Abstract Abstract
Paxillin is a multifunctional and multidomain focal adhesion adaptor protein. It serves as an important scaffolding protein at focal adhesions by recruiting and binding to structural and signaling molecules. Paxillin tyrosine phosphorylation at Y31 and Y118 is important for paxillin redistribution to focal adhesions and angiogenesis. Hepatocyte growth factor (HGF) and sphingosine-1-phosphate (S1P) are potent stimulators of lamellipodia formation, a prerequisite for endothelial cell migration. The role played by paxillin and its tyrosine phosphorylated forms in HGF- or S1P-induced lamellipodia formation and barrier function is unclear. HGF or S1P stimulated lamellipodia formation, tyrosine phosphorylation of paxillin at Y31 and Y118, and c-Abl in human lung microvascular endothelial cells (HLMVECs). Knockdown of paxillin with small interfering RNA (siRNA) or transfection with paxillin mutants (Y31F or Y118F) mitigated HGF- or S1P-induced lamellipodia formation, translocation of p47phox to lamellipodia, and reactive oxygen species (ROS) generation in HLMVECs. Furthermore, exposure of HLMVECs to HGF or S1P stimulated c-Abl-mediated tyrosine phosphorylation of paxillin at Y31 and Y118 in a time-dependent fashion, and down-regulation of c-Abl with siRNA attenuated HGF- or S1P-mediated lamellipodia formation, translocation of p47phox to lamellipodia, and endothelial barrier enhancement. In vivo, knockdown of paxillin with siRNA in mouse lungs attenuated ventilator-induced lung injury. Together, these results suggest that c-Abl-mediated tyrosine phosphorylation of paxillin at Y31 and Y118 regulates HGF- or S1P-mediated lamellipodia formation, ROS generation in lamellipodia, and endothelial permeability.
Keywords: paxillin, tyrosine phosphorylation of paxillin, lamellipodia, c-Abl, endothelial barrier, reactive oxygen species
Acute respiratory distress syndrome, a devastating consequence of sepsis with a mortality of ∼35%, is characterized by increased lung vascular permeability resulting in edema. A variety of edematic agents, such as thrombin, histamine, vascular endothelial growth factor, and reactive oxygen species (ROS), and pathological situations, such as sepsis and hyperoxia, increase endothelial permeability both in vivo and in vitro through regulation of cytoskeletal and junctional proteins, focal adhesions, and redox regulation of the cell1-5 via complex signaling pathways of phosphorylation and dephosphorylation of the effector proteins. Although it is well established that opening of endothelial cell (EC) junctions causes increased permeability, alveolar flooding, and pulmonary edema, it is becoming clearer that ECs are capable of enhancing barrier function in the presence of barrier-enhancing factors, such as sphingosine-1-phosphate (S1P),6,7 hepatocyte growth factor (HGF),8-10 and hyperosmolality.11,12 In rat lung microvascular ECs, oxidant-induced barrier disruption was followed by focal adhesion kinase (FAK)–dependent reestablishment of the barrier.13,14 The effect of oxidants on barrier function is dependent on the dose, time of exposure, and type of oxidant. While higher concentrations of H2O2 have been shown to cause EC hyperpermeability, at lower concentrations barrier dysfunction was followed by barrier recovery that was FAK dependent,15,16 suggesting a role for focal adhesions and focal adhesion proteins in resealing of gaps. Both S1P and HGF stimulate membrane protrusions, lamellipodia formation, membrane ruffling, and barrier enhancement.17-20 We recently identified a role for HGF-induced c-Met/PI3K/Akt signaling and NADPH oxidase–dependent ROS generation in lamellipodia formation and motility of lung ECs ex vivo and in vitro.20 Although HGF- or S1P-mediated barrier enhancement requires FAK-dependent actin cytoskeletal focal adhesion and adherens junction rearrangement,21,22 the role played by lamellipodia and mechanisms of resealing of endothelial junctions by barrier-enhancing agents is not clear.
Paxillin, a multifunctional multidomain focal adhesion adaptor protein of ∼68 kDa, serves as an important scaffolding protein at focal adhesions by recruiting and binding to structural and signaling molecules.23 Paxillin is known to be tyrosine phosphorylated by FAK or the Src family of kinases at Y31 and Y118, which is critical for paxillin redistribution to focal adhesions and angiogenesis.24-27 Paxillin tyrosine phosphorylation at Y31 and Y118 regulates cell migration positively or negatively depending on its location at the leading edge or tail end.28,29 Tyrosine phosphorylation of paxillin also activates the Rho family of GTPases, leading to the formation of filopodia, lamellipodia, and stress fibers, which are actin-based structures primarily localized at the leading edge of migrating cells.30,31 Paxillin has been shown to differentially regulate endothelial barrier function by growth factors via modulation of Rac-Rho signaling.22 Interestingly, genetic deletion of paxillin in mice is embryonically lethal, and increased paxillin expression and mutations are associated with tumor metastasis of many cancers, including lung cancer.32-35 However, the role played by paxillin in lamellipodia formation and endothelial gap closures during barrier restoration remains to be elucidated.
Here, we show that paxillin knockdown attenuates HGF- or S1P-induced lamellipodia formation and barrier enhancement in lung ECs. In addition, our findings demonstrate that c-Abl-mediated tyrosine phosphorylation of paxillin at Y31 and Y118 is essential for HGF- or S1P-mediated lamellipodia formation and endothelial barrier enhancement. In vivo, specific small interfering RNA (siRNA) mediated knockdown of paxillin in the lungs and protected mice from ventilator-induced acute lung injury, showing differential roles of paxillin in barrier dysfunction and restoration.
Methods
Reagents
Human lung microvascular ECs (HLMVECs) and endothelial basal medium (EBM-2) were obtained from Lonza (San Diego, CA). Gold antifade mounting medium, 4′,6‐diamidino‐2‐phenylindole dihydrochloride, Hoechst and precast Tris-glycine polyacrylamide gel electrophoresis (PAGE) gel, Alexa Fluor 488, Alexa Fluor 568, and Alexa Fluor phalloidin 568 were procured from Life Technologies (Eugene, OR). HGF was obtained from PeproTech (Rocky Hill, NJ). Antibodies to phospho-c-Abl, c-Abl, phospho-paxillin, and paxillin as well as cell lysis buffer were obtained from Cell Signaling Technology (Danvers, MA). Antibody to p47 was obtained from Millipore (Bedford, MA). Scrambled siRNA and siRNA for paxillin, c-Abl, and p47phox were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). pHyPer-cyto plasmid was purchased from Evrogen (Moscow, Russia). RNA transfection reagent Gene Silencer was obtained from Genlantis (San Diego, CA). In vivo RNA transfection reagent jetPEI was obtained from Polyplus Transfection (Illkirch, France). FuGENE HD transfection reagent was obtained from Promega (Madison, WI). Electrical cell-substrate impedance sensing electrodes 8W1E were procured from Applied Biophysics (Troy, NY).
