Abstract Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease that puts excessive mechanical loads on the ventricle due to a gradual increase in pulmonary vascular impedance. We hypothesize that the increase in right ventricular (RV) afterload is reflected in the concentration of circulating biochemical markers of ventricular strain and stress (B-type natriuretic peptide [BNP] and N-terminal prohormone BNP [NT-proBNP]). We retrospectively analyzed right heart catheterization (RHC) and serum biochemical analysis data (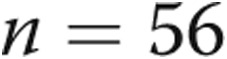 ) for a pediatric PAH cohort with no sign of left ventricular dysfunction. Using RHC data, we computed an estimate of pulmonary vascular resistance (PVR), compliance, and ventricular-vascular coupling. We also compared how the early onset of interventricular decoupling (characterized as septal flattening) impacts serum NT-proBNP concentrations. Our data revealed correlated NT-proBNP expression with both the resistive and reactive components of RV afterload, an estimate of ventricular-vascular coupling, and a significant increase in biomarker expression in patients with a flattened interventricular septum. Furthermore, the strong correlation between PVR and NT-proBNP appears to break down under flat septum morphology. Over 80% of resistive RV afterload variance is reflected in serum NT-proBNP concentration in pediatric patients with PAH with no sign of left ventricular dysfunction. Reactive afterload appears to contribute to myocardial NT-proBNP release at advanced stages of PAH. Therefore, in mild-to-moderate PAH, resistive afterload is likely the greatest contributor to RV wall stress. These findings could also be used to estimate invasive RHC measurements from serum biochemical analysis, but more work is needed to improve correlations and overcome the issue of interventricular decoupling.
) for a pediatric PAH cohort with no sign of left ventricular dysfunction. Using RHC data, we computed an estimate of pulmonary vascular resistance (PVR), compliance, and ventricular-vascular coupling. We also compared how the early onset of interventricular decoupling (characterized as septal flattening) impacts serum NT-proBNP concentrations. Our data revealed correlated NT-proBNP expression with both the resistive and reactive components of RV afterload, an estimate of ventricular-vascular coupling, and a significant increase in biomarker expression in patients with a flattened interventricular septum. Furthermore, the strong correlation between PVR and NT-proBNP appears to break down under flat septum morphology. Over 80% of resistive RV afterload variance is reflected in serum NT-proBNP concentration in pediatric patients with PAH with no sign of left ventricular dysfunction. Reactive afterload appears to contribute to myocardial NT-proBNP release at advanced stages of PAH. Therefore, in mild-to-moderate PAH, resistive afterload is likely the greatest contributor to RV wall stress. These findings could also be used to estimate invasive RHC measurements from serum biochemical analysis, but more work is needed to improve correlations and overcome the issue of interventricular decoupling.
Keywords: pulmonary hypertension, biomarker, NT-proBNP, afterload
Pulmonary arterial hypertension (PAH) is a progressive disease that presents several prognostic challenges. The disease is officially diagnosed by performing right heart catheterization (RHC) and evaluating the mean pulmonary arterial pressure (mPAP) threshold (mPAP <25 mmHg).1 RHC is an invasive procedure that requires anesthetization for pediatric patients and would be unsuitable for regular prognosis. Therefore, it is simply not a practical option for periodic screening,2 which is necessary to properly assess treatment efficacy and gauge disease progression. However, RHC is currently the gold standard in patient monitoring because it can be used to obtain a myriad of other critical measurements that have been correlated with outcome and provide an overall picture of right ventricle (RV) and pulmonary artery (PA) function (e.g., cardiac output [CO], right atrial pressure, pulmonary capillary wedge pressure [PCWP], pulmonary vascular resistance [PVR], and capacitance [C]). Given the logistical limitations of periodically obtaining patient RHC data, particularly in children, plasma protein biomarkers have been recognized as a potential surrogate for direct RV functional measurements.3,4
B-type natriuretic peptide (BNP) is a neurohormone secreted in both the left ventricle (LV) and RV in response to volume expansion5 and pressure overload.6 N-terminal prohormone BNP (NT-proBNP) is a cleaved byproduct of BNP,7 which has a longer in vivo half-life8 and offers numerous logistical advantages for clinical monitoring.2 Serum biomarker expression has been shown to be a better indicator of outcome than mPAP,3 with the diagnostic value of BNP and NT-proBNP well established in symptomatic and asymptomatic9 heart failure.10 These biochemical markers have also revealed enormous prognostic value in pulmonary hypertension7,11 with the potential for assessing treatment efficacy, because serum levels have been shown to decrease with functional improvement.6,12 In the pediatric pulmonary hypertension (PH) population, these peptides are currently used for disease management and have been associated with several functional hemodynamic markers.13 However, in PAH, RV biomarker expression has not been directly associated with the resistive and reactive components for afterload, which would establish a direct link between progressive vascular dysfunction and ventricular response. Nevertheless, directly correlating afterload with NT-proBNP expression requires additional consideration in children, who naturally decrease the amount of circulating biomarker with age,10,14,15 making it an influential variable.
