Abstract
Purpose
To investigate the clinicopathological features and prognosis of signet ring cell carcinoma of the stomach (SRC).
Methods
A total of 1464 gastric cancer patients who underwent curative gastrectomy from 2000 to 2008 at a single center were evaluated. Signet ring cell carcinoma (SRC) was defined as the presence of at least 50% signet ring cells in the pathologic specimen. The clinicopathological parameters and prognosis of SRC were analyzed by comparing with non-signet ring cell carcinoma (NSRC).
Results
Of 1464 patients, 138 patients (9.4%) were classified as SRC. There were significant differences in gender, age, tumor location, TNM stage, p21 expression, and p53 expression between SRC and NSRC. The 5-year survival rates of SRC and NSRC were 36.2% and 49.5%, respectively. The prognosis of SRC was poorer than that of NSRC (P <0.001). Multivariate analysis showed that SRC histology was an independent factor for poor prognosis (P <0.001).
Conclusion
Patients with SRC tend to present with a more advanced stage and poorer prognosis than patients with other types of gastric carcinoma.
Introduction
Although the incidence of gastric cancer has been declining for several decades, it remains the fifth most common cancer and the third most common cause of cancer-related death worldwide [1,2]. Gastric cancer can be classified histologically into various types [3]. Signet ring cell carcinoma is a distinct histological type with cells containing abundant intracytoplasmic mucin [4]. It has been reported that 3.4% to 29% of gastric cancers are signet ring cell carcinomas [5–9]. Although some studies have reported on the clinicopathological features and prognosis of signet ring cell carcinoma of the stomach, results have been inconsistent, with some studies reporting a better prognosis compared with other gastric cancers [6,7,10], and others reporting a worse prognosis [9,11,12]. Therefore, the objective of this study was to investigate differences in clinicopathologic features and survival between signet ring cell carcinoma and other histological types of gastric cancer.
Materials and Methods
Patients
From 2000 to 2008, 1464 patients with histologically confirmed primary gastric adenocarcinoma underwent curative gastrectomy at the Department of Gastric Cancer and Soft Tissue Sarcoma, Fudan University Shanghai Cancer Center. Exclusion criteria for this study were as follows: (1) surgery status unknown; (2) vital status unknown; (3) incomplete pathological data. Signet ring cell carcinoma was defined as an adenocarcinoma with the presence of >50% of tumor cells (signet ring cells) with prominent intracytoplasmic mucins [13]. Data were retrieved from operative and pathological reports, and follow-up data were obtained by phone, outpatient and clinical databases. Written informed consent was obtained from all patients, and the study was approved by the Ethical Committee of Fudan University Shanghai Cancer Center.
Preoperative evaluation and treatment
Preoperative examinations and staging was performed by endoscopic examination and computed tomography scan. Staging was carried out according to the American Joint Committee on Cancer (AJCC) TNM Staging Classification for Carcinoma of the Stomach (Seventh Edition, 2010). Gastrectomy was performed in accordance with the Japanese Classification of Gastric Carcinoma.
Immunohistochemical staining
The expression of p21, p53, c-myc and EGFR in primary lesions were detected by immunohistochemistry. All primary antibodies and mouse monoclonal antibodies were purchased from Dako (Hamburg, Germany). The detailed sources, concentrations of antibody and positive sites were as follows: anti-p21 (clone SX118), 1:50 dilution, nucleus; anti-p53 (clone DO-7), 1:100 dilution, nucleus; anti-c-myc (clone 9E10), 1:100 dilution, cytoplasm; anti-EGFR (clone E30), 1:50 dilution, cytoplasm or membrane. The staining procedures followed supplier’ instructions. Negative controls were subjected to the same procedure except that the first antibody was replaced by PBS.
