Abstract
Objective
Compare respiratory health in children born extremely preterm (EP) or with extremely low birthweight (ELBW) nearly one decade apart, hypothesizing that better perinatal management has led to better outcome.
Design
Fifty-seven (93%) of 61 eligible 11-year old children born in Western Norway in 1999–2000 with gestational age (GA) <28 weeks or birthweight <1000 gram (EP1999–2000) and matched term-controls were assessed with comprehensive lung function tests and standardized questionnaires. Outcome was compared with data obtained at 10 years of age from all (n = 35) subjects born at GA <29 weeks or birthweight <1001 gram within a part of the same region in 1991–92 (EP1991–1992) and their matched term-controls.
Results
EP1999–2000 had significantly reduced forced expiratory flow in 1 second (FEV1), FEV1 to forced vital capacity (FEV1/FVC) and forced expiratory flow between 25–75% of FVC (FEF25–75), with z-scores respectively -0.34, -0.50 and -0.61 below those of the term-control group, and more bronchial hyperresponsiveness to methacholine (dose-response-slope 13.2 vs. 3.5; p<0.001), whereas other outcomes did not differ. Low birthweight z-scores, but not neonatal bronchopulmonary dysplasia (BPD) or low GA, predicted poor outcome. For children with neonatal BPD, important lung-function variables were better in EP1999–2000 compared to EP1991–1992. In regression models, improvements were related to more use of antenatal corticosteroids and surfactant treatment in the EP1999–2000.
Conclusions
Small airway obstruction and bronchial hyperresponsiveness were still present in children born preterm in 1999–2000, but outcome was better than for children born similarly preterm in 1991–92, particularly after neonatal BPD. The findings suggest that better neonatal management not only improves survival, but also long-term pulmonary outcome.
Introduction
Since the 1990s most infants born extremely preterm or with extremely low birthweight (hereafter referred to as EP-born) in high-income countries have survived to discharge [1, 2]. Birth at this stage of pregnancy implies that gas exchange must take place in fetal lungs, often leading to the syndrome of bronchopulmonary dysplasia (BPD) [3]. Life-long pulmonary prospects after EP birth and BPD are unknown, and concerns have been expressed for future functional deficits, such as early onset respiratory insufficiency [4, 5].
Neonatal pulmonary autopsy data suggest that EP birth may lead to severe bronchoalveolar abnormalities, but structural data from later life are scarce [6]. However, a range of functional abnormalities have repeatedly been described in EP-born survivors, such as airway obstruction, bronchial hyperresponsiveness, pulmonary hyperinflation and impaired gas diffusing capacity. Generally, those with neonatal BPD do worse. This scenario applies to EP-born adults from the early era of neonatal intensive care units (NICUs) as well as to children exposed to the far more advanced treatments introduced in the 1990s [7–12]. As lung function seems to track from childhood to adulthood [9, 13, 14], early onset chronic obstructive pulmonary disease (COPD) is a feared scenario in high-risk subgroups [4, 5].
Better NICU management benefits all infants born preterm, but it also facilitates survival of more immature and vulnerable individuals who may be at particular risk of severe long-term morbidity [2, 15]. Therefore, respiratory health and lung function after EP birth have been closely monitored in population-based controlled longitudinal studies in Western Norway since the 1980s [13, 16]. With the present study, we aimed to address respiratory outcomes at 11 years of age in our most recent cohort born EP in 1999–2000 (EP1999–2000) and to compare the findings with those of children born similarly preterm in 1991–1992 (EP1991–1992). We hypothesized that changes in perinatal care during the 1990s were associated with respiratory improvements [16, 17].
Materials and Methods
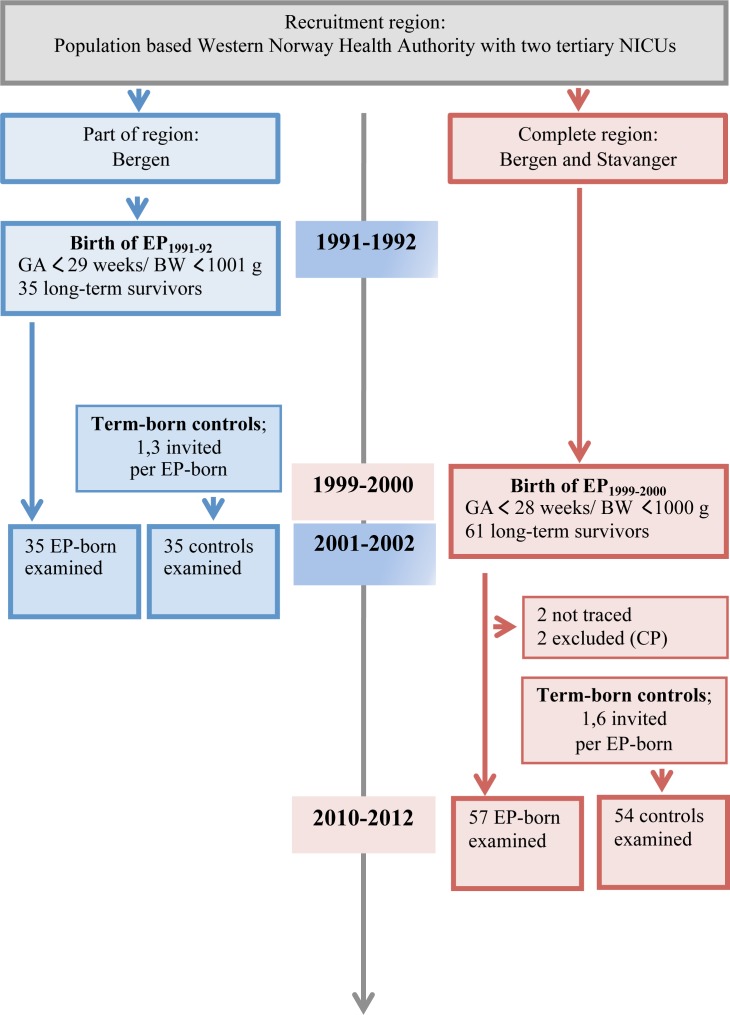
Detailed descriptions are provided in S1 File, and the inclusion process is visualized in Fig 1.
Fig 1. The recruitment of subjects in two cohorts of premature (EP) and matched term-born subjects, one cohort born in 1991–92 and examined in 2001–2002, and one cohort born in 1999–2000 and examined in 2010–2012.
Subjects, definitions and neonatal background data
The EP1999–2000 included all NICU admitted infants born in 1999–2000 with gestational age (GA) <28 weeks or birth weight (BW) <1000 gram within Western Norway Health Authority, which serves a population of approximately 1.1 million. It was a regional selection of a national cohort [2]. The infants were treated at one of the two regional NICUs (Bergen and Stavanger). The EP1999–2000 data were compared with data obtained at 10 years of age from all (n = 35) subjects born with GA <29 weeks or BW <1001 gram within a part of the same region (population 615 000) in 1991–92 and treated at the NICU in Bergen (EP1991–1992). EP1991–1992 data have been published previously in a different context [13, 16] and relevant data are included in the present paper. The acronym ‘EP-born’ is used in this article to represent participants born preterm in both inclusion periods.