EC culture
HLMVECs cultured in complete medium (EBM-2) were maintained at 37°C in 5% CO2 and grown to contact-inhibited monolayers that revealed typical cobblestone morphology. Cells were then detached with 0.05% trypsin, suspended in fresh medium, and cultured on gold electrodes for electrical resistance determinations, on glass coverslips for fluorescent microscopy studies, or in 60–100-mm culture dishes for preparation of cell lysates and Western blot analysis.
Transfection of siRNA and complementary DNA (cDNA)
Endogenous paxillin, c-Abl, and p47 in HLMVECs were depleted using gene-specific siRNA, as described elsewhere.20 In brief, predesigned human paxillin, c-Abl siRNA, p47, or nonspecific/nontargeting siRNA was used to transfect HLMVECs (5–7 passages). Before transfection, cells were starved in 2% fetal bovine serum (FBS) EBM-2 medium overnight. The next day, 50 nM scrambled, paxillin, c-Abl, or p47 siRNA complexes were prepared in Gene Silencer transfection reagent according to the manufacturer’s recommendations, cells were transfected in serum-free medium for 4 hours, and the medium was replaced with fresh complete EBM-2 medium supplemented with 10% FBS and growth factors. Cells were used 72 hours after transfection. To determine intracellular hydrogen peroxide generation, HLMVECs were transfected with 1 μg/mL pHyPer-cyto plasmid. The cDNA of control plasmid or pHyPer-cyto was incubated with FuGene HD transfection reagent in serum-free EGM-2 medium according to the manufacturer’s recommendations. After 3 hours, the medium of transfected cells was replaced with complete EGM-2 medium containing 10% FBS, and the cells were used after 72 hours. Similarly, HLMVECs were transfected with control plasmid or with nonphosphorylatable paxillin mutants for study of the role played by paxillin phosphorylation in lamellipodia formation.
Immunoblotting and immunoprecipitation
Immunoblotting and immunoprecipitation were performed as described elsewhere.36 In brief, after appropriate treatments, cells were pelleted in ice-cold phosphate‐buffered saline, lysed in standard lysis buffer (Cell Signaling, Beverly, MA), and sonicated. Lysates were then centrifuged at 1,000 g for 10 minutes at 4°C, supernatants were collected, and protein was assayed using a Pierce BCA protein assay kit. For immunoprecipitation experiments, equal amounts of protein (1 mg) from each sample were precleared with control immunoglobulin G conjugated to A/G agarose beads at 4°C for 1 hour, and supernatants were collected and incubated overnight with primary antibody conjugated to A/G agarose beads at 4°C. The next day, samples were centrifuged at 1,000 g for 1 minute in a microfuge centrifuge, and the pellets containing agarose beads were washed three times with lysis buffer at room temperature. After centrifugation at 1,000 g for 1 minute, the beads were collected by removing supernatant buffer, and 40 μL of sodium dodecyl sulfate (SDS) sample buffer (100 mM Tris-HCl [pH 6.8], 4% SDS, 0.1% bromophenol blue, 20% glycerol, 200 mM dithiothreitol) was added to the beads and boiled. Lysates were then subjected to 10% SDS-PAGE followed by Western blotting. Proteins were detected by immunoblotting using appropriate primary antibodies and horseradish peroxidase–conjugated anti-rabbit or anti-mouse secondary antibodies. Band intensities were quantified by densitometry using ImageJ software.
Measurement of endothelial permeability by transendothelial electrical resistance
Endothelial permeability changes were measured using the highly sensitive biophysical assay with an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY), as described elsewhere.20 Transendothelial resistance was measured dynamically across the monolayer, and the effect of HGF challenge was monitored over a period of 6 hours. Resistance was normalized to the initial voltage and expressed as a fraction of the normalized resistance value.
Quantification of lamellipodia and colocalization
Lamellipodia were quantified as described elsewhere.20 In brief, for each image background signal was subtracted by drawing a region of interest around the cell periphery of individual cells. All areas outside the cell were cleared to best visualize the leading edges, including cell periphery, and the fluorescence intensity within the entire cell was summed using the MBF ImageJ bundle (Tony Collins, McMaster University, Hamilton, Ontario, and Wayne Rasband, National Institutes of Health, Bethesda, MD). Pearson’s correlation coefficient (PCC) was used as a statistic for quantifying colocalization. PCC was defined using the following formula:
 |
where Ri and Gi refer to the intensity values of the red and green channels, respectively, of pixel i, and 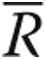 and
and 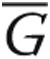 refer to the mean intensities of the red and green channels, respectively, across the entire image. Values near 1 reflect the fluorescence intensities of two images that are perfectly and linearly related to one another. Values near 0 reflect distribution of probes that are uncorrelated with each one another.
refer to the mean intensities of the red and green channels, respectively, across the entire image. Values near 1 reflect the fluorescence intensities of two images that are perfectly and linearly related to one another. Values near 0 reflect distribution of probes that are uncorrelated with each one another.
In vivo transfection of mouse lungs with paxillin siRNA
Mice were housed in a pathogen-free barrier facility maintained by the University of Illinois at Chicago Biologic Resources Laboratory. All experiments involving mice were conducted under protocols approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago. In vivo paxillin knockdown in mouse lungs was achieved by intratracheal paxillin siRNA transfection (5 mg/kg of mouse body weight) using jetPEI as a transfecting agent, as described elsewhere.37 Adult male C57BL/6J mice, 8–10 weeks old with an average weight of 20–25 g, were anesthetized, intubated with a 20-G × 1′′ catheter (Exel International Medical Products), and administered 20–30 μL of siRNA working solution; mice were then analyzed 72 hours after transfection.
In vivo model of ventilator-induced lung injury (VILI)
To explore the effect of paxillin on pulmonary permeability, scrambled RNA (scRNA)– or paxillin siRNA–treated mice were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg). Tracheotomy was performed, and the trachea was cannulated with a 20-G intravenous catheter. Ventilation was performed at a tidal volume of 30 mL/kg, 75 breaths per minute, and 0 positive end-respiratory pressure for 4 hours. Intravenous fluid boluses of 0.1 mL of 0.90% NaCl saline solution were given to maintain a mean arterial pressure >65 mmHg. Bronchial alveolar lavage (BAL) fluid was obtained at the end of the experiment by intratracheal injection of 1 mL of sterile Hanks balanced salt solution followed by gentle aspiration. The BAL fluid was used for measurement of cell count and protein concentration.