Immunohistochemical Staining Scores
All slides were evaluated by two pathologists without knowledge of patients’ clinical data. The percentage of immunoreactive cells was graded on a scale of 0 to 4: no staining was scored as 0, 1–10% of cells stained scored as 1, 11–50% as 2, 51–80% as 3, and 81–100% as 4. Staining intensity was graded from 0 to 3: 0 was defined as negative, 1 as weak, 2 as moderate, and 3 as strong. The raw data were converted to an immunohistochemical score (IHS) by multiplying the quantity and intensity scores. An IHS score of 9–12 was categorized as strong immunoreactivity (+++), 5–8 as moderate (++), 1–4 as weak (+), and 0 as negative (-). On the final analysis, the cases with an HIS of less than 1 were classified as negative, and ≥ 1 as positive. These criteria were based on previously published reports [14].
Follow-up
Follow-up of all patients was carried out according to our hospital’s standard protocol (every three months for at least 2 years, every six months for the next 3 years, and thereafter every 12 months for life) [14]. The check-up items included physical examination, tumor-marker examination, ultrasound, chest radiography, computed tomographic scan, and endoscopic examination. The median follow-up time was 64 months for living patients.
Statistical analysis
The patients’ features and clinicopathological characteristics were analyzed using the χ2 test for categorical variables. Five-year survival rate was calculated by the Kaplan-Meier method, and differences between survival curves were calculated by the long-rank test. Independent prognostic factors were analyzed by multivariate survival analysis using the Cox proportional hazards model. The accepted level of significance was P <0.05. Statistical analyses and graphics were performed using the SPSS 13.0 statistical package (SPSS, Inc., Chicago, IL).
Results
Clinicopathological characteristics
Of 1464 patients, there were 1022 males and 442 females (2.3:1) with a mean age of 58 years. 138 patients (9.4%) had signet ring cell carcinoma and 1326 patients (90.6%) had non-signet ring cell carcinoma. By histological type, there were 35 well-differentiated tumors, 443 moderately-differentiated tumors, and 848 poorly-differentiated tumors in non-signet ring cell patients. By tumor location, 506 patients (34.6%) had tumors located in the upper third of the stomach, 248 (16.9%) had tumors in the middle third, 633 (43.2%) had tumors in the lower third, and 77 (5.3%) had tumors occupying two-thirds or more of the stomach. There were 111 (7.6%) patients with a family history of gastric cancer. The distribution of pathological stage was as follows: 346 (23.6%) stage I, 340 (23.2%) stage II, 778 (53.1%) stage III. Patients demographics were listed in Table 1.
Table 1. Patient Cohort.
| n = 1464 | 100% | |
|---|---|---|
| Tumor subtype | ||
| Signet-ring cell carcinoma | 138 | 9.4 |
| Other adenocarcinoma | 1326 | 90.6 |
| Sex | ||
| Male | 1022 | 69.8 |
| Female | 442 | 30.2 |
| Age (yr) | ||
| <60 | 767 | 52.4 |
| ≥60 | 697 | 47.6 |
| Tumor size (cm) | ||
| <5 | 902 | 61.6 |
| ≥5 | 562 | 38.4 |
| Tumor location | ||
| Upper third | 506 | 34.6 |
| Middle third | 248 | 16.9 |
| Lower third | 633 | 43.2 |
| Two-third or more | 77 | 5.3 |
| Venous tumor emboli | ||
| Yes | 524 | 35.8 |
| No | 940 | 64.2 |
| Nervous invasion | ||
| Yes | 564 | 38.5 |
| No | 900 | 61.5 |
| Serosa invasion | ||
| Yes | 676 | 46.2 |
| No | 788 | 53.8 |
| Lymph node metastasis | ||
| Yes | 501 | 34.2 |
| No | 963 | 65.8 |
| TNM stage | ||
| Stage I | 346 | 23.6 |
| Stage II | 340 | 23.2 |
| Stage III | 778 | 53.1 |
| Family history of gastric cancer | ||
| Yes | 111 | 7.6 |
| No | 1353 | 92.4 |
| P21 expression | ||
| Positive | 949 | 64.8 |
| Negative | 515 | 35.2 |
| P53 expression | ||
| Positive | 1052 | 71.9 |
| Negative | 412 | 28.1 |
| c-myc expression | ||
| Positive | 929 | 63.5 |
| Negative | 535 | 36.5 |
| EGFR expression | ||
| Positive | 581 | 39.7 |
| Negative | 883 | 60.3 |
Clinicopathologic characteristics were compared between signet ring cell carcinoma (SRC) and non-signet ring cell carcinoma (NSRC). Signet ring cell carcinoma presented at a younger age (P = 0.014); presented more frequently in females (P = 0.003). Patients with signet ring cell carcinoma were more likely to present with stage III disease (P = 0.003) (Table 2).
