Abstract
Plant breeding programs in local regions may generate genetic variations that are desirable to local populations and shape adaptability during the establishment of local populations. To elucidate genetic bases for this process, we proposed a new approach for identifying the genetic bases for the traits improved during rice breeding programs; association mapping focusing on a local population. In the present study, we performed association mapping focusing on a local rice population, consisting of 63 varieties, in Hokkaido, the northernmost region of Japan and one of the northern limits of rice cultivation worldwide. Six and seventeen QTLs were identified for heading date and low temperature germinability, respectively. Of these, 13 were novel QTLs in this population and 10 corresponded to the QTLs previously reported based on QTL mapping. The identification of QTLs for traits in local populations including elite varieties may lead to a better understanding of the genetic bases of elite traits. This is of direct relevance for plant breeding programs in local regions.
Keywords: genome-wide association mapping, local population, Oryza sativa L., plant breeding programs, rice
Introduction
The causal relationship between DNA polymorphisms and phenotypes among populations is the fundamental genetic force to improve traits in plant breeding programs. Genetic approaches have become important for identifying the genes for quantitative traits in natural variation. QTL mapping is a powerful method to identify chromosomal regions co-segregating with target traits in populations. Many important genes for domestication and agronomic traits have been identified by QTL analyses (Alonso-Blanco et al. 2009, Yamamoto et al. 2009). However, QTL mapping has some limitations in understanding the genetic architecture of traits involved in local populations. QTL mapping can identify the related genes to biparental variations. Mapping resolution depends on the number of recombinations occurred in the process of the development of mapping populations. Furthermore, suitable mapping populations for study is time consuming.
Genome wide association study (GWAS) has recently become popular for identifying QTLs. GWAS has the potential to overcome the limitations of QTL mapping. It has the ability to detect the genes involved in the population being analyzed. A genetically diverse population involves numerous recombinations. GWAS is advantageous for the identification of causal loci at a higher resolution than that of QTL mapping. Many aspects related to plant growth and development have been approached successfully using GWAS (Ogura and Busch 2015). Some factors must be considered to perform GWAS precisely; sample size, population composition, and statistical methods (Hamblin et al. 2011, Korte and Farlow 2013, Ogura and Busch 2015). The difficulties associated with GWAS have been attributed to genetic confounding and the complexity of genetic bases for traits among the population.
To overcome these genetic cues and identify the genes for traits, we herein proposed a new approach, genome-wide association mapping focusing on a local population derived from breeding programs in local regions. Most varieties in a population are involved in a single pedigree relationship, indicating that they may be genetically identical to the progenies derived from multiple cross combinations with the same genetic relationships. Association mapping focusing on such populations is beneficial for identifying QTLs. Such one advantage is a reduction in the genetic complexity and the genetic architecture of traits among the population, which makes it easy to perform statistical analyses of associations between genotypes and phenotypes. Another advantage is the precise evaluation of phenotypes. The expression of a phenotype is a result of a genotype under environmental conditions. These varieties have been bred in plant breeding programs in local regions and cultivated in the area, and, thus, have better adaptability to local environmental conditions.
In the present study, we performed association mapping focusing on a local rice population from Hokkaido, the northernmost region of Japan and one of the northern limits of rice cultivation worldwide. We previously reported the process underlying the establishment of this local population (Fujino et al. 2015, Shinada et al. 2014), which was divided into six genetic groups over the history of rice breeding programs in Hokkaido (Shinada et al. 2014). In the last two decades, the genetic base of the local population has markedly shifted to the current variety type (Fujino et al. 2015). The trace of insertions and deletions (indels) found in the variety Kitaake compared with the reference Nipponbare revealed that pre-existing mutations in wild rice with the A-genome were continuously introduced into the local population via ancestral populations. The rapid accumulation of pre-existing mutations may play major roles in establishing and shaping adaptability to local regions in current rice breeding programs.
We previously performed association analysis using candidate genes among a local population from Hokkaido. Hd5 for heading date and qLTG3-1 for low temperature germinability played important roles in variations in these traits among the population (Fujino et al. 2013, Fujino and Iwata 2011, Fujino and Sekiguchi 2011). To confirm whether the approach proposed in the present study is suitable for identifying genes, heading date and low temperature germinability were targeted. The results showed that this approach had great potential to identify the QTLs involved in the genetic bases of traits in plant breeding programs in local regions.
Materials and Methods
Plant material
The Hokkaido Rice Core Panel (HRCP) was used for the association analysis (Supplemental Table 1). HRCP included 63 landraces and breeding lines that represented genetic diversity among the local population in Hokkaido (Shinada et al. 2014). HRCP clearly differentiated into six genetic groups over the history of rice breeding programs. Varieties among HRCP were evenly distributed into these groups. Two F2 populations derived from crosses between varieties with different alleles at QTLs for the heading date were developed to confirm the results of the association analysis. One F2 population, HK, was derived from the cross between Hayayuki and Kyouwa. The other F2 population, NH, was derived from the cross between Nanatsuboshi and Honoka224. Seeds were provided by the Local Independent Administrative Agency Hokkaido Research Organization and Hokkaido Agricultural Research Center.
All rice varieties and F2 populations were cultivated in an experimental paddy field at Hokkaido Agricultural Research Center (Sapporo, Hokkaido, Japan, 43°00′N latitude) in 2012 and 2013, respectively. Sowing and transplanting were performed in late April and late May, respectively. Leaf samples of each plant were collected for DNA extraction.
