Abstract Abstract
Pulmonary arterial hypertension (PAH) is characterized by abnormal elaboration of vasoactive peptides, endothelial cell dysfunction, vascular remodeling, and inflammation, which collectively contribute to its pathogenesis. We investigated the potential for high-density lipoprotein (HDL) dysfunction (i.e., proinflammatory effects) and abnormal plasma eicosanoid levels to contribute to the pathobiology of PAH and assessed ex vivo the effect of treatment with apolipoprotein A-I mimetic peptide 4F on the observed HDL dysfunction. We determined the “inflammatory indices” HII and LII for HDL and low-density lipoprotein (LDL), respectively, in subjects with idiopathic PAH (IPAH) and associated PAH (APAH) by an in vitro monocyte chemotaxis assay. The 4F was added ex vivo, and repeat LII and HII values were obtained versus a sham treatment. We further determined eicosanoid levels in plasma and HDL fractions from patients with IPAH and APAH relative to controls. The LIIs were significantly higher for IPAH and APAH patients than for controls. Incubation of plasma with 4F before isolation of LDL and HDL significantly reduced the LII values, compared with sham-treated LDL, for IPAH and APAH. The increased LII values reflected increased states of LDL oxidation and thereby increased proinflammatory effects in both cohorts. The HIIs for both PAH cohorts reflected a “dysfunctional HDL phenotype,” that is, proinflammatory HDL effects. In contrast to “normal HDL function,” the determined HIIs were significantly increased for the IPAH and APAH cohorts. Ex vivo 4F treatment significantly improved the HDL function versus the sham treatment. Although there was a significant “salutary effect” of 4F treatment, this did not entirely normalize the HII. Significantly increased levels for both IPAH and APAH versus controls were evident for the eicosanoids 9-HODE, 13-HODE, 5-HETE, 12-HETE, and 15-HETE, while no statistical differences were evident for comparisons of IPAH and APAH for the determined plasma eicosanoid levels in the HDL fractions. Our study has further implicated the putative role of “oxidant stress” and inflammation in the pathobiology of PAH. Our data suggest the influences on the “dysfunctional HDL phenotype” of increased oxidized fatty acids, which are paradoxically proinflammatory. We speculate that therapies that target either the “inflammatory milieu” or the “dysfunctional HDL phenotype,” such as apoA-I mimetic peptides, may be valuable avenues of further research in pulmonary vascular diseases.
Keywords: pulmonary arterial hypertension, low-density lipoprotein, high-density lipoprotein, inflammatory index, hydroxyeicosatetraenoic acids, hydroxyoctadecadienoic acids, pulmonary arterial endothelial cells, pulmonary arterial smooth muscle cells
Pulmonary arterial hypertension (PAH) comprises a spectrum of diseases, including both “idiopathic” (IPAH) and “associated” (APAH), with a spectrum of underlying etiologies (connective-tissue disease, cirrhosis, congenital heart disease, etc.), as described in the World Health Organization (WHO) classification.1 Irrespective of etiology, PAH is characterized by endothelial cell (EC) dysfunction, vascular remodeling, and inflammation, which collectively contribute to its complex pathogenesis.2 A putative role of inflammation had emerged from a histopathologic observation of perivascular mononuclear cell infiltration, but without associated vasculitis, in plexogenic arteriopathic lesions, comprised of T and B lymphocytes and macrophages.3,4 Increased expression of the inflammatory chemokines fractalkine/CX3CL1, and RANTES in association with CD4+ and CD8+ lymphocytes and pulmonary arterial endothelial cells (PAECs), has further strengthened the potential role of inflammation in PAH.5,6 Our group has recently investigated, in plasma samples from individuals with WHO group 1 PAH, the role of the expression of the specific “type 1 immune response” and “cytokine-chemokine cascade”: interleukin (IL)-18 induces MIG (monokine induced by gamma interferon)/chemokine (C-X-C motif) ligand (CXCL)9, interferon gamma–induced protein (IP)-10/CXCL10, and interferon-inducible T cell alpha chemoattractant (ITAC)/CXCL11. We observed augmented expression of IL-18 and CXCL10 in both subjects’ serum and the pulmonary arterial tunica media.7 In this study, we investigate the potential role of “inflammatory high-density lipoprotein” (inflammatory HDL) as a consequence of “oxidant stress” in the pathogenesis of PAH. Atherosclerosis is the consequence of complex interactions between oxidized lipoproteins, monocytes/macrophages, and endothelial and vascular smooth muscle dysfunction. Oxidation products of arachidonic acid (AA), including prostaglandins (PGs), thromboxanes (TXs), hydroxyeicosatetraenoic acids (HETEs), and hydroxyoctadecadienoic acids (HODEs), contribute to the pathogenesis of atherosclerosis. The biosynthesis of most eicosanoids (HETEs, PGs, TXs) from AA occurs via lipoxygenase (LOX), cyclooxygenase, and the cytochrome P450 pathways. LOXs are classified as 5-, 8-, 12-, or 15-LOX according to the positional specificity to insert molecular oxygen at corresponding positions of AA, resulting in, for example, 5(S)-hydroperoxyeicosatetraenoic acid (5(S)-HPETE) and 8(S)-HPETE. With linoleic acid, 12- and 15-LOX form S-hydroperoxyoctadecadienoic acids 12(S)- and 9(S)-HPODE. These HPETEs and HPODEs are subsequently reduced to their hydroxyl derivatives, HETEs and HODEs.8
