Abstract Abstract
Pulmonary arterial hypertension (PAH) causes right ventricular ischemia, dysfunction, and failure. PAH patients may benefit from antianginal agents based on a shared pathophysiology with left ventricular ischemia. A single-center, randomized, placebo-controlled trial (1∶1) to assess the acute vasoreactivity and safety of ranolazine in PAH was conducted. Plasma samples for pharmacokinetic (PK) studies were drawn during hemodynamic measurements at 0, 60, 90, 120, 240, and 360 minutes from a Swan-Ganz catheter. All patients received 500-mg doses, uptitrated to 1,000 mg at week 4, monthly evaluations, and a complete objective assessment after 12 weeks, followed by an open-label extension. Thirteen patients were randomized and 12 enrolled (6 ranolazine, 6 placebo). All patients completed the acute phase; 10 completed the 12-week study. There were no acute changes in invasive hemodynamics. At 12 weeks ranolazine was well tolerated. Only 1 of the 5 patients on ranolazine had a serum concentration considered to be in the therapeutic range. Two serious adverse events required early withdrawal (both in the ranolazine group); gastrointestinal complaints were the most common adverse event. Efficacy measures did not demonstrate any differences between treatment groups. During the open-label trial, 2 additional patients reached a therapeutic concentration. Ranolazine in PAH appears safe, without acute hemodynamic effects after a 500-mg dose. Ranolazine administrated to PAH patients receiving background PAH therapies did not consistently reach therapeutic levels. Future studies should first perform PK analysis in PAH patients receiving PAH therapies and explore the safety and tolerability of the higher doses perhaps necessary to achieve therapeutic levels in PAH patients. (Trial registration: Clinicaltrials.gov identifier NCT01757808.)
Keywords: pulmonary arterial hypertension, right ventricular failure
Pulmonary arterial hypertension (PAH) is a progressive and ultimately fatal disease of the pulmonary circulation characterized by vasoconstriction, endothelial dysfunction, excessive smooth muscle cell proliferation, and in situ thrombosis of pulmonary arterioles.1 The resultant right ventricular (RV) dysfunction contributes to the progressive signs and symptoms of PAH. Since PAH cannot be cured with current pharmacologic therapies, strategies for improving RV myocardial function in these patients have been proposed to further improve outcomes.
Ranolazine is an approved therapy for the treatment of chronic stable angina. Its antianginal effects do not depend on reductions in heart rate or blood pressure, and it does not affect myocardial work at rest or with exercise.2 At therapeutic concentrations, ranolazine’s effects are related to inhibiting activation of late sodium ion channels, thus preventing calcium overload and producing both lusitropy (ventricular relaxation) and electrical stability.3,4 At higher plasma concentrations (>100 mM), ranolazine alters myocardial muscle cells to shift their substrate use toward carbohydrates rather than fatty acids.2 Ranolazine is cardioprotective by producing more adenosine triphosphate per mole of oxygen (O2), resulting in improved O2 utilization efficiency.5 Treatment with ranolazine improves myocardial blood flow, diminishing ischemia in patients with a high probability of coronary disease on myocardial perfusion imaging.3,4 Ranolazine also appears to improve diastolic hemodynamics and dysfunction. A small, early, proof-of-concept oncology study indicated that ranolazine prevented patients receiving chemotherapy from developing diastolic dysfunction.6
To understand whether ranolazine might have therapeutic benefit in PAH, researchers have investigated its effects in animal models of pulmonary hypertension (PH). Ranolazine given to rodents successfully attenuated the effects of monocrotaline (MCT)-induced PH.7,8 Animals given ranolazine and MCT concomitantly had less elevation in plasma basic natriuretic peptide levels and less decrease in RV ejection fraction (EF) compared with rats treated with MCT alone.7 Animals given ranolazine after 1 week of MCT also had similar benefit, with less elevation in pulmonary arterial systolic pressure (PASP) and less decrease in RVEF at day 28 without systemic effects.
PAH patients with symptomatic RV dysfunction are a high-risk group with a poor prognosis. These patients do not tolerate small decrements in their overall clinical status. In angina studies, ranolazine did not have clinical effects on systemic blood pressure, with only mild adverse effects of nausea, asthenia, and dizziness (ranolazine investigator brochure, March 31, 2010, Gilead Sciences). On the basis of these data and the extensive safety experience already known from ranolazine usage at the approved dosing of 1,000 mg twice daily, we hypothesized that ranolazine would improve RV function and cardiac output in PAH patients. We chose to investigate the safety of giving the medication by determining its effect on acute cardiopulmonary hemodynamics by invasive measurement over the half-life of the drug. Assessment of efficacy included hemodynamics, imaging, and laboratory changes from baseline to 12 weeks.
To investigate this potential, we conducted a randomized, placebo-controlled, single-center pilot study with three primary objectives: (1) to determine whether ranolazine administration had effects on acute cardiopulmonary hemodynamics, (2) to assess the safety of ranolazine acutely and after 3 months of therapy, and (3) to assess changes in RV function after 3 months of therapy. On the basis of current use in early drug development to assess potential efficacy, we investigated changes in pulmonary vascular resistance (PVR), a composite of cardiopulmonary physiological abnormalities in PAH, acutely and over 12 weeks.
Methods
Study design
This was an investigator-initiated, randomized (1∶1), placebo-controlled phase Ib study of acute administration with hemodynamic monitoring of a single dose of study drug (ranolazine or placebo), followed by 12 weeks of daily administration. All patients seen at the University of Chicago (UC) PH clinic who met the inclusion criteria had the opportunity to enroll in the study.
