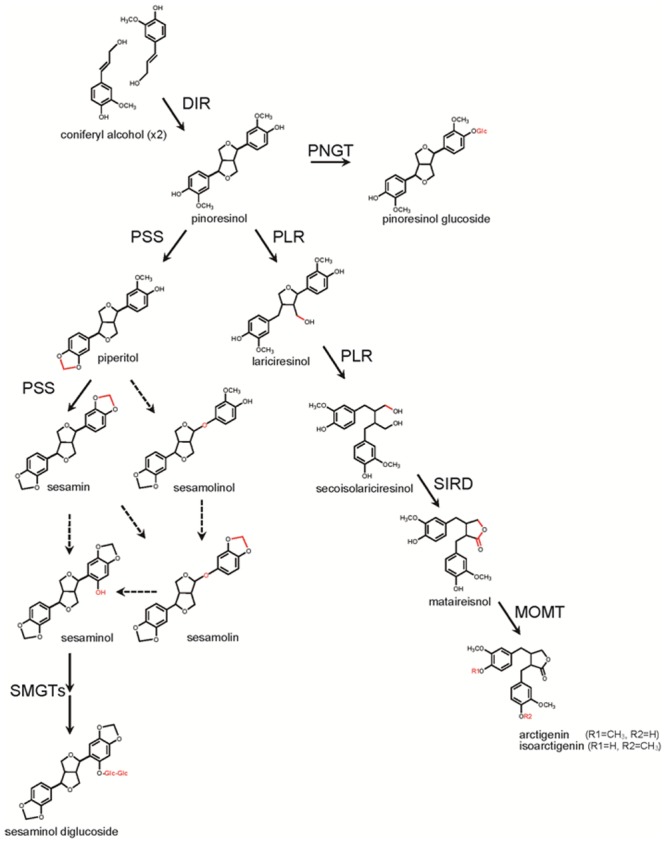
Chemical conversions at each step are indicated in red. Solid and broken lines represent identified and unidentified enzyme-catalyzed reactions, respectively.
Genetic engineering of biosynthetic pathways in plants and in vitro cell or organ cultures either through stable or transient transformation has been attempted in the development of production systems for several plant specialized metabolites, such as alkaloids and flavonoids [13–23]. However, most of transgenic metabolic engineering studies involve merely overexpression of, or RNA interference against, an endogenous biosynthetic enzyme. Furthermore, there have been no reports of the up-regulation of authentic and exogenous beneficial lignan production via either transient or stable gene engineering, except for production of sesamin using Forsythia cells [20]. Wild-type Forsythia koreana plant lacks CYP81Q1 and its functional orthologs, and therefore fails to produce sesamin [1–7]. Instead, this plant abundantly and consistently produces a direct precursor of sesamin, pinoresinol [1–7, 20, 24], which is then either reduced stepwisely into secoisolariciresinol via pinoresinol-lariciresinol reductase (PLR) or glucosylated by UGT71A18 [1–7, 20, 25] in Forsythia (Fig 1). Previously, we showed that sesamin was produced by CPi-Fk, a double-transgenic cell suspension culture line of F. koreana in which a Sesamum sesamin-synthase CYP81Q1 is constitutively expressed and an endogenous PLR is suppressed by RNAi [20]. This was the first report on the exogenous lignan via metabolic engineering, suggesting the potential of CPi-Fk as a prototype of the efficient, stable and sustainable sesamin production system. Unfortunately, CPi-Fk cannot be stored by any procedures, which is a critical drawback of CPi-Fk as a stable and sustainable platform of lignan production. Furthermore, the ability of CPi-Fk to produce sesamin was expected to be markedly improved by introduction of other genes, optimization of culture conditions, and/or the establishment of the procedure for long-term storage. In this paper, we present the development of a triple-transgenic F. koreana cell line, U18i-CPi-Fk, which exhibits a marked increase in production of pinoresinol aglycone and sesamin over that of CPi-Fk and the establishment of an optimized procedure for the long-term cryopreservation and recovery of U18i-CPi-Fk.
Materials and Methods
Cell culture
All cell suspension culture lines were maintained in Gamborg’s B-5 liquid medium supplemented with 6% sucrose and 0.05 mg l-1 2,4-D (Cell Culture Medium; CCM) at 22°C. All suspensions were agitated on a rotary shaker at 110 rpm in the dark and subcultured every 2 weeks with an inoculum of 5 ml of saturated suspension cells. For light treatments, cell cultures were grown in CCM for 2 weeks either under continuous irradiation by blue LED (450–550 nm, 470 nm peak), red LED (600–700 nm, 630 nm peak) (NKsystem, Japan) or white light (white fluorescent tubes) at 100 μmol m-2s-1 photosynthetic photon flux density (PPFD) after an initial 2-week cultivation in the dark.
Binary vector construction
To construct a binary vector for the RNAi of the UTG71A18 gene (Accession: AB524718), the corresponding open reading frame was amplified by PCR using a primer set, RNAi-cacc-UGT71A18-F (5’-CAC CCA GGA ATA GGT CAC TTG ATATCAA-3’) and RNAi-UGT71A18-R (5’-GAA TGG AGC CAA CTA TCC TT-3’). The resultant PCR product was inserted into the pANDA35HK RNAi vector [26] using LR Clonase (Life Technologies).
Transformation of suspension cells
A single colony of Agrobacterium tumefaciens strain EHA105 harboring the UGT71A18-RNAi vector was used to inoculate LB (Luria-Bertani) liquid medium containing 50 mg ml-1 kanamycin and grown at 28°C in a gyratory shaker (180 rpm) until OD600 reached 0.5. The cultures of A. tumefaciens (100 μl) were added to 10 ml of 4-day-old cultures of F. koreana CPi-Fk cells [20] in CCM, followed by co-incubation at 27°C for 2 days in the dark. The suspension cells were then washed four times with 10 ml of fresh CCM, and were plated onto solid CCM medium containing 50 mg ml-1 hygromycin and 100 mg ml-1 cefotaxime. Hygromycin-resistant callus were selected and transferred onto a fresh solid medium containing 50 mg ml-1 hygromycin, and then resuspended in CCM liquid medium to obtain suspensions of UGT71A18-RNAi-transformed CPi-Fk, designated as U18i-CPi-Fk. The resultant U18i-CPi-Fk suspension line was maintained by agitating at 120 rpm in the same medium at 25°C in the dark.
