Abstract
Optimum conditions for obtaining tetraploid were investigated in raphanobrassica, the intergeneric hybrid between radish (Raphanus sativus) and kale (Brassica oleracea var. acephala) by treating in vitro plants with an anti-mitotic agent, amiprophosmethyl (APM). Initially, no tetraploids but hexaploids and octaploids were induced by the treatments. Although the leaves of these polyploids of raphanobrassica showed chlorosis during subcultures in in vitro conditions, the chlorosis could be successfully prevented by the ethylene inhibitors, both AVG and AgNO3. Based on this result, AVG was added into medium used for the culture after the chromosome doubling treatment, which subsequently resulted in increased survival rates of the treated plant materials as well as increased production rates of polyploids including tetraploid. These polyploid plants showed obviously different characters from the original diploid plant. The tetraploid plant had bigger sizes in shoot, flower and leaf, and more number of leaves than the diploid. On the other hand, the hexaploid and octaploid plants had smaller sizes in shoots and leaves, and less number of leaves than the diploid. Concentration of glucosinolates, functional substances of Brassicaceae crops, did not significantly differ between diploid and tetraploid of raphanobrassica, but reduced in hexaploid and octaploid.
Keywords: amiprophos-methyl, aminoethoxyvinylglycine, glucosinolate, inter-generic hybrid, polyploid, Raphanobrassica
Introduction
The genus Raphanus includes two species, R. sativus and R. rapahnistrum. R. sativus is an important vegetable for harvesting root, seed capsule and leaf, whereas R. raphanistrum is a kind of invasive weed (Dellow et al. 2006). The genus Brassica includes many edible species; B. juncea, B. napus, B. oleracea, B. rapa, B. carinata and B. nigra, which are used as vegetables, oil-seeds or functional supplements. These Brassicaceae vegetables are known to have high amounts of functional substances such as glucosinolates (Becerra-Moreno et al. 2013, Cao et al. 1996, Ou et al. 2002). Especially, kale and radish have higher amounts of antioxidants compared to other vegetables (Fahey et al. 2001, Podsędek 2007). Inter-generic hybrid between radish (Raphanus sativus L., 2n = 2× = 18, RR genome) and kale (Brassica oleracea L. var. acephala D.C., 2n = 2× = 18, CC genome) categorized as Raphanobrassica, has been recognized to have two glucosinolates (GSL), i.e., kale specific component glucoraphanin (GRA) and radish specific component glucoraphenin (GRE) (Matthäus and Luftmann 2000, Takahata et al. 2006). GSL is hydrolyzed by myrosinase into isothiocyanate, which suppresses both growth of Helicobacter pylori and H. pylori-induced gastritis, detoxifies carcinogen, and inhibits the cell division of carcinoma (Bonnesen et al. 2001, Kim et al. 2011, Nakamura et al. 2002, Srivastava and Singh 2004, Talalay and Fahey 2001, Zhang et al. 1994). Therefore, demand for new Brassicaceae crops with higher amounts of GSL has now become one of the major concerns of the breeders.
The increase of the secondary metabolite production in induced polyploids was previously demonstrated in some medicinal species such as Atropa belladonna, Solanum khasianum and Artemisia annua (Banyai et al. 2010, Dhawan and Lavania 1996, Zhang et al. 2010). Effectiveness of polyploid production has also been reported for improving the useful characters of plants such as increased resistance against water stress, extended flower longevity and increase in organ size (Dhawan and Lavania 1996, Fox and Duronio 2013, Liu et al. 2011, Saleh et al. 2008). Recently, we confirmed that diploid raphanobrassica plants obtained by crossing radish with kale showed increased contents of GSL compared to both parents (unpublished results). Since these diploid raphanobrassica plants are completely sterile due to the allodiploid nature with RC genome, it is necessary to produce chromosome-doubled plants of the hybrids to restore the fertility for further use as a seed-propagated functional vegetable crop. In the present study, therefore, we aimed to establish the method for producing polyploids in raphanobrassica and to characterize their morphological traits and GSL contents.