Gilead (the manufacturer of ranolazine) permitted use of the drug and supported the conduct of the study at UC by its investigator-initiated-study mechanisms. The Food and Drug Administration exempted the study from an Investigational New Drug requirement. The UC Institutional Review Board approved the consent and protocol. This was in accordance with the ethical standards of the Helsinki Declaration. All subjects provided written informed consent. The risk/benefit was justified on the basis of the poor long-term prognosis of the enrolled patient population, the routine safety surveillance conducted jointly by experts in PAH, and the use of ranolazine in chronic stable angina patients and close surveillance of outpatients familiar with individualized titration of other PAH therapeutics. See the appendix (576.3KB, pdf) for detailed methods and the complete initial approved protocol.
Patients
Inclusion criteria
PAH patients aged 18–72 years, defined as having idiopathic PAH, heritable PAH, or PAH associated with connective-tissue disease, congenital heart disease (repaired), or drug/toxin use,9 in World Health Organization Functional Classes (FC) I–III, with a historic right heart catheterization diagnostic of PAH, defined as mean pulmonary artery pressure (PAP) ≥ 25 mmHg with normal wedge pressure ≤ 15 mmHg at rest and PVR > 3 Wood units, were enrolled (from November 2011 to September 2013). Baseline 6-minute walk (6MW) distance was ≥150 m (without an upper limit).10 All patients had a cardiopulmonary stress test (CPET),11-15 2-dimensional and 3-dimensional echocardiography (2DE and 3DE, respectively),16,17 and administration of the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) quality-of-life questionnaire18,19 performed ≤21 days before enrollment.
Background therapies
Patients were receiving approved PAH monotherapy or a combination of PAH medications, including ambrisentan (5 or 10 mg), sildenafil (60–240 mg), tadalafil (40 mg), epoprostenol, treprostinil, or iloprost at stable doses for >90 days. Patients could also be receiving conventional PAH therapy as clinically indicated (oxygen, calcium channel blockers, digoxin), provided that the dose had not been changed in the 30 days before enrollment. Dosing of anticoagulants (warfarin) was considered stable if it had not been changed in the 30 days before enrollment, except as needed several days before and after cardiac catheterization.
Exclusion criteria
Patients were excluded if they were calcium channel blocker responders receiving monotherapy calcium blockers9,20 or if they were receiving a CY3P4 inducer (i.e., bosentan). Patients with impaired lung, liver, or renal function or systemic hypo- or hypertension also were excluded.
Echocardiography image acquisition
Comprehensive 2DE/3DE and color Doppler evaluation were performed by experienced sonographers using an iE33 imaging system equipped with an S5 transducer (Philips Healthcare, Andover, MA), with digital loops stored and analyzed offline (Xcelera Workstation, Philips). Right and left heart measurements were made according to published guidelines.21 RV peak systolic free-wall longitudinal strain (RVFWLS) was measured with vendor-independent software (Epsilon Imaging). We calculated fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE), the velocity of the tricuspid annular systolic motion, and tricuspid regurgitation. The 3DE images of the RV were acquired from a modified apical 4-chamber view (iE33, Philips Healthcare) with a fully sampled matrix-array transducer (model X7-2t).16
Cardiopulmonary exercise testing (CPET)
All patients were removed from O2 therapy in order to perform the test. The protocol consisted of 5 minutes of rest followed by interval walking, at first for 4 minutes, followed by increases in speed/elevation at 2-minute intervals until maximum tolerance (Naughton-Balke protocol);22 additional data were collected up to 5 minutes into recovery.23 Metabolic equivalents (METs) were estimated and reported with standardized formulas.
6MW testing
The standardized 6MW exercise test was administered by one of three trained technicians in accordance with American Thoracic Society guidelines. The test was administered in a 100-foot hallway within the PH clinic, with 10-foot (3.05-m) demarcations starting at 0.10
Acute hemodynamic testing
Right heart catheterization
Each patient underwent right heart catheterization by standard technique.24 Baseline measurements included mean right atrial pressure (mRAP), systolic, diastolic, and mean PAP, pulmonary artery wedge pressure (measured at end-expiration), cardiac output/cardiac index (CO/CI) by thermodilution, in triplicate, and pulmonary artery O2 saturation. PVR was calculated with the standard formula and expressed in Wood units. After baseline measurements, the catheter was left in place to allow for subsequent measurements after administration of active study drug or placebo.
Acute study treatment
Patients were given either placebo or ranolazine sustained release at a dose of 500 mg. Hemodynamic measurements were repeated at 0, 60, 90, 120, 240, and 360 minutes. Patients were monitored in a telemetry bed during this time in the catheterization lab holding area. Wedge pressure measurement was limited in frequency on the basis of current hospital policies to minimize patient risk. Plasma levels of ranolazine were drawn at each of the above time points via the catheter. Heart rate and systemic blood pressures were recorded at these times and throughout the observation period. After the acute monitoring phase, patients were discharged home.
Pharmacokinetic (PK) analysis
Plasma samples for PK analysis of ranolazine were drawn during hemodynamic measurements at 0, 60, 90, 120, 240, and 360 minutes from the patient’s Swan-Ganz catheter into a syringe and aliquotted by a 20-gauge or larger-bore needle into one 10-mL lavender-top (ethylenediaminetetraacetic acid [EDTA]) tube. The tube was inverted 3 times and placed on ice. Within 30 minutes of collection, plasma was separated by centrifugation at 1,300 g for 15 minutes at 4°C. The separated plasma was aliquotted to cryovials and stored at −70°C until assay. Analyses were performed by Intertek Pharmaceutical Services.