Reverse transcription (RT)-PCR
Total RNA was isolated from 7-day-old suspension cells by the use of RNeasy Plant Mini Kit (Qiagen, CA). First-strand cDNA synthesis was performed using 1 μg of the total RNA with reverse transcriptase SuperScript III (Invitrogen, CA). The endogenous PLR (Accession: AAC49608), UGT71A18 (Accession: AB524718), rRNA (Accession: AJ236041) and introduced CYP81Q1 (Accession: AB194714) were amplified with the following primer sets: PLR-F (5'-ATG GGA AAA AGC AAA GTT TTG ATC ATT GG-3') and PLR-R (5'-CAC GTA ACG CTT GAG GTA CTC TTC CAC-3') for PLR, UGT71A18-F (5'-TAG CAG ATC AAC CCA GTA AAT-3') and UGT71A18-R (5'-TAG CAG ATC AAC CCA GTA AAT-3') and for UGT71A18, rRNA-F (5'-GAA ACC TGC AAA GCA GA-3'), rRNA-R (5'-CTG ACC TGG GGT CGC TGT CGA-3') for rRNA, and CYP81Q1-F (5'-ATG GAA GCT GAA ATG CTA TAT TCA GCT-3') and CYP81Q1-R (5'- AAC GTT GGA AAC CTG ACG AAG AAC TTT TTC-3') for CYP81Q1, respectively. PCR reactions with ExTaq DNA polymerase (Takara Bio, Shiga, Japan) were run at 94°C for 1 min followed by 28 cycles of 94°C for 30 sec, 57°C for 30 sec and 72°C for 1 min, and a final extension at 72°C for 7 min (GeneAmp 9700, Applied Biosystems). PCR products were visualized using 1.5% agarose gel electrophoresis by ethidium bromide staining.
Lignan quantification
Lignan quantification was performed as previously reported [20, 24]. In brief, the suspension cells were cultured for 14 days, and frozen in liquid (LN2) nitrogen and lyophilized followed by extraction by 80% methanol at 4°C and evaporation in vacuo. The residue was dissolved in water, and the remaining aqueous phase, containing the lignan glycoside, was digested at 40°C overnight with 6 units/ml almond β-glucosidase (Sigma) in 0.15 M sodium acetate buffer (pH 5.2). The resulting samples were adjusted with 50% acetonitrile and then centrifuged at 15,000 rpm for 5 min. The supernatant was filtered through a Millex-LH filter (0.45 μm 4 mm-1; Millipore) and then subjected to analysis by reverse-phase high performance liquid chromatography (HPLC) using a Develosil C30-UG-5 column (4.6 × 150 mm, Nomura Chemical, Aichi, Japan). Each sample was eluted with a linear gradient of 35–90% solvent B [90% acetonitrile containing 0.1% (v/v) trifluoroacetic acid] in solvent A [H2O containing 0.1% (v/v) trifluoroacetic acid] for 20 min at a flow rate of 0.6 ml min-1 and then was eluted with 90% solvent B for 7 min. Lignans were monitored by UV absorption at 280 nm, and identified by both retention time and mass spectrum comparison with standard compounds [20].
Stock of cells in liquid nitrogen
Cryopreservation of F. koreana wildtype and transgenic cells was tested under various conditions using sodium alginate based on previous reports with various modifications [27, 28]. U18i-CPi-Fk was centrifuged at 100 x g for 5 min, and was then resuspended in Gamborg’s B-5 medium containing 2% (w/v) sodium alginate, with cell density of 5 x 106 / ml. The cell suspension was dropwisely added to Gamborg’s B-5 medium containing 0.1 M calcium chloride, leading to encapsulation of cells in alginate beads. The resultant beads were washed by Gamborg’s B-5 medium for 10 min, and incubated for 1 h at 25°C in Gamborg’s B-5 medium containing 2 M glycerol, 0.4 M sucrose, and 1% (w/v) proline. Five beads were transferred to a 1-ml cryovial in 0.25 ml of the same medium, followed by 5-hour pre-freezing at -30°C and storage in liquid nitrogen.
Re-culturing of cells from cryopreservation
Cryopreserved cells were rapidly thawed at 40°C, and the beads were stepwisely incubated in Gamborg’s B-5 medium containing 1.2 M sucrose at 25°C for 15 min, Gamborg’s B-5 medium containing 0.5 M sucrose at 25°C for 15 min, and standard Gamborg’s B-5 medium. The recovered cells were re-grown for 55 days as described above and provided for the lignan quantification.
Statistical analysis
Results are expressed as means ± SEM for the indicated number of observations. Data were analyzed by one-way ANOVA with Dunnett’s error protection. Differences were accepted as significant for p < 0.05.
Results
Generation of U18i- CPi-Fk
Initially, we attempted to simultaneously introduce CYP81Q1, PLR-RNAi, and UGT71A18-RNAi cDNA sequences into F. koreana wildtype cells using an expression vector bearing these three sequences. However, this approach failed to obtain triple-transgenic cell cultures, because the expression of either PLR or UGT71A18 was not suppressed by RNAi in the resultant transgenic cells. We therefore introduced the UGT71A18-RNAi sequence into transgenic F. koreana cells harboring CYP81Q1 and PLR-RNAi (CPi-Fk) [20]. CPi-Fk was transformed with A. tumefaciens containing the UGT71A18-RNAi sequence. More than twenty cell lines were initially selected and grown on Gamborg’s B-5 solid media containing hygromycin and cefotaxime. Several independent cell lines, designated as U18i-CPi-Fk, were selected and proliferated in Gamborg’s B-5 liquid medium as suspension cultures.
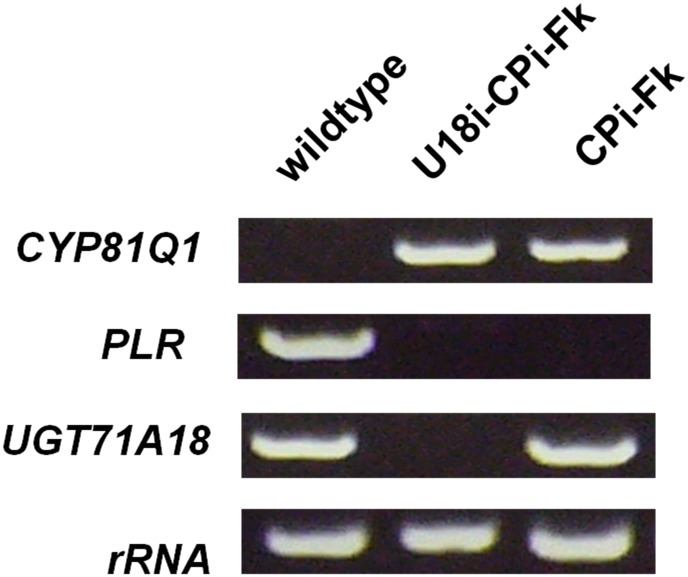
RT-PCR revealed that the expression of both UGT71A18 and PLR was completely suppressed in a U18i-CPi-Fk line, compared to those of F. koreana wildtype cells (Fig 2). CYP81Q1 gene expression was also observed specifically in U18i-CPi-Fk, as seen in CPi-Fk (Fig 2). Altogether, these results provide evidence that all three transgenes, CYP81Q1, PLR-RNAi, and UGT71A18-RNAi, were functionally expressed in U18i-CPi-Fk.
Fig 2. Generation of U18i-CPi-Fk.
Expression of CYP81Q, PLR, and UGT71A18 in F. koreana wildtype, CPi-Fk, and U8i-CPi-Fk.