Materials and Methods
Plant materials
Seeds of diploid raphanobrassica “2009 (10 × 48)” were obtained by crossing a cytoplasmic male sterile radish strain “2008-632” (Raphanus sativus L.) with a normal fertile kale strain “2010-91” (Brassica oleracea L. var. acephala D.C.), which has 20–30 times higher concentration of GRA than ordinary cultivars, at Nagano Vegetable and Ornamental Crops Experiment Station in Japan. These seeds were surface-sterilized with 0.2% sodium hypochlorite solution for 10 min and rinsed with sterile distilled water 3 times. They were then inoculated onto 1/2MS medium (8 g l−1 agar-solidified half-strength MS medium (Murashige and Skoog 1962) supplemented with 20 mg l−1 sucrose) and incubated at 25 ± 2°C under 16 h photoperiod at 30–40 μmol m−2 s−1 with cool white fluorescent lamps. Shoot tips with primary leaves were excised from one of the raphanobrassica seedlings and transferred to fresh 1/2MS medium for regenerating a plantlet, which was then subcultured for multiplication by transferring shoot segments with 2–3 nodes regularly to 1/2MS medium every month.
Induction of polyploids
For chromosome doubling, in vitro plantlets with 3 or 4 leaves obtained 2 weeks after the subculture were used for polyploidization. They were immersed in different concentrations (3, 6 and 9 mg l−1) of autoclaved APM solutions in culture bottles (Nihon Yamamura Glass Co., Ltd., Japan) and kept on the shaking incubator at 120 rpm. After the liquid shaking treatment for 24 hours, plantlets were washed with sterile distilled water three times and transferred to the fresh 1/2MS medium. One month after the treatment, the plantlets were cut into one nodal segments and transferred onto fresh 1/2MS medium for plantlet regeneration. Plantlets with 3–4 leaves thus obtained were sub-cultured for multiplication by cutting into 1 or 2 nodal segments and transferring onto 1/2MS medium every month. Survival of plantlets was confirmed 2 months after the APM treatment, whereas ploidy levels of plantlets were checked with newly growing leaves by flow cytometry 6 months after the treatment as described below. The polyploid plants thus detected were propagated and checked every 6 months for their stability of ploidy levels more than 2 years. Stable polyploids were acclimatized and grown in a greenhouse. To prevent the leaf chlorosis frequently observed during the in vitro subcultures, these polyploid plants were also cultured on 1/2MS medium supplemented with different concentrations of ethylene inhibitors, i.e., AVG at 0, 0.2, 1.0, 2.0 and 4.0 mg l−1 or silver nitrate (AgNO3) at 0, 0.2, 1.0, 2.0, 4.0 and 6.0 mg l−1. After one month, effect of the ethylene inhibitors on prevention of leaf chlorosis was evaluated as the percentage of the total number of chlorotic leaves per total leaves. Based on these results, chromosome doubling with APM treatments was again examined by culturing the plantlets on 1/2MS medium containing 2 mg l−1 AVG. After the treatment, the plantlets were subcultured in vitro, acclimatized and cultivation in greenhouse as described above.
Flow cytometric analysis
After the chromosome doubling treatment, ploidy levels of the treated plants were checked with newly growing leaves by flow cytometry according to the method of Mishiba and Mii (2000) with slight modification using a Partec PA cytometer equipped with a mercury lamp (Partec, GmbH, Münster, Germany). Ploidy levels were determined by comparing the position of dominant peaks at G0–G1 phase of the cell cycle between original diploid plant and the plants treated with APM. For staining released nuclei, about 0.1 g fresh weight tissues were chopped with a razor blade in 1.0 ml of solution composed of 100 mM Tris, 50 mM sodium citrate, 2 mM MgCl2, 1% (w/v) polyvinyl-pyrrolidone (PVP), 0.1% (v/v) Triton X-100 and 2 mg l−1 4,6-diamidino-2-phenylindole (DAPI), pH 7.5, in a plastic Petri dish (Galbraith et al. 1983), and filtered through a 30 μm nylon mesh. The suspension of nuclei was subjected to flow cytometric analysis for determining the relative nuclear DNA contents on a linear scale histogram.
Polyploid cultivation and transplantation
Polyploid plants derived from one seed were maintained in vitro for more than 2 years by subculturing the shoot segments every month on 1/2MS medium supplemented with 0.2 mg l−1 AVG for preventing leaf chlorosis at 25 ± 2°C under 16 h photoperiod. Polyploid plants were sufficiently washed to remove the adhering medium, transferred to commercial soil (Lixil Viva Co., Japan) in 9 cm pots, and grown in the greenhouse. After two weeks of acclimatization, these plants were transferred to 30 cm pots and were further grown in the greenhouse (December 2012 to March 2013). At flowering stage after 3 months of cultivation, plant height, leaf size, petiole length, stem size, and number of leaves were measured for phenotypic characterization.