Phenotype evaluation
Heading date was individually recorded and days to heading (DTH) of the earliest heading panicle among individuals was calculated for each plant as the number of days required from sowing to heading. In addition to DTH at Sapporo in 2012, DTH at Pippu in 2012 (43°51′N latitude) in our previous study (Shinada et al. 2014) was used for the association analysis. To evaluate low temperature germinability, seeds were incubated at 15°C in the dark as described previously (Fujino et al. 2004, 2008). The arc-sine transformation of the average of triplicates in germination rate on sixth day after the start of the incubation was used in the data analysis.
DNA analysis
Total DNA was isolated from young leaves using the CTAB method (Murray and Thompson 1980). Genotypes at 115 SSR marker loci including 63 markers in our previous study (Shinada et al. 2014) were analyzed. Furthermore, two markers linked to Hd5 and qLTG3-1 were used; 19DEL for the Hd5 gene controlling heading date (Fujino et al. 2013) and S103 for the qLTG3-1 gene controlling low temperature germinability (Fujino et al. 2008, Fujino and Sekiguchi 2011). PCR, electrophoresis, and sequencing were performed as described previously (Fujino et al. 2004, 2005).
Data analysis
An association analysis between genotype and phenotype was performed using the program FATESer (http://fateser.ist.hokudai.ac.jp/). The populations used in this study, HRCP, were closely related to each other. FATESer was originally developed to specifically elucidate associations among such populations. The genotypes from 117 markers and phenotypes of three traits were used. Statistical analyses were performed using the t-test, U-test, or Tukey’s test depending on normality/homogeneity of variance of the trait values and number of alleles at the SSR marker loci with the threshold P < 0.001. Minor alleles found in less than 5% were eliminated from the calculation to avoid statistical errors.
Results
Variations in heading date and low temperature germinability among HRCP
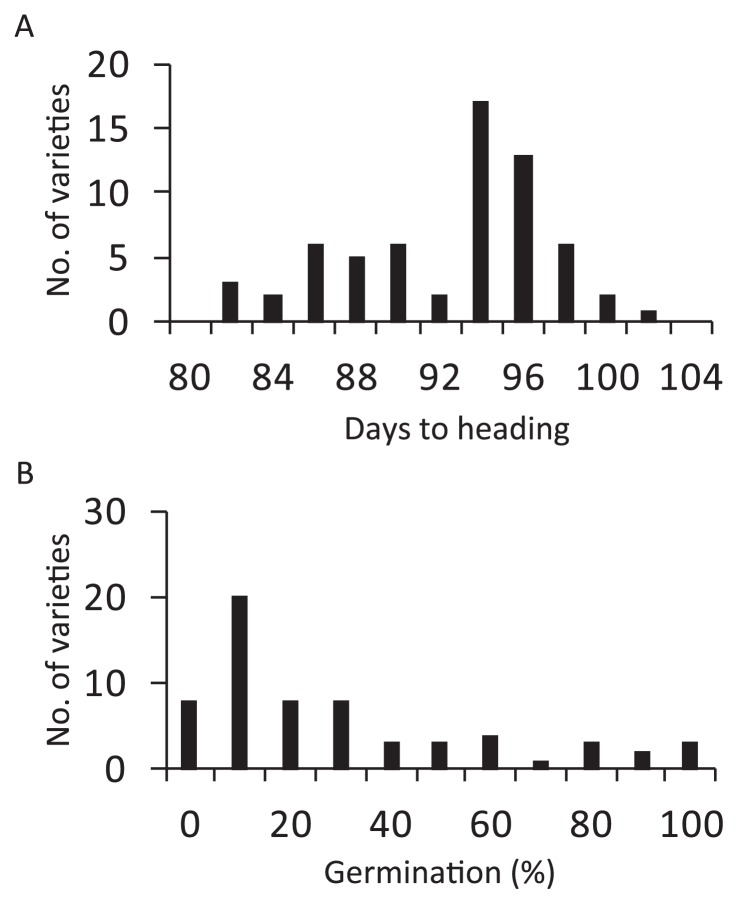
A wide variation in heading date was observed from 82.0 days of Norin No. 11, Norin No. 15, and Norin No. 19 to 101.5 of Minakuchiine (Fig. 1A). A single peak around 94–96 days was observed in HRCP involving the medium maturing variety Hoshinoyume (Fujino 2003), while that of the early maturing variety Kitaibuki was 89.3 days. Sixteen varieties exhibited an earlier heading date than that of Kitaibuki as an extremely early maturing type. The average of DTH in each genetic group were similar, 89.4–93.5 days, without significant difference (Table 1).
Fig. 1.
Frequency distributions of heading date (A) and low temperature germinability (B) among HRCP. The heading date is expressed by days from sowing to heading. Low temperature germinability is expressed by the germination rate at 15°C on sixth day after the start of the incubation.
Table 1.
Changes of average in days to heading (DTH) and low temperature germinability (LTG) during rice breeding programs in Hokkaido
| Group | n | DTH | LTG |
|---|---|---|---|
| I | 11 | 89.4 ± 6.0 a | 43.8 ± 23.9 a |
| II | 10 | 93.5 ± 5.0 a | 42.3 ± 17.9 a |
| IIIa | 12 | 91.9 ± 4.4 a | 36.8 ± 18.7 ab |
| IIIb | 6 | 91.2 ± 5.3 a | 17.9 ± 8.9 bc |
| IV | 10 | 90.7 ± 5.0 a | 7.0 ± 6.2 c |
| V | 13 | 92.6 ± 3.4 a | 7.8 ± 8.5 c |
Different letters indicate a significant difference at P < 0.05 by the Tukey test.