For each EP-born participant of both birth-cohorts, the next-born child in the same maternity ward of the same gender with GA >37 weeks and BW >3000 grams were identified from birth protocols and invited as control. If that individual declined, the next-born eligible child was invited until a match was obtained. Background data were extracted from compulsory notifications to the Medical Birth Registry of Norway, registration-forms developed for the study and questionnaires completed by the parents, including the International Study on Asthma and Allergy in Childhood (ISAAC) questionnaire [18]. Small for gestational age (SGA) was defined as BW <10th percentile for GA according to Norwegian growth curves [19]. Z-scores for BW and later anthropometric measures were calculated with reference to Norwegian growth curves [19, 20]. BPD was defined as still dependent on oxygen supplementation at 36 weeks’ postmenstrual age [3]. Oxygen supplementation was given according to similar department policy in the two inclusion periods in the two participating NICUs, and at 36 weeks’ GA it was generally provided through low flow nasal cannulas guided by pulse oximetry targeting 90–95% saturation. Important changes of treatment between the two inclusion periods were more extensive use of surfactant and antenatal corticosteroids and a change from synthetic (Exosurf®) (31) to natural surfactant (Curosurf®) (Table 1) (32, 33). Most areas of neonatal intensive care medicine had gone through refinements, such as better standardization of antenatal and perinatal care, a higher level of competence among neonatologists and nurses regarding the special needs of these vulnerable infants, and better exploitation of technological advances, such as patient coordinated assisted ventilation and various forms of oscillation.
Table 1. Perinatal data comparing children born preterm in 1991–92 (EP1991–92) and in 1999–2000 (EP1999–2000).
| EP1991–92 cohort | EP1999–2000 cohort | p-value a | ||
|---|---|---|---|---|
| Subjects; n (%) | Control | 35 | 54 | |
| All EP | 35 | 57 | ||
| - non BPD | 23 (66) | 26 (46) | 0.067 | |
| - BPD | 12 (34) | 31 (54) | ||
| Female gender;n (%) | Control | 22 (63) | 25 (46) | 0.135 |
| All EP | 22 (63) | 28 (49) | 0.209 | |
| - non BPD | 17 (74) | 16 (62) | 0.379 | |
| - BPD | 5 (42) | 12 (39) | 0.860 | |
| Birthweight, gram; mean (SD) | Control | 3564 (275) | 3701 (434) | 0.073 |
| All EP | 933 (204) | 850 (175) | 0.039 | |
| - non BPD | 976 (195) | 873 (200) | 0.073 | |
| - BPD | 851 (203) | 831 (151) | 0.722 | |
| Birthweight; sds- score | All EP | -0.36 (0.9) | -0.80 (1.3) | 0.054 |
| - non BPD | -0.37 (0.9) | -0.97 (1.4) | 0.076 | |
| - BPD | -0.32 (0.9) | -0.66 (1.2) | 0.386 | |
| Gestational age, weeks; mean (SD) | All EP | 26.7 (1.7) | 26.8 (1.6) | 0.974 |
| - non BPD | 27.2 (1.7) | 27.3 (1.6) | 0.788 | |
| - BPD | 25.8 (1.5)* | 26.3 (1.4)** | 0.381 | |
| Small for gestational age (SGA); n (%) | All EP | 5 (14) | 20 (35) | 0.030 |
| - non BPD | 3 (13) | 12 (46) | 0.015 | |
| - BPD | 2 (17) | 8 (26) | 0.570 | |
| Postnatal days with oxygen treatment; median (range) | All EP | 49 (2–180) | 65 (0–250) | 0.109 |
| - non BPD | 34 (2–70) | 44 (0–78) | 0.254 | |
| - BPD | 92 (61–180) | 79 (44–250) | 0.080 | |
| Ventilator days; median (range) | All EP | 4.0 (0–55) | 5.0 (0–24) | 0.618 |
| - non BPD | 1.3 (0–40) | 2.5 (0–21) | 0.212 | |
| - BPD | 12.7 (2–55)*** | 8.0 (0–24)*** | 0.011 | |
| Antenatal corticosteroids; n (%) | All EP | 15/34 (44) | 46 (81) | <0.001 |
| - non BPD | 11 (48) | 21 (81) | 0.005 | |
| - BPD | 4/11 (36) | 25 (81) | 0.012 | |
| Surfactant; n (%) | All EP | 17 (49) | 49/56 (88) | <0.001 |
| - non BPD | 7 (30) | 20/25 (80) | 0.001 | |
| - BPD | 10 (83) | 29 (94) | 0.367 | |
| Postnatal corticosteroids; n (%) | All EP | 10 (29) | 18 (32) | 0.772 |
| - non BPD | 2 (9) | 1 (4) | 0.549 | |
| - BPD | 8 (67) | 17 (55)*** | 0.510 | |
| Closing of PDA; n (%) | All EP | 17 (49) | 13 (23) | 0.013 |
| - non BPD | 7 (30) | 3 (12) | 0.122 | |
| - BPD | 10 (83) | 10 (32) | 0.004 | |
| Maternal smoking in pregnancy; n (%) | Control | 9 (26) | - | - |
| All EP | 13 (37) | 13/52 (25) | 0.205 | |
| - non BPD | 10 (43) | 5/24 (21) | 0.111 | |
| - BPD | 3 (25) | 8/28 (29) | 0.957 |
Figures are means (SD), medians (ranges) or counts (%).
a The p-value denotes differences between those born in 1991–92 and in 1999–2000.
* P-values for group differences between EP non BPD vs. EP BPD within each cohort, * p<0.05,
**p<0.01,
***p<0.001.
The studies were approved by the regional committee on medical research ethics in Western Norway Health Authority (REC West), and parents of all participants gave written consent.
Measurements
The two birth-cohorts were examined at 10–11 years of age in 2001–2002 and 2010–2012, respectively, using the same type of equipment and the same examination program, except that nitric oxide was not studied in EP1991–1992. The children were seen on two separate days at the University Hospitals in Bergen or Stavanger according to place of birth. Spirometry, static lung volumes and pulmonary diffusing capacity for carbon monoxide (DLCO/KCO) were measured with Vmax equipment (SensorMedics Inc, Anaheim, USA), applying standard quality criteria [21, 22] with data standardized for age, height and gender [23, 24], except KCO reported as raw data. Fractional exhaled nitric oxide (FeNO) was measured with Exhalyzer CLD-88 (EcoMedics, Switzerland), according to ATS/ERS recommendations [25]. Alveolar NO (ppb) (CANO) and bronchial flux of NO (nl/sec) (Jaw NO) were calculated using three different flows (30, 100 and 300 ml/sec) [26]. Methacholine provocation was performed with an inhalation-synchronised dosimetric nebulizer (Spira Electra, Finland), providing baseline FEV1 ≥65% predicted [27, 28]. The test continued until a fall of 20% or more compared to baseline FEV1, or until the maximum dose of 11.5 μmol methacholine. Dose-response slope (DRS) was calculated as the ratio of maximum percentage decline in FEV1 from baseline to cumulative administered dose of methacholine (%/μmol) [29]. Reversibility to salbutamol was assessed by measuring FEV1 before (baseline) and 10–15 minutes after administering 0.1 mg/10 kg salbutamol (Ventoline) from a metered dose inhaler via a spacer (Volumatic); an increase ≥12% was considered positive response [30]. Methacholine provocation and salbutamol reversibility tests were done on separate days.