Lignan production in U18i-CPi-Fk
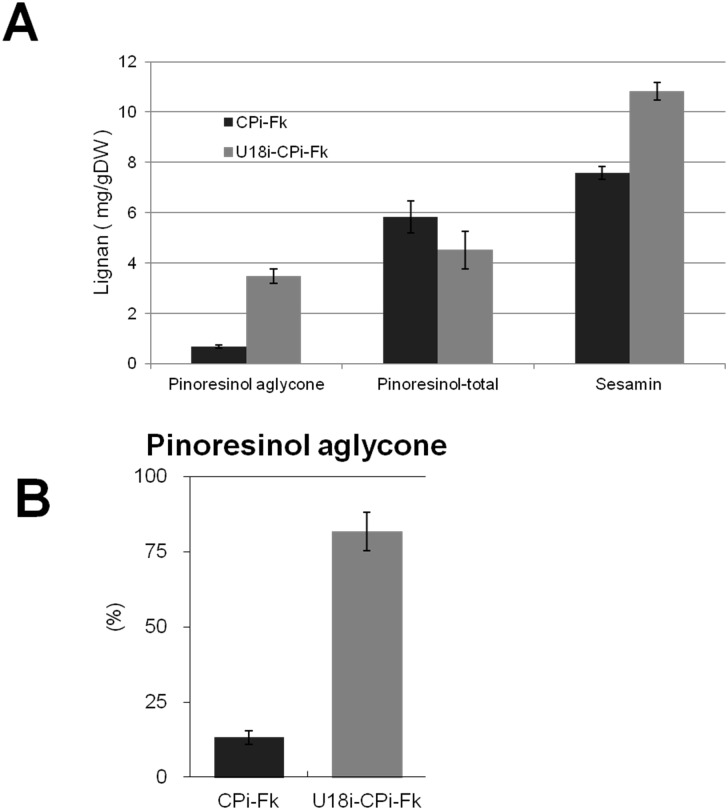
UGT71A18 is responsible for specific glucosylation of pinoresinol [25], and CYP81Q1 converts pinoresinol aglycone, but not its glucosides, into sesamin [7]. Hence, U18i-CPi-Fk, in which UGT71A18 expression is not detected (Fig 2), is expected to accumulate more pinoresinol aglycone and sesamin than CPi-Fk. To evaluate the effect of UGT71A18-RNAi on the production of pinoresinol aglycone and sesamin, we assessed major lignans in CPi-Fk and U18i-CPi-Fk. The lignan mixtures extracted from 2-week cultures of each cell line were either treated or left untreated with β-glycosidase and then subjected to HPLC analysis. As shown in Fig 3A, the amount of total pinoresinol (pinoresinol aglycone and glucoside) was slightly decreased in U18i-CPi-Fk than in CPi-Fk, whereas U18i-CPi-Fk accumulated approximately 5-fold higher amounts of pinoresinol aglycone (2.21 ± 0.72 μg/ g of cell dry weight, DW) than CPi-Fk (0.34 ± 0.09 μg/ g DW). Furthermore, as depicted in Fig 3B, the ratio of pinoresinol aglycone to total pinoresinol in U18i-CPi-Fk is 81.81 ± 6.43%, which is approximately 6.5-fold greater than that in CPi-Fk (13.19 ± 2.35%). These results prove that the introduction of UGT71A18-RNAi construct contributed a great deal to the increase in the ratio of pinoresinol aglycone to total pinoresinol. Consistently, the lignan quantification (Fig 3A) revealed that the amount of sesamin in U18i-CPi-Fk (10.83 ± 0.35 μg/ g DW) was also approximately 1.4-fold greater than that in CPi-Fk (7.57 ± 0.27 μg/ g DW). Collectively, these results verified that U18i-CPi-Fk had considerably higher pinoresinol aglycone and sesamin productivity than the prototypic cell, CPi-Fk, due to the introduction of UGT71A18-RNAi.
Fig 3. Amounts of pinoresinol aglycone, total pinoresinol (aglycone and glucosides), and sesamin.
Comparison of the three lignans between CPi-Fk and U18-CPi-Fk (A). The ratio of pinoresinol aglycone to total aglycone was also calculated (B). Pinoresinol aglycone was quantified separately after a culture period of 15 d under the same conditions. Values (%) in (B) are presented as the ratio of pinoresinol aglycone (2.21 ± 0.72 μg/ g DW and 0.34 ± 0.09 μg/ g DW) to total pinoresinol (3.71 ± 0.40 μg/ g DW and 2.88 ± 0.47 μg/ g DW) in CPi-Fk and U18i-CPi-Fk, respectively. Data are obtained from three independent experiments as the mean ± SEM (P < 0.05).
Effects of light on lignan production in U18i-CPi-Fk
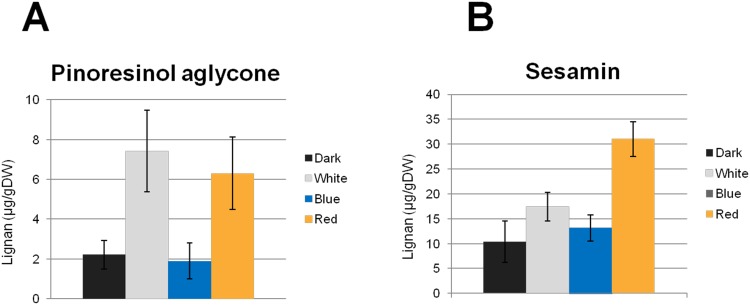
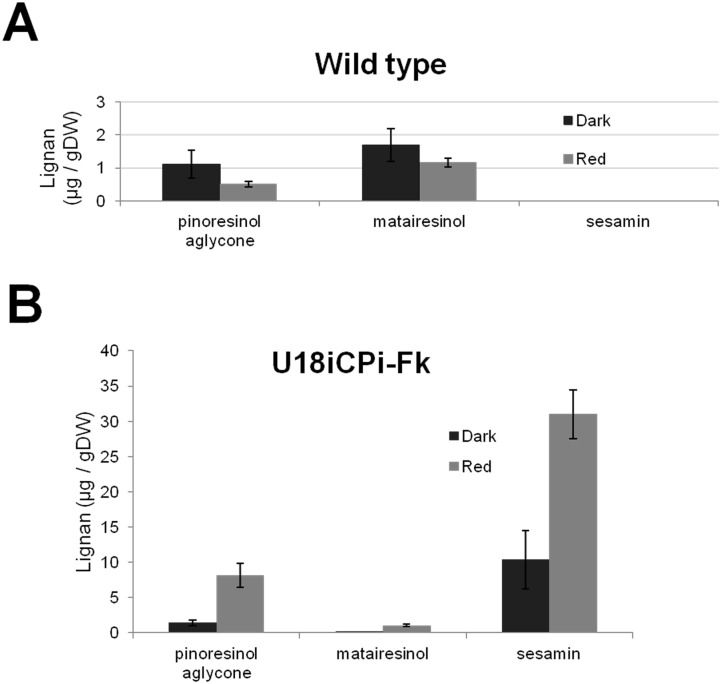
Productivity of several plant specialized metabolites including lignans is affected by the wavelength and the period of light [29–32]. To examine whether the light quality alters lignan production in U18i-CPi-Fk, we compared lignan content in U18i-CPi-Fk cultured either under the dark condition, white fluorescent, blue LED, or red LED light for two weeks following two-week pre-culture under the dark condition. As shown in Fig 4A, pinoresinol aglycone was 3.4-fold (7.41 ± 0.06 μg/ g DW) and 2.8-fold (6.30 ± 1.82 μg/ g DW) greater produced under white fluorescent and red LED, respectively, than under the dark condition (2.21 ± 0.72 μg/ g DW). A striking feature is that sesamin production in U18i-CPi-Fk was approximately 3-fold (31.02 ± 3.45 μg/ g DW) up-regulated specifically under red LED, whereas neither white fluorescent nor blue light resulted in elevation of sesamin production (Fig 4B). To examine the specificity of the effect of red LED on the lignan production in U18i-CPi-Fk, we compared accumulation of pinoresinol aglycone and matairesinol aglycone in the F. koreana wildtype cells and U18i-CPi-Fk. In wild-type cells cultivated under red LED, the levels of pinoresinol aglycone and matairesinol aglycone were decreased to 50% (0.51 ± 0.09 μg/ g DW) and 80% (1.16 ± 0.14 μg/ g DW), respectively, compared to the amounts of pinoresinol (1.02 ± 0.18 μg/ g DW) and matairesinol (1.45 ± 0.20 μg/ g DW) in cells cultured in the dark (Fig 5A). In contrast, red LED light increased the production of these lignans and sesamin in U18i-CPi-Fk relative to production in cells cultivated in the dark; production of pinoresinol aglycone and matairesinol aglycone were approximately 3-fold (8.13 ± 1.68 μg/ g DW) and 2.5-fold (1.07 ± 0.14 μg/ g DW), respectively, under red LED irradiation than those in the dark (Fig 5B). Taken together, these data provide compelling evidence for the specific effect of red LED on the increase of sesamin production in U18i-CPi-Fk.