Although HDL is involved in “reverse cholesterol transport,” its influences additionally include the normal modulation of inflammation in the context of “innate immunity.”9 Oxidized phospholipids and lipoperoxides participate in a continuous cycle of oxidative stress and inflammation through lipid peroxidation chain reaction and activation of lectin-like oxidized low-density lipoprotein (LDL) receptor, scavenger receptor A-1, and oxidized phospholipid receptor.10-12 To counteract this inflammatory milieu, plasma apolipoprotein A-I (apoA-I), the principal apolipoprotein constituent of HDL, normally confers “anti-inflammatory” activity on HDL. However, “pathologic HDL” can occur during “chronic, acute phase responses” that are paradoxically “proinflammatory,”13,14 while inflammation may be regulated by complexes of hemoglobin-haptoglobin.15 Overexpression of apoA-I (which contains 243 amino acid residues) has been shown to ameliorate atherosclerosis in experimental animals.16,17 Widespread clinical application, however, has been deterred because of the formidable cost and lack of availability for mass-production capability; therefore, synthetic apoA-I mimetic peptides, such as 4F (18 amino acids), have been developed that have been demonstrated to attenuate atherosclerosis in experimental animal models and to improve HDL function in humans.18,19 The amphipathic peptide 4F is characterized by 4 phenylalanine residues on a hydrophobic face and demonstrates approximately a million-fold ability to bind to some oxidized lipids, compared with native apoA-I. The observed salutary effects of 4F relate to a preferential removal of oxidation products from lipoproteins and cellular membranes, thereby resulting in a restoration of HDL and LDL function and structure, improved cellular function, and attenuation of inflammation. In our investigation, PAH subjects were characterized by this “pathologic HDL phenotype,” as assessed in a monocyte chemotaxis assay. When PAH subjects’ plasma was treated ex vivo with 4F, a salutary effect was observed on the proinflammatory response, but not to the extent of resuming “normal” HDL function. Further, in our study, PAH subjects also demonstrated significant alterations in plasma HDL-associated eicosanoid levels, potentially contributing to the proinflammatory HDL phenotype. Such eicosanoid alterations may indeed induce proliferation and migration of pulmonary arterial smooth muscle cells (PASMCs) and PAECs in states of pulmonary vascular pathology.
Methods
The study was approved by the Human Subjects’ Institutional Review Board (IRB). All subjects with PAH met accepted criteria for diagnosis as outlined in the Fifth World Symposium (2013) for Pulmonary Arterial Hypertension in Nice, France.1 One sodium heparin–treated 10-mL vial of blood was collected from each subject, and plasma was stored at −80°C before batch analysis.
Cultured human aortic ECs
Aortic ECs were isolated from the trimmings of the donor heart aorta that were not needed during transplantation. They were cultured and exposed to lipoproteins as described.
Chemotactic monocyte assays
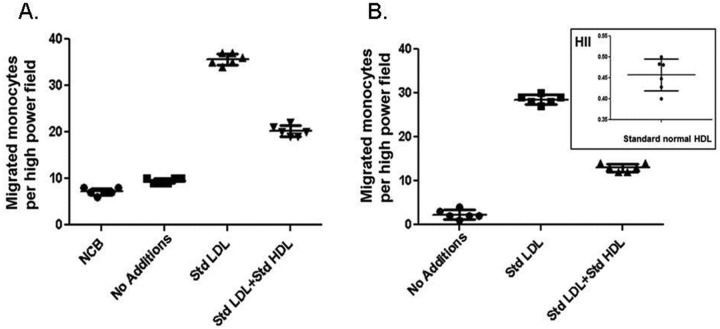
The assays used methods described previously.13,20,21 Human peripheral monocytes and blood samples were obtained from healthy volunteers as described previously.13 Blood was obtained from individuals at Ronald Reagan UCLA Medical Center with informed consent and according to protocols approved by the University of California IRB. Plasma was fractionated by fast-performance liquid chromatography (FPLC), and LDL- and HDL-containing fractions were pooled. Standard LDL and HDL solutions were prepared as reported previously.13 Cultured human aortic ECs were treated with normal LDL alone, LDL plus normal HDL, or LDL plus patient HDL. In subsequent experiments, the cultures received LDL treated with vehicle- or 4F-treated subject LDL or with vehicle- or 4F-treated subject HDL. Briefly, a standard control human LDL, prepared by ultracentrifugation of the plasma of a healthy volunteer, was added as an internal standard to endothelial cultures at a concentration of 100 g LDL cholesterol/mL culture medium. After 8 hours, the supernatants were collected, and the monocyte chemotactic activity (which is largely due to the activity of monocyte chemoattractant protein 1 [MCP-1]) in the supernatant was determined as previously described.13 The values for the control internal standard LDL were normalized to 1.0. For determination of the HDL inflammatory index (HII), a standard control human HDL, prepared by ultracentrifugation of the plasma of a healthy volunteer, was added at 50 g cholesterol/mL culture supernatant, together with the control standard LDL at 100 g LDL cholesterol/mL. After the incubation of lipoproteins with the cells, the cells were washed, incubation continued for 8 hours, and the culture supernatant was collected. The migration of added monocytes across a filter membrane toward the test supernatant containing MCP-1 was determined with standard chemotaxis chambers. Monocyte chemotactic activity was measured as the number of migrated monocytes per high-powered field in triplicate in 6 separate fields. The value obtained by addition of the control internal standard LDL to the test HDL was divided by the monocyte chemotactic activity obtained after adding this LDL to the ECs without HDL. In this assay, anti-inflammatory HDL results in HII values of <1.0, and proinflammatory HDL results in HII values of >1.0. For determination of the LDL inflammatory index (LII), the test LDL was added to the cells at 100 g LDL cholesterol/mL without added HDL, and the resulting monocyte chemotactic activity was divided by the monocyte chemotactic activity obtained after addition of the control internal standard LDL at 100 g/mL without added HDL. In this assay, if the test LDL induced more monocyte chemotactic activity than the control internal standard LDL, the LII will be >1.0. Conversely, if the test LDL produced less monocyte chemotactic activity than the control internal standard LDL, the LII will be <1.0. For treatment of samples with 4F, plasma from IPAH or APAH patients was treated with 1 g/mL of 4F or saline vehicle for 15 minutes at 37°C with gentle mixing. This concentration of 4F (0.43 nM) was earlier found to produce a maximal effect in vitro.20 The samples were then filtered through 100-kDa spin filters. Control or patient plasma was fractionated by FPLC, LDL- and HDL-containing fractions were pooled, cholesterol content was determined, and additions were made to cultures as described above. Figure 1 shows a typical set of data from the chemotactic assay. Chemotaxis was determined by HII and LII bioassay for “healthy controls” (N = 21), as depicted.