The overall objective of our research group is to maximize the clinical utilization of easily measurable serum biochemical properties to offer insight into RV-PA hemodynamics and functional state. The objective of this study was to (1) establish correlations between functional phenotype and RV myocardial NT-proBNP expression in the pediatric PH population and (2) investigate the role of interventricular septal flattening on biomarker expression.
Methods
Patient demographic and study acceptance criteria
Retrospective RHC and biomarker data for 36 patients with PH (mPAP >25 mmHg confirmed by RHC), ranging in age from infancy to young adulthood (Fig. 2), were analyzed for our study. Among the 36 patients, some offered biannual follow-up measurements that were each considered as an individual data set within the sample, resulting in a total data set of 56 sets of data. Longitudinal disease progression was not considered for this study. In addition to RHC, each patient underwent same-day echocardiography to assess ventricular function. Only RHC data sets that corresponded to no LV dysfunction or morphological abnormalities, decreed by a trained cardiologist, were included in the study, leading to the reasonable assumption that the measured serum biomarker expression originates from the RV myocardium. Additional sample patient demographic characteristics are included in Figure 2.
Figure 2.
Quintile plots showing minimum, lower, median, upper, and maximum quintiles of patient demographic characteristics and right heart catheterization hemodynamic data. BPM: beats per minute; BSA: body surface area; CO: cardiac output; HR: heart rate; mPAP: mean pulmonary arterial pressure.
PA function phenotyping (afterload)
The resistive and reactive components of afterload of the PA vasculature is ideally characterized using three-element Windkessel model.16 The distal resistance (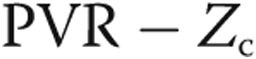 ) and C can be found from typical RHC data,
) and C can be found from typical RHC data,
 |
 |
In equations (1) and (2), mPAP is the mean pulmonary arterial pressure, PCWP is the pulmonary capillary wedge pressure approximated as the pressure in the left atrium, CO is the cardiac output, HR is the heart rate, and PP is the pulse pressure. The characteristic impedance (Zc) can be found using a technique that has been outlined in detail in Kheyfets et al.17 In short, Zc is computed from an estimate of PA distensibility (D; eq. 3), where  is considered PA resistance under zero pressure:18,19
is considered PA resistance under zero pressure:18,19
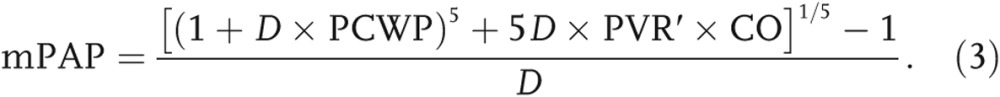 |
The final characteristic impedance is found in equation (4), where ρ is the blood density (1.06 g/mL) and R is the lumen radius assumed proportional to body surface area (BSA),
 |
A measure of afterload that combines all the Windkessel parameters can be computed as the arterial elastance (Ea):
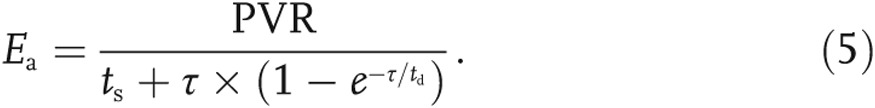 |
In equation (5), ts and td are the systolic and diastolic times, assumed to be 30% and 50% of the cardiac cycle, respectively. The diastolic pressure decay is defined as follows: 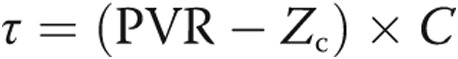 .
.
Phenotyping RV-PA coupling
Ideally, RV-PA coupling would be computed with the aid of ventricular pressure-volume loops,20 which were not available for the present data set. Therefore, we introduce an alternative nondimensional parameter symbolizing the ratio of ventricular to vascular function:
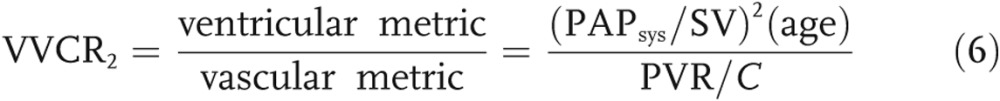 |
The numerator in equation (6) represents the ventricular end-systolic function (assuming that PA pressure at end systole [PAPsys] is equal to RV pressure at end systole [RVPsys]), normalized by the displaced volume. As disease progresses, the stroke volume (SV) decreases, whereas 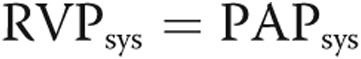 increases, thus increasing the numeration. The denominator of equation (6) represents the ratio of resistive to reactive afterload. As the disease progresses, the denominator also increases, causing the following scenario: when afterload increases at a considerately faster rate than ventricular accommodation, the overall ratio will decrease.
increases, thus increasing the numeration. The denominator of equation (6) represents the ratio of resistive to reactive afterload. As the disease progresses, the denominator also increases, causing the following scenario: when afterload increases at a considerately faster rate than ventricular accommodation, the overall ratio will decrease.