Statistical analysis
Results are expressed as means ± SD of three to five independent experiments. Statistical significance was assessed using analysis of variance, followed by Bonferroni multiple-comparison post hoc tests. Differences with P < 0.05 were considered statistically significant.
Results
Down-regulation of paxillin with siRNA attenuates HGF- and S1P-induced lamellipodia formation and barrier function in lung ECs
We have previously demonstrated that HGF- and S1P-stimulated reorganization and colocalization of actin and cortactin in lamellipodia of lung ECs was dependent on PI3K/Akt signaling.20 As actin regulates formation of cell protrusions at the leading edge of cells and paxillin is a cytoskeletal protein, we therefore determined the role played by paxillin in lamellipodia formation and barrier function in lung ECs. Paxillin was down-regulated (∼80% of expression) in HLMVECs by siRNA (Fig. 1C), and treatment of these cells with HGF or S1P significantly suppressed colocalization of actin and cortactin to lamellipodia (Fig. 1A). In parallel experiments, paxillin depletion with siRNA reduced endothelial barrier enhancement by HGF or S1P (Fig. 1B). Taken together, these results demonstrate a key role for paxillin in HGF- or S1p-induced lamellipodia formation and barrier enhancement in lung ECs.
Figure 1.
Paxillin mediates sphingosine-1-phosphate (S1P)– or hepatocyte growth factor (HGF)–induced endothelial cell lamellipodia formation and barrier function. Human lung microvascular endothelial cells were transfected with scrambled RNA (scRNA) or paxillin small interfering RNA (siRNA; 50 nM) for 72 hours. Cells were then subjected to either immunofluorescent staining for lamellipodia formation with cortactin antibody and phalloidin after S1P (1 μM) or HGF (20 ng/mL) treatment for 15 minutes (A) or electrical cell-substrate impedance measurement for barrier function (B), as described in “Methods.” At least 20 cells were analyzed for each condition; results shown are representative of three independent experiments. Knockdown of paxillin was confirmed by Western blotting, as shown in C. *P < 0.01, compared with vehicle control; #P < 0.05, compared with scRNA plus S1P or scRNA plus HGF. Scale bar = 10 μm. Pxn: paxillin; siPxn: paxillin siRNA.
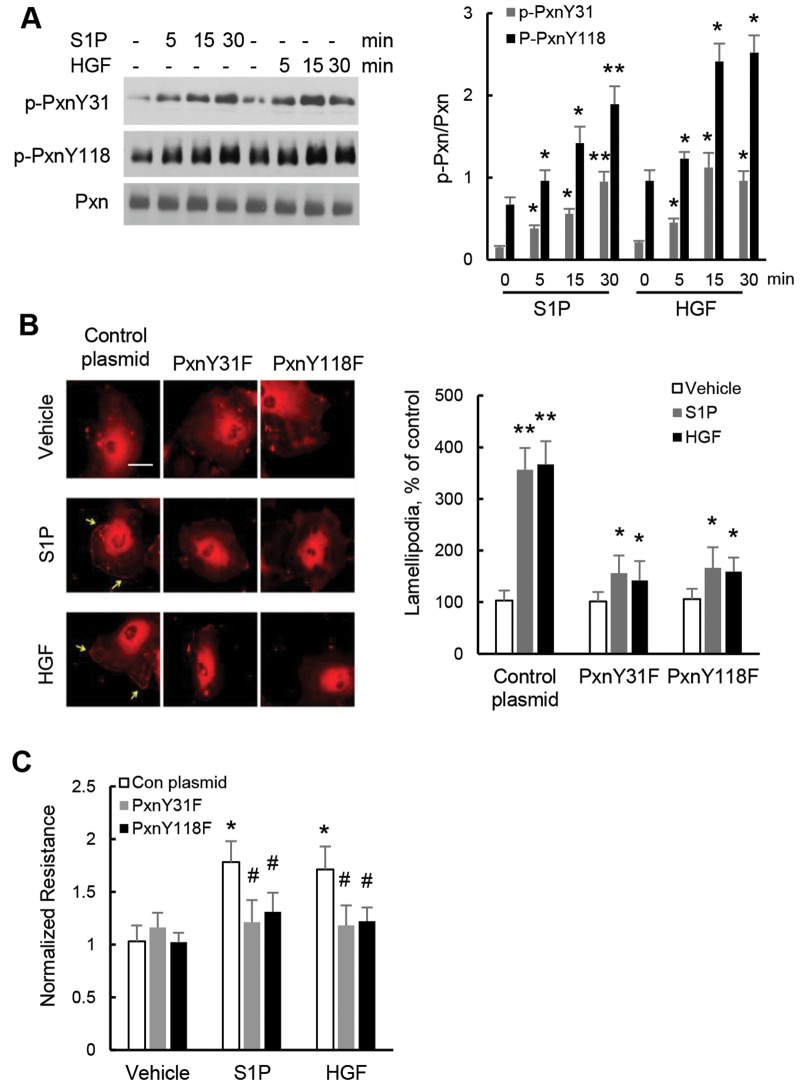
Tyrosine phosphorylation of paxillin at Y31 and Y118 is essential for HGF- or S1P-induced lamellipodia formation and barrier function in lung ECs
Earlier studies have shown that paxillin is tyrosine phosphorylated by growth factors and lipopolysaccharide (LPS) in ECs;37,38 however, the role played by Y31 and Y118 paxillin tyrosine phosphorylation in lamellipodia formation and barrier function is yet to be defined. Exposure of HLMVECs to HGF or S1P stimulated paxillin tyrosine phosphorylation at Y31 and Y118 in a time-dependent manner (Fig. 2A). To address the role played by paxillin tyrosine phosphorylation in lamellipodia formation and endothelial barrier function, HLMVECs were transfected with Y31F and Y118F paxillin mutants (1 μg cDNA/Fugene 6, 72 h) prior to HGF or S1P challenge. As shown in Figure 2B and 2C, overexpression of nonphosphorylatable Y31 and Y118 paxillin mutants significantly reduced HGF- or S1P-mediated redistribution of actin and cortactin to lamellipodia and endothelial barrier enhancement response, respectively. However, overexpression of these two mutants had no effect on HGF- or S1P-induced FAK phosphorylation (data not shown). Interestingly, expression of the Y31 and Y118 paxillin mutants modulated cortactin tyrosine phosphorylation at Y446, suggesting a role for Y31 and Y118 paxillin tyrosine phosphorylation in regulating cortactin phosphorylation (data not shown).