Statistical analysis
Non-paired groups were compared using independent sample t-tests, Mann-Whitney U-test, Fishers exact 2-sided mid-p value [31] or odds ratios (OR), and paired data with the mixed linear model (MLM) of SPSS, as appropriate. For EP1999–2000, multiple backward regression models were constructed in order to address potential associations between neonatal data and selected background data (listed in the Results chapter) vs. current FEV1. Independent variables were included if the correlation with the dependent variable was > 0.3 and the bivariate correlation with other independent variables was < 0.7. Interaction terms were used to test if effects (differences in lung function between EP-born and matched term-controls) differed between EP-born subgroups; i.e. BPD vs. non BPD, GA-categories (GA ≤25 vs. 26–27 vs. ≥28 weeks) and birth-cohort (1991–1992 vs. 1999–2000). The interaction terms were tested for influence from selected neonatal factors that varied between the two preterm born cohorts (see Results). Providing 60 cases were included, the study had 80% power to detect a difference in z-FEV1 of 0.5 between EP1999–2000 and matched term-controls, given a two-sided significance level of 0.05. SPSS (version 21.0) was used for computations.
Results
Subjects (Fig 1)
In EP1999–2000, 61 eligible EP-born were discharged alive, two could not be traced and two were excluded due to cerebral palsy, leaving 57 (93%) participants, all but three Caucasians. In EP1991–1992, all 35 eligible EP-born survivors participated, all Caucasians. On average, respectively 1.6 and 1.3 term-born subjects were approached to recruit a full 1:1 control group for the two inclusion periods. In EP1999–2000, uneven drop-outs eventually caused a numeric gender difference; i.e., 49% EP-born vs. 46% controls were female (p = 0.85).
Perinatal data (Table 1)
For subjects admitted to the NICU, survival rates were 81% (EP1999–2000) vs. 74% (EP1991–92) (p = 0.57). Due to slight differences in the inclusion criteria, mean BW was lower and the proportion born SGA was higher in EP1999–2000. Eleven subjects (19%) were included solely by BW (i.e. GA ≥28 weeks) in EP1999–2000 (mean GA (range) 29.2 (28–31) weeks), and two solely by BW (i.e. GA ≥29 weeks) in EP1991–92 (GA 30 and 31 weeks). The number of participants born at GA ≤27 weeks was 46/57 (81%) and 21/35 (60%), respectively. For subjects with BPD in EP1999–2000 compared to EP1991–1992, mean GA and BW did not differ, but days on ventilator were fewer, a higher proportion had received prenatal corticosteroids and surfactant treatment and fewer had artificial closure of persistent ductus arteriosus (surgery or indomethacin).
Anthropometric data and respiratory symptoms (Table 2)
Table 2. Anthropometric data and respiratory symptoms at age 11 comparing children born preterm in 1991–92 (EP1991–92) and in 1999–2000 (EP1999–2000).
| EP1991–92 cohort n = 35 | EP1999–2000 cohort n = 57 | p-value a | ||
|---|---|---|---|---|
| Age; years | Control | 10.6 (0.4) | 11.7 (0.7) | <0.005 |
| All EP | 10.4 (0.4) | 11.4 (0.6) | <0.005 | |
| - non BPD | 10.4 (0.5) | 11.4 (0.6) | <0.005 | |
| - BPD | 10.4 (0.4) | 11.5 (0.6) | <0.005 | |
| Height; z-score | Control | 0.02 (0.9) | 0.00 (1.1) | 0.925 |
| All EP | -0.51 (1.2)* | -0.41 (1.0)* | 0.654 | |
| - non BPD | -0.52 (1.3) | -0.38 (1.0) | 0.658 | |
| - BPD | -0.49 (1.1) | -0.43 (1.1)) | 0.868 | |
| Weight; z-score | Control | 0.17 (1.0) | -0.14 (1.1) | 0.153 |
| All EP | -0.48 (1.4)* | -0.33 (1.05) | 0.570 | |
| - non BPD | -0.40 (1.7) | -0.43 (1.0) | 0.956 | |
| - BPD | -0.61 (0.8) | -0.25 (1.1)) | 0.303 | |
| BMI; z-score | Control | 0.25 (0.9) | -0.22 (1.0) | 0.033 |
| All EP | -0.28 (1.4) | -0.16 (1.0) | 0.644 | |
| - non BPD | -0.18 (1.6) | -0.35 (1.1) | 0.660 | |
| - BPD | -0.46 (0.9) | 0.00 (1.0) | 0.178 | |
| Asthma ever | Control | 3 (9) | 5 (9) | 0.933 |
| All EP | 12 (34)* | 15 (26)* | 0.427 | |
| - non BPD | 6 (26) | 5 (19) | 0.587 | |
| - BPD | 6 (50) | 10 (32) | 0.309 | |
| Asthma medication last 12 months | Control | 1 (3) | 3 (6) | 0.838 |
| All EP | 5 (14) | 4 (7) | 0.075 | |
| - non BPD | 1 (4) | 3 (12) | 0.775 | |
| - BPD | 4 (33) | 1 (3) | 0.006 | |
| Wheeze last 12 months | Control | 2 (6) | 5 (9) | 0.587 |
| All EP | 11 (31)* | 8 (14) | 0.055 | |
| - non BPD | 6 (26) | 4 (15) | 0.383 | |
| - BPD | 5 (42) | 4 (13) | 0.060 | |
| Atopy | Control | 8 (23) | 20/50 (40) | 0.105 |
| All EP | 9 (26) | 12/55 (22) | 0.675 | |
| - non BPD | 8 (35) | 6/25 (24) | 0.435 | |
| - BPD | 1 (8) | 6/30 (20) | 0.415 |
Figures are means (SD), medians (ranges) or counts (%).
a The p-value denotes differences between those born on 1991–92 and in 1999–2000.
* P-values for group differences between term controls vs. all EP within each cohort, * p<0.05. Atopy registered as minimum one positive SPT (skin prick test) or IgE test in the EP1991–92 cohort and as minimum one positive SPT in the EP1999–2000 cohort.
At follow-up, EP1999–2000 was one year older than EP1991–1992. Anthropometric measures were similar except that the control children for EP1999–2000 had slightly lower BMI. The prevalence of ever having been diagnosed with asthma was similar for the two preterm-born cohorts, and the proportion was significantly higher than for their respective term-born groups. Current respiratory symptoms (wheezing) and use of asthma medication tended to be rarer in EP1999–2000 than in EP1991–92 and not significantly different from term-controls, contrasting EP1991–92. Wheeze was unrelated to airflow-limitation, with one report of current wheeze in the overall 15 subjects with z-FEV1 below -1.64, which is the lower limit of normal [23].