Figure 1.
Inflammatory index. Cultures of human aortic endothelial cells were treated with culture medium alone (No Additions), with low-density lipoprotein (LDL) from a normal healthy individual (Std LDL), or with LDL plus high-density lipoprotein (HDL) from a normal healthy individual (Std LDL + Std HDL). After overnight incubation, cultures were washed, fresh medium was added, incubation continued, and the supernatant was assayed in a standard chemotaxis chamber, as described in “Methods.” A, NCB (No Cell Blank) indicates the values for the number of monocytes migrated toward culture supernatant without incubation with endothelial cells. B, Data were calculated by subtracting the NCB values. The inset shows the inflammatory index for HDL (HII) by taking the value for Std LDL+ Std HDL and dividing it by that for Std LDL.
Levels of free (i.e., not esterified) eicosanoids in plasma or HDL
Liquid chromatography–electrospray ionization, tandem mass spectroscopy (LC-ESI-MS/MS) was performed with a mass spectrometer (4000 QTRAP; Applied Biosystems, Foster City, CA) equipped with an electrospray ionization source as previously described.8
Measurement of 15-HETE and 20-HETE
Plasma 15-HETE was measured with a 15(S)-HETE enzyme-linked immunosorbent assay (ELISA) kit (Cayman). Plasma 20-HETE was measured with a 20-HETE ELISA kit (Detroit R&D). The data on the specificity of each ELISA kit were obtained from the respective manufacturer.
Data analysis
The LII and HII and plasma and HDL eicosanoid levels are expressed as means ± SD. LIIs and HIIs for the sham- and 4F-treated groups were compared in the IPAH and APAH cohorts, and eicosanoid levels were compared in the IPAH and APAH cohorts versus control subjects, with ANOVA and Student’s t test, where appropriate, using SigmaSTAT 3.5 software; P values of <0.05 were considered significant.
Results
We determined the HII and LII for HDL and LDL from plasma of subjects with IPAH (N = 17) or APAH (N = 11) and healthy controls (N = 21). The specific APAH diagnoses, age, sex ratios, WHO/New York Heart Association functional-class distributions, mean pulmonary artery pressures, and PAH-specific pharmacologic therapies are presented in Table 1 for IPAH and APAH cohorts.
Table 1.
IPAH and APAH cohort characteristics
| IPAH (N = 17) | APAH (N = 11) | |
|---|---|---|
| Diagnosis | ||
| SLE | 3 | |
| PSS | 3 | |
| Cirrhosis | 1 | |
| RA | 2 | |
| CHD | 2 | |
| WHO/NYHA functional class | ||
| II | 2 | 2 |
| III | 10 | 8 |
| IV | 5 | 1 |
| Age, mean ± SD, years | 52.2 ± 11.6 | 53.9 ± 17.0 |
| Sex, M∶F | 6∶11 | 3∶8 |
| PAP, mean ± SD, mmHg | 52.1 ± 11.6 | 44.6 ± 25.1 |
| PAH-specific therapies | ||
| ERA | 16 | 11 |
| PDE5 inhibitor | 15 | 10 |
| Prostacyclin analogs | 8 | 5 |
Data are number of patients unless otherwise specified. APAH: associated pulmonary arterial hypertension; CHD: adult congenital heart disease; ERA: endothelin-1 receptor; IPAH: idiopathic pulmonary arterial hypertension; NYHA: New York Heart Association; PAP: pulmonary arterial pressure; PDE5: phosphodiesterase 5; PSS: progressive systemic sclerosis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; WHO: World Health Organization.