Follow-up and treatment
After discharge, patients received phone calls at 24 and 48 hours to assess their clinical status. Patients had visits at weeks 4 and 8 for assessment of clinical status, repeat laboratory testing, the CAMPHOR questionnaire, and adverse events. At week 4, patients were uptitrated from 500 to 1,000 mg twice daily (approved daily dose). At week 12, all objective measures were repeated: FC assessment, laboratory testing, 2DE and 3DE, CPET, 6MW test, cardiac catheterization hemodynamics, and the CAMPHOR questionnaire. A blood sample was drawn for PK analysis.
Withdrawal criteria, Data Safety Monitoring Board (DSMB)
All patients with serious adverse events were discussed with the DSMB before withdrawal. The DSMB consisted of 2 outside PAH experts (RS and RO) with experience in trial design and the acute care of PAH patients. The DSMB received reports of all serious adverse events and updates after each enrollment, with formal updates after 3 patients completed 12 weeks and then monthly as per the DSMB charter (for the charter, see the appendix (576.3KB, pdf) ).
Extension phase
Patients had the option of continuing use of the study drug at the discretion of the physician. The patient and the study team remained blinded until the completion of the 12-week phase by the final patient. Uptitration mimicked the randomized study, and at 12 weeks patients had a blood sample taken for PK analysis. Visits were standard-of-care 3–6-month visits, with procedures as determined by the investigator. Enrolled open-label patients continued to be followed as standard of care.
Sample size and statistical analysis
This was a pilot study planned to assess the acute and 12-week response to treatment and safety in 20 patients. The placebo group was the comparator group. Allowing for a 10% dropout rate, a total of 22 patients was planned to determine adequate safety exposure. However, on the basis of site enrollment feasibility, the principal investigator and the DSMB chose to stop enrollment after 12 patients completed 12 weeks of therapy. All categorical variables are reported as median (interquartile range). Comparisons between groups were made with the Fisher exact test or the Mann-Whitney test.
Results
Patient characteristics
The study consent and protocol were approved on August 31, 2011. Seventeen patients were screened for enrollment; 4 declined to participate. Thirteen patients were randomized to therapy. The first patient enrolled February 20, 2012. The last patient enrolled December 16, 2013, and completed the study 12 weeks later. Upon completion by the final patient, patients and investigators were unblinded to treatment assignment. One patient was found to have a very low CO at catheterization, consistent with shock, and thus was not started on therapy. Twelve patients completed the acute phase (6 ranolazine; 6 placebo), and 10 (4 ranolazine; 6 placebo) completed the 12-week assessments (Fig. 1; Table 1).
Figure 1.
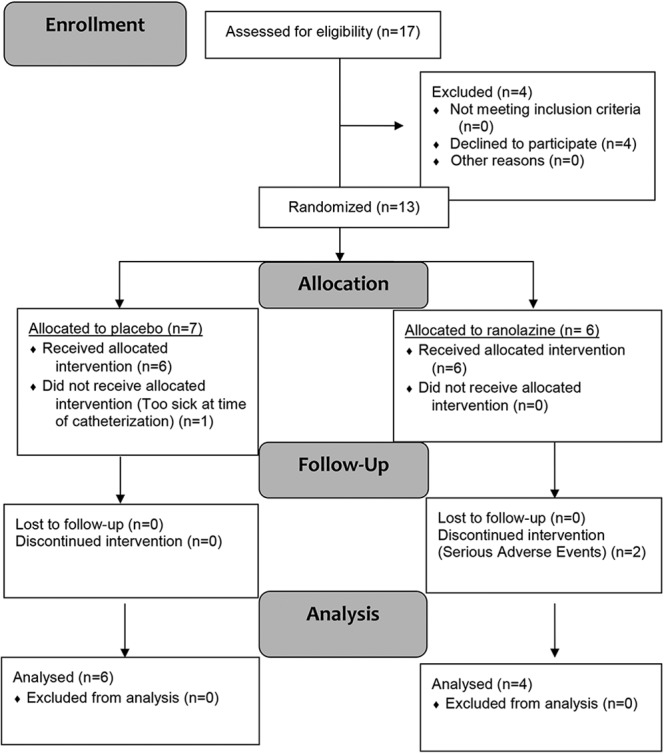
Flow diagram.
Table 1.
Baseline demographics
| Category | Value |
|---|---|
| Age, median, years | 50.5 |
| Age range, years | 29–65 |
| Functional class II | 4 (34) |
| Functional class III | 8 (66) |
| Race | |
| White | 10 (83) |
| African American | 1 (8) |
| Hispanic | 1 (8) |
| Etiology | |
| Idiopathic PAH | 5 (40) |
| Hereditary PAH | 1 (8) |
| Drug/toxin PAH | 1 (8) |
| Connective-tissue disease | 3 (13) |
| Congenital heart disease | 2 (17) |
| PAH medications | |
| Monotherapy | 7 (58) |
| PDE 5I | 3 (25) |
| ERA | 1 (8) |
| IV/SC prostacyclin | 3 (25) |
| Combination therapy | 5 (42) |
| ERA–PDE 5I | 1 (8) |
| PDE 5I–IV/SC prostacyclin | 4 (33) |
Data are no. (%) of patients unless otherwise indicated. ERA: endothelin receptor antagonist; IV: intravenous; PAH: pulmonary arterial hypertension; PDE 5I: phosphodiesterase 5 inhibitor; SC: subcutaneous.