Table 2. Comparison of the Clinicopathological Characteristics of Patients With Signet-Ring Cell Carcinoma (SRC) and non-signet ring cell carcinoma (NSRC).
| Variables | SRC n = 138 | NSRC n = 1326 | P |
|---|---|---|---|
| Gender | 0.003 | ||
| Male | 81 | 941 | |
| Female | 57 | 385 | |
| Age (yr) | 0.014 | ||
| <60 | 86 | 681 | |
| ≥60 | 52 | 645 | |
| Tumor size (cm) | 0.851 | ||
| <5 | 84 | 818 | |
| ≥5 | 54 | 508 | |
| Tumor location | <0.001 | ||
| Upper third | 22 | 484 | |
| Middle third | 34 | 214 | |
| Lower third | 68 | 565 | |
| Two-third or more | 14 | 63 | |
| Venous tumor emboli | 0.501 | ||
| Yes | 53 | 471 | |
| No | 85 | 855 | |
| Nervous invasion | 0.602 | ||
| Yes | 56 | 508 | |
| No | 82 | 818 | |
| Serosa invasion | 0.344 | ||
| Yes | 69 | 607 | |
| No | 69 | 719 | |
| Lymph node metastasis | 0.325 | ||
| Yes | 96 | 867 | |
| No | 42 | 459 | |
| TNM stage | <0.001 | ||
| Stage I | 35 | 311 | |
| Stage II | 13 | 327 | |
| Stage III | 90 | 688 | |
| Family history of gastric cancer | 0.876 | ||
| Yes | 10 | 101 | |
| No | 128 | 1225 | |
| P21 expression | <0.001 | ||
| Positive | 66 | 883 | |
| Negative | 72 | 443 | |
| P53 expression | 0.009 | ||
| Positive | 86 | 966 | |
| Negative | 52 | 360 | |
| c-myc expression | 0.111 | ||
| Positive | 79 | 850 | |
| Negative | 59 | 476 | |
| EGFR expression | 0.292 | ||
| Positive | 49 | 532 | |
| Negative | 89 | 794 |
The expression of p21, p53, c-myc, and EGFR
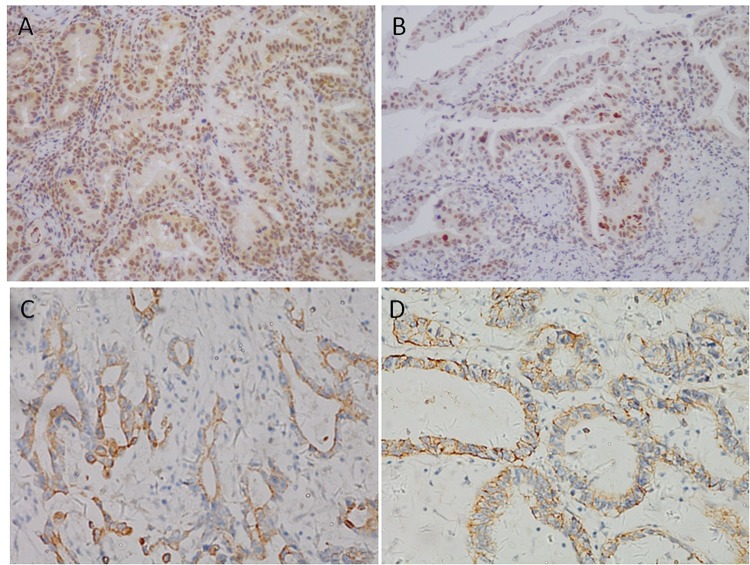
The expression of p21, p53, c-myc, and EGFR were analyzed by immunohistochemical staining. Staining location was predominantly cell cytoplasm for c-myc, cell cytoplasm or membrane for EGFR, and nucleus for p21 and p53 (Fig 1). The positive expression rates of p21, p53, c-myc, and EGFR were 64.8%, 71.9%, 63.5%, and 39.7%, respectively. Relative expression of p21 and p53 was less in SRC than in NSRC, and the difference was significant.
