Abstract
Capsicum baccatum, commonly known as ají, has been reported as a source of variation for many different traits to improve common pepper (C. annuum), one of the most important vegetables in the world. However, strong interspecific hybridization barriers exist between them. A comparative study of two wide hybridization approaches for introgressing C. baccatum genes into C. annuum was performed: i) genetic bridge (GB) using C. chinense and C. frutescens as bridge species; and, ii) direct cross between C. annuum and C. baccatum combined with in vitro embryo rescue (ER). A diverse and representative collection of 18 accessions from four cultivated species of Capsicum was used, including C. annuum (12), C. baccatum (3), C. chinense (2), and C. frutescens (1). More than 5000 crosses were made and over 1000 embryos were rescued in the present study. C. chinense performed as a good bridge species between C. annuum and C. baccatum, with the best results being obtained with the cross combination [C. baccatum (♀) × C. chinense (♂)] (♀) × C. annuum (♂), while C. frutescens gave poor results as bridge species due to strong prezygotic and postzygotic barriers. Virus-like-syndrome or dwarfism was observed in F1 hybrids when both C. chinense and C. frutescens were used as female parents. Regarding the ER strategy, the best response was found in C. annuum (♀) × C. baccatum (♂) crosses. First backcrosses to C. annuum (BC1s) were obtained according to the crossing scheme [C. annuum (♀) × C. baccatum (♂)] (♀) × C. annuum (♂) using ER. Advantages and disadvantages of each strategy are discussed in relation to their application to breeding programmes. These results provide breeders with useful practical information for the regular utilization of the C. baccatum gene pool in C. annuum breeding.
Introduction
Crop relatives have been used for decades for breeding, in particular to transfer genes of resistance or tolerance to pests, diseases or abiotic stress to the cultivated species [1, 2]. Introgression breeding has been extensively used in the genetic improvement of some of the most important Solanaceae crops, like potato (Solanum tuberosum L.) or tomato (Solanum lycopersicum L.). Thus, up to twelve traits have been introgressed in potato from related species like S. demissum, S. stoloniferum, S. chacoense, S. acaule, S. vernei or S. spegazzinii [3] and many more have been transferred to tomato from their wild relatives like S. peruvianum, S. cheesmanii, S. pennellii or S. chilense [4]. However, breeding programmes in the economically important common Capsicum peppers (Capsicum annuum L.) have made little use of related species for breeding as reviewed by Mongkolporn and Taylor [5]. This limitation has been mainly due to the presence of different pre-zygotic barriers which avoid fertilization (e.g. pollen-pistil incompatibilities) and/or post-zygotic barriers, which prevent the achievement of fertile hybrids, e.g. embryo/endosperm abortion, hybrid weakness or sterility [6, 7].
In this sense, C. annuum is related to about other 30 Capsicum species, of which four are also cultivated, C. baccatum L., C. chinense Jacq., C. frutescens L. and C. pubescens R. & P. [8]. By one hand, C. chinense and C. frutescens cultivars have economic importance in America, Africa, and Asia and both are phylogenetically close to C. annuum. In fact, these species make up the annuum-chinense-frutescens complex (or annuum complex), characterized by white flowers and yellow seeds [9]. On the other hand, C. pubescens and C. baccatum represent separate taxons from the annuum complex and, although they have been widely grown in the Andean region and Brazil for millennia, they are very rare outside this area nowadays [9, 10]. The former, mainly known as rocoto (with purple flowers and black rough seeds), is the least economically important [9]. Due to prezygotic barriers which prevent the growth of the pollen tube through the style, and possible postzygotic barriers, it does not cross with any of the other four species [9, 11]. The latter, commonly known as ají (with white flowers and yellow spots), has showed an extremely low/nil cross compatibility with C. annuum [6], although it has been reported as source of variation for a range of traits with potential interest for the genetic improvement of this species. These include resistances to several diseases such as: anthracnose (Colletotrichum spp.), powdery mildew (Leveillula taurica), Rhizoctonia root rot (Rizhoctonia solani), Verticillium wilt (Verticilium dahliae) and bacterial wilt (Ralstonia solanacearum syn. Pseudomonas solanacearum), viruses like PYMV and TSWV or even new flavours [12–21]. However, successful wide hybridization attempts to introgress these traits in C. annuum have been scarce [12, 22].
Postzygotic barriers have been suggested as the main cause of cross compatibility problems between both species, specifically embryo/endosperm abortion and hybrid sterility [23, 24]. In most plant species, the first barrier is caused by abnormal cell division of the zygote or slow endosperm development, which causes an incompatibility with embryo growth [25], while the second is due to a range of factors such as diverged genes, karyotypic changes, gene transposition or gene loss, sequence divergence or dosage imbalance, among others [26].
An alternative to overcome these barriers, known as genetic bridge, is based on the use of species (bridges) phylogenetically close to the two species affected by crossability barriers. The bridge species is used to obtain hybrids with one of the target species, and subsequently these hybrids are crossed to the other target species [27]. Thus, C. chinense and C. frutescens might play this role for wide hybridization between C. annuum and C. baccatum as previously suggested by Pickersgill [28].
Another strategy for wide hybridization between C. annuum and C. baccatum is the in vitro rescue of immature interspecific embryos or embryo rescue before abortion occurs [27]. This approach is technically more complex as it requires embryo excision and in vitro culture. Also, the stage at which embryo abortion occurs after hybridization may depend on the specific genotypes involved in the cross. Thus, for example within Solanaceae, while some authors could rescue interspecific embryos at the latest immature stages [29], there are also examples on which embryos had to be rescued at the earliest stages [30, 31]. However, the earlier the stage at which embryo rescue is done, the more difficult is the procedure and the lower the efficiency [32].
Furthermore, even though hybrid materials could be achieved, hybrid sterility must be also considered as an important postzygotic barrier. Full sterility or different degrees of fertility of the interspecific hybrids may vary depending on the parent genotypes. In extreme cases, when sterility is complete due to the lack of chromosome pairing during meiosis, fertility may be restored by poliploidization, enabling pairing of homologous chromosomes in the allopolyploid hybrid [33].
Unfortunately, studies on wide hybridization between C. baccatum and C. annuum and the overcoming of their compatibility barriers are very scarce [29, 34], especially regarding the range of diversity encompassed in materials used for crosses. Consequently, there is a lack of detailed practical information about the breeding process and levels of sexual compatibility among species, which might also depend on the genotypes involved or the direction of the crosses, among other factors.
Therefore, the development of approaches which allow overcoming these barriers, assessing all the difficulties which can appear during their application, will provide breeders with useful tools, practical information and a comprehensive perspective for the introgression of genes of interest from C. baccatum to C. annuum. Moreover, the use of a wide genetic diversity will contribute to offer a more complete view of these barriers.
The aim of this work was to compare comprehensively two approaches for the achievement of wide hybridization between C. annuum and C. baccatum: i) genetic bridge using C. chinense and C. frutescens as bridge species; and, ii) direct hybridization between C. annuum and C. baccatum in combination with embryo rescue. In both strategies full diallel interspecific crosses were planned and a range of genetically different genotypes were used. The effects of the direction of the crosses, cross compatibility at different levels among the species involved and hybrid viability and fertility are also discussed.
Material and Methods
Plant material and growing conditions
A total of 18 accessions from four cultivated species of Capsicum were utilized in the present study: C. annuum (12 accessions), C. baccatum (3 accessions), and the bridge species C. chinense (2 accessions) and C. frutescens (1 accession). This collection encompassed a comprehensive range of geographical origins and fruit morphological traits (Table 1). New materials (i.e. interspecific hybrids and backcrosses) were included progressively in the experiments as they were obtained during the wide hybridization programme. Plants from parent accessions, and subsequent hybrids and backcrosses were transplanted at the four-leaf stage (about 50–60 days after sowing) to glasshouses at the Universitat Politècnica de València (UPV, Valencia, Spain) along three years (2010, 2011 and 2012). Plants were grown in 10 L pots (coconut coir as substrate), under the spring-summer growing cycle as it provides the most favourable conditions for the development and fruit set of Capsicum in the Mediterranean region [8]. Natural illumination was used for this experiment and temperature control systems (heating and cooling) were activated when temperatures dropped below 18°C (night) or rose above 25°C (day). Plants were pruned to four stems and trained with vertical strings. They were drip irrigated every 8 h for 3 min (4 L/h). Fertilizer was applied with the irrigation water, at a rate of 1 g/L of a commercial 15N-2.2P-24.9K water soluble fertilizer (BASF, Barcelona, Spain).
