Abstract
Catheter-related bloodstream infections are a significant problem in the clinic and may result in a serious infection. Here, we developed a facile and green procedure for buildup of silver nanoparticles (AgNPs) on the central venous catheters (CVCs) surface. Inspired by mussel adhesive proteins, dopamine was used to form a thin polydopamine layer and induce AgNPs formation without additional reductants or stabilizers. The chemical and physicochemical properties of AgNPs coated CVCs were characterized by scanning electron microscopy, X-ray photoelectron spectroscopy, Raman spectroscopy, and water contact angle. The Staphylococcus aureus culture experiment was used to study the antibacterial properties. The cytocompatibility was assessed by water soluble tetrazolium salts (WST-1) assay, fluorescence staining, and scanning electron microscopy analysis. The results indicated that the CVCs surface was successfully coated with compact AgNPs. AgNPs were significantly well separated and spherical with a size of 30–50 nm. The density of AgNPs could be modulated by the concentration of silver nitrate solution. The antibacterial activity was dependent on the AgNPs dose. The high dose of AgNPs showed excellent antibacterial activity while associated with increased cytotoxicity. The appropriate density of AgNPs coated CVCs could exhibit improved biocompatibility and maintained evident sterilization effect. It is promising to design mussel-inspired silver releasing CVCs with both significant antimicrobial efficacy and appropriate biological safety.
Keywords: surface modification, central venous catheter, antibacterial activity, bio-compatibility
Introduction
Central venous catheters (CVCs) are commonly used to provide venous access for drug delivery, intravenous fluid administration, monitoring hemodynamics, and nutritional support in critically ill patients, but they also are a major source of hospital acquired infection.1 Catheter-related bloodstream infections have been associated with increased patient morbidity and mortality in the clinic.2 Generally, bacteria adhere on the surface of CVCs followed by growth under suitable environmental conditions to form biofilms, which is elemental to the onset of pathogenesis. To prevent catheter colonization and catheter-related bloodstream infections, a great deal of approaches have been developed in recent years. Current preventive strategies to decrease the risk of these serious infections include the use of antimicrobial agent impregnated catheters and antimicrobial lock therapy.3 For example, silver-impregnated CVCs and antiseptic CVCs coated with chlorhexidine and silver sulfadiazine have already been used in clinic medicine.4 Silver is widely exploited because of its good antimicrobial action to a wide range of microorganisms.5,6 In the meantime, silver nanoparticles (AgNPs), as a novel form, are applied in a number of products such as medical device and implant coatings. The catheters can be coated internally and externally with silver containing compounds or impregnated with silver.3 Various methods have been used for the synthesis of silver-impregnated CVCs, such as silver iontophoretic technology, solvent casting, electrospinning and electrospraying, plasma based deposition processes, in situ light initiated synthesis,7–10 etc.
Recently, mussel-inspired green synthesis of AgNPs on the surface of various materials has drawn a great attention. It has been found that dopamine, could be auto-oxidized to form adhesive polydopamine films onto a wide range of inorganic and organic materials, including classically adhesion-resistant materials, such as polytetrafluoroethylene (PTFE).11,12 Many researches reveal that the catechol groups in the polydopamine films could in situ generate the AgNPs without using surfactant or reducing agents.13–18 This method has been successfully used for surface modification on various substrate materials, such as microspheres,13 fibers,14 and polymer films.15,16 The polydopamine films also owned strong metal binding ability and adhering capacity to AgNPs. Xu et al reported that polydopamine modified cotton fabrics with AgNPs showed a higher antibacterial activity even after 30 times washes.17 Sureshkumar et al prepared a multilayer of metal nanoparticles on polymer film and found that the surface density of metal nanoparticles could be adjusted by varying the polydopamine coating time.18
The commonly used CVCs in the clinic include polyurethane, silicone, and PTFE CVCs.4 However, the adhesion force of coatings was rather weak owing to the hydrophobic property of the CVCs, and it was difficult to prepare coatings with good adherence on CVCs. In this study, a mild method for preparing AgNPs/polydopamine/CVCs composites with the AgNPs gradients was presented. The dopamine self-polymerization was used to form surface-adherent films on the PTFE, CVCs and then the polydopamine was applied to produce uniformly AgNPs. The relationship of the concentration of silver nitrate solution and the density of AgNPs was investigated. Antibacterial activity was investigated by zones of inhibition (ZoI) assay and fluorescence microscopy using Staphylococcus aureus. The toxicity of the AgNPs/polydopamine/CVCs composites was evaluated by WST-1 and cell adhesion assay.