Fig 1. Positive expression of biological markers by immunohistochemistry in gastric cancer tissue.
A) Positive expression of p21. B) Positive expression of p53. C) Positive expression of c-myc. D) Positive expression of EGFR.
Univariate Analysis
The overall 5-year survival rate was 49% for all patients. The 5-year survival rates of SRC and NSRC were 36.2% and 49.5%, and the differences were statistically significant (Fig 2). In addition to tumor subtype, the significant prognostic factors were age, tumor size, tumor location, venous tumor emboli, nervous invasion, serosa invasion, lymph node metastasis, TNM stage, and EGFR expression (Table 3). In SRC, univariate analysis showed that age, tumor size, tumor location, venous tumor emboli, nervous invasion, serosa invasion, lymph node metastasis, TNM stage, and EGFR expression were significant prognostic factors (Table 4). In NSRC, univariate analysis showed that age, tumor size, tumor location, venous tumor emboli, nervous invasion, serosa invasion, lymph node metastasis, TNM stage, and EGFR expression significantly affected prognosis (Table 5).
Fig 2. Kaplan-Meier survival curves by histological type.
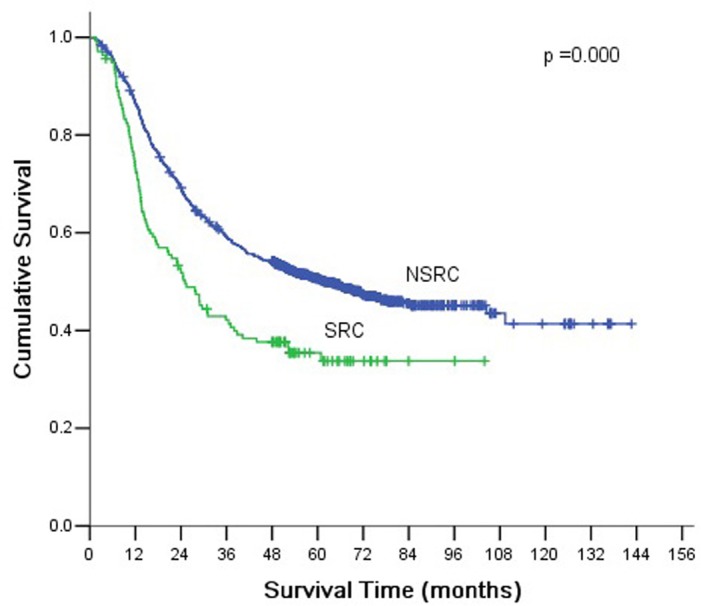
There were significant differences between SRC and NSRC (P <0.001).
Table 3. Univariate analysis of all patients by Kaplan-Meier method.
| Variable | n | 5-Year survival rate (%) | P value |
|---|---|---|---|
| Sex | 0.989 | ||
| Male | 1022 | 48.0 | |
| Female | 442 | 48.6 | |
| Age (yr) | <0.001 | ||
| <60 | 767 | 53.5 | |
| ≥60 | 697 | 42.5 | |
| Tumor subtype | <0.001 | ||
| Signet ring cell carcinoma | 138 | 36.2 | |
| Non-signet ring cell carcinoma | 1326 | 49.5 | |
| Tumor size (cm) | <0.001 | ||
| <5 | 902 | 57.5 | |
| ≥5 | 562 | 33.3 | |
| Tumor location | <0.001 | ||
| Upper third | 506 | 38.1 | |
| Middle third | 248 | 44.4 | |
| Lower third | 633 | 61.3 | |
| Two-third or more | 77 | 19.5 | |
| Venous tumor emboli | <0.001 | ||
| Yes | 524 | 26.7 | |
| No | 940 | 60.2 | |
| Nervous invasion | <0.001 | ||
| Yes | 564 | 28.9 | |
| No | 900 | 60.3 | |
| Serosa invasion | <0.001 | ||
| Yes | 676 | 28.4 | |
| No | 788 | 65.2 | |
| Lymph node metastasis | |||
| Yes | 963 | 30.8 | <0.001 |
| No | 501 | 81.6 | |
| TNM stage | <0.001 | ||
| Stage I | 346 | 93.1 | |
| Stage II | 340 | 58.5 | |
| Stage III | 778 | 23.8 | |
| P21 expression | 0.497 | ||
| Positive | 949 | 47.1 | |
| Negative | 515 | 50.3 | |
| P53 expression | 0.901 | ||
| Positive | 1052 | 48.4 | |
| Negative | 412 | 47.8 | |
| c-myc expression | 0.391 | ||
| Positive | 929 | 47.6 | |
| Negative | 535 | 49.3 | |
| EGFR expression | 0.012 | ||
| Positive | 581 | 43.7 | |
| Negative | 883 | 51.2 |
Table 4. Kaplan-Meier univariate analysis of patients with SRC.