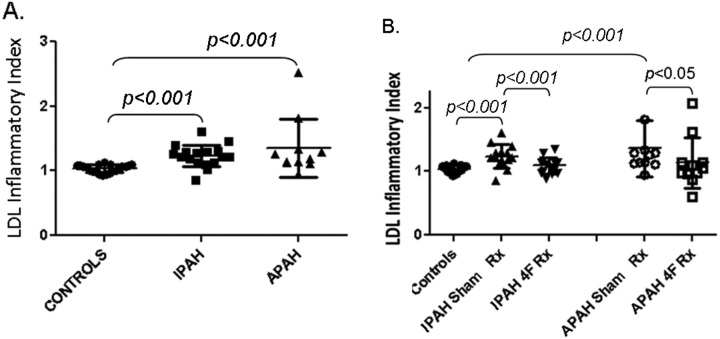
As depicted in Figure 2A and individual-subject data depicted in Table 2, the LIIs for IPAH (1.24 ± 0.05) and APAH (1.34 ± 0.15) were significantly higher (P < 0.001) than that for control LDL (P < 0.001), which had been normalized to 1.0 by convention in our study. The treatment with apoA-I mimetic peptide 4F significantly reduced the LII values, compared with those for sham-treated LDL for IPAH (1.07 ± 0.04, P < 0.001) and APAH (1.08 ± 0.13, P < 0.05; Fig. 2B). The increased LII values reflect significantly increased states of LDL oxidation and thereby increased proinflammatory activity for LDL from both patient cohorts.
Figure 2.
Low-density lipoprotein (LDL) inflammatory index (LII). A, LDL from patients with idiopathic or associated pulmonary arterial hypertension (IPAH or APAH, respectively) was isolated via fast-performance liquid chromatography, and the LII was determined as described in “Methods.” B, To determine the effect of incubation with 4F, LDL samples were treated with either saline (IPAH Sham Rx, APAH Sham Rx) or 4F (IPAH 4F Rx, APAH 4F Rx), and the LII was determined by incubation with cultured aortic endothelial cells. The values for the patient LDL was divided by the value for a standard LDL preparation from a healthy control subject. Values are means ± SD.
Table 2.
Inflammatory indices for high-density lipoprotein (HII) and low-density lipoprotein (LII) and effect of apolipoprotein A-I mimetic peptide 4F treatment
| Controls (N = 21) | Idiopathic pulmonary arterial hypertension (N = 17) | Associated pulmonary arterial hypertension (N = 11) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | HII | LII | HII | HII + 4F | LII | LII + 4F | HII | HII + 4F | LII | LII + 4F |
| 1 | 0.489 | 0.939 | 1.03 | 0.70 | 0.86 | 0.96 | 0.82 | 0.83 | 2.53 | 2.07 |
| 2 | 0.863 | 1.07 | 2.07 | 1.35 | 1.19 | 1.01 | 1.70 | 1.43 | 1.30 | 1.1 |
| 3 | 0.490 | 0.980 | 1.80 | 1.58 | 1.22 | 1.04 | 2.31 | 1.34 | 1.82 | 1.62 |
| 4 | 0.490 | 1.07 | 1.74 | 1.34 | 1.40 | 1.15 | 1.78 | 1.21 | 1.12 | 0.98 |
| 5 | 0.517 | 0.993 | 1.97 | 1.13 | 1.21 | 0.97 | 2.18 | 1.23 | 1.11 | 0.88 |
| 6 | 0.497 | 0.966 | 1.65 | 0.69 | 1.02 | 0.89 | 2.82 | 1.90 | 0.95 | 0.60 |
| 7 | 0.884 | 1.10 | 1.56 | 1.12 | 1.29 | 1.17 | 1.36 | 0.89 | 1.14 | 0.96 |
| 8 | 0.483 | 1.09 | 1.19 | 0.61 | 1.12 | 1.11 | 1.86 | 0.97 | 1.34 | 0.97 |
| 9 | 0.510 | 1.01 | 1.46 | 0.76 | 1.31 | 1.07 | 1.34 | 0.57 | 1.27 | 1.14 |
| 10 | 0.497 | 0.946 | 1.26 | 0.67 | 1.21 | 0.98 | 1.13 | 0.65 | 1.14 | 1.05 |
| 11 | 0.490 | 1.10 | 1.16 | 0.76 | 1.12 | 1.15 | 1.76 | 0.89 | 1.19 | 1.04 |
| 12 | 0.905 | 0.952 | 1.89 | 0.86 | 1.28 | 1.11 | ||||
| 13 | 0.612 | 1.05 | 1.47 | 0.88 | 1.26 | 1.09 | ||||
| 14 | 0.639 | 1.01 | 1.38 | 0.95 | 1.09 | 0.84 | ||||
| 15 | 0.530 | 1.09 | 2.57 | 1.3 | 1.61 | 1.35 | ||||
| 16 | 0.626 | 1.02 | 2.11 | 1.54 | 1.45 | 1.29 | ||||
| 17 | 0.578 | 1.09 | 1.98 | 1.20 | 1.29 | 1.14 | ||||
| 18 | 0.898 | 1.13 | ||||||||
| 19 | 0.592 | 1.07 | ||||||||
| 20 | 0.599 | 0.993 | ||||||||
| 21 | 0.762 | 1.10 | ||||||||
| Mean ± SD | 0.617 ± 0.151 | 1.03 ± 0.009 | 1.68 ± 0.11 | 1.03 ± 0.01 | 1.24 ± 0.05 | 1.07 ± 0.04 | 1.69 ± 0.20 | 1.05 ± 0.14 | 1.34 ± 0.15 | 1.08 ± 0.13 |
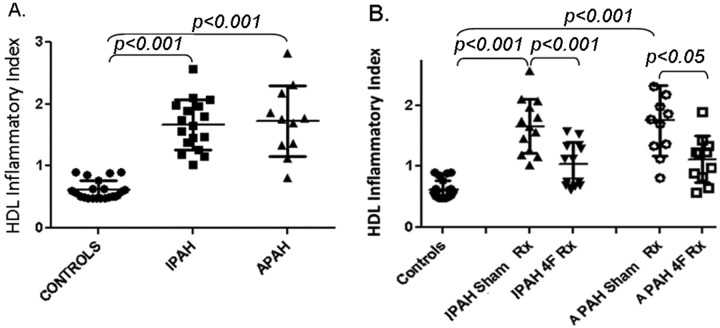
In Figure 3 and individual-subject data depicted in Table 2, the determined HIIs for both PAH cohorts reflected an abnormal or “pathologic HDL” phenotype. In distinct contrast to “normal HDL function” (controls), as represented by HIIs of <1.0 (i.e., anti-inflammatory in nature), for the IPAH and APAH cohorts the determined HIIs were significantly increased (P < 0.001), to 1.68 ± 0.11 and 1.69 ± 0.20 (P < 0.001), respectively, indicating a proinflammatory status.