Materials and methods
Materials
Commercial CVCs (Scw Medicath Ltd, Shenzhen, People’s Republic of China) were cleaned by ultrasound in anhydrous ethanol and deionized (DI) water successively before use. Dopamine hydrochloride was purchased from Sigma-Aldrich, St Louis, MO, USA. Tris(hydroxymethyl)-amino-methane (Tris), silver nitrate, and hydrochloric acid were obtained from Beijing Chemical Plant, Beijing, People’s Republic of China. All commercially available reagents and solvents were used without further purification.
Preparation of polydopamine-AgNPs membrane on the catheters
The CVCs were treated with a dopamine solution (2 mg/mL, 10 mm Tris, pH 8.5) at room temperature with stirring for 20 hours and subsequently ultrasonic washed with DI water, and dried at 60°C for 1 hour. The catheters treated with dopamine solution were denoted as DCVC in the subsequent discussion.
The DCVC samples were then immersed in silver nitrate solutions with three different concentrations (1.0, 0.1, and 0.01 M) for 4 hours at room temperature. Afterward, the samples were ultrasonic cleaned with DI water for 10 minutes and dried at 40°C in a vacuum oven. These samples were denoted as DCVC-Ag1, DCVC-Ag2, and DCVC-Ag3, respectively.
Physicochemical characterization
The surface topography of the specimens was observed by field emission scanning electron microscopy (FE-SEM, Hitachi S4800, Hitachi, Tokyo, Japan). The chemical composition of the surface layer was analyzed using X-ray photoelectron spectroscopy (XPS, VG, Physical Electrons Quantum 2000 scanning ESCA microprobe, Al Kα radiation). The static contact angles (CAs) were measured with an optical contact angle meter system (OCA-20, Dataphysics, Stuttgart, Germany) at ambient humidity and temperature. The DI water droplets used for the CA measurement were 3 µL and the three measurements were performed on each sample for statistical accountability. Raman spectra were recorded using Raman spectroscopy (XploRA, Horiba Jobin Yvon, Longjumeau, France) with a 532 nm air cooled argon ion laser at wavelengths from 50 to 2,000 cm−1.
Antibacterial assay
Samples were cut into small disks with a diameter of 6 mm and sterilized with 75% alcohol for 1 hour and rinsed with phosphate-buffered saline (PBS) for three times. S. aureus strains were grown overnight in Luria-Bertani medium at 37°C for 12 hours. The bacterial concentrations were adjusted to 105 colony-forming units (CFUs)/mL in the antibacterial assay and dispersed uniformly on the surface of Luria-Bertani plate. The sterile samples were laid on the top of the plate and incubated at 37°C for 12 hours. Then the diameters of zones of inhibition against S. aureus were measured.
For the fluorescent staining assay, bacteria were inoculated on the samples mentioned earlier. The concentration of S. aureus was adjusted to 108 CFU/mL. After co-incubated at 37°C for 6 hours, samples were removed and rinsed with PBS, stained using acridine orange for 15 minutes in darkness, and then observed by fluorescence microscopy (Olympus Corporation, Tokyo, Japan).
Biocompatibility assay
MC3T3-E1 cell (Cell Resource Center, PUMC, Beijing, People’s Republic of China) was cultured in α-minimum essential medium (α-MEM) containing 10% fetal bovine serum (FBS) and incubated in a humidified atmosphere of 5% CO2 at 37°C. Before cell seeding, all the samples were cut into 8 mm ×8 mm ×1 mm. The sterile samples were placed in the 24-well microplates, and the cells were seeded onto the samples at a density of 1×104 cells per well.