Biochemical phenotyping
BNP and NT-proBNP plasma concentrations were measured within 5 days of RHC at Children’s Hospital Colorado (Aurora, CO). BNP analysis was done on blood samples collected in ethylenediaminetetraacetic acid tubes, whereas NT-proBNP analysis was performed on blood collected in a serum sample tube. The minimum measurable BNP values were bound to 15 pg/mL, which was assayed using the i-STAT system with a two-site enzyme-linked immunosorbant assay (Abbott Laboratories, Chicago, IL). Serum NT-proBNP was measured using an electrochemiluminescence immunoassay (ProBNP II, Roche Diagnostics, Indianapolis, IN) at Mayo Medical Laboratories (Rochester, MN).
Statistical analysis
We present two types of statistical data: (1) linear correlations and (2) comparisons of the mean of two samples (PH patients with flattened septum vs. normal morphology). For both tests, each data set was confirmed to be normally distributed (Gaussian distribution) with logarithmic operations performed to improve normality (Fig. 1).
Figure 1.
Example of data normalization applied to N-terminal prohormone B-type natriuretic peptide (NT-proBNP) measurements and any data set with a nonnormal distribution. A histogram (A) and quantile-quantile (Q-Q) plot (B) of the nonnormalized NT-proBNP expression are shown, alongside a histogram (C) and Q-Q plot (D) of the same data normalized using a log transform.
An unpaired Student t test was performed to compare the means of two sample groups. An F test was used to assess variance equivalence. For both statistical tests, a 95% confidence interval was considered to be significant, suggesting a 95% probability that the sample result is reflective of the population.21
Results
Data set demographic characteristics
Figure 2 shows relevant study data set demographic and hemodynamic characteristics. Given the large age range considered in our study and the fact that NT-proBNP expression has been shown to vary in development,14 all of the following correlations are normalized by log10(age).
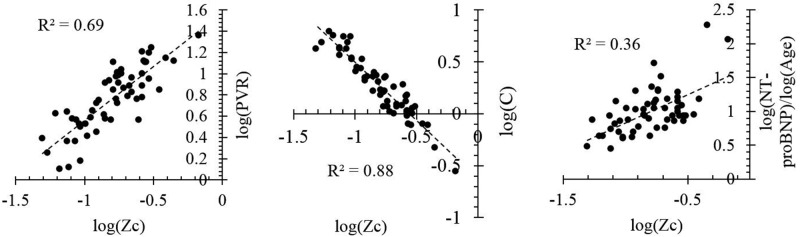
The characteristic impedance (Zc) was found using equation (6), combined with the relationship between pulse wave velocity and distensibility.17 Figure 3 shows a comparison between Zc and PVR, compliance, and NT-proBNP. The close relationship between C and Zc offers an indirect validation of the technique, because the derivation for Zc did not include any aspect of the compliance equation (eq. [2]). As expected, the characteristic impedance appears to increase with increasing PVR and decreasing compliance, reflecting disease progression. Furthermore, Zc explains 36% of the variability in NT-proBNP expression, with both variables increasing concurrently.
Figure 3.
Relationship between estimated characteristic impedance (Zc) and right heart catheterization measured afterload and N-terminal prohormone B-type natriuretic peptide (NT-proBNP) expression ( , by two-tailed test). Zc appears to reflect both the reactive and resistive components of afterload and is increasing concurrently with NT-proBNP expression. C: capitance; PVR: pulmonary vascular resistance.
, by two-tailed test). Zc appears to reflect both the reactive and resistive components of afterload and is increasing concurrently with NT-proBNP expression. C: capitance; PVR: pulmonary vascular resistance.
BNP vs. NT-proBNP expression
Earlier studies have reported on the relationship between BNP and NT-proBNP expression.10,22 In this article, we show that our data set is consistent with earlier findings (see Fig. 4). Given the relationship between the two biomarkers and the aforementioned clinical advantages of measuring NT-proBNP over BNP, the following discussion will focus on NT-proBNP.
Figure 4.
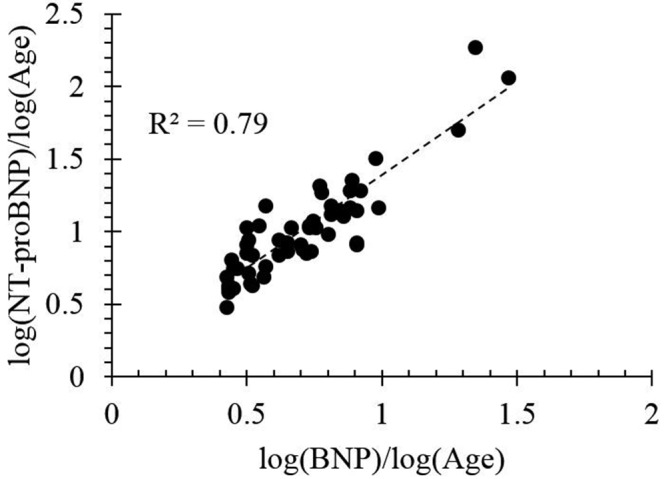
Relationship between log B-type natriuretic peptide (BNP) and log N-terminal prohormone BNP (NT-proBNP) in the considered data set (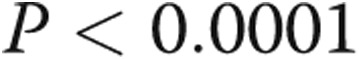 , by two-tailed test) in patients with no evidence of left ventricular morphological or functional abnormalities. Several cardiologists recorded BNP to be <10 pg/mL, which still corresponded to a large variation in NT-proBNP.