Figure 3.
High-density lipoprotein (HDL) inflammatory index (HII). A, Standard low-density lipoprotein (LDL) from a healthy control subject was incubated with cultured human aortic endothelial cells without (data not shown) or with normal HDL (Controls) or with HDL from patients with idiopathic or associated pulmonary arterial hypertension (IPAH or APAH, respectively), and the HII was determined as described in “Methods.” B, To determine the effect of incubation with 4F, HDL from patients was treated with either saline (IPAH Sham Rx, APAH Sham Rx) or 4F (IPAH 4F Rx, APAH 4F Rx) before addition to the cultures. Values are means ± SD.
When associated with “normal HDL function,” treatment with 4F usually yielded HIIs of <1.0, with the HDL being anti-inflammatory. By contrast, in our study, the 4F ex vivo treatment significantly improved the HDL protective function versus sham treatment (Fig. 3B), as reflected by HIIs of 1.03 ± 0.09 (P < 0.001) for IPAH and 1.05 ± 0.14 (P < 0.05) for APAH. Therefore, although a significant “salutary effect” of 4F treatment was observed, this did not entirely normalize the HII and did not render HDL anti-inflammatory for either cohort.
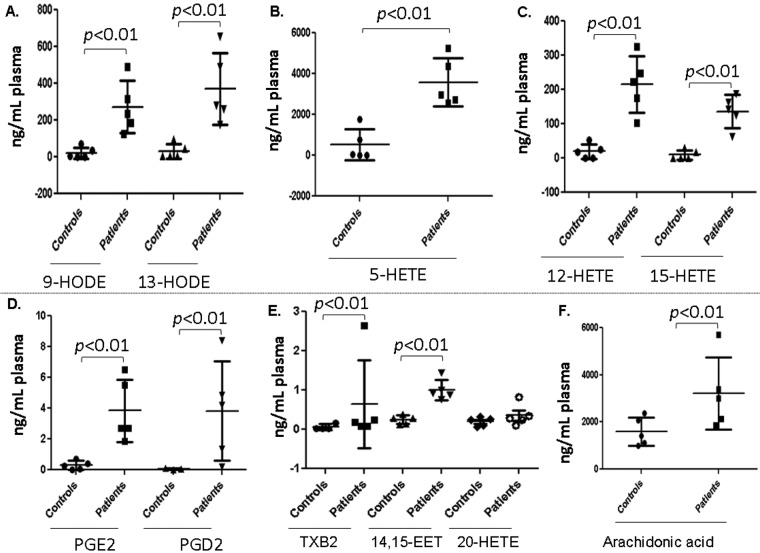
Five plasma samples from each group of healthy controls and IPAH and APAH patients were randomly subjected to LC-ESI-MS/MS analyses. As seen in Figure 4, the levels of plasma 9-HODE, 13-HODE, 5-HETE, 12-HETE, and 15-HETE were orders of magnitude higher in patient plasma than in healthy-control plasma. The levels of PGE2, PGD2, 14–15-EET (epoxyeicosatrienoic acid), and AA also were markedly higher (P < 0.01) in patient plasma than in healthy-control plasma. The data shown in Figure 4 are for patients with IPAH. Similar results were obtained for APAH (data not shown).
Figure 4.
Lipid mediators of inflammation. Plasma samples from 5 healthy controls and 5 patients with idiopathic pulmonary arterial hypertension were analyzed with liquid chromatography–electrospray ionization tandem mass spectrometry, as described in “Methods.” Values are means ± SD. EET: epoxyeicosatrienoic acid; HETE: hydroxyeicosatetraenoic acid; HODE: hydroxyoctadecadienoic acid; PGD2, PGE2: prostaglandin D2 and E2, respectively; TXB2: thromboxane B2.
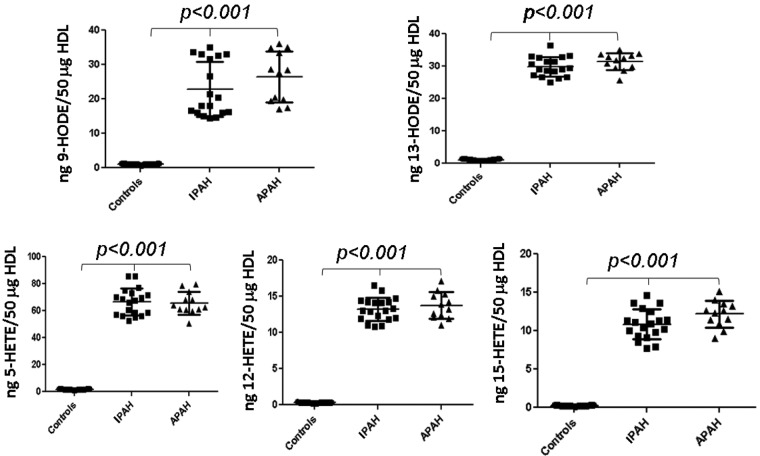
Plasma from healthy controls (N = 21) and the PAH patient cohorts were fractionated by FPLC, and the HDL-containing fractions were analyzed as depicted in Figure 5. Significantly increased levels for both IPAH and APAH patients versus controls (P < 0.001) were observed for 9-HODE, 13-HODE, 5-HETE, 12-HETE, and 15-HETE, while no statistical differences were evident in comparisons of IPAH and APAH patients for the determined plasma eicosanoid levels (P > 0.05).