Low temperature germinability varied between 0% in eight varieties and 96.7% in Kuroge (Fig. 1B). Thirty-six varieties among HRCP exhibited weak low temperature germinability (less than 30%), while eight varieties exhibited vigorous low temperature germinability (more than 80%). The eight vigorous varieties belonged to groups I and II (Supplemental Table 1). The average of low temperature germinability significantly decreased during the rice breeding programs (Table 1).
Association mapping of heading date and low temperature germinability
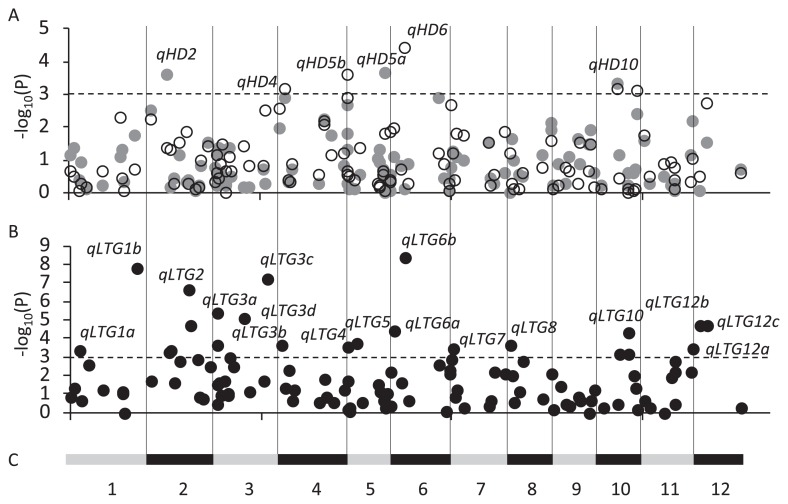
Six QTLs were identified for the heading date (Fig. 2A and Table 2). qHD2 and qHD5a were identified in the heading date at Sapporo, and the allelic difference at both loci was approximately 10 days. qHD4, qHD5b, and qHD6 were identified at Pippu, and allelic differences were 8.6, 4.7, and 4.2 days, respectively. qHD10 was identified at both places, and the allelic difference was approximately 5 days. Hd5 was previously shown to contribute to variations in the heading date among the varieties in Hokkaido (Fujino et al. 2013). However, no significant difference was detected at the marker 19DEL for Hd5 at either place. In this study, 16 varieties with the deletion allele at Hd5 as a nonfunction allele were used. The 16 varieties showed the wide range of DTH, 82.5–97.1 days. Among them, eight were common in the previous study. Because heading date is controlled by complex genetics, the genotype of heading date among the common varieties may be different from that of the remained varieties.
Fig. 2.
Genome-wide P values from FATESer. (A) Heading date. White and gray circles show data for the heading date in Pippu and Sapporo, respectively. (B) Low temperature germinability. (C) Chromosomal locations of the markers examined. Horizontal dotted bar indicates the threshold of probability (0.001%).
Table 2.
QTLs for heading date and low-temperature germinablity by an association analysis
| Trait | QTL | Marker | Chromosome | Position | P-value | Allele | QTLs reported | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||
| A | B | C | D | E | F | G | H | I | ||||||||||||||||
|
| ||||||||||||||||||||||||
| No | Mean ± SD | No | Mean ± SD | No | Mean ± SD | No | Mean ± SD | No | Mean ± SD | No | Mean ± SD | No | Mean ± SD | No | Mean ± SD | No | Mean ± SD | |||||||
| DTH Sapporo | qHD2 | RM6911 | 2 | 9,009,029 | 0.0002484 | 20 | 92.3 ± 4.1 ab | 10 | 90.9 ± 5.3 ab | 9 | 91.6 ± 4.5 ab | 6 | 84.6 ± 1.7 a | 17 | 93.5 ± 3.7 b | |||||||||
| qHD5a | RM3160 | 5 | 20,043,639 | 0.0002320 | 6 | 95.8 ± 2.9 a | 4 | 93.6 ± 3.3 ab | 8 | 88.8 ± 4.9 ab | 12 | 92.6 ± 4.5 ab | 15 | 94.1 ± 2.3 a | 5 | 85.1 ± 2.7 b | ||||||||
| qHD10 | RM3283 | 10 | 12,383,411 | 0.0004819 | 45 | 93.0 ± 4.2 | 18 | 88.5 ± 4.9 * | ||||||||||||||||
| DTH Pippu | qHD4 | RM5414 | 4 | 2,034,676 | 0.0007049 | 34 | 90.7 ± 4 ab | 13 | 91.7 ± 4 ab | 8 | 86.1 ± 3.6 a | 7 | 94.7 ± 4.8 b | |||||||||||
| qHD5b | RM1248 | 5 | 93,969 | 0.0002526 | 13 | 89.1 ± 2.6 ab | 25 | 88.9 ± 3.7 a | 24 | 93.6 ± 4.7 b | ||||||||||||||
| qHD6 | RM1169 | 6 | 7,661,599 | 0.0000368 | 41 | 92.2 ± 4.5 | 21 | 88.0 ± 2.9 * | Hd11) | |||||||||||||||