, by two-tailed test) in patients with no evidence of left ventricular morphological or functional abnormalities. Several cardiologists recorded BNP to be <10 pg/mL, which still corresponded to a large variation in NT-proBNP.
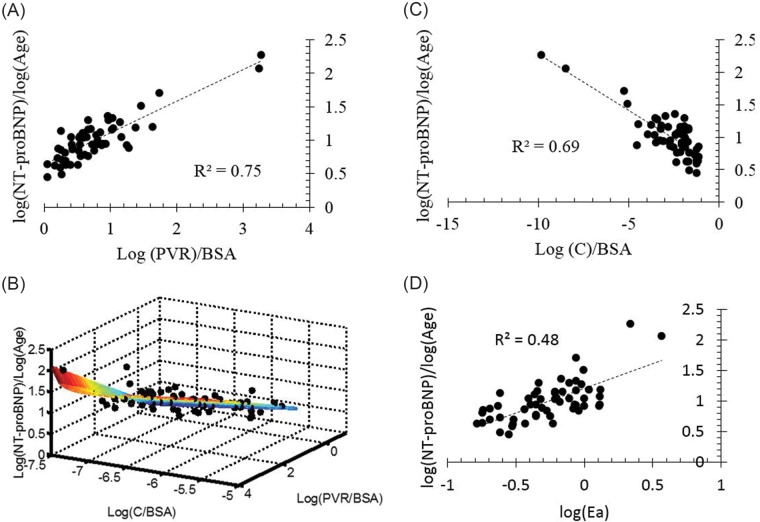
NT-proBNP expression and PA functional phenotype
Figure 5 shows normalized NT-proBNP correlated against resistive (A) and reactive (B) components of impedance, a multivariate model of PVR and compliance (C), and arterial elastance (D). For both the resistive and reactive components of impedance, afterload is associated with an increase in NT-proBNP expression, but the log of PVR index (PVRi, defined as PVR/BSA) explains approximately 75% of the variability in NT-proBNP. A multivariate regression, combing PVRi and C index, results in the following expression: 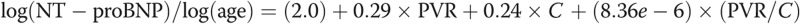 (
(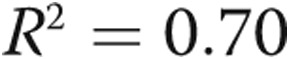 ,
, 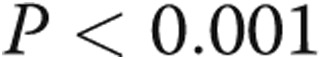 , by two-tailed test). Furthermore, the P values for all three terms are statistically significant (
, by two-tailed test). Furthermore, the P values for all three terms are statistically significant (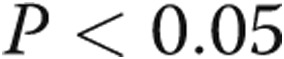 , by two-tailed test). It is noteworthy that the coefficient of the last term is small, but it must be considered in terms of the unit increase in the ratio, and its inclusion improves the statistical significance of the other two terms.
, by two-tailed test). It is noteworthy that the coefficient of the last term is small, but it must be considered in terms of the unit increase in the ratio, and its inclusion improves the statistical significance of the other two terms.
Figure 5.
A, Normalized N-terminal prohormone B-type natriuretic peptide (NT-proBNP) correlated against resistive afterload. B, Normalized NT-proBNP correlated against reactive afterload. C, Nonlinear multivariate regression function of reactive and resistive components of pulmonary vascular impedance correlated against normalized NT-proBNP. D, Normalized NT-proBNP correlated against arterial elastance (Ea). BSA: body surface area; C: capacitance; PVR: pulmonary vascular resistance.
NT-proBNP expression and RV-PA coupling
Because no RV-PA coupling is available for this retrospective data set, a nondimensional estimate was developed (using Buckingham π theorem) to indicate RV response to afterload increase (ventricular-vascular coupling ratio [VVCR2]). Figure 6 shows a linear correlation between VVCR2 and NT-proBNP expression, which suggests that a decrease in the coupling ratio corresponds to an increase in biomarker expression.
Figure 6.

Relationship between age-normalized N-terminal prohormone B-type natriuretic peptide (NT-proBNP) expression and approximated ventricular-vascular coupling ratio (VVCR2; 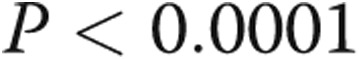 , by two-tailed test).
, by two-tailed test).