The viability of cells on the samples was assessed by WST-1 cell proliferation and cytotoxicity assay kit (Beyotime, Nantong, People’s Republic of China). After 1, 3, and 7 days of culture, 50 µL WST-1 solution was added to each well and incubated at 37°C for 2 hours. After incubation, the absorbance values were measured at 450 nm with a reference wavelength of 690 nm (Infinite M200 Pro, Tecan Schweiz AG, Männedorf, Switzerland).
For the fluorescent staining assay, cells were stained with 3 µM Calcein-AM (Sigma-Aldrich) after 3-day incubation on the samples, and then observed by fluorescence microscopy (Olympus Corporation). The morphology of MC3T3-E1 cells on the samples was investigated by the SEM analysis. After 3 and 7 days of culture, samples were removed from the medium, washed with PBS, fixed in 2.5% glutaraldehyde, and dehydrated in series of ethanol concentrations. After the ethanol was substituted by tert-butanol, samples were lyophilized and sputter coated with gold before examination under the SEM analysis.
Statistical analysis
Triplicate experiments were performed. The data were expressed as the mean ± standard deviation. Analysis of the results was carried out using Student’s t-test, with the significance level of P<0.05 and highly significance level of P<0.01.
Results
Characterization of AgNPs modified CVC surface
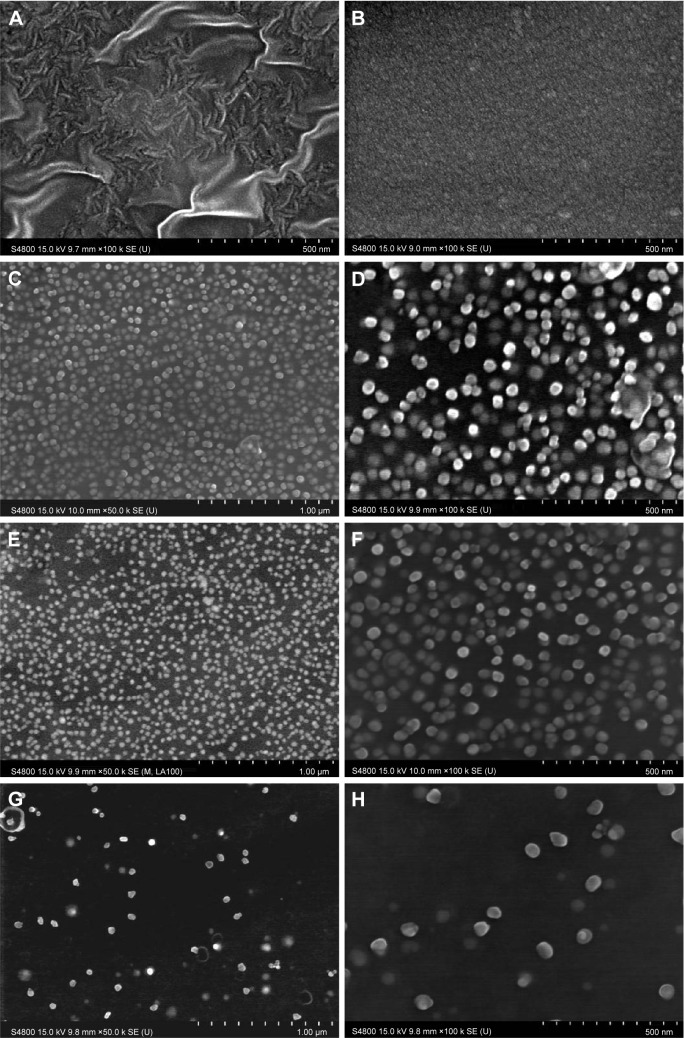
The SEM images for CVC, DCVC, and DCVC-Ag samples are shown in Figure 1. The pristine CVC exhibited an uneven surface, which was fully covered by polydopamine after impregnation in dopamine solution for 24 hours. After immersing into different concentrations of silver nitrate solutions, conductive and monodispersed spherical nanoparticles with a size of 30–50 nm could be found on the DCVC surface, indicating that the polydopamine surface could spontaneously reduce Ag+ ions into AgNPs without requiring additional reducing agent. As shown in Figure 1C and D, DCVC-Ag1 sample has the largest surface density, and the density decreased with silver nitrate solution concentration.