| qHD10 | RM3283 | 10 | 12,383,411 | 0.0007063 | 44 | 92 ± 4.2 | 18 | 87.8 ± 3.8 * | ||||||||||||||||
| LTG | qLTG1a | RM6451 | 1 | 4,797,375 | 0.0003922 | 19 | 40.6 ± 21.0 | 44 | 20.0 ± 20.0 * | qSD-12), Sdr63), qSD14), qDGE15) | ||||||||||||||
| qLTG1b | RM5501 | 1 | 34,548,947 | 0.0000000 | 22 | 43.5 ± 22.0 a | 10 | 31.9 ± 20.0 ab | 30 | 11.6 ± 10.0 b | qSD-16) | |||||||||||||
| qLTG2 | RM6165 | 2 | 19,374,071 | 0.0000002 | 50 | 31.0 ± 22.0 | 13 | 7.8 ± 8.5 * | ||||||||||||||||
| qLTG3a | S103 | 3 | 219,977 | 0.0000043 | 10 | 46.0 ± 25.0 a | 20 | 38.0 ± 18.0 a | 33 | 13.1 ± 13.0 b | qLTG3-17) | |||||||||||||
| qLTG3b | RM6676 | 3 | 14,494,362 | 0.0000071 | 50 | 30.7 ± 22.0 | 13 | 9.2 ± 10.0 * | ||||||||||||||||
| qLTG3c | RM3601 | 3 | 25,959,692 | 0.0000001 | 50 | 30.9 ± 22.0 | 12 | 6.3 ± 8.1 * | ||||||||||||||||
| qLTG3d | RM1038 | 3 | 33,660,363 | 0.0001982 | 34 | 14.8 ± 16.0 a | 6 | 34.4 ± 14.0 ab | 14 | 39.5 ± 22.0 b | 6 | 31.7 ± 15.0 ab | Sd3.28), qSV3-29) | |||||||||||
| qLTG4 | RM5879 | 4 | 35,112,390 | 0.0002563 | 30 | 30.3 ± 22.0 ab | 14 | 38.9 ± 21.0 a | 19 | 10.4 ± 12.0 b | ||||||||||||||
| qLTG5 | RM3777 | 5 | 4,113,575 | 0.0001960 | 8 | 51.4 ± 19.0 | 53 | 22.1 ± 19.0 * | qLTG-510), qPHS-511), Sdr212), qSD-52) | |||||||||||||||
| qLTG6a | RM1369 | 6 | 1,563,617 | 0.0000359 | 30 | 36.1 ± 21.0 a | 5 | 5.0 ± 4.9 ab | 20 | 11.3 ± 10.0 b | 4 | 24.5 ± 15.0 ab | grm6.113) | |||||||||||
| qLTG6b | RM1169 | 6 | 7,661,599 | 0.0000000 | 41 | 35.5 ± 21.0 | 22 | 9.0 ± 8.9 * | Sdr103), qSD614) | |||||||||||||||
| qLTG7 | RM5344 | 7 | 1,905,597 | 0.0003187 | 41 | 20.1 ± 20.0 a | 12 | 46.8 ± 16.0 b | 5 | 42.6 ± 22.0 ab | 5 | 10.8 ± 12.0 ab | ||||||||||||
| qLTG8 | RM5647 | 8 | 2,892,870 | 0.0002175 | 37 | 35.1 ± 23.0 a | 18 | 11.5 ± 12.0 b | 8 | 18.1 ± 16.0 ab | ||||||||||||||
| qLTG10 | RM1125 | 10 | 17,842,706 | 0.0006376 | 12 | 12.4 ± 15.0 a | 21 | 39.2 ± 21.0 b | 27 | 22.1 ± 19.0 ab | qGR-1015) | |||||||||||||
| qLTG12a | RM1880 | 12 | 747,745 | 0.0003380 | 6 | 51.9 ± 18.0 a | 17 | 14.5 ± 18.0 b | 7 | 30.4 ± 23.0 ab | 4 | 46.3 ± 20.0 a | 17 | 15.9 ± 10.0 b | 6 | 39.5 ± 16.0 ab | ||||||||
| qLTG12b | RM7619 | 12 | 4,829,808 | 0.0000191 | 44 | 32.7 ± 21.0 a | 4 | 16.6 ± 12.0 ab | 14 | 5.3 ± 6.8 b | ||||||||||||||
| qLTG12c | RM1036 | 12 | 8,797,117 | 0.0000192 | 13 | 41.4 ± 20.0 a | 22 | 14.7 ± 13.0 b | 15 | 40.7 ± 20.0 a | 12 | 8.8 ± 9.3 b | ||||||||||||
indicates the significant difference at P < 0.001 by t-test. Different alphabets indicate the significant difference at P < 0.001 by Tukey test.
QTLs are defined by the markers showing the lowest P value within the 1 Mb region.
Alleles with more than 3 varieties were used for the calculation.
Seventeen QTLs were identified for low temperature germinability (Fig. 2B and Table 2). The largest allelic difference was detected at qLTG12a, 37.4 between 51.9 of allele A and 14.5 of allele B in an arc-sine transformation value of the germination rate. At other QTLs, allelic differences varied from 20.6 at qLTG1a to 32.6 at qLTG12c. We previously reported that qLTG3-1 contributed to variations in low temperature germinability among the varieties in Hokkaido (Fujino and Iwata 2011). A significant association was detected at the marker S103 for qLTG3-1, qLTG3a.