Samples preparation and LC-MS/MS system for glucosinolate analysis
Glucosinolates were extracted according to the method of Takahata et al. (2006) with slight modifications. In each ploidy level of plants, 200 μg of freeze-dried leaves were powdered by Multi-beads shocker (Yasui Kikai Co., Japan), added with 1 ml of 80% methanol, and incubated for 10 min at 60°C for deactivation of myrosinase. Then samples were centrifuged with 15,000 rpm at 25°C for 5 min and supernatants were collected to new tubes. Extraction was further repeated twice for the residues, and totally 3 ml of methanol extracts were obtained for each ploidy sample. The crude extracts were applied to ion exchange column DEAE–Sephadex® A-25 (GE Healthcare Japan Co., Japan). The column was washed with 5 ml of distilled water and treated with 75 μl sulfatase at 25°C overnight for desulphonation. Samples containing GSL were eluted with 5 ml of distilled water and filtrated through 0.20 μm syringe filter unit (Merck Ltd., USA) before analysis. Contents of glucosinolate were measured by Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS) (LCMS-8030, Shimadzu, Japan). Glucoraphanin (Cayman Chemical Co., USA) and glucoraphenin potassium salt (PhytoLab GmbH & Co. KG) were used as standard substances. TSKgel ODS-100V 3 μm (column size 2.0 mm × 150 mm) (Tosoh Co., Japan) was used as the analytical column. TSKgel Guard Cartridge Holder and TSKgel guardgel ODS-100V 3 μm (column size 2.0 mm I.D. × 1.0 cm) (Tosoh co., Japan) were used as guard holder and cartridge, respectively. The mobile phase selected was (A) water and (B) acetonitrile (20%, v/v) applied at a flow rate of 1 ml min−1 in a gradient mode as follows: (i) 0 min (A/B: 100/0, v/v); (ii) 0–10 min (A/B: 10/90, v/v); (iii) 10–14 min (A/B: 100/0, v/v); (iv) 14–15 min (A/B: 100/0, v/v). The injection volume and column temperature were set at 2 μl and 35°C, respectively. The range of detection wavelength selected after examining the UV spectra collected was 190–340 nm as most of the glucosinolate absorption maxima were at this wavelength range. Full-scan LC–MS spectra were obtained by scanning from m z−1 100 to 1000. Compounds were identified by comparing the PDA, MS and MS/MS data (retention time, spectra, ions, and confirmation transition) with those obtained from pure standard solutions.
Results
Effect of APM on survival rate and chromosome doubling
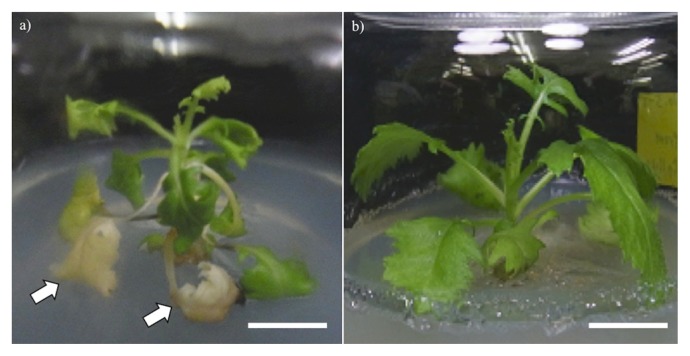
When in vitro plantlets were treated with APM for 24 h, survival rate of the explants evaluated 2 months after the treatment was clearly affected by the concentration of APM; 56% in the control, whereas 9, 1.3 and 0% at 3, 6 and 9 mg l−1, respectively (Table 1, Supplemental Fig. 1). Although no tetraploid was obtained at any APM concentrations, both hexaploid and octaploid plants were induced at the frequencies of 4.0 and 2.7%, respectively at 3 mg l−1, and only 1.3% hexaploid at 6 mg l−1 APM (Table 1). Shorter time treatment (12 h) failed to induce any polyploid (data not shown). These polyploid plants of raphanobrassica showed reduced growth rate with leaf curing and chlorosis compared to the control diploid plant during in vitro subcultures (Fig. 1a). Especially, propagation and maintenance of hexaploid plants were difficult due to slow and dwarf growth compared to octaploid plants.
Table 1.