Table 1. Origin and fruit traits of the accessions utilized in the present experiment.
| Species | Accession | Code | Origin | Color | Weight (g) | Length (cm) | Width (cm) | Pollen viability (%±SD) |
|---|---|---|---|---|---|---|---|---|
| C. annuum | Arnoia | Arn | Centro Investigaciones Agrarias de Magebondo (Galicia, Spain) | Red | 50–90 | 7–11 | 5–7 | 82±3 |
| C. annuum | Bierzo | Bie | Cons. Reg. IGP Pimiento Asado Bierzo, Ponferrada (León, Spain) | Red | 4–7 | 6–7 | 4–8 | 85±5 |
| C. annuum | California Wr. red | CWr | Breeding line, UPV-COMAV | Pale red | 90–150 | 8–11 | 6–10 | 91±4 |
| C. annuum | California Wr. yellow | CWy | Breeding line, UPV-COMAV | Yellow | 108–176 | 8–10 | 7–11 | 94±4 |
| C. annuum | Guindilla | Gui | Guindilla de Ibarra, Ibarra, Spain | Deep red | 8–14 | 8–15 | 1–2 | 98±1 |
| C. annuum | Numex | Num | New Mexico State University, USA | Red | 30–70 | 12–15 | 4–6 | 83±3 |
| C. annuum | Pasilla Bajío | Pas | Mexico, Southern USA | Brown | 20–30 | 15–20 | 2–3 | 89±4 |
| C. annuum | Pimiento de Bola | Bola | Cons. Reg. DOP Pimentón Murcia, Murcia, Spain | Red | 10–14 | 3–5 | 4–6 | 94±2 |
| C. annuum | Pimiento del Piquillo | Piq | Cons. Reg. IGP Piquillo Lodosa, Navarra, Spain | Red | 20–33 | 7–9 | 4–6 | 85±3 |
| C. annuum | PBC534 | P534 | Asian Vegetables Research and Development Center (AVRDC) | Red | 8–15 | 10–15 | 1–2 | 86±4 |
| C. annuum | PBC716 | P716 | Asian Vegetables Research and Development Center (AVRDC) | Red | 2–5 | 4–8 | >0.5 | 93±4 |
| C. annuum | Serrano | Ser | Mexico | Deep red | 3–5 | 3–5 | 1–2 | 89±5 |
| C. chinense | Ají Panca | AjíP | Ecuador | Brown | 10–14 | 7–10 | 2–3 | 81±3 |
| C. chinense | PI-152225 | PI15 | USDA | Deep red | 4–6 | 4–5 | 1–2 | 84±2 |
| C. frutescens | Bol 144 | B144 | Bolivia | Pale red | >1 | 1–2 | >0.5 | 92±4 |
| C. baccatum | Ají Rojo | AjíR | Bolivia | Red | 5–8 | 5–8 | 2–3 | 86±3 |
| C. baccatum | Ají Amarillo | AjíA | Bolivia | Yellow | 3–4 | 4–6 | 1–2 | 91±2 |
| C. baccatum | Brazilian pumpkin | BrP | Brazil | Red | 2–3 | 2–3 | 2–3 | 84±3 |
Hybridization technique
Previously to hybridization, female flowers were emasculated and pollen was extracted from male flowers and released carefully on the stigma. To prevent uncontrolled pollination, female flowers were covered with Scotch tape after hybridization. Each cross was tagged with the genotypes involved in the hybridization and the date at which it was performed. Overall, more than 5000 hybridizations were performed in this study.
Genetic bridge approach
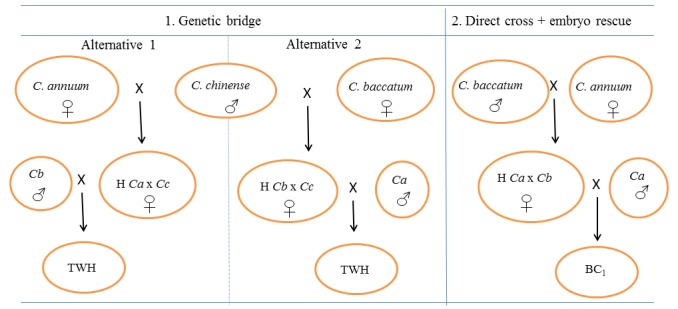
Within the genetic bridge strategy (GB), all possible combinations of full (reciprocal) diallel crosses between the 12 C. annuum and the three C. baccatum accessions on one side and the three accessions of the bridge species C. chinense (2 accessions) and C. frutescens (1 accession) on the other were tested (i.e. a total of 45 hybrid combinations and their reciprocals). F1 hybrid materials obtained were further used for completing the bridge cross (i.e., crossing to C. annuum if C. baccatum had been used to obtain the F1, and viceversa). Between 10 and 30 hybridizations were done for each cross combination and direction of the cross. Two strategies were performed to achieve the bridge cross (Fig 1): i) alternative 1, which firstly consisted in crossing C. annuum with the bridge species and, later the obtained hybrids were crossed with C. baccatum; and, ii) alternative 2, which firstly consisted in crossing C. baccatum with the bridge species and, later the obtained hybrids with C. annuum. As a result, a total of over 2800 hybridizations were done in this GB approach. Fully ripe fruits from each successful cross were harvested and their seeds were extracted and dried. After that, seeds were sown in seedling trays (7×12 cells, 480×300×55 mm) containing cultivation substrate (Humin-substrat N3, Klasmann-Deilmann, Germany). Once plantlets reached the four-leaf stage, they were transferred to glasshouses to perform the second cross (hybrid × parental cross) in order to obtain three-way hybrids (Fig 1).
Fig 1. Diagram of genetic bridge (GB) and embryo rescue (ER) planned approaches to overcome interspecific barriers between C. annuum and C. baccatum.
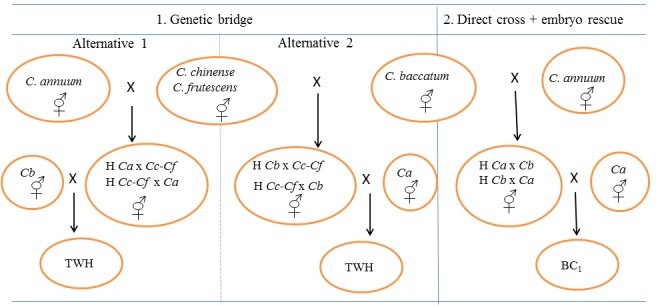
Vertical arrows indicate the hybrid obtained, H = Hybrid, Ca = C. annuum, Cb = C. baccatum, Cc = C. chinense, Cf = C. frutescens, BC1 = First backcross, TWH = Three way hybrid.
Embryo rescue
Within the embryo rescue strategy (ER), a minimum of 10 hybridizations (and up to 47 in some cross combinations) between the 12 C. annuum accessions on one side and the three C. baccatum accessions on the other were performed using a full diallel scheme (i.e., 36 hybrid combinations) with reciprocals. Also several BC1 crosses towards C. annuum were later tried on the basis of available hybrids. As a result, a total of 2500 hybridizations were done in the present ER experiment. About 25–30 days after pollination immature fruits were harvested to perform the embryo rescue following the protocol and culture medium optimized by us in previous studies [35, 36]. The medium included: agar (7 g/L), indole-3-acetic acid (IAA, 0.01 mg/L), gibberellic acid (GA3, 0.01 mg/L), zeatin (0.01 mg/L), sucrose (40 g/L), and MS (2.2 g/L), at pH 5.7. The basal medium (agar, sugars and MS salts) was sterilized at 121°C for 20 min. Hormones were sterilized by microfiltration (0.20 μm Minisart® filters) and were added to the warm (35–40°C) autoclaved medium before solidifying. Embryos were excised carefully and immediately cultured in 90×15 mm Petri dishes, which were sealed with Parafilm® and incubated in a growth chamber (25±10°C; 70% HR; full darkness the first 5 days and a 16h/8h (light/dark) photoperiod since day 6). About 1000 immature seeds were dissected for the ER approach. After 30 days of incubation, seedlings which showed a clear development of root and shoot were transferred to seedling trays and were covered with perforated plastic glasses to prevent dehydration. After one week, plastic glasses were removed. Once F1 plantlets reached the four-leaf stage, they were transferred to 10 L pots at glasshouses for evaluation and to obtain subsequent BC1 generations (Fig 1).