Validation of association mapping results
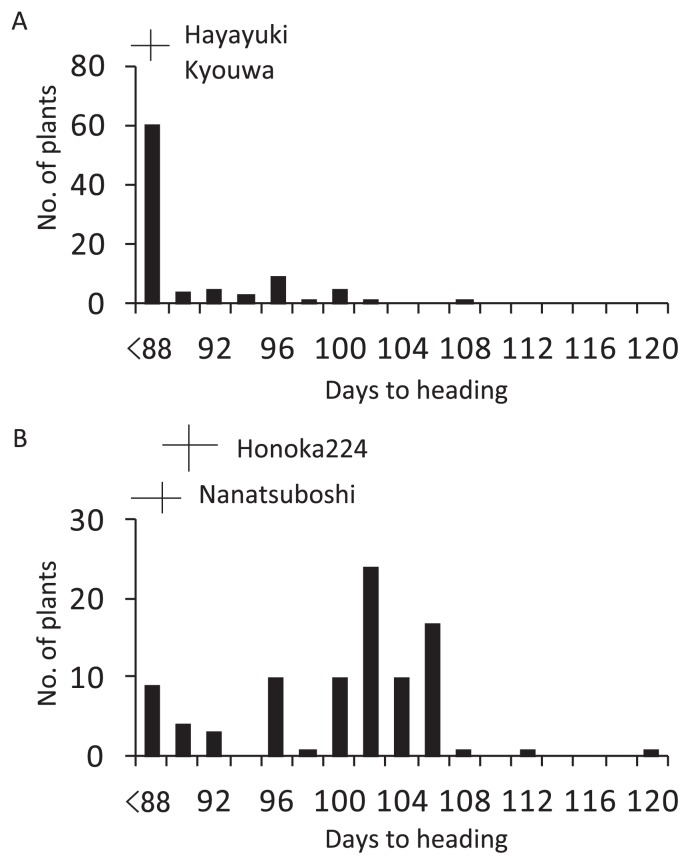
A linkage analysis was carried out using two F2 populations to validate the results of association mapping for the heading date. Two F2 populations were developed based on the genotypes of the six QTLs for the heading date identified in this study (Table 3). In HK F2 population, most plants showed similar heading dates to the parental varieties and a small number of late transgressive segregants were noted (Fig. 3A). Among the six QTLs for the heading date, allelic differences between the parental varieties were observed at a single locus, qHD5b, (Table 3). A significant difference was detected at qHD5b (Table 4). The Hayayuki allele (88.9 days) showed an earlier heading date than that of the Kyouwa allele (93.6 days).
Table 3.
Genotype and DTH of parental varieties for the linkage analysis
| QTL | qHD2 | qHD4 | qHD5a | qHD5b | qHD6 | Hd5 | qHD10 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr | 2 | 4 | 5 | 5 | 6 | 9 | 10 | ||||
| DTH | Marker | RM6911 | RM5414 | RM3160 | RM1248 | RM1169 | 19DEL | RM3283 | |||
|
|
|||||||||||
| Population | Parental varieties | Sapporo | Pippu | ||||||||
| HK | Hayayuki | 85.2 | 89 | C | D | G | B * | A | A | B | |
| Kyouwa | 94.6 | 97 | B | A | E | C | A | A | B | ||
| NH | Nanatsuboshi | 95.4 | 92 | A | A | H | B * | B * | A | A | |
| Honoka224 | 97.1 | 97 | E | B | H | C | A | B | A | ||
indicates alleles with a significant difference by the association analysis.
Fig. 3.
Frequency distributions of the heading date in F2 populations derived from crosses between Hayayuki and Kyouwa (A) and between Nanatsuboshi and Honoka224 (B). The vertical and horizontal bars indicate the mean and range of varieties, respectively.
Table 4.
QTLs of the heading date in F2 populations as determined by the t-test
| Combination (Parent 1/Parent 2) | Marker | QTL | Mean of DTH | Probability | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Parent 1 | Parent 2 | Heterozygous | |||||||
|
|
|
|
|||||||
| n | Mean | n | Mean | n | Mean | ||||
| Hayayuki/Kyouwa | RM1248 | qHD5b | 20 | 88.9 | 18 | 93.6 | 39 | 89.5 | 0.0005 |
| Nanatsuboshi/Honoka224 | RM1248 | qHD5b | 23 | 101.0 | 23 | 101.8 | 41 | 98.4 | 0.6510 |
| RM1169 | qHD6 | 26 | 100.5 | 19 | 99.5 | 45 | 100.3 | 0.5890 | |
| 19DEL | Hd5 | 23 | 102.5 | 30 | 96.1 | 33 | 102.5 | 0.0000 | |
Probability shows the difference between the mean of Parent 1 and 2 types.
Although the parental varieties showed a similar heading date, a wide variation was observed from 88 to 120 days, including many late transgressive segregants in NH F2 population (Fig. 3B). Among the six QTLs for the heading date, allelic differences between the parental varieties were observed at two loci, qHD5b and qHD6 (Table 3). No significant differences were detected at the two loci (Table 4). Hd5 was examined in addition to these two QTLs because Nanatsuboshi and Honoka224 carry wild type and loss-of-function type alleles at Hd5, respectively. The Honoka224 allele at Hd5 locus expressed a significantly earlier heading date (96.1 days) compared with the Nanatsuboshi allele (102.5 days) (Table 4).