Fig 4. Comparison of the production of (A) pinoresinol and (B) sesamin in U18i-CPi-Fk cells under the dark, white, blue, and red continuous light conditions.
Lignans were quantified separately for each sample after a culture period of two weeks in the same medium. Data are presented as the average of three independent experiments (mean ± S.E.M., P < 0.05).
Fig 5. Differential effects of red LED irradiation on the increase of lignan production in Forsythia wildtype and U18i-CP-Fk.
Each indicated lignan in wildtype (A) and U18i-CP-Fk (B) in the dark (black) and red LED (gray) was assessed. Each point represents the mean ± S.E.M (P < 0.05) of three preparations.
Cryopreservation and recovery of U18i-CPi-Fk
Continuous culturing of transgenic plant cells frequently resulted in the loss of transgene functions or reduced cell viability. Recently, cryopreservation procedures for various plant cells have been developed [27, 28]. We thus attempted to establish a procedure for sodium alginate-based cryopreservation of F. koreana cell cultures under various conditions. Although we failed to preserve or revive any Forsythia cells using reported procedures, U18i-CPi-Fk was cryopreserved without significant loss of viability for more than six months as described in the Materials and Methods section. Notably, productivity of both pinoresinol aglycone and sesamin of U18i-CPi-Fk re-cultured for 55 days following six-month cryopreservation was comparable to those before cryopreservation (Fig 6). These data lead to the conclusion that U18i-CPi-Fk is an excellent long-term sustainable platform for lignan production.
Fig 6. Evaluation of lignan production after cryopreservation of U18i-CPi-Fk.
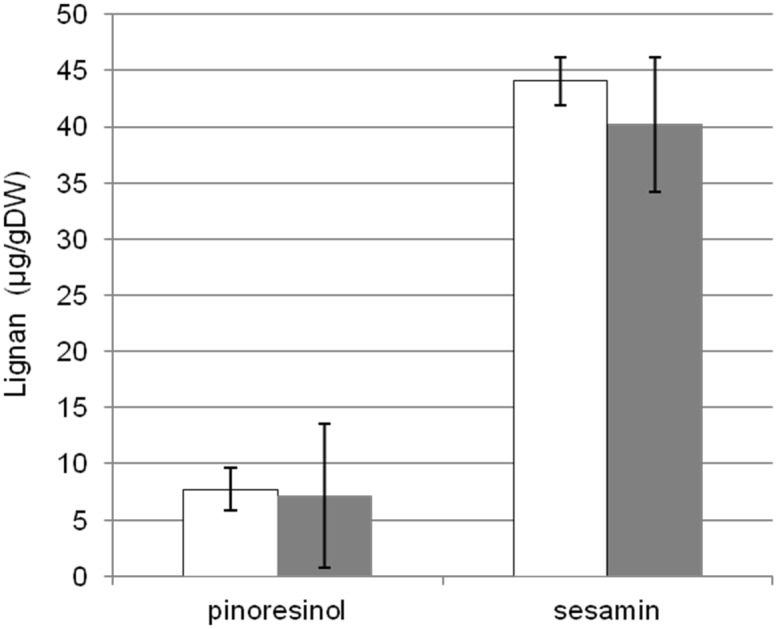
Pinoresinol aglycone and sesamin were extracted from U18-CPi-Fk before cryopreservation (white) and after re-thawing following 6-month cryopreservation (gray). Each lignan was assessed separately after 55-day culture since the recovery under the same condition and expressed as the mean ± SEM of three independent experiments (P < 0.05).
Discussion
Metabolic engineering using transgenic organisms is one of the most promising strategies for efficient and sustainable production of beneficial, but rare, natural compounds in nature. Transgenic plant cells have prominent advantages over transgenic microbes or animals for producing such phytochemicals, including sesamin. Most of the biosynthetic pathways for plant specialized metabolites involve multiple enzymatic steps that are unique to selected plant species. Therefore, transformation of a whole set of the biosynthetic genes would be required for the establishment of transgenic microbial or animal cell lines that could produce phytochemicals [33–36]. In contrast, transgenic plants or their cell lines producing phytochemicals of interest can be generated by transformation of a minimum set of genes using a plant species that originally biosynthesizes precursors or metabolites structurally related to the target compound. Nevertheless, introduction of more than two sets of genes into a plant has been markedly limited, and no such multiple-transgenic plants have yet been created for metabolic engineering of lignan biosynthetic pathways. In the present study, we show stable, efficient and sustainable production of sesamin using novel triple-transgenic Forsythia cells, U18i-CPi-Fk. The triple-transgenic Forsythia cells, U18i-CPi-Fk, produced 1.4-fold greater sesamin and 5-fold greater pinoresinol aglycone than CPi-Fk (Fig 3). To the best of our knowledge, this is the first report on the production of both an endogenous and exogenous plant specialized metabolites by triple-transgenic plants.