Acute administration (primary outcome)
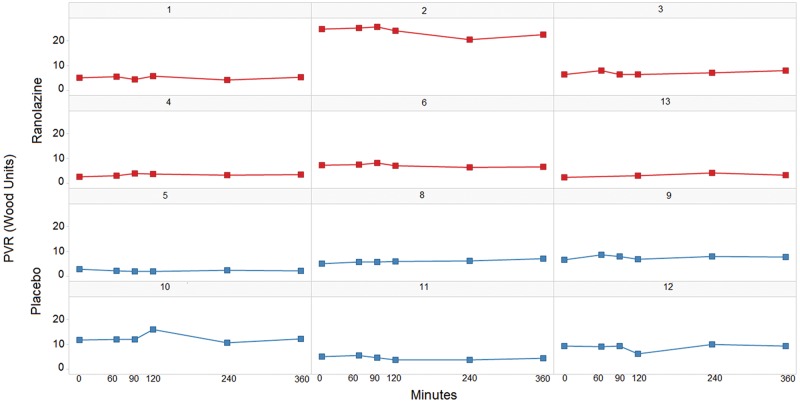
Patients assigned to receive the active drug in the acute study had slightly higher PASP and mean PAP, yet PVR was similar at baseline between groups. There were no significant changes in mRAP and PAP (systolic and diastolic) or in CO/CI during the 360 minutes. There was no change in the primary end point, PVR (P = 0.52; Fig. 2; Table 2). There were no adverse events of hypotension, hypertension, bradycardia, or tachyarrhythmia during the observation period. PK data revealed that only 1 patient reached a therapeutic concentration of 2–6 μM (Table 3).
Figure 2.
Acute pulmonary vascular resistance (PVR) changes for individual patients.
Table 2.
Acute cardiac catheterization hemodynamics
| Baseline | After 360 minutes | |||
|---|---|---|---|---|
| Measurement | Ranolazine | Placebo | Ranolazine | Placebo |
| mRAP, mmHg | 2.0 (3.0) | 3.0 (3.5) | 6.5 (3.3) | 8.0 (3.0) |
| PASP, mmHg | 71.5 (30.0) | 59.5 (33.0) | 75.0 (38.5) | 56.5 (39.8) |
| PADP, mmHg | 30.0 (16.5) | 29.0 (16.3) | 31.0 (16.3) | 28.0 (16.0) |
| mPAP, mmHg | 42.5 (23.8) | 36.5 (20.5) | 47.5 (27.8) | 40.0 (24.5) |
| PCWP, mmHg | 11.0 (5.0) | 11.0 (2.0) | … | … |
| CO, L/min2 | 5.9 (1.0) | 4.6 (0.5) | 5.9 (1.0) | 6.2 (1.7) |
| CI, L/min × m2 | 3.1 (0.6) | 2.4 (0.3) | 2.8 (0.7) | 3.2 (0.9) |
| PVR, Wood units | 5.8 (3.7) | 5.9 (3.5) | 7.4 (3.9) | 6.1 (3.6) |
Data are median (IQR). Legend: CI: cardiac index; CO: cardiac output; IQR: interquartile range; mPAP: mean pulmonary artery pressure; mRAP: mean right atrial pressure; PADP: pulmonary artery diastolic pressure; PASP: pulmonary artery systolic pressure; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance.
Table 3.
Ranolazine concentration (μM/L) over 360 minutes after dosing
| Patient | 60 minutes | 90 minutes | 120 minutes | 240 minutes | 360 minutes |
|---|---|---|---|---|---|
| 1 | 0.62 | 0.87 | 0.77 | 1.19 | 1.14 |
| 2 | BQL | BQL | 0.25 | 2.30 | 3.08 |
| 3 | 0.93 | 0.71 | 0.52 | 0.52 | 1.00 |
| 4 | 0.17 | 0.14 | 0.23 | 0.29 | 0.51 |
| 6 | BQL | BQL | BQL | BQL | 1.42 |
| 13 | BQL | BQL | 0.22 | 0.20 | 0.89 |
Boldface indicates that patient 2 achieved a therapeutic concentration after 360 minutes. BQL: below quantitation limit (50.0 ng/mL).
Tolerability: serious adverse events
Ten of the 12 patients completed all the study evaluations at week 12. The average number of days receiving the study drug per patient was 90 (range: 84–91). Two serious adverse events occurred, prompting withdrawal from study. Both patients had been randomized to ranolazine. After receiving the study drug for 22 days, 1 subject was withdrawn from the study drug because of worsening right heart failure (February 2012); this was considered possibly related to the study drug. The patient developed worsening shortness of breath after catheterization in the setting of volume overload; she was not taking her diuretic as instructed (patient 2). After receiving the study drug for 45 days, a second patient was withdrawn from the study because the patient developed a rise in creatinine in the setting of Varicella zoster infection and hypovolemia. The study was then temporarily placed on hold. The DSMB independently reviewed the data. The patient was withdrawn from the study drug (August 2012) on the basis of this event (possibly related) and other reports of renal dysfunction with ranolazine from a coincident sponsor’s study (patient X). The trial resumed after the consent and protocol were amended to acknowledge the risk of renal failure in patients with baseline renal insufficiency.
Extension phase
Any of the patients could continue on open-label therapy as standard of care if the patient and the investigator perceived a benefit. One patient continues to receive commercial ranolazine therapy; the total drug exposure (longest for trial) as of April 1, 2015, is 595 days.
Adverse events
The most frequent adverse events were diarrhea and nausea. Three patients had upper respiratory infections, and 2 had sinus infections (see Table 4). There were no complications during CPET or catheterization.
Table 4.