| Variable | n | 5-Year survival rate (%) | P value |
|---|---|---|---|
| Sex | 0.319 | ||
| Male | 81 | 34.6 | |
| Female | 57 | 38.6 | |
| Age (yr) | 0.012 | ||
| <60 | 86 | 43.0 | |
| ≥60 | 52 | 25.0 | |
| Tumor size (cm) | <0.001 | ||
| <5 | 84 | 48.8 | |
| ≥5 | 54 | 16.7 | |
| Tumor location | <0.001 | ||
| Upper third | 22 | 18.2 | |
| Middle third | 34 | 29.4 | |
| Lower third | 68 | 51.5 | |
| Two-third or more | 14 | 7.1 | |
| Venous tumor emboli | <0.001 | ||
| Yes | 53 | 15.1 | |
| No | 85 | 49.4 | |
| Nervous invasion | <0.001 | ||
| Yes | 56 | 21.4 | |
| No | 82 | 46.3 | |
| Serosa invasion | <0.001 | ||
| Yes | 69 | 17.4 | |
| No | 69 | 55.1 | |
| Lymph node metastasis | <0.001 | ||
| Yes | 96 | 16.7 | |
| No | 42 | 81.0 | |
| TNM stage | <0.001 | ||
| Stage I | 35 | 91.4 | |
| Stage II | 13 | 53.8 | |
| Stage III | 90 | 12.2 | |
| P21 expression | 0.490 | ||
| Positive | 66 | 36.4 | |
| Negative | 72 | 36.1 | |
| P53 expression | 0.423 | ||
| Positive | 86 | 34.9 | |
| Negative | 52 | 38.5 | |
| c-myc expression | 0.202 | ||
| Positive | 79 | 31.6 | |
| Negative | 59 | 42.4 | |
| EGFR expression | 0.012 | ||
| Positive | 49 | 26.5 | |
| Negative | 89 | 41.6 |
Table 5. Kaplan-Meier univariate analysis of patients with NSRC.
| Variable | n | 5-Year survival rate (%) | P value |
|---|---|---|---|
| Sex | 0.960 | ||
| Male | 941 | 49.2 | |
| Female | 385 | 50.1 | |
| Age (yr) | <0.001 | ||
| <60 | 681 | 54.8 | |
| ≥60 | 645 | 43.9 | |
| Tumor size (cm) | <0.001 | ||
| <5 | 818 | 58.4 | |
| ≥5 | 508 | 35.0 | |
| Tumor location | <0.001 | ||
| Upper third | 484 | 39 | |
| Middle third | 214 | 46.7 | |
| Lower third | 565 | 62.5 | |
| Two-third or more | 63 | 22.2 | |
| Venous tumor emboli | <0.001 | ||
| Yes | 471 | 28.0 | |
| No | 855 | 61.3 | |
| Nervous invasion | <0.001 | ||
| Yes | 508 | 29.7 | |
| No | 818 | 61.7 | |
| Serosa invasion | <0.001 | ||
| Yes | 893 | 33.4 | |
| No | 433 | 82.7 | |
| Lymph node metastasis | <0.001 | ||
| Yes | 867 | 32.4 | |
| No | 459 | 81.7 | |
| TNM stage | <0.001 | ||
| Stage I | 311 | 93.2 | |
| Stage II | 327 | 58.7 | |
| Stage III | 688 | 25.3 | |
| P21 expression | 0.173 | ||
| Positive | 883 | 47.9 | |
| Negative | 443 | 52.6 | |
| P53 expression | 0.924 | ||
| Positive | 966 | 49.6 | |
| Negative | 360 | 49.2 | |
| c-myc expression | 0.510 | ||
| Positive | 850 | 49.1 | |
| Negative | 476 | 50.2 | |
| EGFR expression | 0.037 | ||
| Positive | 532 | 45.3 | |
| Negative | 794 | 52.3 |
Multivariate Analysis
Multivariate analysis was used to determine the independent prognostic factors for gastric cancer patients. Multivariate analysis by the Cox proportional hazard model found that tumor subtype, age, tumor size, venous tumor emboli, nervous invasion, TNM stage, and EGFR expression were independent prognostic factors for all the patients (Table 6). In SRC, multivariate analysis showed that age, TNM stage, and EGFR expression were independent prognostic factors. In NSRC, multivariate analysis showed that age, tumor size, venous tumor emboli, and TNM stage were independent prognostic factors for prognosis.