Lung function for EP1999–2000
Data on EP1991–1992 have been published previously [13, 16], and relevant data are presented in Table C in S1 File. For EP1999–2000, data are presented in Tables 3 and 4 and Table D in S1 File. Flow-volume loops were satisfactorily obtained from all participants. Failure rates were minor also for most other lung function tests, except measures of nitric oxide and lung diffusion (12–14 of 57). The failure rates were similar for EP-born and term-controls on the individual tests; details are presented in S1 File. EP-born had lower z-scores for FEV1, FEV1/FVC and FEF25–75, higher airway resistance (Raw), and higher DRS from the methacholine challenge than term-controls. DRS was negatively and similarly associated with z-FEV1 (p<0.001) in the EP- and term-born groups (test of interaction, p = 0.21). Static lung volumes (z-TLC, z-FRC, z-RV and RV/TLC), FeNO and DLCO did not differ significantly between the EP-born and term-born groups. Nine subjects (7 EP-born; 5 BPD and 2 non-BPD, and 2 term-born) had ≥12% increase in FEV1 on the salbutamol reversibility test (EP vs. term-born, p = 0.18). Mean FEV1 change before vs. after salbutamol did not differ between those born EP and at term, and responses were negatively associated with z-FEV1 (p<0.001) in both groups (test of interaction, p = 0.24).
Table 3. Lung function variables in 11 year old children born preterm (EP) in 1999–2000 and matched term-born controls, split by neonatal bronchopulmonary dysplasia (BPD).
| Controls N = 54 | All EP N = 57 | EP non BPD N = 26 | EP BPD N = 31 | Mean difference (95% CI) All EP vs. Control | * P values EP vs. Control | ||
|---|---|---|---|---|---|---|---|
| FEV1 | Z | -0.31(-0.57, -0.04) | -0.65 (-0.90, -0.41) | -0.56 (-0.91, -0.21) | -0.73 (-1.10, -0.37) | -0.35 (-0.70, 0.01) | 0.04 |
| FVC | Z | -0.16 (-0.42, 0.09) | -0.17 (-0.41, 0.07) | -0.17 (-0.48, 0.13) | -0.17 (-0.54, 0.20) | -0.01 (-0.36, 0.33) | 0.96 |
| FEV1/FVC | Z | -0.30 (-0.54, -0.05) | -0.80 (-1.07, -0.54) | -0.69 (-1.06, -0.31) | -0.90 (-1.29, -0.52) | -0.52 (-0.89, -0.16) | 0.005 |
| FEF25–75 | Z | -0.53 (-0.79, -0.27) | -1.14 (-1.39, -0.89) | -1.04 (-1.40, -0.68) | -1.22 (-1.58, -0.87) | -0.63 (-0.98, -0.27) | 0.001 |
| Raw | Z | 0.68 (0.51, 0.85) | 1.23 (0.77, 1.68) | 0.96 (0.72, 1.21) | 1.45 (0.61, 2.29) | 0.54 (0.05, 1.04) | 0.58 |
| Reversibility a | % change (FEV 1 ) | 5.0 (3.9, 6.1) | 6.6 (4.3, 8.8) | 5.2 (2.9, 7.4) | 7.8 (4.0, 11.5) | 1.6 (-0.9, 4.1) | 0.18 |
| DRS b | Geometric mean | 3.47 (2.19, 5.50) | 13.18 (8.16, 21.37) | 11.48 (5.75, 23.44) | 14.79 (7.24, 29.51) | 3.80 (2.00, 7.24) | <0.001 |
| TLC | Z | 0.45 (0.13, 0.76) | 0.30 (0.05, 0.55) | 0.17 (-0.11, 0.46) | 0.41 (0.00, 0.82) | -0.15 (-0.55, 0.25) | 0.32 |
| FRC | Z | -0.34 (-0.74, 0.06) | 0.07 (-0.27, 0.41) | -0.26 (-0.67, 0.15) | 0.36 (-0.16, 0.89) | 0.41 (-0.11, 0.92) | 0.06 |
| RV | Z | 0.27 (-0.09, 0.63) | 0.003 (-0.30, 0.30) | 0.05 (-0.40, 0.51) | -0.04 (-0.47, 0.38) | -0.27 (-0.73, 0.20) | 0.17 |
| RV/TLC | Ratio | 26.2 (24.4, 27.9) | 25.9 (24.0, 27.9) | 26.9 (24.0, 29.8) | 25.2 (22.4, 27.9) | -0.2 (-2.8, 2.4) | 0.75 |
| DLCO | % predicted | 88.2 (84.3, 91.7) | 86.5 (80.6, 92.4) | 87.4 (81.4, 93.3) | 85.7 (75.0, 96.4) | -1.6 (-8.5, 5.2) | 0.68 |
| VA | % predicted | 97.1 (91.4, 102.9) | 97.8 (91.8, 103.7) | 92.6 (83.7, 101.4) | 102.7 (94.7, 110.8) | 0.6 (-7.5, 8.7) | 0.65 |
| KCO | Mmol/kPa.min | 1.74 (1.66, 1.82) | 1.69 (1.61, 1.77) | 1.77 (1.67, 1.87) | 1.61 (1.48, 1.74) | -0.05 (-1.6, 0.06) | 0.32 |
| % predicted | 80.0 (76.7, 83.3) | 76.3 (72.5, 80.2) | 80.0 (75.3, 84.8) | 72.8 (66.9, 78.8) | -3.6 (-8.6, 1.3) | 0.13 | |
| FeNO 0.05 | G. mean | 11.77 (9.53, 14.54) | 9.65 (7.97, 11.69) | 10.46 (7.59, 14.43) | 9.08 (7.08, 11.65) | -1.22 (-1.62, 1.09) | 0.18 |
| CANO | ppb | 1.48 (1.22, 1.74) | 1.25 (0.97, 1.54) | 1.30 (0.85, 1.74) | 1.22 (0.82, 1.61) | -0.16 (-0.56, 0.25) | 0.24 |
| JawNO | nl/sec | 0.77 (0.57, 0.96) | 0.86 (0.54, 1.17) | 0.83 (0.47, 1.19) | 0.88 (0.36, 1.41) | 0.09 (-0.28, 0.46) | 0.62 |
| FeNO nasal | ppb | 805 (696, 915) | 825 (745, 905) | 901 (758, 1044) | 764 (675, 854) | 20 (-115, 155) | 0.73 |
Figures are observed means (95% confidence intervals), unless otherwise stated. For abbreviations, please see list. Lung diffusion data reported as % predicted and as raw-data, and nitric oxide (NO) data only as raw-data, due to suboptimal reference equations for children.
* Interaction terms testing differences between EP and matched term-born controls in the group with BPD vs. without BPD were non-significant for all variables, and therefore only the p-values for all EP vs. term-born controls were reported.
a Reversibility is given as percentage change in FEV1 after vs. before administration of beta agonist, assuming the pre-value is baseline.
b DRS is the ratio of maximum percentage decline in FEV1 from baseline to cumulative administered dose (μmol) of methacholine (%/μmol), reported as geometric means due to a highly skewed distribution.