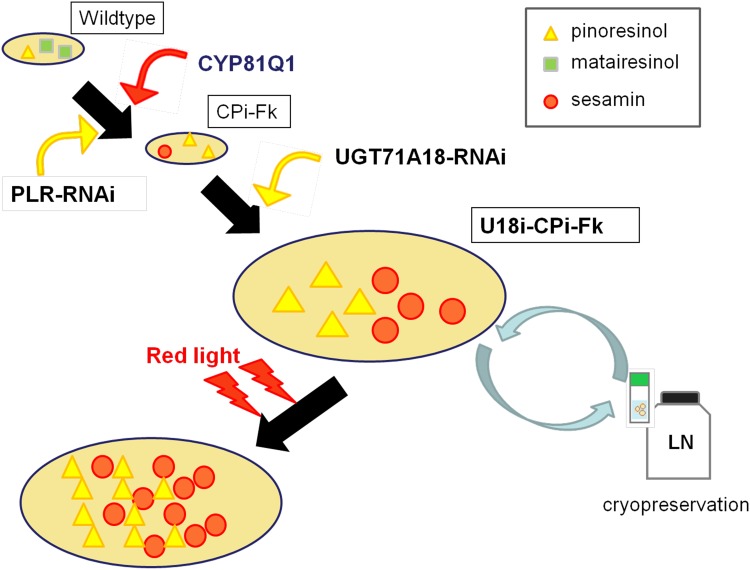
The level of phytochemical production by plants is also regulated by various environmental factors, particularly light wavelengths [29–32]. The responsiveness of plant cell cultures and organs to light stimuli enable the regulation of the production of specialized metabolites, which cannot be used for microbes or animals. Indeed, red LED irradiation specifically increased the production of pinoresinol and sesamin up to 3-fold in U18i-CPi-Fk over production in the dark (Figs 4 and 5). The increase of sesamin is compatible with that of pinoresinol aglycone (Figs 4 and 5). In contrast, red LED irradiation resulted in the reduction of pinoresinol production in F. koreana wildtype cells (Fig 5A). These results suggest that red LED light affect the expression or functions of genes responsible for pinoresinol biosynthesis in F. koreana cells. In addition, in Linum album cell culture, red light reportedly up-regulates expression of enzymes involved in the biosynthesis of phytochemicals upstream of pinoresinol, including phenylalanine ammonia-lyase (PAL) and cinnamoyl-CoA reductase (CCR) [30]. Similarly, production of non-lignan plant specialized metabolites is affected by other light types [30, 37–40]. In this study, the treatment of U18i-CPi-Fk cells with white fluorescent tubes failed to promote sesamin production, while the white light significantly increased pinoresinol aglycone production (Fig 4). These results can be interpreted in two ways. First, the white light tubes include other light wavelengths that might induce metabolic processes or degradation of sesamin. Alternatively, other light wavelengths than red LED might hinder the enzyme activity of CYP81Q1. Although the precise molecular mechanisms await further investigation, these findings clearly demonstrate the importance of the choice of specific light source for maximal production of metabolites of interest in plant cell culture system. Taken together, the present data prove that red LED-directed increase in lignan productivity is a unique and promising advantage of U18i-CPi-Fk for lignan production (Fig 7).
Fig 7. Strategy for efficient, stable, and sustainable lignan production via metabolic engineering of Forsythia cells.
Glycosylation affects the chemical reactivity and water solubility of plant specialized metabolites, frequently leading to changes in their biological activities and the relevant plant phenotypes. For example, changes in the colors of flowers, leaves, and fruits greatly depend on the position and structure of sugars that are attached to the aglycones of various phytochemicals [41–50]. In F. koreana, 90% of total pinoresinol is stored as glucosylated forms in vacuole [2, 20, 24], and pinoresinol glucosides are not converted into sesamin by CYP81Q1 [8]. These findings are in good agreement with the present data demonstrating that RNAi against UGT71A18 led to the marked increase in productivity of sesamin as well as pinoresinol aglycone in U18i-CPi-Fk (Fig 3). In addition, we failed to generate UGT71A18-RNAi-single or UGT71A18-RNAi- and PLR-RNAi-double transgenic Forsythia cells, whereas generation of PLR-RNAi-Forsythia cells was succeeded in our previous study [20]. These results suggest that the accumulation of unreacted pinoresinol aglycone exhibit cytotoxicity that is more lethal to Forsythia than the loss of other lignans downstream of pinoresinol that occurred due to the introduction of PLR-RNAi. In other words, the toxicity of pinoresinol aglycone reinforces the biological significance of pinoresinol glucosylation in Forsythia. Such a view is further supported by previous reports of the toxicity of pinoresinol and its metabolites on plants [51–53]. In contrast, the introduction of CYP81Q1 resulted in increased conversion of pinoresinol aglycone to sesamin and likely contributed to a reduction in the toxicity associated with pinoresinol aglycone accumulation, eventually leading to the generation of U18i-CPi-Fk.
Resilient platforms for the production of beneficial materials in cell culture systems require not only high and efficient productivity but also protocols for long-term and reproducible stocks without significant loss of cell viability. In the present study, we show that, after cryopreservation for six months in liquid nitrogen using a sodium alginate preparation method, U18i-CPi-Fk successfully propagated and produced pinoresinol and sesamin at levels comparable to the original cultures (Fig 6). Universal procedures for long-term stock of plant cell cultures, unlike those of seeds or animal cell cultures, have not been well established. Moreover, cryopreservation procedures for a particular plant species are not readily applicable to other species [27, 28, 54]. In addition, long-term cultures of plant cell cultures frequently result in the loss of cell viability or of inserted genes. In our previous study, we observed a decrease in the growth rate of CPi-Fk cells after two years of culture, and eventually, proliferation loss (data not shown). Consequently, the present establishment of the freeze stocks of U18i-CPi-Fk endorses the usefulness of U18i-CPi-Fk as a stable and sustainable platform of lignan production anytime and anywhere.
Model plants, such as A. thaliana, Nicotiana tabacum, and Oryza sativa, are frequently endowed with only partial or no biosynthetic pathway for specialized metabolites of interest, or, if any, can biosynthesize only very small amounts of most specialized metabolites including lignans [5, 7]. Hence, generation of transgenic model plants competent in lignan production would require complexed transformation of multiple exogenous genes. Indeed, no studies have ever reported efficient and sustainable production of specialized metabolites such as lignans using these model plants. Consequently, a transgenic metabolic engineering strategy using Forsythia, which biosynthesizes large amounts of a basal lignan, pinoresinol (Fig 1), is one of the most promising for efficient and sustainable production of lignans (Fig 7). Moreover, the pinoresinol aglycone to total pinoresinol ratio is high in U18i-CPi-Fk (Fig 3B) owing to the gene-silencing of a pinoresinol-glucosylating enzyme, UGT71A18 (Fig 2). Recent studies of genomes and transcriptomes of non-model, lignan-rich plants such as Sesamum [55–57], Linum [58–60], and Podophyllum [61–63] will enhance not only the elucidation of species-specific total lignan biosynthetic pathways, but also facilitate metabolic engineering of lignan production through transformation of Forsythia plants and cell cultures with newly identified lignan biosynthetic enzyme genes. In addition, a growing body of studies has revealed that the biosynthesis of lignans, including pinoresinol, lariciresinol, and podophyllotoxin, is potentiated by chemical and fungal elicitors in a plant/fungal species- and compound-specific fashion in Linum and Podophyllum cells [6, 7]. Particularly, various elicitors have been found to upregulate the gene expression of early lignan-biosynthetic enzyme genes: CCR, PAL, and PLR, which ultimately contributes to an increase in production of lariciresinol and podophyllotoxin in Linum [6, 7, 64–68]. Although the current sesamin content in U18i-CPi-Fk cells with red LED irradiation (Fig 4) is still low compared to that in sesame seed oil (0.4–0.6% w/w) [1–7], the productivity of sesamin in U18i-CPi-Fk will be more efficient than that of sesame seed oil by combination of the aforementioned multiple approaches for the up-regulation of lignan biosynthesis and the advantages of scalability and year-round sesamin extraction periods of U18i-CPi-Fk. Collectively, we conclude that U18i-CPi-Fk possesses unprecedented functionalities for transgenic metabolic engineering-based platforms for the efficient production of an exogenous lignan, sesamin, and has potential for producing other beneficial lignans via metabolic engineering of the relevant biosynthetic pathways. Consequently, U18i-CPi-Fk is a novel platform that will pave the way for the conversion of conventional agricultural lignan production to innovative bio-industrial lignan production
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing prior to submission.