Adverse events
| Adverse event | Total (N = 12) | Placebo (N = 6) | Ranolazine (N = 6) |
|---|---|---|---|
| Acute right heart failure (SAE) | 1 (8) | 0 (0) | 1 (8) |
| Bloody stool/hemorrhoid | 1 (8) | 1 (8) | 0 (0) |
| Breast tenderness | 1 (8) | 1 (8) | 0 (0) |
| Cough | 1 (8) | 1 (8) | 0 (0) |
| Decreased GFR (SAE) | 1 (8) | 0 (0) | 1 (8) |
| Diarrhea | 5 (42) | 3 (25) | 2 (17) |
| Dizziness | 1 (8) | 1 (8) | 0 (0) |
| Edema | 1 (8) | 0 (0) | 1 (8) |
| Feet tingling/restless | 2 (17) | 1 (8) | 1 (8) |
| Headache | 1 (8) | 0 (0) | 1 (8) |
| Heart racing | 1 (8) | 0 (0) | 1 (8) |
| Hypokalemia | 1 (8) | 0 (0) | 1 (8) |
| Infection (Hickman site) | 1 (8) | 1 (8) | 0 (0) |
| Insomnia | 2 (17) | 2 (17) | 2 (17) |
| Irritability | 1 (8) | 1 (8) | 0 (0) |
| Nausea | 3 (25) | 2 (17) | 1 (8) |
| Rash-herpes zoster | 1 (8) | 0 (0) | 1 (8) |
| Sore throat | 1 (8) | 1 (8) | 1 (8) |
| Upper respiratory infection | 5 (42) | 4 (33) | 1 (8) |
| Upset stomach | 1 (8) | 0 (0) | 1 (8) |
Data are no. (%) of patients. GFR: glomerular filtration rate; SAE: serious adverse event.
Outcome assessments
PK analysis
Only 1 of the 5 patients assigned to active ranolazine had a therapeutic concentration at 12 weeks in the placebo-controlled trial. In the open-label trial, 2 additional patients reached a therapeutic concentration (total: 3 of 10). No patients were receiving contraindicated medications (CYP3A inducers). See Tables 5–7.
Table 5.
12-week secondary outcome measures
| Baseline | 12 weeks | |||
|---|---|---|---|---|
| Ranolazine (N = 4) | Placebo (N = 6) | Ranolazine (N = 4) | Placebo (N = 6) | |
| Hemodynamics | ||||
| mRAP, mmHg | 2.0 (0.8) | 3.0 (3.5) | 4.0 (3.3) | 5.0 (0.8) |
| RVSP, mmHg | 53.5 (21.4) | 63.0 (36.0) | 58.5 (19.6) | 60.5 (37.0) |
| RVDP, mmHg | 4.0 (3.5) | 7.0 (4.3) | 8.0 (7.8) | 8.5 (3.3) |
| PASP, mmHg | 56.5 (31.5) | 59.5 (33.0) | 59.0 (24.0) | 65.0 (34.0) |
| PADP, mmHg | 24.0 (14.8) | 29.0 (16.3) | 25.0 (14.0) | 33.5 (16.5) |
| mPAP, mmHg | 33.5 (20.3) | 36.5 (20.5) | 33.5 (20.5) | 41.5 (19.3) |
| Wedge pressure, mmHg | 11.5 (4.0) | 10.0 (3.5) | 14.0 (3.3) | 12.0 (0.8) |
| CO, L/min | 6.3 (0.5) | 4.6 (0.5) | 6.4 (1.2) | 5.4 (0.6) |
| CI, L/min × m2 | 3.1 (0.3) | 2.4 (0.3) | 3.3 (0.4) | 2.8 (0.5) |
| PVR, Wood units | 3.9 (2.8) | 5.9 (3.5) | 3.0 (2.3) | 6.6 (1.4) |
| 6MWD, m | 467.5 (123) | 419.5 (80.9) | 478 (104.3) | 408.3 (113.6) |
| NT-proBNP, pg/mL | 137 (220) | 161 (101) | 171 (100) | 286 (82) |
| CPET | ||||
| METs | 7.0 (0.8) | 4.5 (0.4) | 8.1 (3.3) | 2.3 (4.6) |
| VE/Vco2 | 35.5 (6.8) | 39.5 (5.8) | 37.0 (10.5) | 42.0 (6.8) |
| Peak Vo2 | 17.6 (2.4) | 12.0 (2.0) | 20.1 (4.8) | 13.3 (18.8) |
| CAMPHOR | ||||
| Symptoms | 5.0 (4.5) | 5.5 (4.8) | 2.0 (4.3) | 3.5 (1.0) |
| Activity | 7.5 (8.5) | 6.5 (4.0) | 8.5 (4.5) | 4.5 (4.0) |
| Quality of life | 7.0 (8.8) | 6.5 (3.3) | 5.0 (6.3) | 3.0 (3.5) |
| Total | 20.5 (22.8) | 17.0 (13.0) | 15.0 (15.5) | 12.5 (6.0) |
Data are median (interquartile range). 10 subjects enrolled at 12 weeks, (ranolazine: n = 4, placebo: n = 6). CAMPHOR: Cambridge Pulmonary Hypertension Outcome Review; CI: cardiac index; CO: cardiac output; CPET: cardiopulmonary exercise testing; METs: metabolic equivalents; mPAP: mean pulmonary artery pressure; mRAP: mean right atrial pressure; NT-proBNP: N-terminal pro brain natriuretic peptide; PADP: pulmonary artery diastolic pressure; PASP: pulmonary artery systolic pressure; PVR: pulmonary vascular resistance; VE/Vco2: ventilator equivalent of carbon dioxide; Vo2: oxygen consumption per unit time; 6MWD: 6-minute walk distance.