Effects of APM on survival rate and chromosome doubling of raphanobrassica plantlets
| APM concentration (mg l−1) | Survival rate (%) | Ploidy level | A + B + C + D (%) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| A: Tetraploid (%) | B: Hexaploid (%) | C: Octaploid (%) | D: Mixoploid (%) | |||
| 0 | 56.0 ± 5.7 a | 0.0 | 0.0 c | 0.0 b | 0.0 | 0.0 c |
| 3 | 18.7 ± 1.9 b | 0.0 | 4.0 ± 0.0 a | 2.7 ± 0.5 a | 0.0 | 6.7 ± 1.9 a |
| 6 | 1.3 ± 1.9 c | 0.0 | 1.3 ± 0.5 b | 0.0 b | 0.0 | 1.3 ± 1.9 b |
| 9 | 0.0 d | 0.0 | 0.0 c | 0.0 b | 0.0 | 0.0 c |
Number of treated plantlets was 10 for each treatment and the experiment was repeated three times. Survival rate was evaluated two months after the treatment. Rate of each ploidy plantlets was calculated as (total number of corresponding ploidy plant-lets / total number of treated plantlets) × 100 four months after the treatment. Different letters indicated significant differences at p < 0.05, as determined by Tukey’s HSD test after arc-sine transformation of the data.
Fig. 1.
Effect of AVG on in vitro growth of hexaploid raphanobrassica plantlets. (a) The plantlet cultured for one month on 1/2MS medium showed leaf curing and chlorosis (arrows), whereas (b) that cultured on the same medium supplemented with 0.2 mg l−1 AVG grew normally. Bar = 10 mm.
Effects of ethylene inhibitors in medium on chlorosis
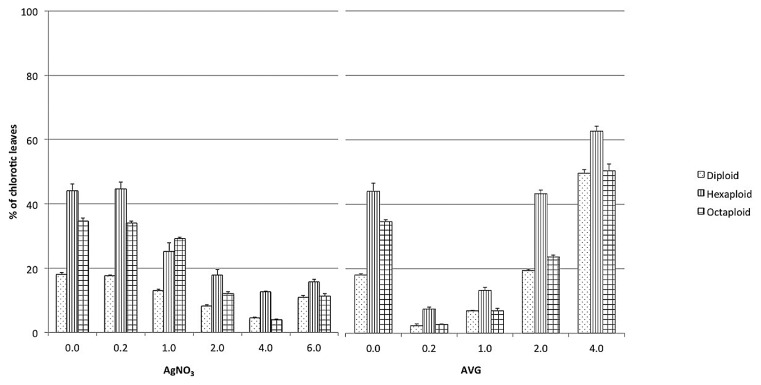
Provided that the leaf curing and chlorosis conspicuously observed in the polyploids were induced by the overproduction of ethylene in these plants, ethylene inhibitors, AgNO3 and AVG were added individually to the medium at different concentrations. As the result, both AgNO3 and AVG successfully prevented leaf chlorosis, and 0.2 mg l−1 AVG was found most effective for the suppression of chlorosis, whereas its higher concentrations (2–4 mg l−1) caused severe chlorosis (Fig. 2). Based on these results, polyploid plants were grown healthy and easily propagated by nodal cuttings on medium containing 0.2 mg l−1 AVG (Fig. 1b).
Fig. 2.
Effect of ethylene inhibitors on leaf chlorosis of in vitro polyploid plantlets. Prevention of leaf chlorosis was evaluated as (total number of chlorotic leaves / total leaves) × 100 (%) one month after transfer onto 1/2MS medium supplemented with (a) AgNO3 at 0, 0.2, 1.0, 2.0, 4.0, 6.0 mg l−1 or (b) AVG at 0, 0.2, 1.0, 2.0, 4.0 mg l−1, respectively. Number of plantlets used for each treatment was 10 and the experiment was repeated 3 times. Each value represents a mean ± S.E. of 3 independent experiments.
Chromosome doubling using medium containing AVG after AMP treatment
Since the growth of subcultured raphanobrassica plantlets was improved by adding ethylene inhibitors at appropriate concentrations, chromosome doubling with APM was also examined by using medium containing 0.2 mg l−1 AVG after the treatments. As the results, survival rates were dramatically increased by using AVG in all the APM treatments and more than half of the explants survived even after the treatment with the highest concentration (9 mg l−1) of APM (Table 2), which gave no survival of the explants without using AVG (Table 1). Polyploids including mixoploids were obtained at all the concentrations of APM treatments and gave 11–33% efficiencies (Table 2), which were almost 5 times higher than those obtained when AVG-free medium was used (Table 1), also clearly increased by using AVG at all the APM concentrations tested. Tetraploid plantlets were obtained for the first time by addition of AVG to the culture medium after APM treatment (Supplemental Fig. 1) and the highest tetraploid production rate (13.9%) was obtained at 6 mg l−1.