Figure 2.
Paxillin tyrosine phosphorylation is essential for sphingosine-1-phosphate (S1P)– or hepatocyte growth factor (HGF)–induced endothelial cell lamellipodia formation. A, Human lung microvascular endothelial cells (HLMVECs) grown in 35-mm dishes (90% confluence) were treated with S1P (1 μM) or HGF (20 ng/mL) for varying time points, as indicated. Phosphorylation of paxillin at Y31 and Y118 was determined by Western blotting using phospho-specific paxillin antibodies. Results shown are representative of three independent experiments. *P < 0.05, compared with vehicle control; **P < 0.01, compared with vehicle control. B, HLMVECs grown on slide chambers (60% confluence) were transfected with either control plasmid or nonphosphorylatable paxillin mutants (Y31F, Y181F), as described in “Methods.” After 72 hours, cells were treated with S1P (1 μM) or HGF (20 ng/mL) for 15 minutes and subjected to immunofluorescent staining for lamellipodia with cortactin antibody. At least 20 cells were analyzed for each condition; examples shown are representative of three independent experiments. *P < 0.05, compared with control plasmid plus S1P or control plasmid plus HGF; **P < 0.01, compared with control plasmid vehicle control. C, HLMVECs grown on electrical cell-substrate impedance sensing plates were transfected with either control plasmid or nonphosphorylable paxillin mutants (Y31F, Y181F), as described in “Methods.” After 72 h, transendothelial resistance reflecting endothelial cell monolayer barrier properties was monitored before and after S1P and HGF treatment. *P < 0.05, compared with plasmid control; #P < 0.05, compared with S1P- or HGF-treated control plasmid–transfected cells. Pxn: paxillin.
Role played by c-Abl in HGF- or S1P-mediated tyrosine phosphorylation of paxillin and cortactin, lamellipodia formation, and endothelial barrier function
There is evidence for the role played by Src family kinases in agonist-mediated tyrosine phosphorylation of cortactin and paxillin in mammalian cells;24,27 however, the role played by c-Abl in tyrosine phosphorylation of paxillin is limited. We have recently shown a role for c-Abl, but not for Src or FAK, in LPS-mediated tyrosine phosphorylation of paxillin in lung ECs. We first investigated whether S1P and HGF treatment activates c-Abl. As shown in Figure 3A, treatment of HLMVECs with S1P or HGF shows obvious phosphorylation of c-Abl at Y245 in a time-dependent manner. To further investigate the role played by c-Abl in tyrosine phosphorylation of paxillin, we down-regulated c-Abl using specific siRNA. Both HGF- and S1P-stimulated Y245 phosphorylation of c-Abl in scRNA-transfected HLMVECs and down-regulation of c-Abl with siRNA attenuated paxillin phosphorylation at Y31 and Y118 residues (Fig. 3B), which was accompanied by decreased localization of actin and cortactin in the lamellipodia (Fig. 4A). Furthermore, knockdown of paxillin with siRNA also attenuated HGF- or S1P-induced barrier enhancement of lung ECs (Fig. 4B). These results demonstrate a key role for c-Abl in paxillin tyrosine phosphorylation, lamellipodia formation, and endothelial barrier function.
Figure 3.
c-Abl tyrosine kinase mediates paxillin phosphorylation and lamellipodia formation in human lung endothelial cells. A, Human lung microvascular endothelial cells (HLMVECs; 90% confluence) were treated with sphingosine-1-phosphate (S1P; 1 μM) or hepatocyte growth factor (HGF; 20 ng/mL) for varying times. Phosphorylation of c-Abl at tyrosine Y245 was analyzed by Western blotting. Results shown are representative of three independent experiments. *P < 0.05, compared with vehicle control; **P < 0.01, compared with vehicle control; ***P < 0.001, compared with vehicle control. B, HLMVECs (60% confluence in 35-mm dishes) were transfected with scrambled RNA (scRNA) or c-Abl small interfering RNA (siRNA; 50 nM) for 72 hours, as described in “Methods.” After 72 hours of transfection, cells were treated with S1P (1 μM) or HGF (20 ng/mL) for 30 minutes, phosphorylation of paxillin was done at Y31 and Y118, and c-Abl expression was analyzed by Western blotting. Results shown are representative of three independent experiments. *P < 0.05, compared with scRNA vehicle; #P < 0.05, compared with scRNA plus S1P or scRNA plus HGF. Pxn: paxillin; sicAbl: c-Abl siRNA.
Figure 4.
c-Abl mediates sphingosine-1-phosphate (S1P)– and hepatocyte growth factor (HGF)–induced lamellipodia formation and barrier enhancement. A, Human lung microvascular endothelial cells (HLMVECs) grown on slide chambers (60% confluence) were transfected with scrambled RNA (scRNA) or c-Abl small interfering RNA (siRNA; 50 nM, 48 hours) prior to S1P (1 μM) or HGF (20 ng/mL) challenge for 30 minutes. Cells were fixed, and lamellipodia formation was visualized by immunofluorescent staining with cortactin antibody or phalloidin for actin. At least 20 cells were analyzed for each condition. Shown is an immunofluorescence image representative of three independent experiments. *P < 0.05, compared with scRNA transfection groups. B, HLMVECs were transfected with scRNA or c-Abl siRNA for 48 hours as described in A. Cells were then reseeded in 8-well electrical cell-substrate impedance sensing plates for another 24 hours, and transendothelial electrical resistance was monitored before and after S1P or HGF treatment and normalized to initial resistance. *P < 0.05, compared with scRNA control; #P < 0.05, compared with scRNA plus S1P or scRNA plus HGF. sicAbl: c-Abl siRNA.
c-Abl siRNA attenuates HGF-induced localization of p47phox and actin in lamellipodia
Having established that HGF- or S1P-mediated paxillin tyrosine phosphorylation is c-Abl dependent in lung ECs, we next determined whether c-Abl regulates localization of p47phox, a NADPH oxidase component, with actin in lamellipodia. As shown in Figure 5, knockdown of c-Abl with siRNA attenuated HGF-mediated redistribution and colocalization of p47phox and actin in lamellipodia.
Figure 5.
c-Abl mediates hepatocyte growth factor (HGF)–induced p47phox localization at lamellipodia. Human lung microvascular endothelial cells were transfected with scrambled RNA (scRNA) or c-Abl small interfering RNA (siRNA; 50 nM) for 72 hours and then treated with HGF (20 ng/mL) for 30 minutes. After treatment, cells were stained with anti-p47phox antibody and phalloidin for actin. At least 20 cells were analyzed for each condition. Shown is an immunofluorescence image representative of three independent experiments. *P < 0.05, compared with scRNA transfection groups. sicAbl: c-Abl siRNA.