Discussion
Genomes in local populations are structured by artificial selection of the genotype × environmental conditions in recurrent cycles of hybridizations among local populations or with an exotic germplasm during plant breeding programs (Shinada et al. 2014). These processes may generate genetic variations that are desirable to local populations and shape adaptability during the establishment of local populations (Fujino et al. 2015). One of the most important objectives in plant breeding programs is the control of adaptability to local environmental conditions. In the present study, we proposed a new approach, association mapping focusing on a local population, to identify the genetic bases for traits improved during rice breeding programs.
Plant breeding programs generate intensive selection pressures that focus on shaping adaptability to local environmental conditions, cultivation methods, and market demands. The establishment of local populations reflects the combination of pre-existing mutations widely distributed throughout wild rice into the local population via cultivated rice over the world (Fujino et al. 2015). These selections have restricted genetic diversity among local populations, establishing an ideotype for the objectives of current breeding programs (Dilday 1990, Fu et al. 2003, Le Clerc et al. 2005, Roussel et al. 2005, Yamamoto et al. 2010). This genetic feature may enhance association analyses to eliminate the complexity of genetic bases, population structure, and genetic architecture of traits among the population.
Association mapping focusing on a local population in this study identified QTLs for heading date and low temperature germinability (Table 2). Of the 17 QTLs for low temperature germinability, nine were novel QTLs in this population and eight corresponded to QTLs previously reported based on QTL analysis. qLTG3a in the present study was co-localized with the gene qLTG3-1 for low temperature germinability (Fujino et al. 2008). This gene is known to play an important role in variations in low temperature germinability among the varieties in Hokkaido (Fujino and Iwata 2011, Fujino and Sekiguchi 2011). qLTG5 in the present study was co-localized with QTLs for low temperature germinability and seed dormancy (Dong et al. 2003, Lin et al. 1998, Miura et al. 2001, 2002). The remaining six loci were co-localized with the QTLs for seed dormancy (Table 2). The allelic difference in qLTG3-1 between Nipponbare and Koshihikari may have been associated with differences in both seed dormancy and low temperature germinability (Hori et al. 2010). These results also demonstrated the potentially close relationship between low temperature germinability and seed dormancy.
Of the six QTLs for the heading date, four were novel QTLs and two corresponded to QTLs previously reported (Table 2). qHD6 in the present study was co-localized with the gene Hd1 for the heading date, rice orthologue CONSTANS (Yano et al. 2000). QTLs for the heading date have been identified at the same region in the population derived from crosses between varieties in Hokkaido (Fujino and Sekiguchi 2008). Furthermore, the existence of qHD5b for the heading date was confirmed by genetic analyses (Table 4). In this chromosomal region, major QTLs for the heading date were identified among Asian rice accessions (Hori et al. 2015). These results strongly indicated that the approach proposed in the present study, association mapping focusing on a local population, is suitable to detect the genes involved in local populations.
This approach proposed herein may identify not only genes for traits improved by rice breeding programs, but also alleles for the traits in each variety among the population. In the present study, two populations were developed based on the genotype of the heading date (Table 3). qHD6 was not confirmed by a genetic analysis and Hd5 was not detected in an association analysis (Tables 2, 3). Based on the segregation pattern, gene interactions such as epistasis were involved in the NH population (Fig. 3). The genotypes of the genes for the resulting traits indicated that association mapping supports screening suitable experimental materials for developing mapping populations.
In rice breeding programs, the selection for desirable traits is completed based on the evaluation of phenotypes. This process may generate a genetic force to change the genetic compositions of local populations. Allelic frequencies at the loci for the heading date clearly correlated with the history of rice breeding programs in Hokkaido. The alleles B at both loci, RM1248 and RM1169 linked to qHD5b and qHD6, respectively, was predominant in group V (Table 5). Allele B at RM1248 was predominant in groups I and II, but all varieties in groups I and II carried allele A at RM1169. Although no significant difference in the average heading date was detected between groups (Table 1), the combinations of favorable alleles in different loci in each group may lead to a desirable phenotype during rice breeding programs.
Table 5.
Distribution of alleles at marker loci RM1248 and RM1169 linked to qHD5b and qHD6, respectively, for the heading date among HRCP
| Group | Allele | ||||
|---|---|---|---|---|---|
|
| |||||
| RM1248 | RM1169 | ||||
|
|
|
||||
| A | B | C | A | B | |
| I | 5 | 6 | 0 | 11 | 0 |
| II | 0 | 4 | 4 | 8 | 0 |
| IIIa | 1 | 1 | 11 | 12 | 1 |
| IIIb | 2 | 0 | 4 | 6 | 0 |
| IV | 5 | 2 | 4 | 4 | 7 |
| V | 0 | 12 | 1 | 0 | 13 |
Many genes/QTLs for agronomic traits have been identified using GWAS in rice (Begum et al. 2015, Huang et al. 2010, 2012, Yang et al. 2014, Zhao et al. 2011). A set of varieties with abundant genetic diversity were used in these studies, including japonica and indica. In contrast, varieties that were not so rich in genetic diversity were used in the proposed approach, which may identify local population-specific genes or alleles related to elite traits. The identification of QTLs for the traits in local populations including elite varieties may provide a clearer understanding of the genetic bases of elite traits. This is of direct relevance for plant breeding programs in local regions. Association mapping focusing on a local population proposed in the present study may identify genes for elite traits at a high resolution using high-density genetic markers such as SNPs.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry) and JSPS KAKENHI Grant Number 25450015.