Table 2.
Effects of APM on survival rate and chromosome doubling efficiency of raphanobrassica plantlets using medium containing AVG after treating with different concentrations of APM
| APM concentration (mg l−1) | Survival rate (%) | Ploidy level | A + B + C + D (%) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| A: Tetraploid (%) | B: Hexaploid (%) | C: Octaploid (%) | D: Mixoploid (%) | |||
| 0 | 97.4 ± 3.6 a | 0.0 c | 0.0 b | 0.0 c | 0.0 c | 0.0 e |
| 3 | 94.7 ± 3.8 a | 5.3 ± 3.8 a | 8.1 ± 0.3 a | 8.1 ± 0.3 b | 10.9 ± 4.1 a | 32.5 ± 3.6 a |
| 6 | 75.6 ± 0.9 b | 13.9 ± 3.9 b | 8.3 ± 1.2 a | 0.0 c | 8.3 ± 0.3 b | 29.7 ± 3.6 a |
| 9 | 51.7 ± 11.6 c | 0.0 c | 0.0 b | 11.1 ± 7.9 a | 0.0 c | 11.1 ± 7.9 b |
After APM treatment, plantlets were cultured on 1/2MS medium containing 0.2 mg/l AVG. Number of plantlets used for each treatment was 10 and the experiment was repeated three times. Survival rate was evaluated two months after the treatment. Rate of each ploidy plantlets was calculated as (total number of corresponding ploidy plantlets / total number of treated plantlets) × 100 four months after the treatment. Different letters indicated significant differences at p < 0.05, as determined by Tukey’s HSD test after arc-sine transformation of the data.
Characteristics of polyploids
In in vitro culture conditions, morphology of tetraploid was not so different but had larger leaves compared to diploid plants. In contrast, hexaploid plant showed rosette-like stunted growth and had the smallest leaves among all the 4 ploidy levels (2x–8x) of plants (Fig. 3a, 3b). The highest frequency of rooting was observed in tetraploid plants, followed by diploid, hexaploid and octaploid plants (Fig. 3c). Three months after transfer to greenhouse, each ploidy level of plants showed similar trends in morphology and growth compared to those in in vitro conditions: tetraploid had larger plant size than diploid and octaploid and hexaploid showed the depressed growth and dwarf morphology (Table 3, Fig. 3d). Almost one month later, diploid plants attained to the flowering stage. However, other ploidy level of plants flowered later, i.e., one week after flowering of diploid plants in tetraploid and one month in both hexaploid and octaploid plants. Although octaploid had almost equal plant size to the original diploid, it had larger flower size than the diploid and comparable to the tetraploid (Fig. 3e, 3f). All the induced polyploids (4, 6, 8x) showed male sterility like as the male sterile female radish parent used for producing diploid raphanobrassica in the present study.
Fig. 3.
Morphology of polyploid plants. a) In vitro plantlets 2 weeks after the subculture. b) Leaves of in vitro plants two weeks after the subculture. c) Root formation of in vitro plantlets one month after the subculture. d) Three month-old plants after transfer the acclimatized plantlets to the greenhouse. e) and f) Flowers 4 months after transfer the acclimatized plantlets to the greenhouse. From left to right (a, b, d, e, f): diploid, tetraploid, hexaploid and octaploid plant, respectively. In (c), diploid (upper left), tetraploid (upper right), hexaploid (lower left) and octaploid (lower right).
Table 3.
Characterization of polyploid plants
| Ploidy level | Length of leaf blade (cm) | Width of leaf blade (cm) | Length of petiole (cm) | Width of petiole (cm) | Plant height (cm) | Width of stem (cm) | Number of leaves |
|---|---|---|---|---|---|---|---|
| Diploid | 22.0 ± 0.4 b | 11.2 ± 0.3 b | 3.1 ± 0.1 b | 1.2 ± 0.0 b | 12.6 ± 0.5 c | 3.4 ± 0.1 b | 13.0 ± 0.8 b |
| Tetraploid | 31.4 ± 1.2 a | 19.5 ± 2.5 a | 4.3 ± 0.1 a | 1.9 ± 0.4 a | 17.6 ± 0.5 b | 4.6 ± 0.0 a | 15.7 ± 0.5 a |
| Hexaploid | 8.9 ± 0.5 d | 4.5 ± 0.1 d | 2.0 ± 0.1 c | 1.1 ± 0.0 c | 11.0 ± 0.8 c | 4.3 ± 0.2 a | 5.3 ± 0.5 d |
| Octaploid | 13.7 ± 0.8 c | 5.8 ± 0.3 c | 1.5 ± 0.1 d | 1.9 ± 0.0 b | 33.3 ± 0.9 a | 4.1 ± 0.2 a | 9.7 ± 0.5 c |
Length and width of leaf blade, length and width of petiole, plant height and width of stem were measured 3 months after acclimatization. For each ploidy level, 10 plants were used for the measurements. Different letters indicate significant differences at p < 0.05, as determined by Tukey’s HSD test after arc-sine transformation of the data.