Paxillin and paxillin tyrosine phosphorylation are involved in HGF- or S1P-induced p47phox/ROS localization in lamellipodia of lung ECs
We have previously shown that HGF stimulated p47phox translocation and ROS generation in lamellipodia of lung ECs.39 Having established a role for paxillin and paxillin tyrosine phosphorylation in HGF- or S1P-mediated lamellipodia formation and endothelial barrier function, we next determined the potential link between paxillin and paxillin tyrosine phosphorylation in accumulation of ROS in lamellipodia. To elucidate the role played by paxillin in HGF- or S1P-induced ROS in lamellipodia, ECs were transfected with p-Hyper-cytosol, and living cells were analyzed by confocal microscopy. As shown in Figure 6A, HGF or S1P stimulated hydrogen peroxide accumulation in the cell periphery, which was significantly inhibited by paxillin siRNA. Next, to determine the role played by paxillin tyrosine phosphorylation in ROS accumulation in lamellipodia, HLMVECs were transfected with the paxillin mutant Y118F for 48 h prior to S1P challenge. As expected, S1P enhanced colocalization of actin and p47phox in lamellipodia, which was attenuated by the paxillin Y118F mutant (Fig. 6B). These results establish a key role for HGF- or S1P-mediated paxillin and paxillin tyrosine phosphorylation in ROS accumulation in lamellipodia.
Figure 6.
Paxillin tyrosine phosphorylation regulates hepatocyte growth factor (HGF)– or sphingosine-1-phosphate (S1P)–induced p47phox/reactive oxygen species accumulation in lamellipodia of human lung endothelial cells. A, Human lung microvascular endothelial cells (HLMVECs; 60% confluence) were transfected with pHyPer-cyto plasmid (1 μg/mL) for 24 hours and then transfected with scrambled RNA (scRNA) or paxillin small interfering RNA (siRNA; 50 nM) for 48 hours. At the end of transfection, cells were treated with S1P (1 μM) or HGF (20 ng/mL) for 15 minutes. Accumulation of pHyPer-cyto signal at the cell periphery is indicated by arrows. At least 20 cells were analyzed for each condition. Shown is an image representative of three independent experiments. *P < 0.01, compared with scRNA vehicle; #P < 0.05, compared with scRNA plus S1P or scRNA plus HGF. B, HLMVECs were transfected with control plasmid or PxnY118F mutant plasmid (1 μg/mL) for 72 hours, and cells were treated with S1P (1 μM) for 15 minutes. After treatment, cells were stained with anti-p47phox antibody and phalloidin for actin. At least 20 cells were analyzed for each condition. Shown is an image representative of three independent experiments. *P < 0.05, compared with control vector. siPxn: paxillin siRNA.
Knockdown of paxillin with siRNA in mouse lungs inhibits VILI
Our in vitro results suggested a role for paxillin and its tyrosine phosphorylation in HGF- or S1P-mediated lamellipodia formation and endothelial barrier enhancement in HLMVECs; therefore, we next examined the role played by paxillin in vivo in VILI. Paxillin in mouse lungs was down-regulated by intratracheally instilling paxillin siRNA for 72 h prior to ventilator challenge. As shown in Figure 7A and 7B, LPS challenge induced significant leakage of protein in scRNA-treated mice and infiltration of inflammatory cells into the alveolar space. The above indicators of pulmonary leak and inflammation were significantly attenuated in paxillin knockdown lungs. Furthermore, histochemical analysis of paraffin-embedded mouse lungs revealed inflammatory cell recruitment and areas of alveolar hemorrhage indicative of vascular disruption in scrambled siRNA–treated mice. In contrast, there was a marked attenuation of lung injury in paxillin siRNA–treated mice (Fig. 7C). Knockdown of paxillin in mouse lungs was confirmed by Western blotting of lung tissue lysates obtained from mice administered scrambled and paxillin siRNA (Fig. 7A). These results suggest that paxillin is an important regulator of ventilator-induced pulmonary vascular permeability in vivo.
Figure 7.
Knockdown of paxillin in mouse lungs attenuates ventilator-induced lung permeability and injury. A, B, C57BL/6J mice were instilled with scrambled or paxillin small interfering RNA (siRNA; 5 mg/kg of body weight) for 48 hours prior to ventilation challenge. Lung permeability and injury were assessed by measurement of bronchial alveolar lavage (BAL) fluid cell count and BAL protein concentration. Values are means ± SD from 5 animals. *P < 0.01, compared with scrambled RNA (scRNA) control; **P < 0.05, compared with scRNA plus ventilator-induced lung injury (VILI). C, Hematoxylin-eosin staining of lung sections from scRNA- or paxillin siRNA–transfected mice after 4 hours of ventilation. Images are representative lung sections from 5 mice per group. Magnification = ×20; scale bar = 100 μm. Down-regulation of paxillin was confirmed by Western blotting, as shown in A. siPxn: paxillin siRNA.
Discussion
Lamellipodia are membrane protrusions formed at the leading edge of cells that play a key role in motility, angiogenesis, and morphogenesis.40-44 The basic mechanisms underlying the process of lamellipodia formation that requires participation of cytoskeleton, paxillin, and actin microfilaments have been extensively studied; however, the role played by paxillin in endothelial lamellipodia formation and barrier restoration and integrity remains poorly understood. Here, we show that knockdown of paxillin using siRNA decreases HGF- or S1P-mediated lamellipodia formation and barrier enhancement in human lung ECs. Moreover, we observed that c-Abl-dependent paxillin tyrosine phosphorylation at Y31 and Y118 is essential for HGF- or S1P-induced p47phox colocalization and ROS generation in the lamellipodia and endothelial barrier enhancement. In addition, we show that in vivo paxillin depletion in lungs by siRNA protects mice against VILI. These findings suggest that paxillin is essential for growth factor– or S1P-mediated lamellipodial-dependent lung endothelial barrier enhancement.