NT-proBNP expression and interventricular coupling (septal flattening)
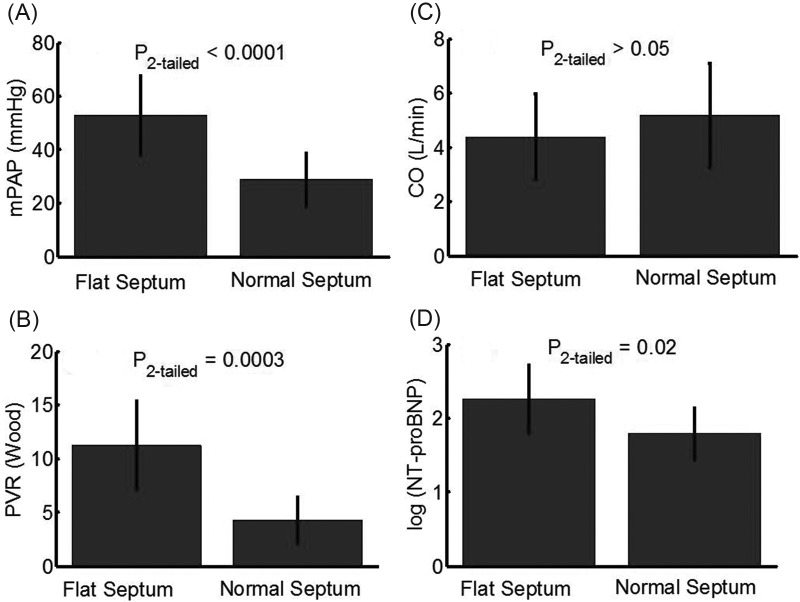
The interventricular septum is known to flatten in response to a decreased pressure difference between the LV and the RV. Here, we show that interventricular septal flattening occurs concurrently with RV-PA functional degradation and RV distress biomarker expression, before a notable decrease in pump (RV pumping) function. Within the entire data set considered, the sample was divided into two groups, (1) moderate-to-severe septal flattening and (2) mild-to-normal septal flattening, which were categorized on the basis of echocardiographic findings within 24 h of RHC by a trained cardiologist. Figure 7 shows bar plots (with error bars indicating standard deviation) comparing differences between patients with a notably flat interventricular septum and normal septal motion. As expected, an increased mPAP and PVR cause a flattened septum, which does not appear to contribute to a decreased CO. However, patients with a notably flattened septum do have a significantly higher plasma NT-proBNP concentration. It is worth noting that the group with a flattened septum, which showed an increased PVR and NT-proBNP, did not reveal any difference in systemic vascular resistance when compared with the normal septal morphology group.
Figure 7.
Bar plots (with standard deviation represented by error bars) showing statistical difference in mean pulmonary arterial pressure (mPAP; A), resistive afterload (B), ventricular pump function (C), and N-terminal prohormone B-type natriuretic peptide (NT-proBNP; D) expression between patients with normal and flattened interventricular septums. In scenarios of increased afterload and mPAP, with preserved cardiac output (CO), right ventricular distress biomarker expression is significantly increased in patients with a flattened interventricular septum. PVR: pulmonary vascular resistance.
Considering only patients with a normal interventricular septum
Performing the same correlations as above while excluding patients with a flattened septum significantly improves most of the presented correlations, notably the correlations between PVR and 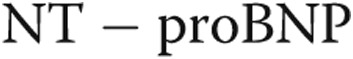 (
(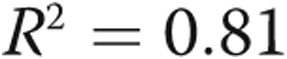 ), C and
), C and 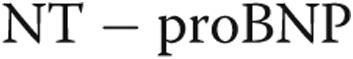 (
(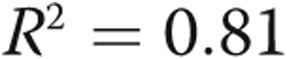 ), and
), and 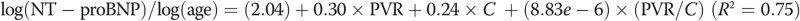
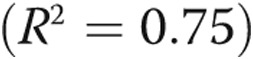 . However, the correlation between PVR and C revealed an
. However, the correlation between PVR and C revealed an  (plots not shown).
(plots not shown).
Discussion
Note on study demographics and data manipulation
The patients considered in this study vary in age from infants several months old to young adults. Nevertheless, pediatric PH patients were the primary target for this study, and an explicit comparison should be done involving adults to confirm the resulting trends. In fact, NT-proBNP expression has been reported to be lower in pediatric patients, compared with adults.11
Due to the fact that NT-proBNP appeared to change with age in the pediatric population, we divided measured concentration levels by age. PVR, a computation that is a function of a volume measurement (CO) that is proportional to size, is traditionally indexed by multiplying BSA.23 However, in this study, we found that it better correlated with NT-proBNP when dividing it by BSA. This is not an attempt to change convention, but rather simply to show that NT-proBNP is indeed governed by a function of (1) resistive afterload and (2) a variable indicative of patient body size, which is a simple, noninvasive measurement. Therefore, if the present results are corroborated in larger future studies and the considerations presented in this article are accounted for (septal inversion, discussed below), circulating biomarkers could be used to compute afterload and other important clinical characteristics.
Relationship between RV NT-proBNP expression and vascular functional phenotype
Gradual degradation of vascular function in PH is characterized by distal remodeling,24,25 attributed to a complex network of biochemical pathways and hemodynamically appearing as an increase in PVR and a decrease in vascular compliance (increasing the reactive component of impedance). The increased transmural pressure leads to an elevation in vascular circumferential stress in the proximal PA,26 thus triggering an up-regulation of load-bearing extracellular matrix proteins. Unlike the adult vasculature, which sees an up-regulation of collagen, a stiff protein with a short half-life, the developing circulation sees an increase in elastin. Elastin is more compliant than collagen, has a half-life on the order of decades,27 and allows for a more uniform stress distribution within the vessel wall.28 This effectively reduces the reactive afterload acting on the RV, which would undoubtedly impact biomarker expression.