Figure 5.
High-density lipoprotein (HDL)-oxidized lipids. HDL was fractionated from the plasma of healthy controls or patients with idiopathic or associated pulmonary arterial hypertension (IPAH or APAH, respectively) and analyzed with liquid chromatography–electrospray ionization tandem mass spectrometry, as described in “Methods.” Values are means ± SD. HETE: hydroxyeicosatetraenoic acid; HODE: hydroxyoctadecadienoic acid.
Discussion
In this study, we have demonstrated a “pathologic HDL phenotype” in subjects with PAH. This observation may reasonably be expected in our subjects with APAH as related to connective-tissue diseases; however, a similar magnitude of HDL dysfunction was noted in the IPAH cohort. This effect was further compounded by a significant increase in the normal proinflammatory influence of LDL in both PAH cohorts, as reflected by the increased LII values. A 25–50-fold increase in plasma levels of oxidation products of both arachidonic acid (HETEs) and linoleic acid (HODEs) therein suggested a significant increase in “oxidant stress” contributing to the proinflammatory HDL dysfunction in PAH. In this study, the proinflammatory effects of both LDL and HDL were mitigated after treatment ex vivo with the apoA-I mimetic peptide 4F. Although 4F did not entirely normalize the HII in our studies, a significant “salutary effect” of 4F treatment was observed that may represent a potential future “therapeutic target” in PAH. The recent report that the 4F peptide induces microRNA-193–3p in rodent models of PAH provides further support to the notion that apoA-I mimetic peptides may have potential in the treatment of these disorders.22
The mechanism(s) remain unclear regarding a putative role for inflammation in the complex pathobiology of PAH. Our group has recently reported the expression, in plasma samples from individuals with WHO group 1 PAH, of the specific “type 1 immune response” and “cytokine-chemokine cascade”: IL-18 induces MIG/CXCL9, IP-10/CXCL10, and ITAC/CXCL11. We observed augmented expression of IL-18 and CXCL10 in both subjects’ serum and the pulmonary arterial tunica media.7
Our observation of increased “oxidant stress” contributing to pathogenesis in PAH may potentially relate to an inhibitory influence of oxidized LDL (oxLDL) on endothelial prostacyclin (PGI2) generation, which has previously been described in rat aorta in vitro. Preincubation of aorta with a mixture of “normal HDL” and oxLDL, as compared to preincubation with only oxLDL, resulted in significant recovery of PGI2 generation and, hence, a potential “protective role” for HDL.23 Our observation of a “dysfunctional HDL phenotype” may partially explain the abnormally reduced PGI2 generation that is pivotal in the pathogenesis and treatment of experimental and clinical PAH.24-28 Further, in studies of pleiotropic HMG CoA (3-hydroxy-3-methyl-glutaryl coenzyme A) reductase inhibitor medications (“statins”) which, despite effectuating a reduction in “oxidant stress,” a reduction in Rho/Rho-kinase (ROCK) signaling, enhanced endothelial nitric oxide production, and increased expression of bone morphogenetic protein receptor type 2, have been disappointing in both the treatment of PAH and experimental models of vascular PGI2 production.29-34 “Oxidant stress” has been reported in PAH by Cracowski et al.,35 who described increased isoprostane urinary levels that correlated with worsened patient survival. Further, using oligonucleotide microarray gene expression, Geraci et al.36 have described an increase in pulmonary expression of oxidative genes in PAH patients.
Our observation of increased plasma levels of eicosanoids, such as 15-HETE, in PAH may also contribute to pathogenesis via influences on ROCK, a downstream target of the guanosine triphosphatase RhoA. ROCK has been described in pathologic processes, including endothelial dysfunction, vasoconstriction, and inflammation.37,38 Ma et al.39 have described a key role for 15-HETE in pulmonary vascular remodeling and vascular angiogenesis in a hypoxia model of PAH. In their investigation, 15-HETE regulated cell cycle progression and induced EC and PASMC proliferation via increase in messenger RNA and protein expression of ROCK in PASMCs.40
Potential pitfalls in our investigation may relate to confounding conditions with the associated inflammation potentially contributing to HDL dysfunction, particularly in our IPAH cohort. Viral respiratory infection or sepsis was not clinically evident at time of sample collection from our subjects.41 No type 1 diabetes mellitus or advanced renal dysfunction (less than chronic kidney disease stage 2) characterized our PAH subjects.21,42 In addition, although our bioassay of monocyte chemotaxis has been extensively validated and standardized in our prior investigations using cultured aortic ECs, we cannot discount the inherent differences between aortic and pulmonary arterial endothelium, which may have influenced our findings. Nevertheless, subjects’ LDL and HDL demonstrated a pathologic phenotype for proinflammatory monocyte chemotaxis in our assay.