Paxillin is a scaffold and adaptor protein with LD motifs, LIM domains, and SH2-binding sites that mediate binding to other proteins, such as Crk, p130Cas, and Dock180,45-49 and together these interactions appear to play a key role in cell migration and adhesion. In addition, paxillin can be phosphorylated at Y31 and Y118 by FAK or Src kinases.25-27 While tyrosine phosphorylation of paxillin correlates with enhanced cell motility38,50 and cell growth in high-metastatic cancer, impaired tyrosine phosphorylation of paxillin retards formation of focal adhesions and stress fibers. However, the role played by paxillin Y31 and Y118 phosphorylation in lamellipodia formation and endothelial barrier function is unclear. Our present findings clearly show that HGF or S1P, a stimulus for EC motility and barrier enhancement, promote c-Abl-mediated phosphorylation of paxillin at Y31 and Y118; however, the present study does not rule out a role for FAK or Src in HFG- or S1P-induced paxillin tyrosine phosphorylation. We also recently demonstrated that LPS-mediated Y31 and Y118 phosphorylation of paxillin was not mediated by FAK or Src but requires c-Abl kinase activation in human lung ECs.37 Our experiments using Y31F/Y118F paxillin mutants support a critical role for this protein in HGF- or S1P-mediated localization of cortactin in lamellipodia and endothelial barrier enhancement. Interestingly, cortactin is also tyrosine phosphorylated by c-Abl and Src family kinases, thereby suggesting a central role for c-Abl kinase in the modulation of paxillin function through phosphorylation, especially in lamellipodia formation in lung ECs. There is evidence supporting the requirement of c-Abl for normal actin polymerization and lamellipodia formation51 via phosphorylation of Wiskott-Aldrich syndrome protein verprolin homologous 3 (WAVE3) by c-Abl in cell migration.52 However, c-Abl may also exert a negative effect on lamellipodia formation and cell motility. In 3T3 cells, c-Abl facilitated sequestration of Rac-GTP to dorsal membranes and thereby reduced the pool of Rac-GTP available for lamellipodia formation, cell spreading, and migration.53 Thus, the role played by c-Abl in lamellipodia formation and cell motility may be cell and context specific.
In addition to stimulating paxillin tyrosine phosphorylation, our data also show a role for c-Abl in cortactin phosphorylation and increased association between c-Abl and paxillin, c-Abl and cortactin, and cortactin and paxillin after HGF or S1P challenge, suggesting assembly of these targets in a multiprotein complex for lamellipodia formation. In fact, c-Abl has been shown to bind to phospho-HS1 cortactin via its SH2 domain, which was necessary for complete tyrosine phosphorylation of HS1 during T cell activation.51 Cortactin, a cortical actin-binding protein, plays an important role in EC motility and barrier enhancement.54-56 In human lung ECs, S1P and hyperoxia induced translocation of cortactin to cell periphery and membrane ruffles,39,57 and interaction between phosphocortactin and p47phox was critical for hyperoxia-induced NADPH oxidase activation and ROS generation.39 Furthermore, HGF or S1P stimulated cortactin tyrosine phosphorylation and colocalization of cortactin with actin in lamellipodia (Fig. 1), and down-regulation of cortactin with siRNA attenuated HGF-induced p47phox/cortactin/rac1 translocation to lamellipodia, ROS accumulation in lamellipodia, and cell motility in human lung ECs,20 suggesting a key role for cortactin. In a recent study, E-cadherin was involved in myosin-dependent tension development in the cortical actin cytoskeleton, suggesting that cortactin may play a role in inducing the adhesive contacts of membrane protrusions to reseal the adherens junctions barrier.58 However, the role played by cortactin and tyrosine phosphorylation of cortactin in lamellipodia formation is somewhat controversial.
We also demonstrated here that tyrosine phosphorylation of paxillin was essential for p47phox localization in lamellipodia (Fig. 6). Down-regulation of paxillin with siRNA or transfection of lung ECs with paxillin Y31F and Y118F mutants blocked HGF- or S1P-induced translocation of p47phox as well as ROS accumulation in lamellipodia, indicating a role for paxillin and paxillin tyrosine phosphorylation in lamellipodia formation. Given that paxillin and cortactin are substrates for c-Abl, we have shown in the present study that blocking paxillin or tyrosine phosphorylation of paxillin attenuates HGF- or S1P-mediated colocalization of cortactin with p47phox in lamellipodia. These results suggest that c-Abl-mediated tyrosine phosphorylation of paxillin serves as a mechanism to regulate the translocation of cortactin and p47phox to lamellipodia, activation of NADPH oxidase, and ROS generation. The role played by spatiotemporal enrichment of ROS in lamellipodia in cell migration and/or restoration of endothelial barrier integrity is unclear. As the balance between opening and resealing of the endothelial adherens junctions is essential for maintenance of barrier integrity, it is reasonable to assume that ROS generated in the lamellipodia regulates either directly or indirectly VE-cadherin trafficking and adherens junctions resealing. Further studies are necessary to determine the mechanism(s) of lamellipodial ROS in regulating endothelial barrier restoration.
In summary, we have demonstrated a novel role for c-Abl-mediated paxillin and paxillin tyrosine phosphorylation in HGF- or S1P-induced lamellipodia formation in lung ECs, which is dependent on recruitment of cortactin and p47phox and localized ROS generation. The interactions between paxillin, cortactin, and p47phox may form the basis for localized ROS generation in the lamellipodia and subsequent restoration of the adherens junction barrier.
Acknowledgments
We are grateful to Dr. Prasad Kanteti for suggestions and careful editing of the manuscript.
Source of Support: This work was supported by National Institutes of Health grant P01 HL 98050 to VN.
Conflict of Interest: None declared.