Cross compatibility evaluation and confirmation of hybridity
In both strategies the compatibility of each cross was evaluated at four levels: i) number of fruits set per number of hybridizations performed, ii) percentage of germinated seeds (or rescued embryos in the case of ER) 1 month after sowing, iii) plant hybrid appearance, and iv) pollen viability (n = 5 mesurements), estimated according to Rodríguez-Riaño and Dafni [37] with 1% (w/v) of thiazolyl blue tetrazolium bromide (MTT) dye (M-2128, Sigma-Aldrich). To confirm the hybrid nature of new materials, we used a set of six polymorphic SSRs (Table 2). These SSRs markers were scored according to the method described by Minamiyama et al. [38].
Table 2. SSRs utilized to confirm the hybridity of individuals obtained after interspecific hybridization [37].
| SSRs | Primer forward | Primer reverse | Size (pb) | Linkage group |
|---|---|---|---|---|
| CAMS-679 | TTTGCATGTTTTACCCATTCC | ATGTGAAACACATAGGTAGCACTGA | 200 | 1 |
| CAMS-460 | CCTTTCACTTCAGCCCACAT | ACCATCCGCTAAGACGAGAA | 230 | 7 |
| CAMS-405 | TTCTTGGGTCCCACACTTTC | AGGTTGAAAGGAGGGCAATA | 260 | 11 |
| CAMS-644 | CGCATGAAGCAAATGTACCA | ACCTGCAGTTTGTTGTTGGA | 200 | 4 |
| CAMS-806 | TGTCACAAGTGTCAAGGTAGGAG | CCCCAAAAATTTTCCCTCAT | 140 | 10 |
| CAMS-398 | ATGGTCCATGGTCAGCAGAT | GGGCAGAACAGTGGATGATT | 180 | 7 |
Results and Discussion
Both approaches (GB and ER) enabled to successfully achieve the wide hybridization between C. annuum and C. baccatum, including obtaining F1 hybrids, three-way-hybrids, and even backcross generations within the ER strategy. On the basis of the SSRs analysis, all new materials were confirmed as hybrids of their corresponding parents and, therefore, no uncontrolled pollination was present in the experiment. This is an important step for C. annuum breeding using C. baccatum as donor species for traits present in the latter. In the case of the ER approach first backcross (BC1) generations towards C. annuum were obtained; and, in the case of the GB approach three-way hybrids using C. chinense as bridge species from both cross alternatives (i.e. starting with crosses between C. annuum and C. chinense and between C. baccatum and C. chinense) were achieved. However, a range of results, efficiency rates and crossability barriers was found in both strategies depending on the direction and the genotypes involved in the crosses. Consequently, all these factors must be considered to plan breeding programs which include interspecific crosses between these four species.
Genetic bridge approach
C. chinense as genetic bridge species
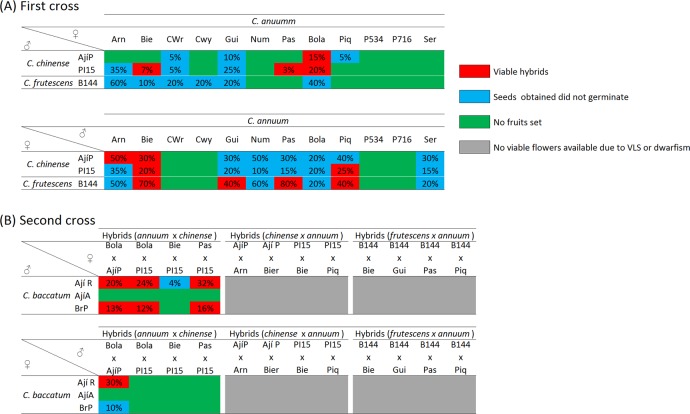
Despite a high crossability between the species of the annuum complex has been widely assumed [6, 8, 9], many crosses of C. annuum accessions with both C. chinense or C. frutescens were unsuccessful (Fig 2). Thus, in the C. annuum (♀) × C. chinense (♂) cross scheme, fruit set occurred in 10 of the 24 possible combinations and only three C. annuum accessions (California Wr. red, Bola and Guindilla) were able to set fruit with any of the C. chinense accessions used (Fig 2). Furthermore, only the seeds of four combinations germinated and developed in normal hybrids, which also showed pollen viability estimates comprised between 17–31% (Table 3), considerably lower than those from their parent accessions, always higher than 80% (Table 1). Capsicum annuum accession Bola was the only one which produced hybrid plants with both C. chinense accessions (Table 3). Moreover, one of these (Bola × P15) was the hybrid with the highest pollen viability (31%). Therefore, this accession appears as suitable germplasm for the GB strategy, as well as for the transfer of genes from C. chinense to commercial peppers. Although Zijlstra et al. [11] discarded prezygotic barriers between species belonging to the annuum complex, the low or even nil fruit set rates obtained in our experiment suggest strong prezygotic incompatibility between C. annuum and C. chinense. Even more, as observed in some cases by Inai et al. [39], our low germination rates could be due to embryo abortion, which also suggest postzygotic barriers.
Fig 2. Cross diagram showing the results of the different crosses performed within the genetic bridge approach starting with crosses between C. annuum and the bridge species.
(A) First cross table indicates the crossability degree of C. annuum with C. chinense or C. frutescens, (B) Second cross table indicates the crossability degree of hybrids obtained in A with C. baccatum. Numbers over the cells indicate percentage of fruit set over an average of 25 artificial pollinations. Green indicates non-viable crosses, where no fruits were obtained (0% of fruits set, not indicated as number); blue indicates fruit set but non-viable seeds; red indicates fertile hybrids with normal development; and grey indicates hybrid inviable plants.
Table 3. Descriptive results of the genetic bridge (GB) strategy using the alternative of obtaining hybrids between C. annuum (Ca) and C. chinense (Cch) for being subsequently crossed with C. baccatum to obtain three-way hybrids.
Cross combinations that did not set fruit or set fruit but seeds did not germinate are not included in the table.
| Pistillate (♀) accessions | × | Staminate (♂) accessions | No. crosses | No. set fruit (fruit/crosses %) | Germination (%) | Hybrid appearance | Pollen viability (% ± SD) | |