We attempted to correlate NT-proBNP expression against four measures of afterload: (1) PVR, (2) C, (3) Ea, and (4) multivariate regression, considering both the resistive and reactive components of afterload. NT-proBNP correlated against the resistive component of afterload (PVR) produced a better association than Ea, compliance, or the multivariate regression. In the multivariate regression, the predictive capability of the model was close to that of PVR alone, but C did not appear to have a significant impact on NT-proBNP variability other than in several patients with extremely stiffened PAs. This is supported by (1) the three-dimensional mesh plot, which shows a sudden increase at extremely low compliance but is otherwise flat across the 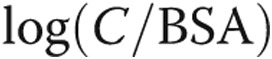 axis, and (2) in the linear regression, which shows
axis, and (2) in the linear regression, which shows  vs. NT-proBNP. In the latter, the strong correlation is mostly driven by patients with extremely low compliance, with the remainder of the sample poorly distributed. The resistive component of afterload explains over 70% of the variability in NT-proBNP expression. Interestingly, the association found here in the pediatric population is notably more significant than that found in the adult population.29 PVR is a measure of the dissipated energy of blood flow,30 resulting from distal constriction, that must be replaced by a source. Given the aforementioned remodeling differences between pediatric and adult patients, it is possible that the reactive component of impedance plays a lesser role in pediatrics, with PVR contributing to the majority of ventricular distress.
vs. NT-proBNP. In the latter, the strong correlation is mostly driven by patients with extremely low compliance, with the remainder of the sample poorly distributed. The resistive component of afterload explains over 70% of the variability in NT-proBNP expression. Interestingly, the association found here in the pediatric population is notably more significant than that found in the adult population.29 PVR is a measure of the dissipated energy of blood flow,30 resulting from distal constriction, that must be replaced by a source. Given the aforementioned remodeling differences between pediatric and adult patients, it is possible that the reactive component of impedance plays a lesser role in pediatrics, with PVR contributing to the majority of ventricular distress.
An increased PVR also decreases the normal pressure difference between the ventricles and is partially responsible for the flattened septum. In fact, PVR offers the most notable difference between patients with normal and flat interventricular septal walls and would be a rational explanation for the RV-PA pressure buildup. Within the current sample, patients with moderate to severe septal flattening presented elevated serum biomarker expression, with relatively preserved CO. Therefore, the physical shift of the septum does not directly impact CO, but it could be directly influencing increased LV pressure and falsifying NT-proBNP readings. Within our data set, the strength of the correlation between PVRi and NT-proBNP expression is notably decreased in patients isolated with a flat interventricular septum. However, it is increased when considering only those patients with a normal septum. This could be explained in two ways: (1) the septal shift works to absorb the increased afterload and take stress off the myocardial wall, thus decreasing distress biomarker expression; and/or (2) the septal shift causes an increase in LV myocardial stress, which could be resulting in LV myocardium biomarker expression, thus supplying blood with circulating peptides that originated from both ventricles. The latter explanation is likely more descriptive of the current findings, given that the overall level of biomarker expression increased in the group with a flattened septum. Therefore, serum NT-proBNP levels in patients with even mild LV dysfunction or interventricular septal flattening might not be ideal estimates of PA vascular function.
Relationship between RV NT-proBNP expression and ventricular-vascular coupling
Ventricular-vascular coupling is typically computed as the ratio of end-systolic elastance to Ea.20,31 Initiatively, it is a measure of the RV functional reserve, beginning to decrease in manifest PH as the RV is no longer able to accommodate an increase in afterload through adaptive remodeling mechanisms. Ventricular-vascular coupling has been shown to decrease concurrently with BNP in a developing PH pig model32 but has not been adequately investigated in pediatric patients with regard to RV-PA decoupling. Properly computing ventricular-vascular coupling requires a simultaneous measure of ventricular pressure and volume under baseline and challenge conditions. In animal studies, this can be achieved with a vena-cava occlusion, with is obviously not an option for human studies. Numerous estimates have been proposed to circumvent this challenge,33 but validating these techniques remains an ongoing process. Regardless, the current retrospective data set considered for this study did not offer adequate data for reliably computing ventricular-vascular coupling. Therefore, we sought to arrive at a nondimensional equation that relates the available hemodynamic measurements of ventricular function to vascular function. For this purpose, we considered ventricular function to be represented as the maximum pressure generated by the RV, normalized by the volume of blood displaced in each stroke. As the ventricle remodels, it is able to generate higher pressures with frequent strokes of decreased volume, suggesting that the numerator of equation (6) would increase with disease. It is worth noting that some studies have used the ratio of  to estimate systemic vascular afterload (Ea),34 whereas a recent review suggested the estimate was using mPAP20 in the pulmonary circulation. However, we feel systolic PAP is more characteristic of ventricular performance, because it is more reflective of the maximum RV pressure. In our estimate (eq. [6]), vascular performance was taken as the ratio of resistive to reactive afterload (
to estimate systemic vascular afterload (Ea),34 whereas a recent review suggested the estimate was using mPAP20 in the pulmonary circulation. However, we feel systolic PAP is more characteristic of ventricular performance, because it is more reflective of the maximum RV pressure. In our estimate (eq. [6]), vascular performance was taken as the ratio of resistive to reactive afterload ( ). As the disease progresses, PVR increases while C decreases, thus increasing the ratio of the two measurements and the denominator of equation (6). In this study, we find that the ratio of RV ventricular function increase to the rate of PA vascular function increase is significantly correlated to ventricular NT-proBNP expression. In other words, as the rate of ventricular accommodation to a deteriorating vascular function is decreased, the concentration NT-proBNP is increased. Interestingly, the cardiac marker is not correlated to NT-proBNP expression and is relatively constant within our sample.