Literature Cited
- Alonso-Blanco, C., Aarts, M.G.M., Bentsink, L., Keurentjes, J.J.B., Reymond, M., Vreugdenhil, D. and Koornneef, M. (2009) What has natural variation taught us about plant development, physiology, and adaptation? Plant Cell 21: 1877–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum, H., Spindel, J.E., Lalusin, A., Borromeo, T., Gregorio, G., Hernandez, J., Virk, P., Collard, B. and McCouch, S.R. (2015) Genome-wide association mapping for yield and other agronomic traits in an elite breeding population of tropical rice (Oryza sativa). PLoS One 10: e0119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilday, R.H. (1990) Contribution of ancestral lines in the development of new cultivars of rice. Crop Sci. 30: 905–911. [Google Scholar]
- Dong, Y., Tsuzuki, E., Kamiunten, H., Terao, H., Lin, D., Matsuo, M. and Zheng, Y. (2003) Identification of quantitative trait loci associated with pre-harvest sprouting resistance in rice (Oryza sativa L.). Field Crops Res. 81: 133–139. [Google Scholar]
- Fu, Y.B., Peterson, G.W., Scoles, G., Rossnagel, B., Schoen, D.J. and Richards, K.W. (2003) Allelic diversity changes in 96 Canadian oat cultivars released from 1886 to 2001. Crop Sci. 43: 1989–1995. [Google Scholar]
- Fujino, K. (2003) Photoperiod sensitivity gene controlling heading date in rice cultivars in the northernmost region of Japan. Euphytica 131: 97–103. [Google Scholar]
- Fujino, K., Sekiguchi, H., Sato, T., Kiuchi, H., Nonoue, Y., Takeuchi, Y., Ando, T., Lin, S.Y. and Yano, M. (2004) Mapping of quantitative trait loci controlling low-temperature germinability in rice (Oryza sativa L.). Theor. Appl. Genet. 108: 794–799. [DOI] [PubMed] [Google Scholar]
- Fujino, K., Sekiguchi, H. and Kiguchi, T. (2005) Identification of an active transposon in intact rice plants. Mol. Genet. Genomics 273: 150–157. [DOI] [PubMed] [Google Scholar]
- Fujino, K. and Sekiguchi, H. (2008) Mapping of quantitative trait loci controlling heading date among rice cultivars in the northernmost region of Japan. Breed. Sci. 58: 367–373. [Google Scholar]
- Fujino, K., Sekiguchi, H., Matsuda, Y., Sugimoto, K., Ono, K. and Yano, M. (2008) Molecular identification of a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Proc. Natl. Acad. Sci. USA 105: 12623–12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino, K. and Sekiguchi, H. (2011) Origins of functional nucleotide polymorphisms in a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Plant Mol. Biol. 75: 1–10. [DOI] [PubMed] [Google Scholar]
- Fujino, K. and Iwata, N. (2011) Selection for low-temperature germinability on the short arm of chromosome 3 in rice cultivars adapted to Hokkaido, Japan. Theor. Appl. Genet. 123: 1089–1097. [DOI] [PubMed] [Google Scholar]
- Fujino, K., Yamanouchi, U. and Yano, M. (2013) Roles of Hd5 gene controlling heading date for adaptation to the northern limits of rice cultivation. Theor. Appl. Genet. 126: 611–618. [DOI] [PubMed] [Google Scholar]
- Fujino, K., Obara, M., Ikegaya, T. and Tamura, K. (2015) Genetic shift in local rice populations during rice breeding programs in the northern limit of rice cultivation in the world. Theor. Appl. Genet. 128: 1739–1746. [DOI] [PubMed] [Google Scholar]
- Gu, X.Y., Kianian, S.F. and Foley, M.E. (2004) Multiple loci and epistases control genetic variation for seed dormancy in weedy rice (Oryza sativa). Genetics 166: 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, X.Y., Kianian, S.F. and Foley, M.E. (2006) Isolation of three dormancy QTLs as Mendelian factors in rice. Heredity 96: 93–99. [DOI] [PubMed] [Google Scholar]
- Hamblin, M.T., Buckler, E.S. and Jannink, J.L. (2011) Population genetics of genomics-based crop improvement methods. Trends Genet. 27: 98–106. [DOI] [PubMed] [Google Scholar]
- Hori, K., Sugimoto, K., Nonoue, Y., Ono, N., Matsubara, K., Yamanouchi, U., Abe, A., Takeuchi, Y. and Yano, M. (2010) Detection of quantitative trait loci controlling pre-harvest sprouting resistance by using backcrossed populations of japonica rice cultivars. Theor. Appl. Genet. 120: 1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, K., Nonoue, Y., Ono, N., Shibaya, T., Ebana, K., Matsubara, K., Ogiso-Tanaka, E., Tanabata, T., Sugimoto, K., Taguchi-Shiobara, F.et al. (2015) Genetic architecture of variation in heading date among Asian rice accessions. BMC Plant Biol. 15: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X., Wei, X., Sang, T., Zhao, Q., Feng, Q., Zhao, Y., Li, C., Zhu, C., Lu, T., Zhang, Z.et al. (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 42: 961–967. [DOI] [PubMed] [Google Scholar]
- Huang, X., Zhao, Y., Wei, X., Li, C., Wang, A., Zhao, Q., Li, W., Guo, Y., Deng, L., Zhu, C.et al. (2012) Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat. Genet. 44: 32–39. [DOI] [PubMed] [Google Scholar]