|---|---|---|---|---|---|---|---|---|
| C. annuum | × | C. chinense | ||||||
| Bie | × | P15 | 30 | 2 | (7%) | 10/15 (67%) | Normal | 21 ± 4 |
| Bola | × | AjíP | 20 | 3 | (15%) | 3/14 (21%) | Normal | 17 ± 5 |
| Bola | × | P15 | 20 | 4 | (20%) | 4/15 (27%) | Normal | 31 ± 6 |
| Pas | × | P15 | 30 | 1 | (3%) | 4/14 (29%) | Normal | 18 ± 8 |
| C. chinense | × | C. annuum | ||||||
| AjíP | × | Arn | 20 | 10 | (50%) | 9/14 (64%) | VLS 1 | - |
| AjíP | × | Bie | 20 | 6 | (30%) | 6/15 (40%) | VLS | - |
| PI15 | × | Bie | 20 | 4 | (20%) | 1/15 (7%) | VLS | - |
| PI15 | × | Piq | 20 | 5 | (25%) | 6/15 (40%) | VLS | - |
| (Ca × Cch) | × | C. baccatum | ||||||
| Bola × AjíP | × | AjíR | 15 | 3 | (20%) | 6/14 (43%) | Normal | 83 ± 6 |
| Bola × AjíP | × | BrP | 15 | 2 | (13%) | 8/14 (57%) | Normal | 28 ± 5 |
| Bola × PI15 | × | AjíR | 25 | 6 | (24%) | 1/14 (7%) | Normal | 33 ± 6 |
| Bola × PI15 | × | BrP | 25 | 3 | (12%) | 7/14 (50%) | Normal | 12 ± 4 |
| Pas × PI15 | × | AjíR | 25 | 8 | (32%) | 11/14 (79%) | Normal | 15 ± 7 |
| Pas × PI15 | × | BrP | 25 | 4 | (16%) | 3/14 (21%) | Normal | 4 ± 3 |
| C. baccatum | × | (Ca × Cch) | ||||||
| AjíR | × | Bola × AjíP | 10 | 3 | (30%) | 1/5 (20%) | Normal | 95 ± 2 |
1 VLS = virus-like syndrome.
The reciprocal cross C. chinense (♀) × C. annuum (♂) enabled a higher number of successful combinations in terms of fruit set, with 16 out of the 24 possible combinations (Fig 2). However, only hybrid seeds from four combinations germinated (Table 3) and, moreover, hybrids showed stunted growth, filiform leaves, short internodes and did not enter into the reproductive phase (Fig 3). These symptoms were not observed in other plants grown in the same trial and all samples were negative to ELISA tests for Tobamoviruses or tomato spotted wilt virus (TSWV). Thus, the most likely reason for this abnormal development was the virus-like syndrome (VLS), which might be due to the interaction between cytoplasm and nuclear genes of C. chinense and C. annuum respectively [6, 39]. Therefore, the results suggest that C. chinense should always be utilised as male parent to achieve viable hybrids with C. annuum.
Fig 3. C. chinense (♀) × C. annuum (♂) hybrids showing virus-like syndrome (VLS).
(A) at 50 days after sowing, (B) 100 days after sowing, and (C) 150 days after sowing. Stunted growth, filiform leaves and short internodes can be observed.
The four viable C. annuum (♀) × C. chinense (♂) hybrids available were then utilized to complete the bridge cross with C. baccatum. Crosses involving C. baccatum Aji amarillo did not set any fruit (Fig 2), suggesting a low cross compatibility of this accession. Then, regarding the rest of combinations, the best results were obtained using hybrids as female parents, with seven out of the eight possible combinations setting fruit, and viable seeds being obtained from six combinations (Fig 2 and Table 3). All these materials showed a normal appearance, although a wide range of pollen viability was observed, which was comprised between 4% and 83% in (Pas × PI15) (♀) × BrP (♀) and (Bola × AjíP) (♀) × AjíR (♂) respectively (Table 3). On the contrary, when C. annuum (♀) × C. chinense (♂) hybrids were utilized as pollen donors, fruit set and successful hybrid plants were only achieved in AjíR (♀) × (Bola × AjíP) (♂) (Fig 2). Surprisingly, this hybrid showed the highest pollen viability (95%) among all hybrid materials of the GB approach involving C. chinense as bridge species, which suggests that the involved genotypes Bola (C. annuum), AjíP (C. chinense), and AjíR (C. baccatum) have a high compatibility. Consequently, given the number of successful combinations (6/8), the following scheme [C. annuum (♀) × C. chinense (♂)] (♀) × C. baccatum (♂) is recommended when using the GB approach.
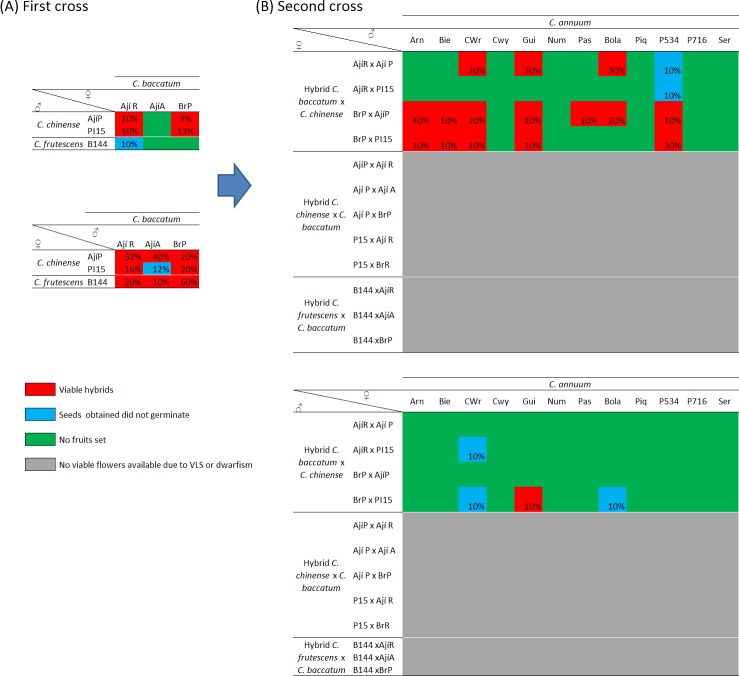
Regarding the bridge cross strategy that involved C. baccatum to obtain the first set of interspecific hybrids, it was possible to achieve normal C. baccatum (♀) × C. chinense (♂) hybrids from all combinations, with the only exception of those combinations involving the Ají Amarillo accession (C. baccatum), which did not to set any fruit (Fig 4) and confirmed its low aptitude for interspecific crosses. Despite these hybrids had low pollen viability (19–33%) (Table 4), similarly to C. annuum (♀) × C. chinense (♂) materials, it was possible to obtain subsequent crosses with C. annuum.
Fig 4. Cross diagram showing the results of the different crosses performed within the genetic bridge approach starting with crosses between C. baccatum and the bridge species.
(A) First cross tables indicate the crossability degree of C. baccatum with C. chinense and C. frutescens, (B) Second cross tables indicate the crossability degree of the hybrids obtained with C. annuum. Numbers over the cells indicate percentage of fruit set over an average of 25 artificial pollinations. Green indicates non-viable crosses, where no fruits were obtained (0% of fruits set, not indicated as number); blue indicates fruit set but non-viable seeds; red indicates fertile hybrids with normal development; and grey indicates hybrid inviable plants.
Table 4. Descriptive results of the genetic bridge (GB) strategy using the alternative of obtaining hybrids between C. baccatum (Cb) and C. chinense (Cch) for being subsequently crossed with C. annuum to obtain three-way hybrids.
Cross combinations that did not set fruit or set fruit but seeds did not germinate are not included in the table.