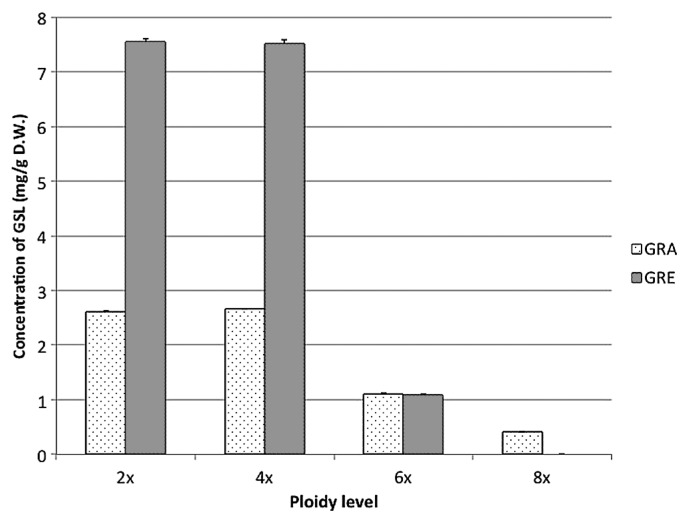
Original diploid raphanobrassica had both radish-derived GRE and kale-derived GRA as GSL, and the former showed 3 times higher concentration (760 μg/100 mg DW) than the latter (270 μg/100 mg DW) (Fig. 4). These values are almost comparable to those of parental materials: slightly lower content of GRE than that of radish (984 μg/100 mg DW) and slightly higher content of GRA than that of kale genotype used in the present study (195 μg/100 mg DW). Although tetraploid had almost the same concentrations of GSL as diploid, hexaploid and octaploid showed drastic reduction in the concentrations of GSL and no GRE was detected in octaploid (Fig. 4).
Fig. 4.
GSL contents in polyploid plants. GSL: glucoraphanin (GRA) and glucoraphenin (GRE) contents were measured with leaves of 3 month-old plants by LC-MS/MS. The data were analyzed from at least 8 samples of each polyploid plant.
Discussion
Based on the results for production of tetraploid kale (Niimi et al., submitting), in vitro cultured raphanobrassica plantlets were treated with APM for obtained polyploids in this study. Consequently, hexaploid and octaploid plants were induced at 3 and 6 mg l−1 APM but no tetraploid could be induced in this experiment. Although the reason for the unexpected hexaploid production is still unclear, we suspected that treatment time of 24 hours was too long and caused extra-round of DNA replications. Therefore, we also tried shorter time treatment (12 hours) but polyploid could not be induced (data not shown). The polyploid raphanobrassica plantlets showed leaf chlorosis during subcultures and were difficult to maintain and propagate in vitro. Especially, propagation and maintenance of hexaploid plants were difficult due to slow and stunted growth compared to diploid and octaploid plants. Chlorosis and abscission of leaves were sometimes reported to occur by ethylene production from the in vitro plants (Magdalita et al. 1997, Santana-Buzzy et al. 2006). In the present study, therefore, we added either AgNO3 or AVG to the culture medium and found that leaf chlorosis could be prevented by these ethylene inhibitors at appropriate concentrations, especially by AVG at 0.2 mg l−1. Moreover, AVG was found to be effective for the suppression of chlorosis for longer time compared to AgNO3 (data not shown). Consequently, AVG-treated plants were grown healthy and easily propagated by nodal cuttings. We therefore considered that the addition of AVG into a medium after chromosome doubling treatment might have favored for the survival and recover of the chromosome-doubled cells through the suppression of putative ethylene production from the APM-treated tissues, which resulted in the higher rates of both survival and polyploid production. Since hexa- and octaploid plants were obtained without adding AVG into the medium after APM treatment, it is possible that tetraploid cells are more sensitive to the ethylene production than hexa- and octaploid cells although the reason for the difference in the sensitivity is not clear. Although AVG treatment also induced chimeric plant (mixoploid) formation, flow cytometric analysis might be efficiently used to eliminate the mixoploids or to obtain stabilized polyploid plants by monitoring the ploidy level through the successive subcultures. These results suggest that the use of AVG after chromosome doubling treatment might be effective strategy for obtaining polyploids not only in the other Brassicaceae plants but also in the wide range of recalcitrant species.