Table 4. Lung function variables reflecting airway flow before and after administration of salbutamol, and bronchial responsiveness to methacholine, in 11 year old children born preterm (EP) in 1999–2000, split by gestational age (GA) at birth.
| Controls N = 54 | GA ≤ 25 weeks N = 10 | GA 26–27 weeks N = 36 | GA ≥ 28 weeks N = 11 | ||
|---|---|---|---|---|---|
| Gestational age (SD) | weeks | - | 24.4 (0.5) | 26.7 (0.5) | 29.2 (1.0) |
| Birth weight (SD) | grams | - | 741.2 (107) | 904.1 (177) | 770.1 (146) |
| BW z-scores (SD) | - | 0.09 (0.9) | -0.54 (1.0) | -2.46 (0.7) *** | |
| No. with SGA a | (% of group) | 1 (10) | 8 (22) | 11 (100) | |
| No. with BPD | (% of group) | - | 8 (80) | 20 (56) | 3 (27) *** |
| FEV1 | z | -0.27 (-0.52, -0.01) | -0.46 (-1.28, 0.37) | -0.54 (-0.82, -0.27) | -1.22 ** (-1.84, -0.59) |
| z-Post-β 2 | -0.07 (-0.34, 0.21) | 0.05 (-1.04, 1.13) | -0.41 (-0.82, 0.01) | -0.67 (-1.29, -0.04) | |
| FEV1/FVC | z | -0.30 (-0.54, -0.05) | -0.13 (-0.98, 0.73) | -0.82 * (-1.12, -0.52) | -1.38 ** (-1.93, -0.83) |
| z- Post-β 2 | 0.22 (0.00, 0.44) | 0.39 (-0.39, 1.18) | -0.23 (-0.58, 0.13) | -0.31 (-0.89, 0.28) | |
| FEF25–75 | z | -0.52 (-0.78, -0.27) | -0.92 (-1.59, -0.25) | -1.07 * (-1.37, -0.77) | -1.78 ** (-2.34, -1.22) |
| z- Post-β 2 | 0.06 (-0.20, 0.31) | -0.07 (-0.88, 0.74) | -0.58 (-0.91, 0.24) | -0.50 (-1.17, 0.17) | |
| DRS | geometric mean | 3.48 (2.21, 5.47) | 3.58 (1.11, 14.19) | 12.74 ** (6.95, 23.33) | 38.54 ** (1.74, 85.31) |
Figures are observed means (95% confidence intervals) split by GA categories for those lung function variables that differed between the EP and term-born groups. For abbreviations, please see list. Interaction terms, testing if differences between EP and matched term-born controls were different over the three GA categories, were non-significant for all variables. * p-values testing differences between EP-born subgroups, or (when possible) EP-born subgroups vs. control subjects,
* p<0.05,
** p<0.01,
*** p<0.001.
a SGA = small for gestational age.
There was no effect of neonatal BPD vs. no BPD on any of the assessed lung function variables (non-significant interaction-terms). Lung function variables that differed between the EP- and term-born groups were analyzed also by GA-category, and the deficits were numerically most pronounced for the GA-category ≥28 weeks, although interaction terms were non-significant (Table 4).
Lung function related to perinatal and background variables
For EP1999–2000, BW z-score was the only significant predictor of z-FEV1 at 11 years (B = 0.24; p = 0.04; R2 = 0.10) in a regression model including the following perinatal variables: GA, BW z-scores, days on ventilator, days on oxygen treatment, use of antenatal and postnatal corticosteroids, surfactant treatment and BPD vs. no-BPD. Applying the same model on the EP1991–1992, BW z-scores similarly predicted z-FEV1 (B = 0.35; p = 0.02) in a final model; however, including also days of oxygen (B = -0.011; p<0.001) and antenatal corticosteroids (B = 0.59; p = 0.03) (R2 = 0.48, all three variables). Maternal smoking in pregnancy was not associated with z-FEV1 at age 11. Regressing the background variables asthma ever, asthma medication last 12 months, wheeze last 12 months, atopy and maternal smoking in pregnancy on z-FEV1 at age 11, revealed no significant associations for either EP or term-born in any birth-cohort.
Differences between EP1991–1992 and EP1999–2000
Paired differences between EP-born and matched term-controls for the inclusion periods 1991–1992 and 1999–2000 are given in Table 5 and illustrated in Figs 2 and 3. The differences were significantly smaller for the variables z-FEV1, z-FVC and z-FEF25–75 and RV/TLC for the group with BPD born in 1999–2000 compared to the group with BPD born in 1991–1992 (tests of interaction). Adjusting these interaction terms for differences between the study periods regarding GA, BW, days on ventilator and PDA management did not influence conclusions, whereas the effect disappeared for z-FEV1 when surfactant and/or antenatal corticosteroids were included in the model. Differences between EP-born without BPD and their matched term-controls did not differ between two inclusion periods.
Table 5. Comparison of lung function indices in two cohorts of children born preterm in 1991–92 (EP1991–92) and in 1999–2000 (EP1999–2000).
| EP1991–1992 cohort | EP1999–2000 cohort | ||||||
|---|---|---|---|---|---|---|---|
| Control vs. all-EP | Control vs. EP no-BPD | Control vs. EP BPD | Control vs. all-EP | Control vs. EP no-BPD | Control vs. EP BPD | * p-value, control vs. EP BPD | |
| FEV1; z-score | 0.86 (0.44, 1.26) | 0.53 (-0.00, 1.07) | 1.46 (0.80, 2.13) | 0.35 (-0.01, 0.70) | 0.25 (-0.30, 0.79) | 0.43 (-0.08, 0.94) | 0.02 |
| FVC; z-score | 0.44 (0.07, 0.82) | 0.41 (-0.11, 0.93) | 0.51 (-0.14, 1.16) | 0.01 (-0.33, 0.36) | 0.01 (-0.53, 0.54) | 0.01 (-0.49, 0.52) | < 0.001 |
| FEV1/FVC; z-score | 0.57 (-0.03, 1.18) | 0.08 (-0.60, 0.77) | 1.51 (0.65, 2.36) | 0.52 (-0.16, 0.89) | 0.41 (-0.15, 0.97) | 0.62 (0.09, 1.15) | 0.03 |
| FEF25–75; z-score | 0.94 (0.47, 1.42) | 0.54 (-0.07, 1.14) | 1.72 (0.96, 2.47) | 0.63 (0.27, 0.98) | 0.52 (-0.02, 1.06) | 0.72 (0.20, 1.23) | 0.04 |
| TLC; z-score | 0.10 (-0.26, 0.45) | 0.24 (-0.24, 0.72) | -0.18 (-0.76, 0.43) | 0.15 (-0.25, 0.55) | 0.28 (-0.33, 0.88) | 0.04 (-0.54, 0.62) | 0.74 |
| RV; z-score | -0.17 (-0.56, 0.21) | 0.16 (-0.33, 0.65) | -0.79 (-1.39, -0.19) | 0.27 (-0.20, 0.73) | 0.21 (-0.49, 0.92) | 0.31 (-0.37, 0.99) | 0.02 |
| RV/TLC | -1.27 (-4.94, 0.11) | -0.59 (-3.93, 2.74) | -5.77 (-9.87, -1.66) | 0.21 (-2.40, 2.82) | -0.69 (-4.67, 3.28) | 1.01 (-2.82, 4.84) | 0.03 |
| DRS; geometric mean | 5.01 (2.19, 11.22) | 3.47 (1.17, 10.47) | 11.22 (2.69, 47.86) | 3.80 (2.00, 7.24) | 3.31 (1.20, 8.71) | 4.27 (1.62, 10.96) | 0.36 |
Figures are mean differences (95% confidence intervals) between EP born subgroups and their respective matched term-born control subjects. The units are z-scores, except the ratio RV/TLC and the variable DRS where figures are differences in the dose response slope to methacholine. Positive figures indicate higher z-scores for the control group or higher DRS for the EP-born group.