). As the disease progresses, PVR increases while C decreases, thus increasing the ratio of the two measurements and the denominator of equation (6). In this study, we find that the ratio of RV ventricular function increase to the rate of PA vascular function increase is significantly correlated to ventricular NT-proBNP expression. In other words, as the rate of ventricular accommodation to a deteriorating vascular function is decreased, the concentration NT-proBNP is increased. Interestingly, the cardiac marker is not correlated to NT-proBNP expression and is relatively constant within our sample.
Clinical and research translation and future work
The basic research implications of this work suggest that, in the mild-to-moderate phases of the disease, the resistive component is primarily responsible for elevated RV wall stress (characterized by an increase in NT-proBNP). However, as the PA becomes extremely stiff, this appears to cause a sudden increase in NT-proBNP expression (probably accompanied by RV wall stress). Future work will utilize computational modeling to confirm that NT-proBNP release correlates with RV myocardial stress in pediatric PAH.
The clinical implications of this work suggest that noninvasive NT-proBNP measurements could be used to approximate PVR at early stages of the disease and possibly to approximate vascular stiffness and ventricular-vascular coupling. However, additional work is needed to arrive at a multivariate algorithm that adequately predicts the variability of vascular (and vascular-ventricular) function from noninvasive serum biochemical analysis. The implications of such an algorithm would be invaluable, particularly for pediatric PAH patients, who must be placed under general anesthesia during RHC.
Conclusions
The overall objective of this study was to show preliminary correlations between serum NT-proBNP measurements and ventricular-vascular function in pediatric PH. Assuming little-to-no circulating NT-proBNPs originating from the LV, afterload explains more than 80% of the variability in RV NT-proBNP expression, mostly dominated by the resistive components of impedance. However, the reactive component of afterload does appears to contribute to biomarker expression at extreme stiffness, which might further be compromised due to NT-proBNP expression from the LV. Finally, NT-proBNP appears to correlate with an estimate of RV-PA decoupling, but additional work is needed to compute coupling directly.
Source of Support: National Institutes of Health (R01 HL114753) and the Jayden DeLuca Foundation.
Conflict of Interest: None declared.
References
- 1.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiéry JL, Barbera JA, Beghetti M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493–2537. [DOI] [PubMed]
- 2.Foris V, Kovacs G, Tscherner M, Olschewski A, Olschewski H. Biomarkers in pulmonary hypertension: what do we know? Chest 2013;144:274–283. [DOI] [PubMed]
- 3.Nagaya N, Nishikimi T, Okano Y, Uematsu M, Satoh T, Kyotani S, Kuribayashi S, et al. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol 1998;31:202–208. [DOI] [PubMed]
- 4.Reesink HJ, Tulevski II, Marcus JT, Boomsma F, Kloek JJ, Vonk Noordegraaf A, Bresser P. Brain natriuretic peptide as noninvasive marker of the severity of right ventricular dysfunction in chronic thromboembolic pulmonary hypertension. Ann Thorac Surg 2007;84:537–543. [DOI] [PubMed]
- 5.Liang F, Gardner DG. Mechanical strain activates BNP gene transcription through a p38/NF-κB-dependent mechanism. J Clin Invest 1999;104:1603–1612. [DOI] [PMC free article] [PubMed]
- 6.Esteves WA, Lodi-Junqueira L, Neto CP, Tan TC, Nascimento BR, Mehrotra P, Barbosa MM, Ribeiro AL, Nunes MC. The impact of right ventricular stroke work on B-type natriuretic peptide levels in patients with mitral stenosis undergoing percutaneous mitral valvuloplasty. J Interv Cardiol 2013;26:501–508. [DOI] [PubMed]
- 7.Fijalkowska A, Kurzyna M, Torbicki A, Szewczyk G, Florczyk M, Pruszczyk P, Szturmowicz M. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest 2006;129:1313–1321. [DOI] [PubMed]
- 8.Holmes SJ, Espiner EA, Richards AM, Yandle TG, Frampton C. Renal, endocrine, and hemodynamic effects of human brain natriuretic peptide in normal man. J Clin Endocrinol Metab 1993;76:91–96. [DOI] [PubMed]
- 9.Pfister R, Schneider CA. Natriuretic peptides BNP and NT-pro-BNP: established laboratory markers in clinical practice or just perspectives? Clin Chim Acta 2004;349:25–38. [DOI] [PubMed]