| Pistillate (♀) accessions | × | Staminate (♂) accessions | No. crosses | No. set fruit (fruit/crosses %) | Germination (%) | Hybrid appearance | Pollen viability (% ± SD) | |
|---|---|---|---|---|---|---|---|---|
| C. baccatum | × | C. chinense | ||||||
| AjíR | × | AjíP | 40 | 4 | (10%) | 14/14 (100%) | Normal | 24 ± 9 |
| AjíR | × | PI15 | 40 | 4 | (10%) | 14/14 (100%) | Normal | 30 ± 12 |
| BrP | × | AjíP | 35 | 3 | (9%) | 10/10 (100%) | Normal | 33 ± 10 |
| BrP | × | PI15 | 40 | 5 | (13%) | 2/15 (13%) | Normal | 19 ± 3 |
| C. chinense | × | C. baccatum | ||||||
| AjíP | × | AjíR | 25 | 8 | (32%) | 7/15 (47%) | VLS 1 | - |
| AjíP | AjíA | 25 | 10 | (40%) | 10/15 (67%) | VLS | - | |
| AjíP | BrP | 10 | 2 | (20%) | 1/15 (7%) | VLS | - | |
| PI15 | × | AjíR | 25 | 4 | (16%) | 6/15 (40%) | VLS | - |
| PI15 | BrP | 10 | 2 | (20%) | 1/15 (7%) | VLS | - | |
| (Cb × Cch) | × | C. annuum | ||||||
| AjíR × AjíP | × | CWr | 10 | 2 | (20%) | 3/6 (50%) | Normal | 34 ± 12 |
| AjíR × AjíP | × | Bola | 10 | 3 | (30%) | 1/6 (17%) | Normal | 10 ± 5 |
| AjíR × AjíP | × | Gui | 10 | 3 | (30%) | 2/3 (67%) | Normal | 84 ± 6 |
| BrP × AjíP | × | CWr | 10 | 2 | (20%) | 10/29 (35%) | Normal | 83 ± 10 |
| BrP × AjíP | × | Arn | 10 | 4 | (40%) | 12/45 (27%) | Normal | 94 ± 1 |
| BrP × AjíP | × | Bie | 10 | 1 | (10%) | 2/15 (13%) | Normal | 83 ± 5 |
| BrP × AjíP | × | Bola | 10 | 2 | (20%) | 4/11 (36%) | Normal | 55 ± 12 |
| BrP × AjíP | × | Gui | 10 | 1 | (10%) | 3/18 (17%) | Normal | 55 ± 8 |
| BrP × AjíP | × | P534 | 10 | 1 | (10%) | 1/4 (25%) | Normal | 11 ± 3 |
| BrP × AjíP | × | Pas | 10 | 1 | (10%) | 1/8 (13%) | Ms 2 | 0 |
| BrP × PI15 | × | CWr | 10 | 1 | (10%) | 5/7 (71%) | Normal | 87 ± 6 |
| BrP × PI15 | × | Arn | 10 | 1 | (10%) | 3/4 (75%) | Normal | 58 ± 9 |
| BrP × PI15 | × | Bie | 10 | 1 | (10%) | 3/3 (100%) | Normal | 68 ± 10 |
| BrP × PI15 | × | Gui | 10 | 1 | (10%) | 7/11 (64%) | Normal | 10 ± 2 |
| BrP × PI15 | × | P534 | 10 | 3 | (30%) | 5/22 (23%) | Ms | 0 |
| C. annuum | × | (Cb × Cch) | ||||||
| Gui | × | BrP × PI15 | 10 | 1 | (10%) | 1/10 (10%) | Normal | 3 ± 2 |
1 VLS = virus-like syndrome
2Ms = male sterility.
By contrast, within the reciprocal crosses C. chinense (♀) × C. baccatum (♂), fruit set and viable seeds were observed in all possible combinations (Fig 4). Unfortunately, as observed in C. chinense (♀) × C. annuum (♂) plants, all these hybrids also showed VLS, indicating the presence of detrimental genes in C. chinense cytoplasm that may also induces detrimental effects in hybrids with C. baccatum (Table 4). This is consistent with other works related to interspecific hybridization in pepper [6, 7, 39, 40]. Therefore, as suggested for crosses with C. annuum, C. chinense should be also utilized as pollen donor in crosses with C. baccatum to prevent detrimental effects in the hybrid offspring.
Finally, the use of C. baccatum (♀) × C. chinense (♂) hybrids as male parents for finishing the GB approach only succeed in 1 out of 48 possible combinations (and in one single plant), which also showed a 3% pollen viability (Fig 4, Table 4). Such findings are in agreement with those observed in the C. baccatum (♀) × [C. annuum (♀) × C. chinense (♂)](♂) scheme, confirming that due to the frequently low pollen viability of both interspecific hybrids, C. annuum (♀) × C. chinense (♂) and C. baccatum (♀) × C. chinense (♂), they should not be used as male parents. On the contrary, the best results were achieved utilizing C. baccatum (♀) × C. chinense (♂) hybrids as pistillate parent, which allowed finishing the GB in 15 out of the 48 possible combinations (Fig 4 and Table 4). In addition, many of these hybrids showed high levels of pollen viability (>50%). Therefore, this alternative is more efficient for the GB when starting the cross scheme crossing C. baccatum with C. chinense.
As a whole, the comparison between the different alternatives for GB showed that the use of [C. annuum (♀) × C. chinense (♂)] (♀) × C. baccatum (♂) crossing schemes allowed obtaining plants with a range of pollen viability (4–83%) in six cross combinations. The use of the [C. baccatum (♀) × C. chinense (♂)] (♀) × C. annuum (♂) scheme provided 13 fertile combinations with up to 94% pollen viability. Therefore, according to these findings, we recommend to utilize the latter alternative to introgress genes from C. baccatum to C. annuum. Moreover, this strategy provides 50% C. annuum genome, while three-way hybrids from the other scheme will only carry 25% of C. annuum genome. Nevertheless, it should be also considered that this strategy, depending on the character to introgress, may require checking the resulting phenotypes at the first step from crosses between C. baccatum and C. chinense before performing the second cross involved in the bridge cross. In fact, Yoon and Park (2005) [12] utilized the other alternative, despite they also found low pollen viability.
C. frutescens as genetic bridge species
In comparison to C. chinense, C. frutescens showed more difficulties to be used as a bridge species. Thus, although C. frutescens is phylogenetically close to C. annuum and it is generally supposed that hybridization between both species can be done easily [9], results suggest that such compatibility is not so obvious. In this way, when C. frutescens was utilised as pollen donor with C. annuum, hybrid seeds did not germinate (Fig 2), which is consistent with other authors who have reported some cases of embryo abortion and even after using embryo rescue they obtained partially sterile hybrids [41].
Otherwise, when C. frutescens was utilized as pistillate parent, only 4 out of 12 possible combinations were successful, in addition, once germinated they showed dwarfism (Table 5 and Fig 5). In this regard, while some authors found partial embryo abortion [41], Yazawa et al. [40] obtained dwarfism in all hybrids obtained and concluded that dwarfism is controlled by two complementary dominant genes. Thus, C. frutescens (♀) × C. annuum (♂) crosses seem to follow the Bateson–Dobzhansky–Muller (BDM) model, which explains the simplest scenario for generating genetic incompatibilities without detriment to either of two diverging lineages. The ‘classical’ BDM model involves two epistatic loci aaBB and AAbb wich are innocuous in their native context but interact negatively in hybrids [42].
Table 5. Descriptive results of the bridge cross technique using C. frutescens as genetic bridge between C. annuum and C. baccatum.
Those F1 combinations which did not set fruit, or set fruit but seeds did not germinate, are not included in the table.
| Pistillate (♀) accessions | × | Staminate (♂) accessions | No. crosses | No. set fruit (fruit/crosses %) | Germination (%) | Hybrid appearance | |
|---|---|---|---|---|---|---|---|
| Alternative 1: First C. annuum × C. frutescens hybrids, followed by hybridization with C. baccatum | |||||||
| C. frutescens | × | C. annuum | |||||
| B144 | × | Piq | 10 | 4 | (40%) | 3/10 (30%) | Dwarfism |
| B144 | Pas | 10 | 8 | (80%) | 1/10 (10%) | Dwarfism | |
| B144 | Bie | 10 | 7 | (70%) | 1/10 (10%) | Dwarfism | |
| B144 | Gui | 10 | 4 | (40%) | 1/10 (10%) | Dwarfism | |
| Alternative 2: First C. baccatum × C. frutescens hybrids, followed by hybridization with C. annuum | |||||||
| C. frutescens | × | C. baccatum | |||||
| B144 | × | AjíR | 10 | 2 | (20%) | 9/10 (90%) | VLS 1 |
| B144 | AjíA | 10 | 1 | (10%) | 1/10 (10%) | VLS | |
| B144 | BrP | 10 | 6 | (60%) | 3/10 (30%) | VLS | |
1VLS = virus-like sindrome.
Fig 5. C. frutescens (♀) × C. annuum (♂) hybrids showing dwarfism and poor root development at 100 days after germination.
Regarding the crossing between C. baccatum and C. frutescens, hybrids were only obtained when the latter was utilized as pistillate parent (Fig 4) as was early observed by Rao et al. [43]. However, in agreement with Pickersgill [44] and the results of this experiment with C. chinense, all hybrids were also affected by virus-like syndrome (VLS), probably due to C. frutescens cytoplasmic genes, which interact with C. baccatum nuclear genes. In addition, although Rao et al. [43] were able to recover normal hybrids in appearance, they were sterile. Because of that, despite more C. frutescens genotypes could be tested for compatibility with C. annuum or C. baccatum, in our opinion C. frutescens should not be used as bridge between both species.