Abbreviations
- CCR
cinnamoyl-CoA reductase
- DIR
dirigent protein
- LC-MS
liquid chromatography-mass spectrometry
- PAL
phenylalanine ammonia-lyase
- PLR
pinoresinol/lariciresinol reductase
- RNAi
RNA interference
- RT-PCR
reverse transcription-PCR
- SDG
secoisolariciresinol diglucoside
Data Availability
All relevant data are within the paper.
Funding Statement
This work is supported by the Plant Factory project of the Ministry of Economy, Trade and Industry, Japan. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Davin LB, Lewis NG. A historical perspective on lignan biosynthesis: monolignol, allylphenol and hydroxycinnamic acid coupling and downstream metabolism. Phytochem Rev. 2003; 2: 257–288. 10.1023/B:PHYT.0000046175.83729.b5 [DOI] [Google Scholar]
- 2. Umezawa T. Diversity in lignan biosynthesis. Phytochem Rev. 2003; 2: 371–390. 10.1023/B:PHYT.0000045487.02836.32 [DOI] [Google Scholar]
- 3. Suzuki S, Umezawa T. Biosynthesis of lignans and norlignans. J Wood Sci. 2007; 53: 273–284. 10.1007/s10086-007-0892-x [DOI] [Google Scholar]
- 4. Davin LB, Jourdes M, Patten AM, Kim KW, Vassão DG, Lewis NG. Dissection of lignin macromolecular configuration and assembly: comparison to related biochemical processes in allyl/propenyl phenol and lignan biosynthesis. Nat Prod Rep. 2008. 25, 1015–1090. 10.1039/b510386j [DOI] [PubMed] [Google Scholar]
- 5. Satake H, Ono E, Murata J. Recent advances in metabolic engineering of lignan biosynthesis pathways for the production of transgenic plant-based foods and supplements. J Agric Food Chem 2013; 61, 11721–11729. 10.1021/jf4007104 [DOI] [PubMed] [Google Scholar]
- 6. Malik S, Biba O, Grúz J, Arroo RRJ, Strnad M. Biotechnological approaches for producing aryltetralin lignans from Linum species. Phytochem Rev. 2014; 13, 893–913. 10.1007/s11101-014-9345-5 [DOI] [Google Scholar]
- 7. Satake H, Koyama T, Bahabadi SE, Matsumoto E, Ono E, Murata J. Essences in metabolic engineering of lignan biosynthesis. Metabolites. 2015; 5, 270–90. 10.3390/metabo5020270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ono E, Nakai M, Fukui Y, Tomimori N, Fukuchi-Mizutani M, Saito M et al. Formation of two methylenedioxy bridges by a Sesamum CYP81Q protein yielding a furofuran lignan, (+)-sesamin. Proc Natl Acad Sci U S A. 2006;103:10116–10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sirato-Yasumoto S, Katsuta M, Okuyama Y, Takahashi Y, Ide T. Effect of sesame seeds rich in sesamin and sesamolin on fatty acid oxidation in rat liver. J Agric. Food Chem. 2001; 49: 2647–2651. [DOI] [PubMed] [Google Scholar]
- 10. Truan JS, Chen JM, Thompson LU. Comparative effects of sesame seed lignan and flaxseed lignan in reducing the growth of human breast tumors (MCF-7) at high levels of circulating estrogen in athymic mice. Nutr Cancer. 2012; 64: 65–71. 10.1080/01635581.2012.630165 [DOI] [PubMed] [Google Scholar]
- 11. Nakai M, Harada M, Nakahara K, Akimoto K, Shibata H, Miki W et al. Novel antioxidative metabolites in rat liver with ingested sesamin. J Agric Food Chem. 2003; 51: 1666–1670 [DOI] [PubMed] [Google Scholar]
- 12. Nakano D, Itoh C, Takaoka M, Kiso Y, Tanaka T, Matsumura Y. Antihypertensive effect of sesamin. IV. Inhibition of vascular superoxide production by sesamin. Biol Pharm Bull. 2002; 25: 1247–1249. [DOI] [PubMed] [Google Scholar]
- 13. Ogita S, Uefuji H, Yamaguchi Y, Koizumi N, Sano H. Producing decaffeinated coffee plants. Nature 423: 823 [DOI] [PubMed] [Google Scholar]
- 14. Allen RS, Millgate AG, Chitty JA, Thisleton J, Miller JA, Fist AJ et al. RNAi-mediated replacement of morphine with the nonnarcotic alkaloid reticuline in opium poppy. Nat Biotechnol. 2004; 22: 1559–1566. [DOI] [PubMed] [Google Scholar]
- 15. Hemmati S, Schmidt TJ, Fuss E. (+)-pinoresinol/(-)-lariciresinol reductase from Linum perenne Himmelszelt involved in the biosynthesis of justicidin B. FEBS Lett. 2007; 581: 603–610. [DOI] [PubMed] [Google Scholar]
- 16. Ayella AK, Trick HN, Wang W. Enhancing lignan biosynthesis by over-expressing pinoresinol lariciresinol reductase in transgenic wheat. Mol. Nutr. Food Res. 2007; 51: 1518–1526. [DOI] [PubMed] [Google Scholar]
- 17. Bayindir U, Alfermann W, Fuss E. Hinokinin Biosynthesis in Linum corymbulosum Reichenb. Plant J. 2008; 55: 810–820. 10.1111/j.1365-313X.2008.03558.x [DOI] [PubMed] [Google Scholar]
- 18. Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol. 2008; 26: 1301–1308. 10.1038/nbt.1506 [DOI] [PubMed] [Google Scholar]
- 19. Badejo AA, Eltelib HA, Fukunaga K, Fujikawa Y, Esaka M. Increase in ascorbate content of transgenic tobacco plants overexpressing the acerola (Malpighia glabra) phosphomannomutase gene. Plant Cell Physiol. 2009; 50: 423–428. 10.1093/pcp/pcn206 [DOI] [PubMed] [Google Scholar]
- 20. Kim HJ, Ono E, Morimoto K, Yamagaki T, Okazawa A, Kobayashi A et al. Metabolic engineering of lignan biosynthesis in Forsythia cell culture. Plant Cell Physiol. 2009; 50, 2200–2209. 10.1093/pcp/pcp156 [DOI] [PubMed] [Google Scholar]
- 21. Leonard E, Runguphan W, O'Connor S, Prather KJ. Opportunities in metabolic engineering to facilitate scalable alkaloid production. Nat Chem Biol. 2009; 5: 292–300. 10.1038/nchembio.160 [DOI] [PubMed] [Google Scholar]
- 22. Farhi M, Marhevka E, Ben-Ari J, Algamas-Dimantov A, Liang Z, Zeevi V et al. Generation of the potent anti-malarial drug artemisinin in tobacco. Nat Biotechnol. 2011; 29:1072–1074. 10.1038/nbt.2054 . [DOI] [PubMed] [Google Scholar]