Table 6. Multivariate analysis of patients by Cox model.
| Variable | P value | RR | 95% CI |
|---|---|---|---|
| Age | 0.000 | 1.310 | 1.132–1.516 |
| Signet ring cell carcinoma | 0.000 | 1.798 | 1.432–2.257 |
| Tumor size | 0.003 | 1.249 | 1.079–1.445 |
| Tumor location | 0.281 | 0.960 | 0.892–1.034 |
| Venous tumor emboli | 0.000 | 1.460 | 1.248–1.707 |
| Nervous invasion | 0.013 | 1.227 | 1.045–1.442 |
| Serosa invasion | 0.983 | 1.002 | 0.840–1.195 |
| Lymph node metastasis | 0.398 | 1.169 | 0.814–1.679 |
| TNM stage | 0.000 | 2.918 | 2.284–3.727 |
| EGFR | 0.038 | 1.168 | 1.009–1.352 |
Comparison of Survival According to Stage Between SRC and NSRC Groups
According to the AJCC/TNM staging, gastric cancer patients are classified into stage I, II, or III. Histopathologically, tumors in each stage are classified as SRC or NSRC. There were significant differences of overall survival rates between SRC and NSRC in stage III (P <0.001, Fig 3).
Fig 3. Comparison of survival according to tumor stage.
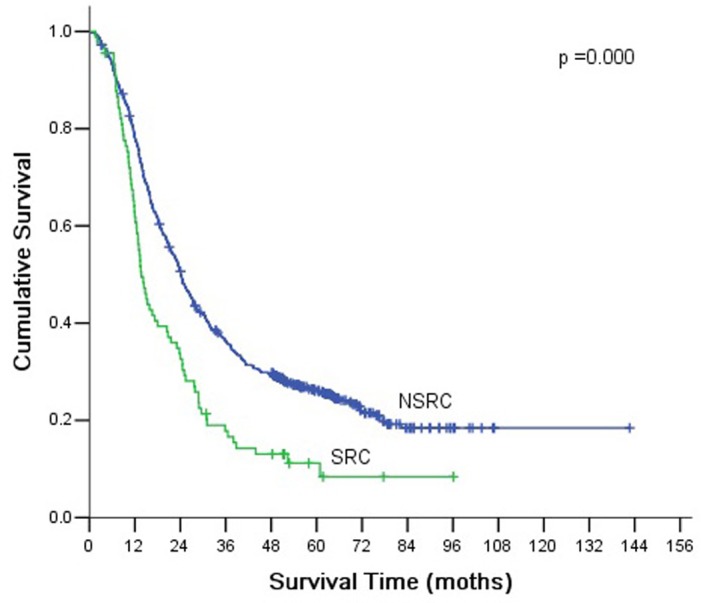
There were significant differences between SRC and NSRC according to stage III (P <0.001).