* Interaction terms, testing if differences between EP and matched term-born controls were different in the two study periods (1991–92 vs. 1999–2000) were non-significant, except for the groups with neonatal BPD, for which p-values are given.
Fig 2. Z-FEV1 in 11 year old term and preterm-born (EP) subjects born in 1991–92 and 1999–2000, EP-born split by the presence of BPD.
Fig 3. Z-FEF25–75 in 11 year old term and preterm-born (EP) subjects born in 1991–92 and 1999–2000, EP-born split by the presence of BPD.
Discussion
This study showed that respiratory outcomes in mid-childhood were encouraging in children born at extremely low GAs or BWs in 1999–2000. Still, the preterm born participants had more small-airway obstruction and bronchial hyperresponsiveness than matched term-controls, but static lung volumes, diffusing capacity and exhaled nitric oxide did not differ. Outcome data were in most respects unrelated to BPD, and those born most immature did surprisingly well. Compared to children born similarly preterm in the same region in 1991–1992, lung function data were generally better, and particularly for those with neonatal BPD, where significant improvements had occurred for important variables.
Strengths and limitations
The major strengths of this study were the population-based design with almost complete attendance, and recruitment of matched control groups that followed a strict algorithm based on the ‘next-born-subject’ principle, minimizing risks of selection bias. An age difference of approximately one year at follow-up between the two birth-cohorts was adjusted for by reference equations or by statistical models. Potential bias introduced by a two-center design was limited by statistical analyses performed according to the matched preterm versus term-born structure. The algorithm for inclusion was based on a combined use of GA and BW, initially preferred in 1991–1992 to ensure inclusion of all children perceived to be extremely immature at birth. We continued this approach in 1999–2000, although changing the GA criterion from <29 weeks to <28 weeks. Due to the inclusion criteria the data may not be generalizable to subjects born extremely preterm in general. A diagnosis of BPD must reflect oxygen treatment algorithms, which to some extent is subject to department policy. In this study, the same senior neonatologists operated the same algorithms for oxygen supplementation in both inclusion periods, making systematic changes unlikely. The study could not discriminate moderate from severe BPD since oxygen treatment at 36 weeks GA was provided through low flow nasal cannulas without recording the exact fraction of inspired oxygen (FiO2) [3]. By nature, unfavourable events or conditions tend to be linked in neonatal intensive care medicine, often setting up vicious circles that require treatments with long-term side effects. Observational statistical models struggle with these scenarios due to collinearity between variables. To limit spurious associations we therefore excluded the independent neonatal variable considered least meaningful if a correlation coefficient was ≥ 0.7 in the same analysis. This situation calls for cautious interpretation of regression models that aim to address statistical associations between such variables and outcome. The study may be criticized for having few participants. Longitudinal studies over decades are by nature difficult to perform as demonstrated by the paucity of similar data. The power calculations indicate reasonable detection limits.
Lung function in the children born preterm in 1999–2000
The lung function abnormalities in the 11 year old children born preterm in 1999–2000 were minor. FEV1 was mostly within normal limits and they had no signs of abnormal volume distribution or diffusing capacity for carbon monoxide. However, forced mid-expiratory flow (FEF25–75) in the range of 75% predicted and significant methacholine hyperresponsiveness indicate that small airway airflow limitation and bronchial abnormalities were still present. Those born most immature did surprisingly well, in that lung function variables did not differ significantly from those of the term-controls. Moreover, the presence of neonatal BPD was not significantly associated with outcome, contrasting some [12, 32], and consistent with one previous study [11]. Most of the differences between preterm and term-born groups seemed to be explained by the highest GA-category; i.e. basically SGA infants with GA ≥28 weeks who were included on the basis of BW <1000 gram, in whom significant airway obstruction and remarkably high bronchial hyperresponsiveness were observed. In adjusted regression models, BW z-score was the only remaining neonatal variable associated with FEV1 at age 11. Thus, within the frames of todays advanced NICU management, the paradigm that neonatal BPD or extreme immaturity is inevitably linked to poor long-term pulmonary outcome may need to be revised. However, statements regarding causal pathways for novel findings are bound to be speculations within the frames of a study of this kind.
Interestingly, administration of salbutamol increased all airflow variables to within 0.5 z-score of zero; i.e. clearly within normal limits. Salbutamol responses were negatively and similarly associated with FEV1 in the EP-born and term-control group, further reducing the group differences. Few subjects had salbutamol responses considered clinically significant (≥12% from baseline) [30], and on average the responses did not differ between the EP- and term-born group. The question to what extent airway obstruction after EP birth is a fixed or reversible phenomenon therefore remains unanswered.
Pulmonary abnormalities after EP birth may clinically mimic pediatric asthma, and studies have reported increased respiratory symptoms and more hospital admissions, particularly during the first few years of life [33–35]. In this group of 11 year old children born EP in 1999–2000, current respiratory symptoms and use of asthma medication were reassuringly rare, also in those who were most immature at birth, contrasting some previous studies (8, 12, 36). Childhood asthma is generally characterized by eosinophilic airway inflammation (17), which may be assessed by fractional exhaled nitric oxide (FeNO) (25), with extended FeNO analyses as a new promising tool (26). We found that neither FeNO nor alveolar NO differed between the preterm and term-born group, providing support for the notion that eosinophilic airway inflammation is not involved in lung disease after EP birth (10, 37). However, active inflammatory mechanisms may still be involved, and recent studies have indicated increased oxidative stress in the respiratory system after EP birth, pathways this study was not set up to explore (38, 39).