Figure 1.
SEM images of (A) CVC, (B) DCVC, (C and D) DCVC-Ag1, (E and F) DCVC-Ag2, and (G and H) DCVC-Ag3.
Abbreviations: CVC, central venous catheter; DCVC, central venous catheters coated with polydopamine films; SEM, scanning electron microscopy.
The relative surface wettability was determined by the static water CA. As shown in Table 1, the pristine catheter showed hydrophobic property with the CA value of 113.3°±2.2°. After modifying with surface-adherent polydopamine films, the DCVC showed a hydrophilic surface with the CA value of 80.4°±1.8°. The following in situ generation of AgNPs on the DCVC surface resulted in slightly improved hydrophilicity. The CA value decreased as the AgNPs number increased, and that of DCVC-Ag1 samples were nearly constant (52.2°±2.4°).
Table 1.
Surface chemical composition and water contact angle of pristine CVC, DCVC, and DCVC-Ag samples (atomic percentage according to XPS analysis)
| Atomic concentration (at%)
|
Contact angle (°) | ||||||
|---|---|---|---|---|---|---|---|
| C1s | N1s | O1s | F1s | Si2p | Ag3d | ||
| CVC | 46.21 | – | 8.92 | 37.56 | 7.31 | – | 113.3±2.2 |
| DCVC | 61.25 | 5.38 | 23.95 | 0.77 | 8.65 | – | 80.4±1.8 |
| DCVC-Ag1 | 52.43 | 4.20 | 21.87 | 5.60 | 6.26 | 9.64 | 52.2±2.4 |
| DCVC-Ag2 | 56.25 | 5.66 | 25.58 | 0.97 | 4.80 | 6.74 | 59.3±3.9 |
| DCVC-Ag3 | 55.29 | 5.46 | 27.02 | 1.45 | 9.61 | 1.19 | 66.3±2.7 |