In vitro rescue of immature embryos from crosses between C. annuum and C. baccatum
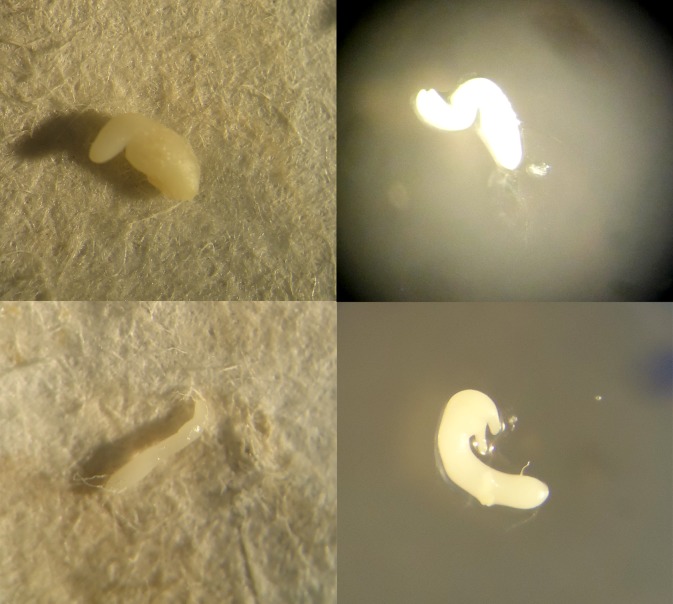
In a preliminary experiment, a diallel cross scheme was done between eight accessions of C. annuum (California Wr. both red and yellow, Arnoia, Bierzo, Guindilla, Pasilla, Piquillo, and Serrano) and the three C. baccatum accessions. The few set fruits achieved were hasvested at the fully ripe stage and their seeds were removed and evaluated. All seeds were empty and showed necrotic points in the center (Fig 6), which is frequently related to embryo abortion. In fact, as expected, germination tests demonstrated that these seeds were not viable. Those results were in agreement with other authors [6, 23] and confirmed that embryo abortion is an important barrier in crosses between C. annuum and C. baccatum. In addition, further anatomical studies of interespecific embryos close to abortion revealed that the main cause of abortion was an early hardening of the endosperm, which limited the development of the embryos and caused morphological deformities and, eventually, embryo collapse and death (Fig 7). Therefore, embryo rescue was the only alternative to achieve these interspecific hybrids.
Fig 6. Comparison between normal and aborted seeds from crosses between C. annuum and C. baccatum.
(A) aborted seeds from interspecific cross between Bierzo (♀) × Aji Rojo (♂), and (B) normal seeds from self-pollinated C. annuum cv. Bierzo.
Fig 7. Isolated embryos from interspecific crosses between C. baccatum and C. annuum before abortion.
Different deformities, due to early hardening of endosperm, can be observed.
As a whole, the use of C. baccatum accessions as male parents provided the highest efficiency in terms of both fruit set and number of regenerated hybrids. Thus, fruit set was recorded in 67% of C. annuum (♀) × C. baccatum (♂) combinations (26 out of 36), although the efficiency rate ranged from 4% to 30%, depending on the genotypes involved (Table 6). Interspecific embryos could be excised and cultured in vitro from half of these combinations, while no embryos were found in the other half, which might be due to embryo abortion at very early stages (globular or earlier), whose rescue and regeneration would be extremely difficult or unfruitful [29, 36]. Finally, plantlets from 10 C. annuum (♀) × C. baccatum (♂) combinations were obtained and reached the adult stage. The efficiency of this in vitro technique varied among combinations, ranging from 6% to 75% in P534 × AjíR and P716 × AjíR, respectively (Table 6). Furthermore, combinations involving Ají Rojo and Brazilian Pumpkin C. baccatum accessions yielded the highest number of C. annuum (♀) × C. baccatum (♂) individuals: 36 and 16 hybrid plants, respectively, while only 3 hybrid plants from a single combination were obtained with Ají Amarillo (Table 6).
Table 6. Descriptive results of the direct cross and embryo rescue technique to obtain hybrids between C. annuum and C. baccatum.
F1 combinations that did not set fruit are not included in the table.
| Pistillate (♀) accessions | × | Staminate (♂) accessions | No. crosses | No. set fruit (fruit/crosses %) | In vitro germination (%) | Pollen viability (% ± SD) | |
|---|---|---|---|---|---|---|---|
| C. annuum | × | C. baccatum | |||||
| Arn | × | AjíA | 22 | 3 | (14%) | embryo not available | - |
| Arn | × | AjíR | 50 | 6 | (12%) | embryo not available | - |
| Arn | × | BrP | 45 | 4 | (9%) | embryo not available | - |
| Bie | × | AjíA | 20 | 3 | (15%) | 0/5 (0%) | - |
| Bie | × | AjíR | 47 | 3 | (6%) | 1/12 (8%) | 15 ± 1 |
| Bie | × | BrP | 45 | 6 | (13%) | 7/14 (50%) | 5 ± 4 |
| Bola | × | AjíA | 10 | 2 | (20%) | embryo not available | - |
| Bola | × | AjíR | 35 | 2 | (6%) | embryo not available | - |
| Bola | × | BrP | 35 | 11 | (31%) | 19/30 (63%) | 12 ± 6 |
| CWr | × | AjíA | 20 | 1 | (5%) | embryo not available | - |
| CWr | × | AjíR | 45 | 7 | (16%) | 0/12 (0%) | - |
| CWr | × | BrP | 45 | 6 | (13%) | embryo not available | - |
| CWy | × | AjíR | 22 | 2 | (9%) | 0/15 (0%) | - |
| Gui | × | AjíA | 20 | 6 | (30%) | embryo not available | - |
| Gui | × | AjíR | 48 | 8 | (17%) | 5/15 (33%) | 14 ± 8 |
| Gui | × | BrP | 40 | 4 | (10%) | 1/12 (8%) | 19 ± 8 |
| Num | × | BrP | 25 | 1 | (4%) | embryo not available | - |
| P534 | × | AjíR | 35 | 2 | (6%) | 1/18 (6%) | 10 ± 7 |
| P534 | × | BrP | 25 | 1 | (4%) | 4/18 (22%) | 14 ± 4 |
| P716 | × | AjíA | 10 | 1 | (10%) | 3/12 (25%) | 0 |
| P716 | × | AjíR | 35 | 4 | (11%) | 9/12 (75%) | 20 ± 5 |
| P716 | × | BrP | 25 | 2 | (8%) | 5/14 (35%) | 6 ± 2 |
| Pas | × | AjíA | 25 | 1 | (4%) | embryo not available | - |
| Pas | × | AjíR | 40 | 2 | (5%) | embryo not available | - |
| Piq | × | AjíR | 40 | 2 | (5%) | embryo not available | - |
| Ser | × | AjíA | 25 | 1 | (4%) | embryo not available | - |
| C. baccatum | × | C. annuum | |||||
| AjíR | × | Arn | 35 | 4 | (11%) | 0/4 (0%) | - |
| AjíR | × | Bie | 35 | 4 | (11%) | 1/3 (33%) | 33 ± 6 |
| AjíR | × | Bola | 10 | 1 | (10%) | embryo not available | - |
| AjíR | × | Gui | 25 | 3 | (12%) | 0/10 (0%) | - |
| AjíR | × | Num | 25 | 1 | (4%) | embryo not available | - |
| AjíR | × | Pas | 25 | 2 | (8%) | 0/12 (0%) | - |
| AjíR | × | Piq | 25 | 2 | (8%) | 2/12 (17%) | 21 ± 8 |
| AjíR | × | Ser | 25 | 2 | (8%) | embryo not available | - |
| AjíA | × | Num | 10 | 4 | (40%) | embryo not available | - |
| AjíA | × | Piq | 25 | 1 | (4%) | embryo not available | - |
| BrP | × | Arn | 35 | 1 | (3%) | embryo not available | - |
| BrP | × | Pas | 35 | 1 | (3%) | embryo not available | - |
| BrP | × | Piq | 40 | 1 | (3%) | embryo not available | - |
| BrP | × | Ser | 40 | 2 | (5%) | embryo not available | - |
On the other hand, a considerably lower efficiency was found in C. baccatum (♀) × C. annuum (♂) hybridizations and fruit set fruit was only observed in 14 out of the 36 possible combinations (Table 6). In addition, only three hybrid plants from two combinations, Ají R × Bie and Aji R × Piq, were eventually obtained (Table 6), consequently, breeders are advised to use C. annuum as female parent in crosses with C. baccatum to achieve the highest efficiency.