References
- 1.Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res 2010;87(2):262–271. [DOI] [PMC free article] [PubMed]
- 2.Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW 2nd, Duran WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol 2003;284(1):H92–H100. [DOI] [PubMed]
- 3.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 2006;8(11):1223–1234. [DOI] [PubMed]
- 4.Marumo T, Noll T, Schini-Kerth VB, Harley EA, Duhault J, Piper HM, Busse R. Significance of nitric oxide and peroxynitrite in permeability changes of the retinal microvascular endothelial cell monolayer induced by vascular endothelial growth factor. J Vasc Res 1999;36(6):510–515. [DOI] [PubMed]
- 5.Qian Y, Ducatman A, Ward R, Leonard S, Bukowski V, Lan Guo N, Shi X, Vallyathan V, Castranova V. Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: role in endothelial permeability. J Toxicol Environ Health A 2010;73(12):819–836. [DOI] [PMC free article] [PubMed]
- 6.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001;108(5):689–701. [DOI] [PMC free article] [PubMed]
- 7.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem 2004;92(6):1075–1085. [DOI] [PubMed]
- 8.Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J 2007;21(11):2776–2786. [DOI] [PubMed]
- 9.Gille J, Khalik M, Konig V, Kaufmann R. Hepatocyte growth factor/scatter factor (HGF/SF) induces vascular permeability factor (VPF/VEGF) expression by cultured keratinocytes. J Invest Dermatol 1998;111(6):1160–1165. [DOI] [PubMed]
- 10.Martin TA, Mansel RE, Jiang WG. Antagonistic effect of NK4 on HGF/SF induced changes in the transendothelial resistance (TER) and paracellular permeability of human vascular endothelial cells. J Cell Physiol 2002;192(3):268–275. [DOI] [PubMed]
- 11.Ragette R, Fu C, Bhattacharya J. Barrier effects of hyperosmolar signaling in microvascular endothelium of rat lung. J Clin Invest 1997;100(3):685–692. [DOI] [PMC free article] [PubMed]
- 12.Safdar Z, Wang P, Ichimura H, Issekutz AC, Quadri S, Bhattacharya J. Hyperosmolarity enhances the lung capillary barrier. J Clin Invest 2003;112(10):1541–1549. [DOI] [PMC free article] [PubMed]
- 13.Usatyuk PV, Natarajan V. Regulation of reactive oxygen species–induced endothelial cell-cell and cell-matrix contacts by focal adhesion kinase and adherens junction proteins. Am J Physiol Lung Cell Mol Physiol 2005;289(6):L999–L1010. [DOI] [PubMed]
- 14.Usatyuk PV, Parinandi NL, Natarajan V. Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem 2006;281(46):35554–35566. [DOI] [PubMed]
- 15.Pan Q, Qiu WY, Huo YN, Yao YF, Lou MF. Low levels of hydrogen peroxide stimulate corneal epithelial cell adhesion, migration, and wound healing. Invest Ophthalmol Vis Sci 2011;52(3):1723–1734. [DOI] [PMC free article] [PubMed]
- 16.Yang S, Yip R, Polena S, Sharma M, Rao S, Griciene P, Gintautas J, Jerome H. Reactive oxygen species increased focal adhesion kinase production in pulmonary microvascular endothelial cells. Proc West Pharmacol Soc 2004;47:54–56. [PubMed]
- 17.Lee JF, Ozaki H, Zhan X, Wang E, Hla T, Lee MJ. Sphingosine-1-phosphate signaling regulates lamellipodia localization of cortactin complexes in endothelial cells. Histochem Cell Biol 2006;126(3):297–304. [DOI] [PubMed]
- 18.Maceyka M, Alvarez SE, Milstien S, Spiegel S. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol Cell Biol 2008;28(18):5687–5697. [DOI] [PMC free article] [PubMed]
- 19.Morimura S, Suzuki K, Takahashi K. βPIX and GIT1 regulate HGF-induced lamellipodia formation and WAVE2 transport. Biochem Biophys Res Commun 2009;382(3):614–619. [DOI] [PubMed]
- 20.Usatyuk PV, Fu P, Mohan V, Epshtein Y, Jacobson JR, Gomez-Cambronero J, Wary KK, et al. Role of c-Met/phosphatidylinositol 3-kinase (PI3k)/Akt signaling in hepatocyte growth factor (HGF)–mediated lamellipodia formation, reactive oxygen species (ROS) generation, and motility of lung endothelial cells. J Biol Chem 2014;289(19):13476–13491. [DOI] [PMC free article] [PubMed]
- 21.Belvitch P, Dudek SM. Role of FAK in S1P-regulated endothelial permeability. Microvasc Res 2011;83(1):22–30. [DOI] [PMC free article] [PubMed]
- 22.Birukova AA, Cokic I, Moldobaeva N, Birukov KG. Paxillin is involved in the differential regulation of endothelial barrier by HGF and VEGF. Am J Respir Cell Mol Biol 2009;40(1):99–107. [DOI] [PMC free article] [PubMed]
- 23.Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci 2008;121:2435–2444. [DOI] [PMC free article] [PubMed]
- 24.Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell 2005;16(9):4316–4328. [DOI] [PMC free article] [PubMed]
- 25.Roy S, Ruest PJ, Hanks SK. FAK regulates tyrosine phosphorylation of CAS, paxillin, and PYK2 in cells expressing v-Src, but is not a critical determinant of v-Src transformation. J Cell Biochem 2002;84(2):377–388. [DOI] [PubMed]
- 26.Takayama Y, Tanaka S, Nagai K, Okada M. Adenovirus-mediated overexpression of C-terminal Src kinase (Csk) in type I astrocytes interferes with cell spreading and attachment to fibronectin: correlation with tyrosine phosphorylations of paxillin and FAK. J Biol Chem 1999;274(4):2291–2297. [DOI] [PubMed]
- 27.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 2004;6(2):154–161. [DOI] [PubMed]
- 28.Iwasaki T, Nakata A, Mukai M, Shinkai K, Yano H, Sabe H, Schaefer E, et al. Involvement of phosphorylation of Tyr-31 and Tyr-118 of paxillin in MM1 cancer cell migration. Int J Cancer 2002;97(3):330–335. [DOI] [PubMed]
- 29.Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Valles AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol 2000;148(5):957–970. [DOI] [PMC free article] [PubMed]
- 30.Chen GC, Turano B, Ruest PJ, Hagel M, Settleman J, Thomas SM. Regulation of Rho and Rac signaling to the actin cytoskeleton by paxillin during Drosophila development. Mol Cell Biol 2005;25(3):979–987. [DOI] [PMC free article] [PubMed]
- 31.Wang F, Nobes CD, Hall A, Spiegel S. Sphingosine 1-phosphate stimulates rho-mediated tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 fibroblasts. Biochem J 1997;324:481–488. [DOI] [PMC free article] [PubMed]
- 32.Chen DL, Wang DS, Wu WJ, Zeng ZL, Luo HY, Qiu MZ, Ren C, et al. Overexpression of paxillin induced by miR-137 suppression promotes tumor progression and metastasis in colorectal cancer. Carcinogenesis 2013;34(4):803–811. [DOI] [PMC free article] [PubMed]