Note: These data is expressed as the mean ± SD (n=3).
Abbreviations: CVC, central venous catheter; DCVC, central venous catheters coated with polydopamine films; XPS, X-ray photoelectron spectroscopy; SD, standard deviation.
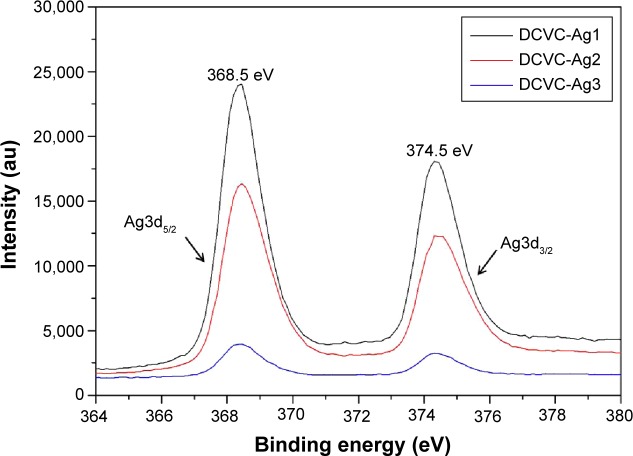
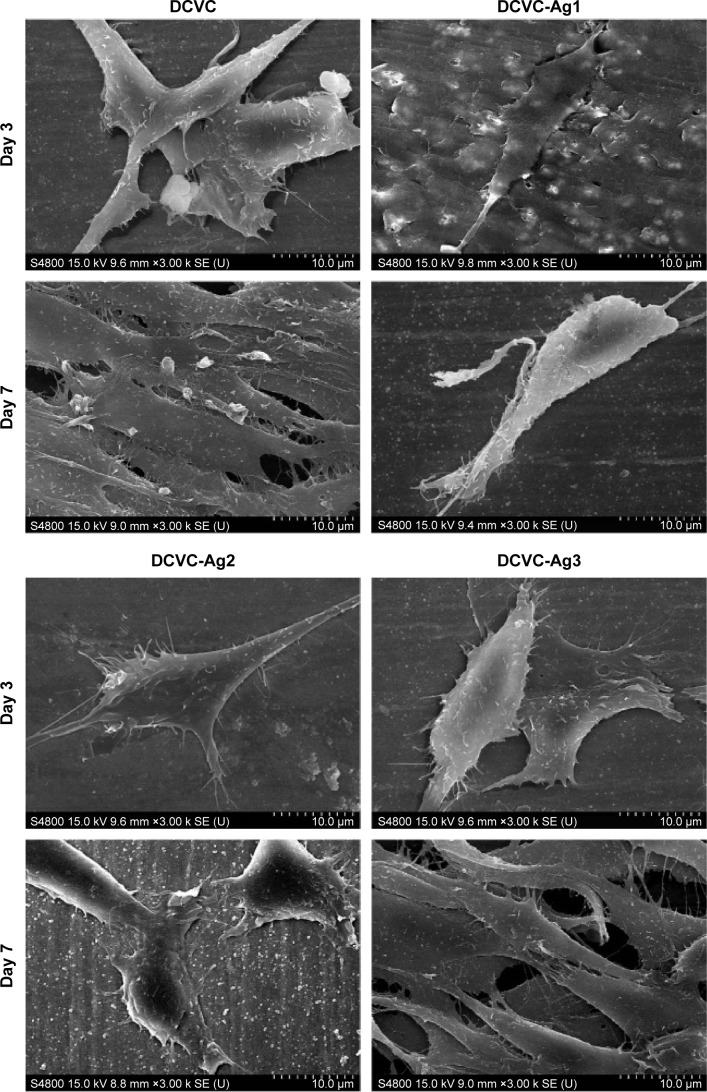
The surface chemical compositions of the CVCs films before and after surface modification were determined by XPS. As shown in Figure 2, the sharp peak of F1s developed from pristine CVCs vanished after polydopamine modification. Meanwhile, no peak corresponding to N1s could be observed on the pristine CVCs, which indicated the nitrogen atom was derived from the polydopamine membrane on DCVC. This result suggests that the thickness of the deposited polydopamine layer is higher than the probing depth of the XPS technique (approximately 7.5 nm in an organic matrix).19 The Ag concentrations determined from the XPS survey spectra of DCVC-Ag1, DCVC-Ag2, and DCVC-Ag3 are 9.64, 6.74 and 1.19 at%, respectively (Table 1). The high-resolution XPS spectra of Ag obtained from DCVC-Ag1, DCVC-Ag2, and DCVC-Ag3 are shown in Figure 3. The binding energies of Ag3d doublet peaks located at 368.5 and 374.5 eV can be assigned to Ag3d5/2 and Ag3d3/2 of metallic Ag0, indicating that Ag mainly exists in the Ag0 state on the DCVC surface.20,21
Figure 2.