In both cases, pollen fertility was relatively low, comprised between 0% and 33% of P716 (♀) × Aji A (♂) and Ají R (♀) × Bie (♂) (Table 6), which was comparatively lower than the values recorded in the GB strategy. In any case, such values are in agreement with those reported by other authors [24, 45].
Finally, also supported by in vitro rescue, BC1 generations towards C. annuum were attempted. Each hybrid was backcrossed with the corresponding C. annuum parent for compatibility reasons. All BC1 crosses involving hybrids as male parents failed and no fruit was obtained. As a result, fruit set was only achieved when hybrids were utilised as female parents. Furthermore, BC1 materials were only achieved with C. annuum (♀) × C. baccatum (♂) hybrids, while all BC1 crosses using C. baccatum (♀) × C. annuum (♂) hybrids failed (Table 7). Despite fruit set was low and, consequently, only three BC1 combinations were obtained, in vitro rescue enabled to regenerate a high number of embryos (Table 7).
Table 7. Descriptive results of the backcross and embryo rescue technique to obtain hybrids between C. annuum (Ca) and C. baccatum (Cb).
BC1 combinations that did not set fruit are not included in the table.
| Pistillate (♀) accessions | × | Staminate (♂) accessions | No. crosses | No. set fruit (fruit/crosses %) | In vitro germination (%) | Pollen viability (% ± SD) | |
|---|---|---|---|---|---|---|---|
| (Ca × Cb) | × | C. annuum | |||||
| Bie × AjíR | × | Bie | 20 | 0 | (0%) | - | - |
| Bie × BrP | × | Bie | 20 | 2 | (10%) | 8/16 (50%) | 35 ± 4 |
| Bola × BrP | × | Bola | 20 | 4 | (20%) | 7/16 (44%) | 91 ± 4 |
| Gui × AjíR | × | Gui | 20 | 0 | (0%) | - | - |
| Gui × BrP | × | Gui | 20 | 0 | (0%) | - | - |
| P534 × AjíR | × | P534 | 20 | 0 | (0%) | - | - |
| P534 × BrP | × | P534 | 20 | 0 | (0%) | - | - |
| P716 × AjíA | × | P716 | 20 | 0 | (0%) | - | - |
| P716 × AjíR | × | P716 | 20 | 0 | (0%) | - | - |
| P716 × BrP | × | P716 | 20 | 4 | (20%) | 8/16 (50%) | 40 ± 4 |
| (Cb × Ca) | × | C. annuum | |||||
| AjíR × Bie | × | Bie | 20 | 0 | (0%) | - | - |
| AjíR × Piq | × | Piq | 30 | 0 | (0%) | - | - |
Moreover, these BC1 materials showed a normal appearance and their pollen viability (35–91%) was considerably higher than the values showed by their corresponding F1 parents. Thus, for example Bola (♀) × BrP (♂) had 12% pollen viability, while the corresponding BC1 (Bola (♀) × BrP(♂)) (♀) × Bola (♂) had a pollen viability of 91% (Tables 6 and 7). These results demonstrate that pollen fertility in crosses between C. annuum and C. baccatum can be recovered fast in 1–2 backcrosses, which may facilitate breeding programs.
Conclusions
According to the results, wide hybridization between C. annuum and C. baccatum is possible using both the GB and ER approaches, although the degree of success is highly dependent on the genotype to obtain interspecific hybrids and subsequent generations. The best crossing schemes to obtain successful hybridization and introgression from C. baccatum to C. annuum have been identified (Fig 8), and the genotypes with the best performance in these experiments are good candidates for introgression breeding from C. baccatum to C. annuum. Ultimately, these results provide breeders with relevant information on wide hybridization approaches and on appropriate plant material to be used for successfully incorporate the C. baccatum gene pool as a source of variation for introgression breeding in C. annuum breeding programmes. Additionally, those breeders interested in introgressing genes of interest in C. annuum from C. chinense or C. frutescens should consider the prezygotic and postzygotic barriers between these species identified here in order to achieve a high efficiency.
Fig 8. Recommended diagram for wide hybridization between different species for introgression breeding of C. baccatum to C. annuum based on the results of this work.
Vertical arrows indicate the hybrid obtained, H = Hybrid, Ca = C. annuum, Cb = C. baccatum, Cc = C. chinense, BC1 = First Backcross, TWH = Three way hybrid.
Acknowledgments
Juan P. Manzur thanks Universitat Politècnica de València for a research predoctoral grant (2011-S2-4264, programa para la formación de personal investigador). Authors are grateful to Centro Inv. Agr. Mabegondo, S. Larregla from NEIKER, P.W. Bosland from NMSU and the Consejos Reguladores of IGP Pimiento Asado del Bierzo, DOP Pimentón de Murcia, and IGP Piquillo de Lodosa for providing us with seeds from Arnoia, Guindilla de Ibarra, Numex, Bierzo, Bola and Piquillo, respectively.
Data Availability
All relevant data are within the paper.
Funding Statement
Juan P. Manzur thanks Universitat Politècnica de València for a research predoctoral grant (2011-S2-4264, programa para la formación de personal investigador). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hajjar R, Hodgkin T. The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica. 2007; 156: 1–13. [Google Scholar]
- 2. Honnay O, Jacquemyn H, Aerts R. Crop wild relatives: more common ground for breeders and ecologists. Front Ecol Environ. 2012; 10: 121–121. [Google Scholar]
- 3. Hirsch CN, Hirsch CD, Felcher K, Coombsm J, Zarka D, Van Deynze. Retrospective view of North American potato (Solanum tuberosum L.) breeding in the 20th and 21st centuries. G3. 2013; 3: 1003–1013. 10.1534/g3.113.005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin T, Zhu G, Zhang J, Xu X, Yu Q, Zheng Z. Genomic analyses provide insights into the history of tomato breeding. Nat Genet. 2014; 46: 1220–1226. 10.1038/ng.3117 [DOI] [PubMed] [Google Scholar]
- 5. Mongkolporn O Taylor PWJ. Capsicum In: Kole C, editor. Wild Crop Relatives: Genomic and Breeding Resources. Berlin Heidelberg: Springer-Verlag; 2011. pp. 43–57. 10.1007/978-3-642-20450-0_4 [DOI] [Google Scholar]
- 6. Yoon JB, Do JW, Yang DC, Park HG. Interspecific cross compatibility among five domesticated species of Capsicum genus. J Kor Soc Hort Sci. 2004; 45: 324–329. [Google Scholar]
- 7. Kamvorn W, Techawongstien S, Techawongstien S, Theerakulpisut P. Compatibility of inter-specific crosses between Capsicum chinense Jacq. and Capsicum baccatum L. at different fertilization stages. Sci Hort. 2014; 179: 9–15. [Google Scholar]
- 8. Nuez F, Gil-Ortega R, Costa J. El cultivo de pimientos, chiles y ajíes Madrid: Mundiprensa; 2003. [Google Scholar]
- 9. Bosland PW, Votava E. Peppers: vegetable and spice capsicums New York: CABI Publishing; 2000. [Google Scholar]
- 10. do Rêgo ER, do Rego MM, Finger FL, Cruz CD, Dias Casali VW. A diallel study of yield components and fruit quality in chilli pepper (Capsicum baccatum). Euphytica. 2009; 168: 275–287. [Google Scholar]
- 11. Zijlstra S, Purimahua C, Lindhout P. Pollen tube growth in interspecific crosses between Capsicum species. HortScience. 1991; 26: 585–586. [Google Scholar]