- Ji, S.L., Jiang, L., Wang, Y.H., Zhang, W.W., Liu, X., Liu, S.J., Chen, L.M., Zhai, H.Q. and Wan, J.M. (2009) Quantitative trait loci mapping and stability for low temperature germination ability of rice. Plant Breed. 128: 387–392. [Google Scholar]
- Korte, A. and Farlow, A. (2013) The advantages and limitations of trait analysis with GWAS: a review. Plant Methods 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clerc, V., Bazante, F., Baril, C., Guiard, J. and Zhang, D. (2005) Assessing temporal changes in genetic diversity of maize varieties using microsatellite markers. Theor. Appl. Genet. 110: 294–302. [DOI] [PubMed] [Google Scholar]
- Lee, S.J., Oh, C.S., Suh, J.P., McCouch, S.R. and Ahn, S.N. (2005) Identification of QTLs for domestication-related and agronomic traits in an Oryza sativa × O. rufipogon BC1F7 population. Plant Breed. 124: 209–219. [Google Scholar]
- Li, W., Xu, L., Bai, X. and Xing, Y. (2011) Quantitative trait loci for seed dormancy in rice. Euphytica 178: 427–435. [Google Scholar]
- Lin, S.Y., Sasaki, T. and Yano, M. (1998) Mapping quantitative trait loci controlling seed dormancy and heading date in rice, Oryza sativa L., using backcross inbred lines. Theor. Appl. Genet. 96: 997–1003. [Google Scholar]
- Marzougui, S., Sugimoto, K., Yamanouchi, U., Shimono, M., Hoshino, T., Hori, K., Kobayashi, M., Ishiyama, K. and Yano, M. (2012) Mapping and characterization of seed dormancy QTLs using chromosome segment substitution lines in rice. Theor. Appl. Genet. 124: 893– 902. [DOI] [PubMed] [Google Scholar]
- Miura, K., Lin, S.Y., Yano, M. and Nagamine, T. (2001) Mapping quantitative trait loci controlling low temperature germinability in rice (Oryza sativa L.). Breed. Sci. 51: 293–299. [Google Scholar]
- Miura, K., Lin, S.Y., Yano, M. and Nagamine, T. (2002) Mapping quantitative trait loci controlling seed longevity in rice (Oryza sativa L.). Theor. Appl. Genet. 104: 981–986. [DOI] [PubMed] [Google Scholar]
- Murray, M.G. and Thompson, W.F. (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8: 4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura, T. and Busch, W. (2015) From phenotypes to causal sequences: using genome wide association studies to dissect the sequence basis for variation of plant development. Curr. Opin. Plant Biol. 23: 98–108. [DOI] [PubMed] [Google Scholar]
- Roussel, V., Leisova, L., Exbrayat, F., Stehno, Z. and Balfourier, F. (2005) SSR allelic diversity changes in 480 European bread wheat varieties released from 1840 to 2000. Theor. Appl. Genet. 111: 162–170. [DOI] [PubMed] [Google Scholar]
- Shinada, H., Yamamoto, T., Yamamoto, E., Hori, K., Yonemaru, J., Matsuba, S. and Fujino, K. (2014) Historical changes in population structure during rice breeding programs in the northern limits of rice cultivation. Theor. Appl. Genet. 127: 995–1004. [DOI] [PubMed] [Google Scholar]
- Thomson, M.J., Tai, T.H., McClung, A.M., Lai, X.H., Hinga, M.E., Lobos, K.B., Xu, Y., Martinez, C.P. and McCouch, S.R. (2003) Mapping quantitative trait loci for yield, yield components and morphological traits in an advanced backcross population between Oryza rufipogon and the Oryza sativa cultivar Jefferson. Theor. Appl. Genet. 107: 479–493. [DOI] [PubMed] [Google Scholar]
- Wan, J.M., Cao, Y.J., Wang, C.M. and Ikehashi, H. (2005) Quantitative trait loci associated with seed dormancy in rice. Crop Sci. 45: 712–716. [Google Scholar]
- Yamamoto, T., Yonemaru, J. and Yano, M. (2009) Towards the understanding of complex traits in rice: substantially or superficially? DNA Res. 16: 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, T., Nagasaki, H., Yonemaru, J., Ebana, K., Nakajima, M., Shibaya, T. and Yano, M. (2010) Fine definition of the pedigree haplotypes of closely related rice cultivars by means of genome-wide discovery of single-nucleotide polymorphisms. BMC Genomics 11: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W., Guo, Z., Huang, C., Duan, L., Chen, G., Jiang, N., Fang, W., Feng, H., Xie, W., Lian, X.et al. (2014) Combining high-throughput phenotyping and genome-wide association studies to reveal natural genetic variation in rice. Nat. Commun. 5: 5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano, M., Katayose, Y., Ashikari, M., Yamanouchi, U., Monna, L., Fuse, T., Baba, T., Yamamoto, K., Umehara, Y., Naganura, Y.et al. (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12: 2473–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z.H., Qu, X.S., Wan, S., Chen, L.H. and Zhu, Y.G. (2005) Comparison of QTL controlling seedling vigour under different temperature conditions using recombinant inbred lines in rice (Oryza sativa). Ann. Bot. 95: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, K., Tung, C.W., Eizenga, G.C., Wright, M.H., Ali, M.L., Price, A.H., Norton, G.J., Islam, M.R., Reynolds, A., Mezey, J.et al. (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat. Commun. 2: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.