Discussion
The main findings of this study were as follows: (1) Signet ring cell carcinoma was an independent prognostic factor for five year gastric cancer. In particular, there was a significant difference in the survival of patients with stage III between SRC and NSRC. (2) There were differences in prognostic factors between SRC and NSRC, and EGFR expression was an independent predictor of poor prognosis for patients with SRC, but not for those with NSRC.
According to the Japanese gastric cancer classification system, tumors are classified by histological subtype as classical adenocarcinomas, signet ring cell carcinoma, mucinous adenocarcinoma, or other rare types [15]. Although some studies have shown that histological subtype is a key factor for tumor biology and prognosis, published results have been inconsistent [6, 7, 9–12]. In contrast to TNM staging, histological subtype is not incorporated in clinical classification systems.
In view of the inconsistent literature, we decided to evaluate the clinicopathological features and prognostic significance of SRC in our large single-center sample. In the current study, the incidence of SRC was 9.4% of gastric cancers, which was similar to incidence in previous reports [5–9]. We found that signet ring cell carcinoma had different clinicopathological features compared to other types of gastric carcinoma. SRC occurred more frequently in female and in younger patients than NSRC. This was also similar to the demographics reported in the previous studies [6, 8], though the exact reason remains unclear. It has been reported that histology may be influenced by sex hormones [16], but more research is needed to investigate the association between age, sex and gastric cancer histopathological type. Our sample also showed that SRC and NSRC tended to present at different anatomic locations, with SRC occurring more frequently in the middle third of the stomach. This was consistent with the findings of Ostuji et al. [7] and Zhang et al. [12]. Some previous studies have shown that SRC develops from the fundic glands, which are predominantly located in the fundus and body of the stomach [7, 17]. In contrast, Kim et al. [18] did not find differences in tumor location between SRC and NSRC. We also found that patients with signet ring cell carcinoma were more likely to present at more advanced stages, including a greater proportion of patients with stage III. Finally, on IHC biomarker analysis we found that the expression of p21 and p53 were significantly different between SRC and NSRC. In aggregate, the differences we identified between SRC and NSRC indicate that SRC may present a distinct and more aggressive disease.
In the current study, the 5-yr survival rate of patients with SRC was 36.2%, significantly shorter than patients with NSRC. Multivariate analysis showed that signet ring cell was an independent prognostic factors. However, this result could be related to the higher proportion of advanced stage tumors among SRC patients. In order to exclude the influence of disease stage at the time of presentation, we performed a subgroup analysis by tumor stage, which showed no significant differences in overall survival rates between SRC and NSRC in stage I and II. However, in stage III tumors, the prognosis was poorer in SRC than NSRC. These results were similar to previous studies [17, 19, 20]. Kim et al. [17] reported that the prognosis of early gastric carcinoma with SRC was similar to other types of gastric carcinoma. Li et al. [19] and Piessen et al. [20] found that the 5-yr survival rate of SRC was significantly poorer than that of NSRC in advanced gastric carcinoma.
In addition, we found that EGFR expression was an independent prognostic factor for patients with SRC by multivariate analysis, while it was not for those with NSRC. Given that SRC was not sensitive to common chemotherapeutic agents, the results of this study, indicating the association of EGFR expression and poor prognosis in SRC, may facilitate further development of agents targeting EGFR expression and clinical trials evaluating the role of those agents in SRC.
Conclusion
SRC is a distinct type of gastric carcinoma in terms of clinicopathological features and prognosis. SRC presents with more advanced stage than NSRC, and carries a worse prognosis.
Acknowledgments
The authors thank Ben Liotta for editing our manuscript’s English language style, and the patients for their participation in this study.
Data Availability
Data are available from the Department of Gastric Cancer and Soft Tissue Sarcoma, Fudan University Shanghai Cancer Center, Shanghai Medical College, Fudan University, for researchers who meet the criteria for access to confidential data. Interested researchers may contact the corresponding author with queries related to data access.
Funding Statement
This research is supported by grants from the Shanghai Committee of Science and Technology Funds (Contract grant numbers: 14ZR1407800, 124119a4302), and the National Natural Science Foundation of China (81502027). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript.