- 23. Renouard S, Tribalatc MA, Lamblin F, Mongelard G, Fliniaux O, Corbin C et al. RNAi-mediated pinoresinol lariciresinol reductase gene silencing in flax (Linum usitatissimum L.) seed coat: consequences on lignans and neolignans accumulation. J Plant Physiol. 2014; 171: 1372–1377. 10.1016/j.jplph.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 24. Morimoto K, Satake H. Seasonal alteration in amounts of lignans and their glucosides and gene expression of the relevant biosynthetic enzymes in the Forsythia suspense leaf. Biol Pharm Bull. 2013; 36: 1519–1523. 10.1248/bpb.b13-00437 [DOI] [PubMed] [Google Scholar]
- 25. Ono E, Kim HJ, Murata J, Morimoto K, Okazawa A, Kobayashi A et al. Molecular and functional characterization of novel furofuran-class lignan glucosyltransferases from Forsythia . Plant Biotechnol. 2010; 27: 317–324. 10.5511/plantbiotechnology.27.317 [DOI] [Google Scholar]
- 26. Miki D, Shimamoto K. Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol. 2004; 45: 490–495. . [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi T, Niino T, Kobayashi M. Simple cryopreservation protocol with an encapsulation technique for tobacco BY-2 suspension cell cultures. Plant Biotechnol. 2005; 22, 105–112. 10.5511/plantbiotechnology.22.105 [DOI] [Google Scholar]
- 28. Ogawa Y, Sakurai N, Oikawa A, Kai K, Morishita Y, Mori K et al. High-throughput cryopreservation of plant cell cultures for functional genomics. Plant Cell Physiol. 2012; 53: 943–952. 10.1093/pcp/pcs038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morimoto K, Kim HJ, Ono E, Kobayashi A, Okazawa A, Satake H. Effects of light on production of endogenous and exogenous lignans by Forsythia koreana wildtype and transgenic cells. Plant Biotechnol. 2011; 28: 331–337. 10.5511/plantbiotechnology.11.0420a [DOI] [Google Scholar]
- 30. Yousefzadi M, Sharifi M, Behmanesh M, Ghasempour A, Moyano E, Palazon J. The effect of light on gene expression and podophyllotoxin biosynthesis in Linum album cell culture. Plant Physiol. Biochem. 2012; 56: 41–46. 10.1016/j.plaphy.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 31. Hata N., Hayashi Y., Okazawa A., Ono E., Satake H., Kobayashi A. Effect of photoperiod on growth of the plants, and sesamin content and CYP81Q1 gene expression in the leaves of sesame (Sesamum indicum L.). Envir Exp Bot 2012; 75: 212–219. 10.1016/j.envexpbot.2011.07.004 [DOI] [Google Scholar]
- 32. Hata N, Hayashi Y, Ono E, Satake H, Kobayashi A, Muranaka T et al. Differences in plant growth and leaf sesamin content of the lignan-rich sesame variety ‘Gomazou’ under continuous light of different wavelengths. Plant Biotechnol. 2013; 30: 1–8. 10.5511/plantbiotechnology.12.1021a [DOI] [Google Scholar]
- 33. Winzer T, Kern M, King AJ, Larson TR, Teodor RI, Donninger SL et al. Plant science. Morphinan biosynthesis in opium poppy requires a P450-oxidoreductase fusion protein. Science. 2015; 349: 309–312. 10.1126/science.aab1852 [DOI] [PubMed] [Google Scholar]
- 34. Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013; 496(7446):528–532. 10.1038/nature12051 . [DOI] [PubMed] [Google Scholar]
- 35. Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006; 440: 940–943. . [DOI] [PubMed] [Google Scholar]
- 36. Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci U S A. 2012;109(3):E111–118. 10.1073/pnas.1110740109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bharti AK, Khurana JP. Mutants of Arabidopsis as tools to understand the regulation of phenylpropanoid pathway and UVB protection mechanisms. Photochem Photobiol. 1997. 65: 765–776. [DOI] [PubMed] [Google Scholar]
- 38. von Lintig J, Welsch R, Bonk M, Giuliano G, Batschauer A Kleinig H. Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. Plant J 1997; 12: 625–634. [DOI] [PubMed] [Google Scholar]
- 39. Yazaki K, Matsuoka H, Ujihara T and Sato F. Shikonin biosynthesis in Lithospermum erythrorhizon: Light-induced negative regulation of secondary metabolism. Plant Biotechnol. 1999; 16: 335–342. 10.5511/plantbiotechnology.16.335 [DOI] [Google Scholar]
- 40. Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011; 6: 1720–31. 10.4161/psb.6.11.17613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bowles D, Isayenkova J, Lim EK, Poppenberger B. Glycosyltransferases: managers of small molecules. Curr Opin Plant Biol. 2005; 8: 254–263. [DOI] [PubMed] [Google Scholar]
- 42. Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 2008; 54: 733–749. 10.1111/j.1365-313X.2008.03447.x [DOI] [PubMed] [Google Scholar]
- 43. Griesser M, Hoffmann T, Bellido ML, Rosati C, Fink B, Kurtzer R et al. Redirection of flavonoid biosynthesis through the down-regulation of an anthocyanidin glucosyltransferase in ripening strawberry fruit. Plant Physiol. 2008; 146: 1528–1539. 10.1104/pp.107.114280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee C, Teng Q, Huang W, Zhong R, Ye ZH. Down-regulation of PoGT47C expression in poplar results in a reduced glucuronoxylan content and an increased wood digestibility by cellulase. Plant Cell Physiol. 2009; 50:1075–1089. 10.1093/pcp/pcp060 [DOI] [PubMed] [Google Scholar]
- 45. Kim I-A, Heo J-O, Chang K-S, Lee S-E, Lee M-H, Lim C-E et al. (2010) Overexpression and Inactivation of UGT73B2 Modulate Tolerance to Oxidative Stress in Arabidopsis . J. Plant Biol. 53, 233–239 10.1007/s12374-010-9110-2 [DOI] [Google Scholar]
- 46. Montefiori M, Espley RV, Stevenson D, Cooney J, Datson PM, Saiz A et al. Identification and characterisation of F3GT1 and F3GGT1, two glycosyltransferases responsible for anthocyanin biosynthesis in red-fleshed kiwifruit (Actinidia chinensis). Plant J. 2011; 65(1): 106–118. 10.1111/j.1365-313X.2010.04409.x . [DOI] [PubMed] [Google Scholar]