Table 6.
12-week ranolazine concentration
| Patient | Ranolazine, μmol/L |
|---|---|
| 1 | 3.11 |
| 3 | 0.94 |
| 4 | 1.53 |
| 5 | 4.23 |
| 6 | BQL |
| 8a | 1.53 |
| 10a | 1.33 |
| 11a | 1.65 |
| 12a | 2.62 |
| 13 | 0.13 |
Boldface indicates a therapeutic concentration. BQL: below quantitation limit (50.0 ng/mL).
Patients in 12-week open-label extension.
Table 7.
Echocardiographic (2DE/3DE) outcome measures
| Baseline | 12 weeks | |||
|---|---|---|---|---|
| Ranolazine (N = 4) | Placebo (N = 6) | Ranolazine (N = 4) | Placebo (N = 6) | |
| LV EF, % | 66.3 (8.8) | 66.0 (8.0) | 67.3 (6.0) | 64.0 (4.0) |
| LV EDD, cm | 4.2 (0.9) | 4.5 (0.8) | 4.3 (0.2) | 4.4 (0.3) |
| IVSd, cm | 0.9 (0.1) | 1.1 (0.2) | 1.0 (0.1) | 1.0 (0.1) |
| PWd, cm | 0.9 (0.1) | 1.0 (0.2) | 0.9 (0.2) | 1.0 (0.1) |
| RV base, cm | 4.7 (1.1) | 4.4 (1.1) | 4.7 (0.8) | 4.5 (0.6) |
| RV mid, cm | 2.8 (0.5) | 3.1 (1.0) | 3.2 (0.6) | 3.5 (0.7) |
| RV length, cm | 8.0 (1.2) | 7.7 (2.0) | 7.8 (1.9) | 8.1 (2.2) |
| RA/IVC pressure, mmHg | 15.0 (10.0) | 7.5 (7.5) | 7.5 (0) | 7.5 (0) |
| TR max gradient, mmHg | 58.7 (16.4) | 60.0 (33.0) | 61.0 (20.0) | 61.1 (20.0) |
| TAPSE, cm | 1.9 (0.7) | 1.7 (0.6) | 1.9 (0.8) | 2.3 (0.5) |
| S′, cm/s | 11.6 (1.9) | 11.5 (2.4) | 12.3 (3.3) | 12.7 (2.8) |
| RV ED area, cm2 | 23.5 (6.5) | 29.9 (7.2) | 26.1 (4.5) | 26.7 (7.9) |
| FAC, % | 28.1 (4) | 27.6 (16.5) | 31.1 (8.8) | 27.6 (6.9) |
| RVFWLS, % | −16.7 (3.3) | −23.7 (7.9) | −14.4 (8.9) | −21.9 (8.2) |
| 3DE EF, % | 30.7 (4.3) | 17.9 (12.2) | 32.0 (0) | 23.4 (13.9) |
Data are median (interquartile range). ED: end-diastolic; EDD: end-diastolic dimension; EF: ejection fraction; FAC: fractional area change; IVC: inferior vena cava; IVSd: interventricular septum dimension; LV: left ventricle; PWd: posterior wall dimension, RA: right atrium; RV: right ventricle; RVFWLS: right ventricular free-wall longitudinal strain; S′: maximal systolic excursion velocity, from pulsed tissue Doppler; TAPSE: tricuspid annular plane systolic excursion; TR max: tricuspid regurgitation maximum; 2DE: 2-dimensional echocardiography, 3DE: 3-dimensional echocardiography.
FC/CPET/6MW/lab/CAMPHOR
There were no changes in FC (P = 0.67), CPET (minute ventilation/CO2 production [P = 0.67], peak oxygen consumption [P = 0.52], and calculated METs [P = 0.24]), 6MW distance (P = 0.67), N-terminal pro brain natriuretic peptide (P = 0.29), or CAMPHOR (P = 1.0) from baseline to week 12.
2DE/3DE
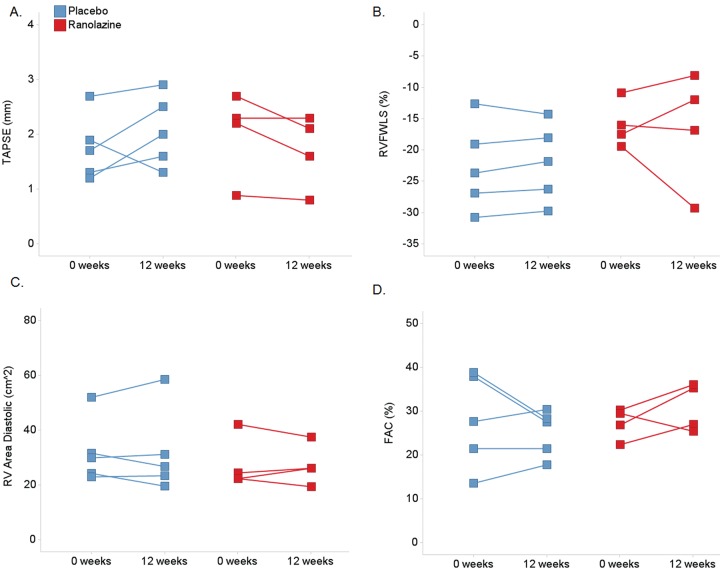
There were no changes from baseline to week 12 in left ventricular or RV dimensions or volumes in 2DE or 3DE (see Tables 5, 7). TAPSE, RV area, and FAC, as measures to detect change, had both positive and negative variability. RVFWLS had the least variability (see Fig. 3).
Figure 3.