Some authors have expressed optimism regarding long-term pulmonary outcomes in children who were born EP more recently [11, 36]. Kotecha et al. found in their review milder impairments in FEV1 for preterm born children with neonatal BPD (defined by need of oxygen treatment at 28 days of age) in recent compared to earlier studies [36]. However, two research groups reported data indicating that respiratory abnormalities are still present after EP birth; one regional study of children born at GA < 28 weeks or BW < 1000 grams in 1997 in the state of Victoria, Australia [12, 32], and another based on the EPICure study of children born atGA < 26 weeks in 1995 in the UK or Ireland [10, 32]. Both studies reported significantly lower z-FEV1 and z-FEF25–75 in preterm compared to term-born children. The Australian study [12] compared children born in 1997 with a group born in 1991–92, and found overall airflow limitation in both groups, but for those with no history of neonatal BPD, the children born in 1997 had better z-FEV1 and z-FVC than those born in 1991–92. In light of the possibility that more children at risk of unfavorable outcome may have survived in the most recent cohort, this may be interpreted as a positive development. These findings are in line with ours, although we found this positive development particularly evident in the group with neonatal BPD. The EPICure study had more extreme inclusion criteria, with mean GA at birth almost two weeks lower (24.9) and BW approximately 100 gram lower (750 gram) than the EP1999–2000 cohort of our study, and also a higher rate of neonatal BPD; i.e. 70% compared to 50%. They found no differences between preterm and term-born participants regarding FRC, TLC and alveolar volume, which is comparable to our findings, but elevated RV/TLC and some impairments in DLCO and DLCO/VA; differences that we did not see as clearly in our EP1999–2000 cohort but resembling our findings at ten years of age in the EP1991–1992 cohort [16]. One may speculate that more infants born at the limits of viability in the EPICure study may have contributed to these differences in findings.
Comparing lung function of children born preterm in 1991–1992 and 1999–2000
There were significant improvements for z-FEV1 and other important lung function variables from 1991–1992 to 1999–2000. This was particularly evident for the group of children with neonatal BPD, who presumably had the most turbulent neonatal history. The interaction terms used to assess these improvements were robust for adjustment for most perinatal variables, except that use of antenatal corticosteroids and surfactant eliminated the improvements in z-FEV1. Thus, statistical modeling of the dataset suggested that more extensive use of these modalities may partly explain the observed improvement with time. However, a limitation that applies to studies of this kind is that a variety of known and unknown influences of possible significance for outcome cannot be accounted for in the applied regression models. Thus, more and larger studies are required to resolve this issue.
The inclusion algorithms varied slightly between the two preterm-born cohorts. Despite this, the two groups had fairly similar BWs and GAs, although with more SGA children included in 1999–2000. More SGA children and more extreme criteria as regards GA in 1999–2000 should theoretically lead to worse outcomes, whereas the opposite was in fact observed. Thus, one may argue that this strengthens the notion that outcome did in fact improve for the average infant born preterm during the 1990s.
The field of neonatal intensive care is in constant change, and it has been suggested that pushing the limits of viability might have masked improvements in outcome [4], although some recent studies have indicated otherwise [12, 37]. We have previously reported respiratory health and lung function data for subjects born EP in the early 1980s and 1990s and suggested improvements with time in groups without neonatal BPD [13]. The present study which also included children born in this millennium, indicates that improvements has also occurred in children with BPD, i.e. the infants with the most turbulent neonatal history.
Conclusion
Eleven year old children born at extremely low GAs or BWs in 1999–2000 had more small-airway obstruction and bronchial hyperresponsiveness than matched term-controls, but lung volumes and diffusing capacity were similar. Outcome was mostly unrelated to BPD, and those born most immature did surprisingly well. Compared to a group born similarly preterm in the same region nearly one decade earlier, lung function was generally better, particularly after neonatal BPD. The findings indicate that infants born preterm in this millennium may have better pulmonary prognosis than previously assumed, and that BPD or extreme immaturity may not necessarily be linked to poor lung function in mid-childhood.
Supporting Information
(DOC)
Acknowledgments
We are indebted to Dr. P. B. Juliusson, Department of Pediatrics, Haukeland University Hospital and Department of Clinical Science, University of Bergen, for helping us converting height and weight data into z-scores for Norwegian children and adolescents, and to Prof. G.E. Eide, Center for Clinical Research, Haukeland University Hospital, and Department of Global Public Health and Primary Care, Lifestyle Epidemiology Research Group, University of Bergen, for help with statistical methods and interpretation.
Abbreviations
- BW
birthweight
- CANO
alveolar NO
- COPD
chronic obstructive pulmonary disease
- CPAP
continuous positive airway pressure
- DLCO
lung diffusion capacity for carbon monoxide
- DRS
dose-response-slope
- EP
preterm born participants of the present study
- ERS
European Respiratory Society
- FENO
fractional exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- FEF25–75
forced expiratory flow between 25 and 75% of vital capacity
- FRC
functional residual capacity
- FVC
forced vital capacity
- GA
gestational age
- Hb
haemoglobin
- JawNO
bronchial flux of NO
- KCO
diffusion capacity corrected for alveolar volume
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- PDA
persistent ductus arteriosus
- PD20
the dose methacholine required to lower FEV1 ≥ 20% of baseline level
- Raw
airway resistance
- ROP
retinopathy of prematurity
- RV
residual volume
- SGA
small for gestational age
- SPT
skin prick test
- TLC
total lung capacity
- VA
alveolar volume
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Major funding institutions were University of Bergen, Western Norway Regional Health Authority and Haukeland University Hospital. Minor support was provided by Pediatric Lung Research Fund, Haukeland University Hospital.