In summary, our study has further implicated the putative role of “oxidant stress” and inflammation in the complex pathobiology of PAH. We speculate that therapies that target either the “inflammatory milieu” or the “dysfunctional HDL phenotype,” such as apoA-I mimetic peptides,43-46 may be valuable avenues of further research in pulmonary vascular diseases.
Acknowledgments
We wish to acknowledge the UCLA Pulmonary Hypertension Research Coordinator, Michaela Jacquet CCRC, for her invaluable assistance with the study.
Source of Support: This work was supported in part by US Public Health Service grants HL30568 and HL34343 and a Network Grant from the Leducq Foundation.
Conflict of Interest: SR, MN, and AMF are principals in Bruin Pharma, and AMF is an officer in Bruin Pharma. All other authors have no conflicts of interest to declare.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl.):D34–D41. [DOI] [PubMed]
- 2.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 2010;121(18):2045–2066. [DOI] [PMC free article] [PubMed]
- 3.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144(2):275–285. [PMC free article] [PubMed]
- 4.Voelkel NF, Tuder R. Interleukin-1 receptor antagonist inhibits pulmonary hypertension induced by inflammation. Ann NY Acad Sci 1994;725:104–109. [DOI] [PubMed]
- 5.Balabanian K, Foussat A, Dorfmüller P, Durand-Gasselin I, Capel F, Bouchet-Delbos L, Portier A, et al. CX3C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med 2002;165(10):1419–1425. [DOI] [PubMed]
- 6.Dorfmüller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G, Capron F, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2002;165(4):534–539. [DOI] [PubMed]
- 7.Ross DJ, Strieter RM, Fishbein MC, Ardehali A, Belperio JA. Type I immune response cytokine-chemokine cascade is associated with pulmonary arterial hypertension. J Heart Lung Transplant 2012;31(8):865–873. [DOI] [PubMed]
- 8.Imaizumi S, Grijalva V, Navab M, Van Lenten BJ, Wagner AC, Anantharamaiah GM, Fogelman AM, Reddy ST. L-4F differentially alters plasma levels of oxidized fatty acids resulting in more anti-inflammatory HDL in mice. Drug Metab Lett 2010;4(3):139–148. [DOI] [PMC free article] [PubMed]
- 9.Davidson MH, Toth PP. High-density lipoprotein metabolism: potential therapeutic targets. Am J Cardiol 2007;100(11 suppl. 1):S32–S40. [DOI] [PubMed]
- 10.Deigner HP, Hermetter A. Oxidized phospholipids: emerging lipid mediators in pathophysiology. Curr Opin Lipidol 2008;19(3):289–294. [DOI] [PubMed]
- 11.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res 2006;69(1):36–45. [DOI] [PubMed]
- 12.Lähteenmäki TA, Seppo L, Laakso J, Korpela R, Vanhanen H, Tikkanen MJ, Vapaatalo H. Oxidized LDL from subjects with different dietary habits modifies atherogenic processes in endothelial and smooth muscle cells. Life Sci 2000;66(5):455–465. [DOI] [PubMed]
- 13.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation 2001;103(18):2283–2288. [DOI] [PubMed]
- 14.Imaizumi S, Navab M, Morgantini C, Charles-Schoeman C, Su F, Gao F, Kwon M, et al. Dysfunctional high-density lipoprotein and the potential of apolipoprotein A-1 mimetic peptides to normalize the composition and function of lipoproteins. Circ J 2011;75(7):1533–1538. [DOI] [PMC free article] [PubMed]
- 15.Watanabe J, Grijalva V, Hama S, Barbour K, Berger FG, Navab M, Fogelman AM, Reddy ST. Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J Biol Chem 2009;284(27):18292–18301. [DOI] [PMC free article] [PubMed]
- 16.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 2011;8(4):222–232. [DOI] [PubMed]
- 17.Navab M, Anantharamaiah GM, Fogelman AM. The role of high-density lipoprotein in inflammation. Trends Cardiovasc Med 2005;15(4):158–161. [DOI] [PubMed]
- 18.Navab M, Reddy ST, Van Lenten BJ, Buga GM, Hough G, Wagner AC, Fogelman AM. High-density lipoprotein and 4F peptide reduce systemic inflammation by modulating intestinal oxidized lipid metabolism: novel hypotheses and review of literature. Arterioscl Thromb Vasc Biol 2012;32(11):2553–2560. [DOI] [PMC free article] [PubMed]
- 19.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res 2008 49(6):1344–1352. [DOI] [PMC free article] [PubMed]
- 20.Van Lenten BJ, Wagner AC, Jung CL, Waring AJ, Ruchala P, Lehrer RI, Watson AD, et al. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res 2008;49(11):2302–2311. [DOI] [PMC free article] [PubMed]
- 21.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int 2009;76(4):437–444. [DOI] [PMC free article] [PubMed]
- 22.Sharma S, Umar S, Potus F, Iorga A, Wong G, Meriwether D, Breuils-Bonnet S, et al. Apolipoprotein A-I mimetic peptide 4F rescues pulmonary hypertension by inducing microRNA-193-3p. Circulation 2014;130(9):776–785. [DOI] [PMC free article] [PubMed]