Change in right ventricular echocardiographic parameters for individual patients from baseline to 12 weeks, categorized by treatment assignment: A, TAPSE: tricuspid annular plane systolic excursion; B, RVFWLS: right ventricular free-wall longitudinal strain; C, RV: right ventricle; D, FAC: fractional area change.
Discussion
On the basis of the potential effects of ranolazine on RV myocardial function, the known safety profile of ranolazine, and the positive preclinical data from MCT PAH animal models, there is rationale for cross-purposing ranolazine for PAH. In our study, ranolazine did not acutely lower PAP or demonstrate any measurable efficacy across multiple indices of PAH severity. However, although lower doses were used in the acute phase, even at the higher, approved dose used in the open-label phase of the study (1,000 mg twice daily), most patients did not reach a therapeutic level. Thus, the lack of an efficacy signal in this study could have been due to subtherapeutic ranolazine dosing.
Hemodynamic parameters are a central part of PAH diagnosis and management, as they are the gold standard for measuring pressure (to confirm the diagnosis) as well as important for measuring flow and resistance. While it appears that many PAH drugs can continue to improve hemodynamics over time, acute improvement may be an optimal initial screening for drug efficacy for direct vasodilatory drugs.24-28 This continues to be the case when evaluating the additive hemodynamic effects of many combinations of PAH drugs. The best diagnostic test to screen a drug with an alternative mechanism of action in PAH is unknown, and therefore acute vasodilatory testing is still completed. In addition, in trials with a small sample size, the placebo arm enables investigators to better decipher “noise,” variability in the measurements, to determine whether the observed effects were valid. This is why we did not conduct a single-arm phase I open-label study.
Limitations
The major limitation was the lack of therapeutic dosing of ranolazine. Our PK data illustrate that a more complete PK study in PAH patients receiving PAH background therapy should be done before evaluating acute vasoreactivity and longer-term safety and efficacy in a phase I study.
Our study was from a single center with an established PH practice, which may have biased patient enrollment. The small sample size used, with only 5 active-therapy patients for 12 weeks, may have been underpowered to detect a “signal” and increases the chances for Type II error. Because of institutional policy, we were not able to perform pulmonary capillary wedge pressure measurements with the Swan-Ganz catheter without the use of fluoroscopy. Thus, repeating wedge measurements over time was not possible, since subsequent hemodynamic monitoring occurred in the catheterization lab holding area, where fluoroscopy was not available. We do not believe that ranolazine increased wedge pressure, because none of the patients had symptoms or signs of congestive heart failure (hypoxemia, orthopnea, rales, dyspnea) consistent with an acutely elevated wedge pressure. On the basis of reports of ranolazine use in PH associated with left heart disease, we also do not believe that the wedge pressure decreased and falsely elevated PVR. Finally, limitations in outcome measurements include measurement quality and operational aspects of data collection. Patients were not enrolled if they were in FC IV because of safety concerns; however, this is a group that may have the most potential to benefit.
Conclusions
Cross-purposing drugs for an orphan disease utilizes an approved therapy in a new indication (i.e., PAH) with predetermined knowledge of pharmacodynamic, PK, and adverse-event drug profiles.20,29,30 Ranolazine at doses approved to treat angina appears to be safe in PAH patients receiving background PAH therapies, with no acute hemodynamic effects after a 500-mg dose. Because ranolazine in this study did not consistently reach therapeutic levels, the lack of an efficacy signal at the approved 1,000-mg twice-daily dose may have been due to underdosing. PK studies of candidate drugs for patients receiving background PAH therapies should be completed before initiation of more comprehensive studies such as ours, in order to determine the minimally effective dose. Future work will have to explore the safety and tolerability of the higher doses perhaps necessary to achieve therapeutic levels in patients with PAH.
Source of Support: This project was supported through the investigator-initiated-funding mechanisms at Gilead in contract with the University of Chicago.
Conflict of Interest: The University of Chicago receives research grant support from Actelion, Gilead, Novartis, Medtronic, Lung Biotechnology, and Reata for MG-M to be a principal investigator on research grants. MG-M has served as a consultant for Actelion, Bayer, Gilead, Medtronic, Merck, Bellerophon (formerly known as Ikaria), and United Therapeutics as a member of steering committees, DSMBs, and event committees. She has received honoraria for continuing medical education from Medscape and ABComm. MG-M is a member of the Patient-Centered Outcomes Research Institute Advisory Panel on Rare Diseases and a special government employee for the Food and Drug Administration Cardio-Renal division. RS has received research grants from Actelion, Bayer, GeNO, Gilead, Ikaria, Novartis, Reata, and United Therapeutics; he is on the speakers’ bureaus for Actelion, Bayer, Gilead, and United Therapeutics and is a consultant for Gilead. SC is on the speakers’ bureau for United Therapeutics and the consultant/advisory boards for Bayer, Gilead, and United Therapeutics. HG is an employee of Gilead. RJO has received research grants from Actelion, Bayer, Gilead, Ikaria, Lung Biotechnology, Reata, and United Therapeutics; he has received advisory board and/or speaking fees from Actelion, Bayer, Gilead, Lung Biotechnology, Medtronic, Pfizer, Reata, and United Therapeutics. All other authors declare no conflict of interest.