References
- 1. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–56. Epub 2010/08/25. 10.1542/peds.2009-2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Markestad T, Kaaresen PI, Ronnestad A, Reigstad H, Lossius K, Medbo S, et al. Early death, morbidity, and need of treatment among extremely premature infants. Pediatrics. 2005;115(5):1289–98. Epub 2005/05/04. 10.1542/peds.2004-1482 . [DOI] [PubMed] [Google Scholar]
- 3. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–9. Epub 2001/06/13. . [DOI] [PubMed] [Google Scholar]
- 4. Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–55. Epub 2007/11/09. 357/19/1946 [pii] 10.1056/NEJMra067279 . [DOI] [PubMed] [Google Scholar]
- 5. Krauss-Etschmann S, Bush A, Bellusci S, Brusselle GG, Dahlen SE, Dehmel S, et al. Of flies, mice and men: a systematic approach to understanding the early life origins of chronic lung disease. Thorax. 2013;68(4):380–4. Epub 2012/07/12. 10.1136/thoraxjnl-2012-201902 . [DOI] [PubMed] [Google Scholar]
- 6. Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):179–84. Epub 2006/07/25. 10.1053/j.semperi.2006.05.004 . [DOI] [PubMed] [Google Scholar]
- 7. Doyle LW. Respiratory function at age 8–9 years in extremely low birthweight/very preterm children born in Victoria in 1991–1992. Pediatr Pulmonol. 2006;41(6):570–6. Epub 2006/04/18. 10.1002/ppul.20412 . [DOI] [PubMed] [Google Scholar]
- 8. Korhonen P, Laitinen J, HyoUdynmaa E, Tammela O. Respiratory outcome in school-aged, very-low-birth-weight children in the surfactant era. Acta Paediatrica. 2007;93(3):316–21. 10.1111/j.1651-2227.2004.tb02954.x [DOI] [PubMed] [Google Scholar]
- 9. Filippone M, Bonetto G, Cherubin E, Carraro S, Baraldi E. Childhood course of lung function in survivors of bronchopulmonary dysplasia. JAMA. 2009;302(13):1418–20. Epub 2009/10/08. 10.1001/jama.2009.1419 . [DOI] [PubMed] [Google Scholar]
- 10. Lum S, Kirkby J, Welsh L, Marlow N, Hennessy E, Stocks J. Nature and severity of lung function abnormalities in extremely pre-term children at 11 years of age. Eur Respir J. 2011;37(5):1199–207. Epub 2010/10/16. 10.1183/09031936.00071110 . [DOI] [PubMed] [Google Scholar]
- 11. Kaplan E, Bar-Yishay E, Prais D, Klinger G, Mei-Zahav M, Mussaffi H, et al. Encouraging pulmonary outcome for surviving, neurologically intact, extremely premature infants in the postsurfactant era. Chest. 2012;142(3):725–33. Epub 2012/03/17. 10.1378/chest.11-1562 . [DOI] [PubMed] [Google Scholar]
- 12. Hacking DF, Gibson AM, Robertson C, Doyle LW. Respiratory function at age 8–9 after extremely low birthweight or preterm birth in Victoria in 1997. Pediatr Pulmonol. 2013;48(5):449–55. Epub 2012/07/25. 10.1002/ppul.22619 . [DOI] [PubMed] [Google Scholar]
- 13. Vollsaeter M, Roksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax. 2013. Epub 2013/06/12. 10.1136/thoraxjnl-2012-202980 . [DOI] [PubMed] [Google Scholar]
- 14. Gibson AM, Reddington C, McBride L, Callanan C, Robertson C, Doyle LW . Lung function in adult survivors of very low birth weight, with and without bronchopulmonary dysplasia. Pediatr Pulmonol. 2014. Epub 2014/09/10. 10.1002/ppul.23093 . [DOI] [PubMed] [Google Scholar]
- 15. Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147 e1–8. Epub 2007/02/20. 10.1016/j.ajog.2006.09.014 . [DOI] [PubMed] [Google Scholar]
- 16. Halvorsen T, Skadberg BT, Eide GE, Roksund OD, Markestad T. Better care of immature infants; has it influenced long-term pulmonary outcome? Acta Paediatr. 2006;95(5):547–54. Epub 2006/07/11. . [DOI] [PubMed] [Google Scholar]
- 17. Halvorsen T, Skadberg BT, Eide GE, Roksund O, Aksnes L, Oymar K. Characteristics of asthma and airway hyper-responsiveness after premature birth. Pediatr Allergy Immunol. 2005;16(6):487–94. Epub 2005/09/24. 10.1111/j.1399-3038.2005.00314.x . [DOI] [PubMed] [Google Scholar]
- 18. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351(9111):1225–32. Epub 1998/06/27. . [PubMed] [Google Scholar]
- 19. Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta obstetricia et gynecologica Scandinavica. 2000;79(6):440–9. Epub 2000/06/17. . [PubMed] [Google Scholar]
- 20. Juliusson PB, Roelants M, Nordal E, Furevik L, Eide GE, Moster D, et al. Growth references for 0–19 year-old Norwegian children for length/height, weight, body mass index and head circumference. Annals of human biology. 2013. Epub 2013/02/19. 10.3109/03014460.2012.759276 . [DOI] [PubMed] [Google Scholar]
- 21. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–22. Epub 2005/09/02. 10.1183/09031936.05.00035005 . [DOI] [PubMed] [Google Scholar]
- 22. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. Epub 2005/08/02. 10.1183/09031936.05.00034805 . [DOI] [PubMed] [Google Scholar]
- 23. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. Epub 2012/06/30. 10.1183/09031936.00080312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenthal M, Cramer D, Bain SH, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years: II—Single breath analysis and plethysmography. Thorax. 1993;48(8):803–8. Epub 1993/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–30. Epub 2005/04/09. 10.1164/rccm.200406-710ST . [DOI] [PubMed] [Google Scholar]
- 26. Hogman M. Extended NO analysis in health and disease. Journal of breath research. 2012;6(4):047103 Epub 2012/06/09. 10.1088/1752-7155/6/4/047103 . [DOI] [PubMed] [Google Scholar]
- 27. Nieminen MM, Lahdensuo A, Kellomaeki L, Karvonen J, Muittari A. Methacholine bronchial challenge using a dosimeter with controlled tidal breathing. Thorax. 1988;43(11):896–900. Epub 1988/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):309–29. Epub 2000/01/05. 10.1164/ajrccm.161.1.ats11-99 . [DOI] [PubMed] [Google Scholar]
- 29. O'Connor G, Sparrow D, Taylor D, Segal M, Weiss S. Analysis of dose-response curves to methacholine. An approach suitable for population studies. The American review of respiratory disease. 1987;136(6):1412–7. Epub 1987/12/01. 10.1164/ajrccm/136.6.1412 . [DOI] [PubMed] [Google Scholar]
- 30. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. Epub 2005/11/03. 10.1183/09031936.05.00035205 . [DOI] [PubMed] [Google Scholar]
- 31. Lydersen S, Fagerland MW, Laake P. Recommended tests for association in 2 x 2 tables. Statistics in medicine. 2009;28(7):1159–75. Epub 2009/01/27. 10.1002/sim.3531 . [DOI] [PubMed] [Google Scholar]
- 32. Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182(2):237–45. Epub 2010/04/10. 10.1164/rccm.200912-1806OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skromme K, Leversen KT, Eide GE, Markestad T, Halvorsen T. Respiratory illness contributed significantly to morbidity in children born extremely premature or with extremely low birth weights in 1999–2000. Acta Paediatr. 2015. Epub 2015/08/26. 10.1111/apa.13165 . [DOI] [PubMed] [Google Scholar]
- 34. Lamarche-Vadel A, Blondel B, Truffer P, Burguet A, Cambonie G, Selton D, et al. Re-hospitalization in infants younger than 29 weeks' gestation in the EPIPAGE cohort. Acta Paediatr. 2004;93(10):1340–5. Epub 2004/10/27. . [DOI] [PubMed] [Google Scholar]
- 35. Broughton S, Roberts A, Fox G, Pollina E, Zuckerman M, Chaudhry S, et al. Prospective study of healthcare utilisation and respiratory morbidity due to RSV infection in prematurely born infants. Thorax. 2005;60(12):1039–44. Epub 2005/10/18. 10.1136/thx.2004.037853 ; PubMed Central PMCID: PMCPmc1747273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kotecha SJ, Edwards MO, Watkins WJ, Henderson AJ, Paranjothy S, Dunstan FD, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax. 2013. Epub 2013/04/23. 10.1136/thoraxjnl-2012-203079 . [DOI] [PubMed] [Google Scholar]
- 37. Serenius F, Sjors G, Blennow M, Fellman V, Holmstrom G, Marsal K, et al. EXPRESS study shows significant regional differences in 1-year outcome of extremely preterm infants in Sweden. Acta Paediatr. 2014;103(1):27–37. Epub 2013/09/24. 10.1111/apa.12421 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.