- 10.Takatsuki S, Wagner BD, Ivy DD. B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide in pediatric patients with pulmonary arterial hypertension. Congenit Heart Dis 2012;7:259–267. [DOI] [PMC free article] [PubMed]
- 11.Colvin KL, Dufva MJ, Delaney RP, Ivy DD, Stenmark KR, Yeager ME. Biomarkers for pediatric pulmonary arterial hypertension: a call to collaborate. Front Pediatr 2014;2:7. [DOI] [PMC free article] [PubMed]
- 12.Nawaytou H, Bernstein HS. Biomarkers in pediatric heart disease. Biomark Med 2014;8:943–963. [DOI] [PubMed]
- 13.Bernus A, Wagner BD, Accurso F, Doran A, Kaess H, Ivy DD. Brain natriuretic peptide levels in managing pediatric patients with pulmonary arterial hypertension. Chest 2009;135:745–751. [DOI] [PMC free article] [PubMed]
- 14.Nir A, Lindinger A, Rauh M, Bar-Oz B, Laer S, Schwachtgen L, Koch A, Falkenberg J, Mir TS. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol 2009;30:3–8. [DOI] [PubMed]
- 15.Leuchte HH, El Nounou M, Tuerpe JC, Hartmann B, Baumgartner RA, Vogeser M, Muehling O, Behr J. N-terminal pro-brain natriuretic peptide and renal insufficiency as predictors of mortality in pulmonary hypertension. Chest 2007;131:402–409. [DOI] [PubMed]
- 16.Lankhaar JW, Westerhof N, Faes TJ, Marques KM, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol 2006;291:H1731–H1737. [DOI] [PubMed]
- 17.Kheyfets V, Rios L, Evans D, Smith T, Schroederb T, Mueller T, Muralib S, et al. The role of wall shear stress in the assessment of right ventricle hydraulic workload. Pulm Circ 2015;5:90–100. [DOI] [PMC free article] [PubMed]
- 18.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: an important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ 2011;2:212–223. [DOI] [PMC free article] [PubMed]
- 19.Linehan JH, Haworth ST, Nelin LD, Krenz GS, Dawson CA. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol 1992;73:987–994. [DOI] [PubMed]
- 20.Bellofiore A, Chesler NC. Methods for measuring right ventricular function and hemodynamic coupling with the pulmonary vasculature. Ann Biomed Eng 2013;41:1384–1398. [DOI] [PMC free article] [PubMed]
- 21.Winters R, Winters A, Amedee RG. Statistics: a brief overview. Ochsner J 2010;10:213–216. [PMC free article] [PubMed]
- 22.Tsutamoto T, Sakai H, Nishiyama K, Tanaka T, Fujii M, Yamamoto T, Horie M. Direct comparison of transcardiac increase in brain natriuretic peptide (BNP) and N-terminal proBNP and prognosis in patients with chronic heart failure. Circ J 2007;71(12):1873–1878. [DOI] [PubMed]
- 23.Chemla D, Castelain V, Herve P, Lecarpentier Y, Brimioulle S. Haemodynamic evaluation of pulmonary hypertension. Eur Respir J 2002;20:1314–1331. [DOI] [PubMed]
- 24.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2012;122:4306–4313. [DOI] [PMC free article] [PubMed]
- 25.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2008;118:2372–2379. [DOI] [PMC free article] [PubMed]
- 26.Humphrey JD. Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension 2008;52:195–200. [DOI] [PMC free article] [PubMed]
- 27.Taylor CA, Humphrey JD. Open problems in computational vascular biomechanics: hemodynamics and arterial wall mechanics. Comput Methods Appl Mech Eng 2009;198:3514–3523. [DOI] [PMC free article] [PubMed]
- 28.Tian L, Lammers SR, Kao PH, Albietz JA, Stenmark KR, Qi HJ, Shandas R, Hunter KS. Impact of residual stretch and remodeling on collagen engagement in healthy and pulmonary hypertensive calf pulmonary arteries at physiological pressures. Ann Biomed Eng 2012;40:1419–1433. [DOI] [PMC free article] [PubMed]
- 29.Leuchte HH, Holzapfel M, Baumgartner RA, Ding I, Neurohr C, Vogeser M, Kolbe T, Schwaiblmair M, Behr J. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 2004;43:764–770. [DOI] [PubMed]
- 30.Nichols WW, McDonald DA. McDonald’s blood flow in arteries: theoretic, experimental, and clinical principles. London: Hodder Arnold, 2011.
- 31.Kass DA, Kelly RP. Ventriculo-arterial coupling: concepts, assumptions, and applications. Ann Biomed Eng 1992;20:41–62. [DOI] [PubMed]
- 32.Guihaire J, Haddad F, Boulate D, Capderou A, Decante B, Flecher E, Eddahibi S, et al. Right ventricular plasticity in a porcine model of chronic pressure overload. J Heart Lung Trans 2014;33:194–202. [DOI] [PubMed]
- 33.Brimioulle S, Wauthy P, Ewalenko P, Rondelet B, Vermeulen F, Kerbaul F, Naeije R. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol 2003;284:H1625–H1630. [DOI] [PubMed]
- 34.Razzolini R, Ramondo A, Isabella G, Cardaioli P, Vaccari D, Carasi M, De Leo A, Chioin R, Suga H, Dalla-Volta S. Analytical expression of effective afterload in aortic and mitral regurgitation. Jpn Heart J 1999;40:295–309. [DOI] [PubMed]