Supplements
Appendix (576.3KB, pdf)
References
- 1.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl.):D4–D12. [DOI] [PMC free article] [PubMed]
- 2.Thadani U, Ezekowitz M, Fenney L, Chiang YK. Double-blind efficacy and safety study of a novel anti-ischemic agent, ranolazine, versus placebo in patients with chronic stable angina pectoris. Circulation 1994;90(2):726–734. [DOI] [PubMed]
- 3.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart 2006;92(suppl. 4):iv6–iv14. [DOI] [PMC free article] [PubMed]
- 4.Venkataraman R, Belardinelli L, Blackburn B, Heo J, Iskandrian AR. A study of the effects of ranolazine using automated quantitative analysis of serial myocardial perfusion images. JACC Cardiovasc Imaging 2009;2(11):1301–1309. [DOI] [PubMed]
- 5.Gralinski MR, Black SC, Kilgore KS, Chou AY, McCormack JG, Lucchesi BR. Cardioprotective effects of ranolazine (RS-43285) in the isolated perfused rabbit heart. Cardiovasc Res 1994;28(8):1231–1237. [DOI] [PubMed]
- 6.Minotti G. Pharmacology at work for cardio-oncology: ranolazine to treat early cardiotoxicity induced by antitumor drugs. J Pharmacol Exp Ther 2013;346(3):343–349. [DOI] [PubMed]
- 7.Liles JT, Oliver J, Forrer SA, Belardinelli L, Dhalla AK. Beneficial effects of ranolazine in a model of pulmonary hypertension and right-sided heart failure. Am J Respir Crit Care Med 2011;183(meeting abstracts):A4977. doi:10.1164/ajrccm-conference.2011.183.1_MeetingAbstracts.A4977.
- 8.Liles J, Oliver J, Chi L, Dhalla A, Belardinelli L. Ranolazine reduces monorotaline-induced pulmonary hypertension when administered following disease induction. Circulation 2012;126(21 suppl.):A11990.
- 9.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl.):D34–D41. [DOI] [PubMed]
- 10.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166(1):111–117. [DOI] [PubMed]
- 11.Hansen JE, Sun XG, Yasunobu Y, Garafano RP, Gates G, Barst RJ, Wasserman K. Reproducibility of cardiopulmonary exercise measurements in patients with pulmonary arterial hypertension. Chest 2004;126(3):816–824. [DOI] [PubMed]
- 12.Yasunobu Y, Oudiz RJ, Sun XG, Hansen JE, Wasserman K. End-tidal Pco2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest 2005;127(5):1637–1646. [DOI] [PubMed]
-
13.Weltman A, Snead D, Stein P, Seip R, Schurrer R, Rutt R, Weltman J. Reliability and validity of a continuous incremental treadmill protocol for the determination of lactate threshold, fixed blood lactate concentrations, and
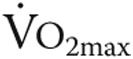 . Int J Sports Med 1990;11(1):26–32. [DOI] [PubMed]
. Int J Sports Med 1990;11(1):26–32. [DOI] [PubMed] - 14.Dickstein K, Barvik S, Aarsland T, Snapinn S, Millerhagen J. Validation of a computerized technique for detection of the gas exchange anaerobic threshold in cardiac disease. Am J Cardiol 1990;66(19):1363–1367. [DOI] [PubMed]
- 15.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986;60(6):2020–2027. [DOI] [PubMed]
- 16.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 2012;13(1):1–46. [DOI] [PubMed]
- 17.Abramson SV, Burke JB, Pauletto FJ, Kelly JJ Jr. Use of multiple views in the echocardiographic assessment of pulmonary artery systolic pressure. J Am Soc Echocardiogr 1995;8(1):55–60. [DOI] [PubMed]
- 18.McKenna SP, Doughty N, Meads DM, Doward LC, Pepke-Zaba J. The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR): a measure of health-related quality of life and quality of life for patients with pulmonary hypertension. Qual Life Res 2006;15(1):103–115. [DOI] [PubMed]
- 19.Gomberg-Maitland M, Thenappan T, Rizvi K, Chandra S, Meads DM, McKenna SP. United States validation of the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR). J Heart Lung Transplant 2008;27(1):124–130. [DOI] [PubMed]
- 20.Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013;62(25 suppl.):D60–D72. [DOI] [PubMed]
- 21.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16(7):777–802. [DOI] [PubMed]
- 22.Patterson JA, Naughton J, Pietras RJ, Gunnar RM. Treadmill exercise in assessment of the functional capacity of patients with cardiac disease. Am J Cardiol 1972;30(7):757–762. [DOI] [PubMed]
- 23.Wasserman K, Hansen JE, Sue DY, Casaburi R, Whipp BJ. Principles of exercise testing and interpretation. 3rd ed. Philadelphia, PA: Lippincott, Williams, & Wilkins, 1999.
- 24.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl.):D42–D50. [DOI] [PubMed]
- 25.Nootens M, Schrader B, Kaufman E, Vestal R, Long W, Rich S. Comparative acute effects of adenosine and prostacyclin in primary pulmonary hypertension. Chest 1995;107(1):54–57. [DOI] [PubMed]
- 26.Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension. Circulation 2002;105(20):2398–2403. [DOI] [PubMed]
- 27.Grimminger F, Weimann G, Frey R, Voswinckel R, Thamm M, Bölkow D, Weissmann N, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J 2009;33(4):785–792. [DOI] [PubMed]
- 28.Grünig E, Michelakis E, Vachiéry JL, Vizza CD, Meyer FJ, Dölberg M, Bach D, Dingemanse J, Galiè N. Acute hemodynamic effects of single-dose sildenafil when added to established bosentan therapy in patients with pulmonary arterial hypertension: results of the COMPASS-1 study. J Clin Pharmacol 2009;49(11):1343–1352. [DOI] [PubMed]
- 29.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369(4):330–340. [DOI] [PubMed]
- 30.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani HA, Jansa P, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369(9):809–818. [DOI] [PubMed]