It has been well known that polyploids generally show the alterations in plant morphology from its diploid counterpart (Otto and Whitton 2000), which was also found in raphanobrassica in the present study. For example, tetraploid showed the highest frequency of rooting and the biggest plant size, whereas hexaploid showed the most inferior growth. Since hexaploid plants were obtained irrespective of the use of AVG for the post-APM treatment culture medium, hexaploid induction by the mitotic inhibitor treatment might be a common phenomenon in raphanobrassica. To clarify the mechanism involved in the production of hexaploid, some experiments are now in progress to reveal the genomic constitution using GISH analysis and precise nuclear DNA contents with flow cytometric analysis of the hexaploid.
Raphanobrassica plants produced in the present study were revealed to have both GRE derived from radish and GRA from kale as GSL at almost comparable concentrations to those of both parents, suggesting the possibility that they could be utilized as a functional crop with these two important GSLs. The increase in the contents of secondary metabolite products in induced polyploids was previously demonstrated in Datura (Berkov 2001) and Solanum commersonii (Caruso et al. 2011). However, in the present study on raphanobrassica, GSL concentration per dry weight did not differ between diploid and tetraploid, and both hexaploid and octaploid showed reduced concentrations. These results suggest that GSL concentration could not be increased by increasing the ploidy level in raphanobrassica, although it might be necessary to evaluate other polyploids derived from different genotypes that are produced by the same intergeneric hybridization, for obtaining the general conclusion on the relationship between ploidy level and GSL concentration.
In conclusion, polyploid plants ranging from tetraploid to octaploid could be efficiently produced by APM treatment with the aid of AVG added to the culture medium used for the post-APM treatment. Among the 3 different ploidy levels of plants, tetraploid might be useful as functional vegetable due to larger biomass than and comparable GSL content to the original diploid plant. Although tetraploid raphanobrassica strain produced in the present study is male sterile due to the use of radish with cytoplasmic male sterility as a female parent, it might be utilized as the female parental line for F1 seed production of a novel functional vegetable crop at the tetraploid level although restoration of female fertility in the tetraploid needs to be confirmed by crossing with other tetraploid raphanobrassica with normal pollen fertility. By utilizing the chromosome doubling method established in the present study, production of the other tetraploid raphanobrassica strains with normal fertility is now in progress by utilizing the diploid radish strain with normal fertility for producing diploid raphanobrassica.
Supplementary Material
Literature Cited
- Banyai, W., Sangthong, R., Karaket, N., Inthima, P., Mii, M. and Supaibulwatana, K. (2010) Overproduction of artemisinin in tetraploid Artemisia annua L. Plant Biotechnol. 27: 427–433. [Google Scholar]
- Becerra-Moreno, A., Alanís-Garza, P.A., Mora-Nieves, J.L., Mora-Mora, J.P. and Jacobo-Velázquez, D.A. (2013) Kale: An excellent source of vitamin C, pro-vitamin A, lutein and glucosinolates. CyTA - J. Food 12: 298–303. [Google Scholar]
- Berkov, S. (2001) Size and alkaloid content of seeds in induced autotetraploids of Datura innoxia, Datura stramonium and Hyoscyamus niger. Pharm. Biol. 39: 329–331. [Google Scholar]
- Bonnesen, C., Eggleston, I.M. and Hayes, J.D. (2001) Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 61: 6120–6130. [PubMed] [Google Scholar]
- Cao, G., Sofic, E. and Prior, R.L. (1996) Antioxidant capacity of tea and common vegetables. J. Agric. Food Chem. 44: 3426–3431. [Google Scholar]
- Caruso, I., Lepore, L., De Tommasi, N., Dal Piaz, F., Frusciante, L., Aversano, R., Garramone, R. and Carputo, D. (2011) Secondary metabolite profile in induced tetraploids of wild solanum commersonii Dun. Chem. Biodivers. 8: 2226–2237. [DOI] [PubMed] [Google Scholar]