References
- 1. Shibata A, Parsonnet J, Stomach cancer (2006) In: Schottenfeld D, Fraumeni JF, eds. Cancer epidemiology and prevention, 3 rd edn. New York: Oxford University Press; Pp 707–720. [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015, 65(2): 87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 3. Yamamichi N, Inada K, Ichinose M, Yamamichi-Nishina M, Mizutani T, Watanabe H, et al. (2007) Frequent loss of Brm expression in gastric cancer correlates with histological features and differentiation state. Cancer Res 67: 10727–10735. [DOI] [PubMed] [Google Scholar]
- 4. Chu PG, Weiss LM (2004) Immunohistochemical characterization of signet-ring cell carcinoma as of the stomach, breast, and colon. Am J Clin Pathol 121: 884–892. [DOI] [PubMed] [Google Scholar]
- 5. Antonioli DA, Goldman H. (1982) Changes in the location and type of gastric adenocarcinoma. Cancer 50(4):775–781. [DOI] [PubMed] [Google Scholar]
- 6. Maehara Y, Sakaguchi Y, Moriguchi S, Orita H, Korenaga D, Kohnoe S, Sugimachi K. (1992) Signet ring cell carcinoma of the stomach. Cancer 69 (7): 1645–1650. [DOI] [PubMed] [Google Scholar]
- 7. Otsuji E, Yamaguchi T, Sawai K, Takahashi T. (1998) Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol 67:216–220. [DOI] [PubMed] [Google Scholar]
- 8. Taghavi S, Jayarajan SN, Davey A, Willis AI. (2012) Prognostic significance of signet ring gastric cancer. J Clin Oncol 30:3493–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK, Jung SA. (2014) Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer 17:43–53. 10.1007/s10120-013-0234-1 [DOI] [PubMed] [Google Scholar]
- 10. Jiang CG, Wang ZN, Sun Z, Liu FN, Yu M, Xu HM. (2011) Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: results from a Chinese mono-institutional study. J Surg Oncol 103: 700–703. 10.1002/jso.21878 [DOI] [PubMed] [Google Scholar]
- 11. Lee JH, Choi IJ, Kook MC, Nam BH, Kim YW, Ryu KW. (2010) Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br J Surg 97: 732–736. 10.1002/bjs.6941 [DOI] [PubMed] [Google Scholar]
- 12. Zhang M, Zhu G, Zhang H, Gao H, Xue Y. (2010) Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg 14:601–606. 10.1007/s11605-009-1127-9 [DOI] [PubMed] [Google Scholar]
- 13. Hamilton SR, Nakamura S, Bosman FT, Quirke P, Boffetta P, Riboli E, IIyas M, Sobin LH, Morreau H. (2010) Carcinoma of the colon and rectum In: Bosman FT, Carneiro F, Hruban RH, Theise ND eds. WHO classification of tumours of the digestive organs. IARC; Lyon, pp: 134–146. [Google Scholar]
- 14. Liu X, Cai H, Huang H, Long Z, Shi Y, Wang Y. The prognostic significance of apoptosis-related biological markers in Chinese gastric cancer patients. PloS One 2011;6:e29670 10.1371/journal.pone.0029670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112. 10.1007/s10120-011-0041-5 [DOI] [PubMed] [Google Scholar]
- 16. Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, et al. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol 2002; 128:319–324. [DOI] [PubMed] [Google Scholar]
- 17. Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol 1994;3:221–227. [DOI] [PubMed] [Google Scholar]
- 18. Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, Kim SK, Lee JH. (2004) Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg 74:1060–1064. [DOI] [PubMed] [Google Scholar]
- 19. Li C, Kim S, Lai JF, Hyung WJ, Choi WH, Choi SH, et al. (2007) Advanced gastric carcinoma with signet ring cell histology. Oncology 72:64–68. [DOI] [PubMed] [Google Scholar]
- 20. Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. (2009) Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 250:878–887. 10.1097/SLA.0b013e3181b21c7b [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Department of Gastric Cancer and Soft Tissue Sarcoma, Fudan University Shanghai Cancer Center, Shanghai Medical College, Fudan University, for researchers who meet the criteria for access to confidential data. Interested researchers may contact the corresponding author with queries related to data access.