- 47. Babst BA, Chen HY, Wang HQ, Payyavula RS, Thomas TP, Harding SA et al. Stress-responsive hydroxycinnamate glycosyltransferase modulates phenylpropanoid metabolism in Populus. J Exp Bot. 2014; 65: 4191–4200. 10.1093/jxb/eru192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lao J, Oikawa A, Bromley JR, McInerney P, Suttangkakul A, Smith-Moritz AM et al. The plant glycosyltransferase clone collection for functional genomics. Plant J. 2014; 79: 517–529. 10.1111/tpj.12577 . [DOI] [PubMed] [Google Scholar]
- 49. Sugimoto K, Matsui K, Iijima Y, Akakabe Y, Muramoto S, Ozawa R et al. Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proc Natl Acad Sci U S A. 2014; 111: 7144–7149. 10.1073/pnas.1320660111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dima O, Morreel K, Vanholme B, Kim H, Ralph J, Boerjan W. Small glycosylated lignin oligomers are stored in Arabidopsis leaf vacuoles. Plant Cell. 2015; 27: 695–710. 10.1105/tpc.114.134643 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oliva A, Moraes RA, Watson SB, Duke SO, Dayan FE. Aryltertralin lignans inhibit plant growth by affecting formation of mitotic microtubular organizing centers. Pestic Biochem Phys. 2002; 72: 45–54. 10.1006/pest.2002.2582 [DOI] [Google Scholar]
- 52. Harmatha J, Dinan L. Biological activities of lignans and stilbenoids associated with plant-insect chemical interaction. Phytochem Rev. 2003; 2: 321–330. 10.1023/B:PHYT.0000045494.98645.a3 [DOI] [Google Scholar]
- 53. Cutillo F, D'Abrosca B, DellaGreca M, Fiorentino A, Zarrelli A. Lignans and neolignans from Brassica fruticulosa: effects on seed germination and plant growth. J Agric Food Chem. 2003; 51: 6165–6172. [DOI] [PubMed] [Google Scholar]
- 54. Sarasan V, Cripps R, Ramsay MM, Atherton C, Mcmichen M, Prendergast G. Conservation in vitro of threatened plants—progress in the past decade. In Vitro Cell Dev -Pl. 2006; 42: 206–214. 10.1079/IVP2006769 [DOI] [Google Scholar]
- 55. Wang L, Yu S, Tong C, Zhao Y, Liu Y, Song C et al. Genome sequencing of the high oil crop sesame provides insight into oil biosynthesis. Genome Biol. 2014; 15: R39 10.1186/gb-2014-15-2-r39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang L, Han X, Zhang Y, Li D, Wei X, Ding X et al. Deep resequencing reveals allelic variation in Sesamum indicum . BMC Plant Biol. 2014; 14: 225 10.1186/s12870-014-0225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu K, Yang M, Liu H, Tao Y, Mei J, Zhao Y. Genetic analysis and molecular characterization of Chinese sesame (Sesamum indicum L.) cultivars using insertion-deletion (InDel) and simple sequence repeat (SSR) markers. BMC Genet. 2014; 15: 35 10.1186/1471-2156-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Z, Hobson N, Galindo L, Zhu S, Shi D, McDill J et al. The genome of flax (Linum usitatissimum) assembled de novo from short shotgun sequence reads. Plant J. 2012; 72: 461–473. 10.1111/j.1365-313X.2012.05093.x [DOI] [PubMed] [Google Scholar]
- 59. Barvkar VT, Pardeshi VC, Kale SM, Kadoo NY, Gupta VS. Phylogenomic analysis of UDP glycosyltransferase 1 multigene family in Linum usitatissimum identified genes with varied expression patterns. BMC Genomics. 2012; 13,175 10.1186/1471-2164-13-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Babu PR, Rao KV, Reddy VD. Structural organization and classification of cytochrome P450 genes in flax (Linum usitatissimum L.). Gene. 2013; 513: 156–162. 10.1016/j.gene.2012.10.040 [DOI] [PubMed] [Google Scholar]
- 61. Bhattacharyya D, Sinha R, Hazra S, Datta R, Chattopadhyay S. De novo transcriptome analysis using 454 pyrosequencing of the Himalayan Mayapple, Podophyllum hexandrum . BMC Genomics. 2013; 14: 748 10.1186/1471-2164-14-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Marques JV, Kim KW, Lee C, Costa MA, May GD, Crow JA et al. Next generation sequencing in predicting gene function in podophyllotoxin biosynthesis. J Biol Chem. 2013; 288: 466–479. 10.1074/jbc.M112.400689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Marques JV, Dalisay DS, Yang H, Lee C, Davin LB, Lewis NG.A multi-omics strategy resolves the elusive nature of alkaloids in Podophyllum species. Mol Biosyst. 2014; 10: 2838–2849. 10.1039/c4mb00403e [DOI] [PubMed] [Google Scholar]
- 64. Hano C, Addi M, Bensaddek L, Crônier D, Baltora-Rosset S, Doussot J et al. Differential accumulation of monolignol-drived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta. 2006; 223:975–989. 10.1007/s00425-005-0156-1 [DOI] [PubMed] [Google Scholar]
- 65. Yousefzadi M., Sharifi M, Behmanesh M, Ghasempour A, Moyano E, Palazon J. Salicylic acid improves podophyllotoxin production in cell cultures of Linum album by increasing the expression of genes related with its biosynthesis. Biotechnol Lett. 2010; 32: 1739–1743. 10.1007/s10529-010-0343-4 [DOI] [PubMed] [Google Scholar]
- 66. Esmaeilzadeh Bahabadi S, Sharifi M, Safaie N, Murata J, Yamagaki T, Satake H. Increased lignan biosynthesis in the suspension cultures of Linum album by fungal extracts. Plant Biotechnol Rep. 2011; 5: 367–373. 10.1007/s11816-011-0190-3 [DOI] [Google Scholar]
- 67. Esmaeilzadeh Bahabadi S, Sharifi M, Behmanesh M, Safaie N, Murata J, Araki R et al. Time-course changes in fungal elicitor-induced lignan synthesis and expression of the relevant genes in cell cultures of Linum album . J Plant Physiol. 2012; 169: 487–491. 10.1016/j.jplph.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 68. Esmaeilzadeh Bahabadi S, Sharifi M, Murata J, Satake H. The effect of chitosan and chitin oligomers on gene expression and lignan production in Linum album cell cultures. J Med Plant. 2014; 13: 46–53. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.