- 33.Chen DL, Wang ZQ, Ren C, Zeng ZL, Wang DS, Luo HY, Wang F, et al. Abnormal expression of paxillin correlates with tumor progression and poor survival in patients with gastric cancer. J Transl Med 2013;11:277. [DOI] [PMC free article] [PubMed]
- 34.German AE, Mammoto T, Jiang E, Ingber DE, Mammoto A. Paxillin controls endothelial cell migration and tumor angiogenesis by altering neuropilin 2 expression. J Cell Sci 2014;127:1672–1683. [DOI] [PMC free article] [PubMed]
- 35.Salgia R, Li JL, Ewaniuk DS, Wang YB, Sattler M, Chen WC, Richards W, et al. Expression of the focal adhesion protein paxillin in lung cancer and its relation to cell motility. Oncogene 1999;18(1):67–77. [DOI] [PubMed]
- 36.Usatyuk PV, Burns M, Mohan V, Pendyala S, He D, Ebenezer DL, Harijith A, et al. Coronin 1B regulates S1P-induced human lung endothelial cell chemotaxis: role of PLD2, protein kinase C and Rac1 signal transduction. PLoS ONE 2013;8(5):e63007. [DOI] [PMC free article] [PubMed]
- 37.Fu P, Usatyuk PV, Lele A, Harijith A, Gregorio CC, Garcia JG, Salgia R, Natarajan V. c-Abl mediated tyrosine phosphorylation of paxillin regulates LPS-induced endothelial dysfunction and lung injury. Am J Physiol Lung Cell Mol Physiol 2015;308(10):L1025–L1038. [DOI] [PMC free article] [PubMed]
- 38.Romanova LY, Hashimoto S, Chay KO, Blagosklonny MV, Sabe H, Mushinski JF. Phosphorylation of paxillin tyrosines 31 and 118 controls polarization and motility of lymphoid cells and is PMA-sensitive. J Cell Sci 2004;117:3759–3768. [DOI] [PubMed]
- 39.Usatyuk PV, Romer LH, He D, Parinandi NL, Kleinberg ME, Zhan S, Jacobson JR, et al. Regulation of hyperoxia-induced NADPH oxidase activation in human lung endothelial cells by the actin cytoskeleton and cortactin. J Biol Chem 2007;282(32):23284–23295. [DOI] [PubMed]
- 40.Frigault MM, Naujokas MA, Park M. Gab2 requires membrane targeting and the Met binding motif to promote lamellipodia, cell scatter, and epithelial morphogenesis downstream from the Met receptor. J Cell Physiol 2008;214(3):694–705. [DOI] [PubMed]
- 41.Khurana S, Tomar A, George SP, Wang Y, Siddiqui MR, Guo H, Tigyi G, Mathew S. Autotaxin and lysophosphatidic acid stimulate intestinal cell motility by redistribution of the actin modifying protein villin to the developing lamellipodia. Exp Cell Res 2008;314(3):530–542. [DOI] [PMC free article] [PubMed]
- 42.Majumder S, Sowden MP, Gerber SA, Thomas T, Christie CK, Mohan A, Yin G, Lord EM, Berk BC, Pang J. G-protein-coupled receptor-2-interacting protein-1 is required for endothelial cell directional migration and tumor angiogenesis via cortactin-dependent lamellipodia formation. Arterioscler Thromb Vasc Biol 2014;34(2):419–426. [DOI] [PMC free article] [PubMed]
- 43.McDougall AR, Hooper SB, Zahra VA, Cole TJ, Lo CY, Doran T, Wallace MJ. Trop2 regulates motility and lamellipodia formation in cultured fetal lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 2013;305(7):L508–L521. [DOI] [PubMed]
- 44.Yang Y, Lundquist EA. The actin-binding protein UNC-115/abLIM controls formation of lamellipodia and filopodia and neuronal morphogenesis in Caenorhabditis elegans. Mol Cell Biol 2005;25(12):5158–5170. [DOI] [PMC free article] [PubMed]
- 45.Chen S, Wang R, Li QF, Tang DD. Abl knockout differentially affects p130 Crk-associated substrate, vinculin, and paxillin in blood vessels of mice. Am J Physiol Heart Circ Physiol 2009;297(2):H533–H539. [DOI] [PMC free article] [PubMed]
- 46.Flinn HM, Ridley AJ. Rho stimulates tyrosine phosphorylation of focal adhesion kinase, p130 and paxillin. J Cell Sci 1996;109:1133–1141. [DOI] [PubMed]
- 47.Lamorte L, Rodrigues S, Sangwan V, Turner CE, Park M. Crk associates with a multimolecular Paxillin/GIT2/β-PIX complex and promotes Rac-dependent relocalization of paxillin to focal contacts. Mol Biol Cell 2003;14(7):2818–2831. [DOI] [PMC free article] [PubMed]
- 48.Valles AM, Beuvin M, Boyer B. Activation of Rac1 by paxillin-Crk-DOCK180 signaling complex is antagonized by Rap1 in migrating NBT-II cells. J Biol Chem 2004;279(43):44490–44496. [DOI] [PubMed]
- 49.Yano H, Uchida H, Iwasaki T, Mukai M, Akedo H, Nakamura K, Hashimoto S, Sabe H. Paxillin α and Crk-associated substrate exert opposing effects on cell migration and contact inhibition of growth through tyrosine phosphorylation. Proc Natl Acad Sci USA 2000;97(16):9076–9081. [DOI] [PMC free article] [PubMed]
- 50.Ito A, Kataoka TR, Watanabe M, Nishiyama K, Mazaki Y, Sabe H, Kitamura Y, Nojima H. A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. EMBO J 2000;19(4):562–571. [DOI] [PMC free article] [PubMed]
- 51.Huang Y, Comiskey EO, Dupree RS, Li S, Koleske AJ, Burkhardt JK. The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse. Blood 2008;112(1):111–119. [DOI] [PMC free article] [PubMed]
- 52.Sossey-Alaoui K, Li X, Cowell JK. c-Abl-mediated phosphorylation of WAVE3 is required for lamellipodia formation and cell migration. J Biol Chem 2007;282(36):26257–26265. [DOI] [PubMed]
- 53.Jin H, Wang JY. Abl tyrosine kinase promotes dorsal ruffles but restrains lamellipodia extension during cell spreading on fibronectin. Mol Biol Cell 2007;18(10):4143–4154. [DOI] [PMC free article] [PubMed]
- 54.Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol 2005;15(14):1276–1285. [DOI] [PubMed]
- 55.Gov NS, Bernheim-Groswasser A. Releasing the brakes while hanging on: cortactin effects on actin-driven motility. Bioarchitecture 2012;2(1):11–14. [DOI] [PMC free article] [PubMed]
- 56.Siton O, Ideses Y, Albeck S, Unger T, Bershadsky AD, Gov NS, Bernheim-Groswasser A. Cortactin releases the brakes in actin-based motility by enhancing WASP-VCA detachment from Arp2/3 branches. Curr Biol 2011;21(24):2092–2097. [DOI] [PubMed]
- 57.Usatyuk PV, He D, Bindokas V, Gorshkova IA, Berdyshev EV, Garcia JG, Natarajan V. Photolysis of caged sphingosine-1-phosphate induces barrier enhancement and intracellular activation of lung endothelial cell signaling pathways. Am J Physiol Lung Cell Mol Physiol 2011;300(6):L840–L850. [DOI] [PMC free article] [PubMed]
- 58.de Rooij J. Cadherin adhesion controlled by cortical actin dynamics. Nat Cell Biol 2014;16(6):508–510. [DOI] [PubMed]