- 12. Yoon B, Park HG. Trispecies bridge crosses (Capsicum annuum x C. chinense) x C. baccatum, as an alternative for introgression of anthracnose resistance from C. baccatum into C. annuum . J Kor Soc Hort Sci. 2005; 46: 5–9. [Google Scholar]
- 13. Kim SH, Yoon BJ, Park HG. Inheritance of anthracnose resistance in a new genetic resource, Capsicum baccatum PI594137. J Crop Sci Biotech. 2008; 11: 13–16. [Google Scholar]
- 14. Mahasuk P, Taylor PWJ, Mongkolporn O. Identification of Two New Genes Conferring Resistance to Colletotrichum acutatum in Capsicum baccatum . Phytopathology. 2009; 9: 1100–1104. [DOI] [PubMed] [Google Scholar]
- 15. Souza VL, Café-Filho AC. Resistance to Leveillula taurica in the genus Capsicum . Plant Pathol. 2003; 52: 613–619. [Google Scholar]
- 16. Muhyi R, Bosland PW. Evaluation of Capsicum germplasm for sources of resistance to Rhizoctonia solani . HortScience. 1995; 30: 341–342. [Google Scholar]
- 17. González-Salán MM, Bosland PW. Sources of resistance to Verticillium wilt in Capsicum . Euphytica. 1992; 59: 49–53. [Google Scholar]
- 18. Matsunaga H, Monma S. Varietal differences in resistance to bacterial wilt in related species of Capsicum annuum . Capsicum and Eggplant Newsletter. 1995; 14: 60–61. [Google Scholar]
- 19. Bento CS, Rodrigues R, Gonçalves LS, Oliveira HS, Santos MH, Pontes MC, et al. Inheritance of resistance to Pepper yellow mosaic virus in Capsicum baccatum var. pendulum. Genet Mol Res. 2013; 12: 1074–1082. 10.4238/2013.April.10.3 [DOI] [PubMed] [Google Scholar]
- 20. Soler S, Debreczeni DE, Vidal E, Aramburu J, López C, Galipienso L, et al. New Capsicum baccatum accession shows tolerance to wild‐type and resistance‐breaking isolates of Tomato spotted wilt virus. Ann Appl Biol. 2015; In press. 10.1111/aab.12229 [DOI] [Google Scholar]
- 21. Kollmannsberger H, Rodriguez-Burruezo A, Nitz S, Nuez F. Volatile and capsaicinoid composition of aji (Capsicum baccatum) and rocoto (Capsicum pubescens), two Andean species of chile peppers. J Sci Food Agric. 2011; 91: 1598–1611 10.1002/jsfa.4354 [DOI] [PubMed] [Google Scholar]
- 22. Eggink PM, Tikunov Y, Maliepaard C, Haanstra JPW, de Rooij H, Vogelaar, et al. Capturing flavors from Capsicum baccatum by introgression in sweet pepper. Theor Appl Genet. 2014; 127: 373–390. 10.1007/s00122-013-2225-3 [DOI] [PubMed] [Google Scholar]
- 23.Yang DC. Interespecific hybridization for the breeding of anthracnose-resistant hot Peppers lines. M.Sc. Thesis. Seoul National University. 2001.
- 24. Egawa Y, Tanaka C. Cytogenetical study of the interspecific hybrid between Capsicum annuum and C. baccatum . Jpn J Breed. 1986; 36: 16–21. [Google Scholar]
- 25. Nowack MK, Ungru A, Bjerkan KN, Grini PE, Schnittger A. Reproductive cross-talk: seed development in flowering plants. Biochem Soc Trans. 2010; 38: 604–612. 10.1042/BST0380604 [DOI] [PubMed] [Google Scholar]
- 26. Maheshwari S, Barbash DA. The genetics of hybrid incompatibilities. Annu Rev Genet. 2011; 45: 331–355. 10.1146/annurev-genet-110410-132514 [DOI] [PubMed] [Google Scholar]
- 27. Shivanna KR, Bahadur B. Efficacy of Biotechnological Approaches to Raise Wide Sexual Hybrids In: Bahadur B, Rajam MV, Sahijram L, Krishnamurthy KV, editors. Plant Biology and Biotechnology vol II: Plant genomics and biotechnology New Dehli: Springer India; 2015. pp. 347–362. [Google Scholar]
- 28. Pickersgill B. The genus Capsicum: a multidisciplinary approach to the taxonomy of cultivated and wild plants. Biol Zent BI. 1988; 107: 381–389. [Google Scholar]
- 29. Yoon JB, Yang DC, Do JW, Park HG. Overcoming two post-fertilization genetic barriers in interspecific hybridization between Capsicum annuum and C. baccatum for introgression of anthracnose resistance. Breed Sci. 2006; 56: 31–38. [Google Scholar]
- 30. Barbano PP, Topoleski LD. Postfertilization hybrid seed failure in Lycopersicon esculentun Lycopersicon peruvianium ovules. J Amer Soc Hort Sci. 1984; 109: 95–100. [Google Scholar]
- 31. Chen LZ, Adachi T. Efficient hybridization between Lycopersicon esculentum and L. peruvianum via 'embryo rescue' and In vitro propagation. Plant Breed. 1996; 115: 251–256. [Google Scholar]
- 32. Shen X, Gmitter FG Jr, Grosser JW. Immature embryo rescue and culture In Thorpe TA, Young EC, editors. Plant Embryo Culture. New York: Humana Press; 2011. pp. 75–92. [DOI] [PubMed] [Google Scholar]
- 33. Lafon-Placette C, Köhler C. Epigenetic mechanisms of postzygotic reproductive isolation in plants. Curr Opin Plant Biol. 2015; 23: 39–44. 10.1016/j.pbi.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 34. Pickersgill B. Genetic resources and breeding of Capsicum spp. Euphytica. 1997; 96: 129–133. [Google Scholar]
- 35. Manzur JP, Calvache-Asensio MN, Rodríguez-Burruezo A. Effect of growth regulators and initial dark incubation on the in vitro culture efficiency of immature zygotic embryos from peppers (Capsicum annuum). Sci Agric. 2013; 71: 488–493. [Google Scholar]
- 36. Manzur JP, Penella C, Rodríguez-Burruezo A. Effect of the genotype, developmental stage and medium composition on the in vitro culture efficiency of immature zygotic embryos from genus Capsicum . Sci Hortic. 2013; 161: 181–187. [Google Scholar]
- 37. Rodríguez–Riaño T, Dafni A. A new procedure to assess pollen viability. Sex Plant Reprod. 2000; 12: 241–244. [Google Scholar]
- 38. Minamiyama Y, Tsuro M, Hirai M. An SSR-based linkage map of Capsicum annuum . Mol Breed. 2006; 18: 157–169. [Google Scholar]
- 39. Inai S, Ishikawa K, Nunomura O, Ikehashi H. Genetic analysis of stunted growth by nuclear-cytoplasmic interaction in interspecific hybrids of Capsicum by using RAPD markers. Theor Appl Genet. 1993; 87: 416–422. 10.1007/BF00215086 [DOI] [PubMed] [Google Scholar]
- 40. Yazawa S, Sato T, Namiki T. Interspecific Hybrid Dwarfism and Geographical Distribution of the Dwarfness Gene in Capsicum . J Japan Soc Hort Sci. 1989; 58: 609–618. [Google Scholar]
- 41. Hossain A, Minami M, Nemoto K. Immature embryo culture and interspecific hybridization between Capsicum annuum L. and C. frutescens L. via embryo rescue. Jpn J Trop Agr. 2003; 47: 9–16. [Google Scholar]
- 42. Orr HA. Dobzhansky, Bateson, and the genetics of speciation. Genetics. 1996; 144: 1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rao BN, Valli TS, Lakshmi N. Cytogenetic studies on the interspecific hybrid Capsicum baccatum L.–C. frutescens L. and its progeny. Euphytica. 1992; 59: 135–140. [Google Scholar]
- 44. Pickersgill B. Relationships between weedy and cultivated forms in some species of chili peppers (genus Capsicum). Evolution. 1971; 25: 683–691. [DOI] [PubMed] [Google Scholar]
- 45. Silva Monteiro CE, Santana Pereira T, Pereira de Campos K. Reproductive characterization of interspecific hybrids among Capsicum species. Crop Breeding and Applied Biotechnology. 2011; 11: 241–249. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.