- Dellow, J.J., Storrie, A., Cheam, A.H., King, W.McG., Jacobs, S. and Kemp, D.R. (2006) Major brassicaceous weeds in Australian agriculture. In: Cheam, A.H. (ed.) Wild Radish and other Cruciferous Weeds: proceedings of a symposium held at the Department of Agriculture and Food Western Australia, South Perth, pp. 1–10. [Google Scholar]
- Dhawan, O.P. and Lavania, U.C. (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: A review. Euphytica 87: 81–89. [Google Scholar]
- Fahey, J.W., Zalcmann, A.T. and Talalay, P. (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56: 5–51. [DOI] [PubMed] [Google Scholar]
- Fox, D.T. and Duronio, R.J. (2013) Endoreplication and polyploidy: insights into development and disease. Development 140: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith, D.W., Harkins, K.R., Maddox, J.M., Ayres, N.M., Sharma, D.P. and Firoozabady, E. (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220: 1049–1051. [DOI] [PubMed] [Google Scholar]
- Kim, S.H., Nagalingam, A., Saxena, N.K., Singh, S.V. and Sharma, D. (2011) Benzyl isothiocyanate inhibits oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3. Carcinogenesis 32: 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., Chen, S., Chen, Y., Guan, Z., Yin, D. and Chen, F. (2011) In vitro induced tetraploid of Dendranthema nankingense (Nakai) Tzvel. shows an improved level of abiotic stress tolerance. Sci. Hortic. 127: 411–419. [Google Scholar]
- Magdalita, P.M., Godwin, I.D., Drew, R.A. and Adkins, S.W. (1997) Effect of ethylene and culture environment on development of papaya nodal cultures. Plant Cell Tiss. Org. Cult. 49: 93–100. [Google Scholar]
- Matthäus, B. and Luftmann, H. (2000) Glucosinolates in members of the family Brassicaceae: separation and identification by LC/ESI-MS-MS. J. Agric. Food Chem. 48: 2234–2239. [DOI] [PubMed] [Google Scholar]
- Mishiba, K. and Mii, M. (2000) Polysomaty analysis in diploid and tetraploid Portulaca grandiflora. Plant Sci. 156: 213–219. [DOI] [PubMed] [Google Scholar]
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497. [Google Scholar]
- Nakamura, Y., Kawakami, M., Yoshihiro, A., Miyoshi, N., Ohigashi, H., Kawai, K., Osawa, T. and Uchida, K. (2002) Involvement of the mitochondrial death pathway in chemopreventive benzyl isothiocyanate-induced apoptosis. J. Biol. Chem. 277: 8492–8499. [DOI] [PubMed] [Google Scholar]
- Otto, S.P. and Whitton, J. (2000) Polyploid incidence and evolution. Annu. Rev. Genet. 34: 401–437. [DOI] [PubMed] [Google Scholar]
- Ou, B., Huang, D., Hampsch-Woodill, M., Flanagan, J.A. and Deemer, E.K. (2002) Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 50: 3122–3128. [DOI] [PubMed] [Google Scholar]
- Podsędek, A. (2007) Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT - Food Sci. Technol. 40: 1–11. [Google Scholar]
- Saleh, B., Allario, T., Dambier, D., Ollitrault, P. and Morillon, R. (2008) Tetraploid citrus rootstocks are more tolerant to salt stress than diploid. CR Biol. 331: 703–710. [DOI] [PubMed] [Google Scholar]
- Santana-Buzzy, N., Canto-Flick, A., Iglesias-Andreu, L.G., Montalvo-Peniche, M.D.C., López-Puc, G. and Barabona-Pérez, F. (2006) Improvement of in vitro culturing of Habanero pepper by inhibition of ethylene effects. HortScience 41: 405–409. [Google Scholar]
- Srivastava, S.K. and Singh, S.V. (2004) Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis 25: 1701–1709. [DOI] [PubMed] [Google Scholar]
- Takahata, Y., Watanabe, M. and Watanabe, Y. (2006) Concentration and distribution pattern of glucosinolates in cruciferous vegetables. HortRes. 60: 63–66. [Google Scholar]
- Talalay, P. and Fahey, J.W. (2001) Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 131: 3027S–3033S. [DOI] [PubMed] [Google Scholar]
- Zhang, X.Y., Hu, C.G. and Yao, J.L. (2010) Tetraploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J. Plant Physiol. 167: 88–94. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Kensler, T.W., Cho, C.G., Posner, G.H. and Talalay, P. (1994) Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA 91: 3147–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.