- 23.Mahfouz MM, Kummerow FA. High density lipoprotein can modulate the inhibitory effect of oxLDL on prostacyclin generation by rat aorta in vitro. Prostaglandins Other Lipid Mediat 2003;72(3–4):91–114. [DOI] [PubMed]
- 24.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 1999;159(6):1925–1932. [DOI] [PubMed]
- 25.Archer SL, Chesler E, Cohn JN, Weir EK. ZK 36–374, a stable analog of prostacyclin, prevents acute hypoxic pulmonary hypertension in the dog. J Am Coll Cardiol 1986;8(5):1189–1194. [DOI] [PubMed]
- 26.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. New Engl J Med 1996;334(5):296–301. [DOI] [PubMed]
- 27.Barst RJ, Rubin LJ, McGoon MD, Caldwell EJ, Long WA, Levy PS. Survival in primary pulmonary hypertension with long-term continuous intravenous prostacyclin. Ann Int Med 1994;121(6):409–415. [DOI] [PubMed]
- 28.Simonneau G, Barst RJ, Galiè N, Naeije R, Rich S, Bourge RC, Keogh A, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002;165(6):800–804. [DOI] [PubMed]
- 29.Mahfouz MM, Kummerow FA. Atorvastatin reduces the plasma lipids and oxidative stress but did not reverse the inhibition of prostacyclin generation by aortas in streptozotocin diabetic rats. Prostaglandins Other Lipid Mediat 2005;76(1–4):59–73. [DOI] [PubMed]
- 30.McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin-atorvastatin-simvastatin study. Am J Physiol Lung Cell Mol Physiol 2007;293(4):L933–L940. [DOI] [PubMed]
- 31.Wilkins MR, Ali O, Bradlow W, Wharton J, Taegtmeyer A, Rhodes CJ, Ghofrani HA, et al. Simvastatin as a treatment for pulmonary hypertension trial. Am J Respir Crit Care Med 2010;181(10):1106–1113. [DOI] [PMC free article] [PubMed]
- 32.Watson G, Oliver E, Zhao L, Wilkins MR. Pulmonary hypertension: old targets revisited (statins, PPARs, beta-blockers). In: Humbert M, Evgenov OV, Stasch JP, eds. Pharmacotherapy of pulmonary hypertension. Handb Exp Pharmacol 2013;218:531–548. doi:10.1007/978-3-642-38664-0_21. [DOI] [PubMed]
- 33.Robbins IM, Kawut SM, Yung D, Reilly MP, Lloyd W, Cunningham G, Loscalzo J, Kimmel SE, Christman BW, Barst RJ. A study of aspirin and clopidogrel in idiopathic pulmonary arterial hypertension. Eur Respir J 2006;27(3):578–584. [DOI] [PubMed]
- 34.Zeng WJ, Xiong CM, Zhao L, Shan GL, Liu ZH, Xue F, Gu Q, et al. Atorvastatin in pulmonary arterial hypertension (APATH) study. Eur Respir J 2012;40(1):67–74. [DOI] [PubMed]
- 35.Cracowski JL, Cracowski C, Bessard G, Pepin JL, Bessard J, Schwebel C, Stanke-Labesque F, Pison C. Increased lipid peroxidation in patients with pulmonary hypertension. Am J Respir Crit Care Med 2001;164(6):1038–1042. [DOI] [PubMed]
- 36.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res 2001;88(6):555–562. [DOI] [PubMed]
- 37.Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med 2005;171(5):494–499. [DOI] [PubMed]
- 38.McLoughlin P, Hyvelin JM, Howell K. Pulmonary hypertension. New Engl J Med 2005;352(4):418–419. [DOI] [PubMed]
- 39.Ma C, Li Y, Ma J, Liu Y, Li Q, Niu S, Shen Z, Zhang L, Pan Z, Zhu D. Key role of 15-lipoxygenase/15-hydroxyeicosatetraenoic acid in pulmonary vascular remodeling and vascular angiogenesis associated with hypoxic pulmonary hypertension. Hypertension 2011;58(4):679–688. [DOI] [PubMed]
- 40.Ma J, Zhang L, Li S, Liu S, Ma C, Li W, Falck JR, et al. 8,9-Epoxyeicosatrienoic acid analog protects pulmonary artery smooth muscle cells from apoptosis via ROCK pathway. Exp Cell Res 2010;316(14):2340–2353. [DOI] [PMC free article] [PubMed]
- 41.Barlage S, Gnewuch C, Liebisch G, Wolf Z, Audebert FX, Glück T, Fröhlich D, Krämer BK, Rothe G, Schmitz G. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med 2009;35(11):1877–1885. [DOI] [PubMed]
- 42.Moradi H, Vaziri ND, Kashyap ML, Said HM, Kalantar-Zadeh K. Role of HDL dysfunction in end-stage renal disease: a double-edged sword. J Renal Nutr 2013;23(3):203–206. [DOI] [PMC free article] [PubMed]
- 43.Reddy ST, Navab M, Anantharamaiah GM, Fogelman AM. Apolipoprotein A-I mimetics. Curr Opin Lipidol 2014;25(4):304–308. [DOI] [PMC free article] [PubMed]
- 44.Reddy ST, Navab M, Anantharamaiah GM, Fogelman AM. Searching for a successful HDL-based treatment strategy. Biochim Biophys Acta Mol Cell Biol Lipids 2014;1841(1):162–167. [PubMed]
- 45.Lüscher TF, Landmesser U, von Eckardstein A, Fogelman AM. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res 2014;114(1):171–182. [DOI] [PubMed]
- 46.Fogelman AM. Trying to harness the potential of HDL: wishful thinking or sound strategy? Eur Heart J 2014;35(46):3248–32479. [DOI] [PubMed]