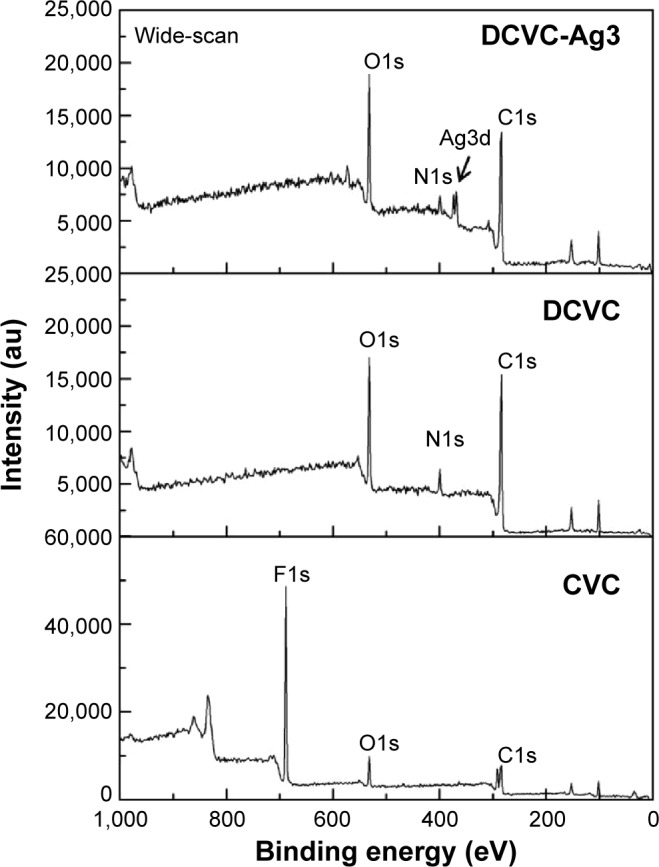
The survey XPS spectra of CVC, DCVC, and DCVC-Ag3.
Abbreviations: au, arbitrary unit; CVC, central venous catheter; DCVC, central ve nous catheters coated with polydopamine films; XPS, X-ray photoelectron spectroscopy.
Figure 3.
The high-resolution XPS spectra of DCVC-Ag1, DCVC-Ag2, and DCVC-Ag3.
Abbreviations: au, arbitrary unit; DCVC, central venous catheters coated with polydopamine films; XPS, X-ray photoelectron spectroscopy.
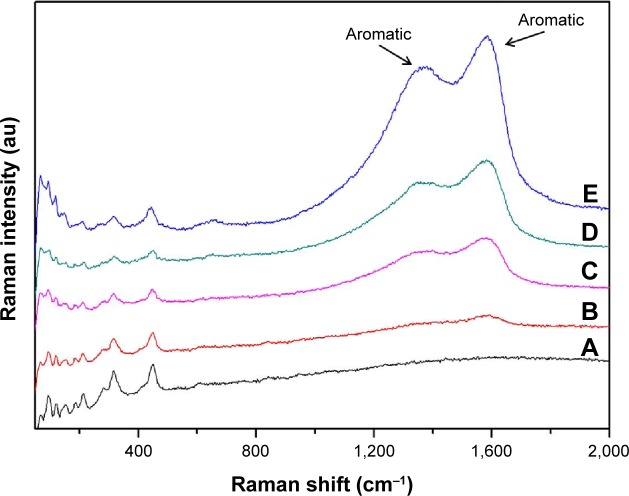
Silver is one of the best materials for making surface enhanced Raman scattering active surfaces, and a great deal of effort has been put into perfecting methods of forming silver surfaces for strong surface enhanced Raman scattering signals.22 The chemical composition on the surface was further investigated by Raman spectroscopy. As shown in Figure 4, the peak at 1,330 cm−1 (amide III) is assigned to the vibrational mode of dopamine, which is a distortion of the C-C bond connecting the two oxygen-bearing carbon atoms, and the peak at 1,595 cm−1 (amide II) is related to in plane N-H bending and C-N stretching of dopamine, both of which corresponding to characteristic aromatic groups in polydopamine. The intensity of characteristic peaks of aromatic groups was quite weak in DCVC, and no peaks could be detected in CVC. However, the intensities of the characteristic peaks were obviously increased in the DCVC-Ag samples, and proportional to the density of AgNPs. SEM, XPS, and Raman spectra provide unequivocal proofs that metallic AgNPs have been successfully coated on the CVCs surface.
Figure 4.
Raman spectra of various samples: (A) CVC, (B) DCVC, (C) DCVC-Ag3, (D) DCVC-Ag2, and (E) DCVC-Ag1.
Abbreviations: au, arbitrary unit; CVC, central venous catheter; DCVC, central venous catheters coated with polydopamine films.
Antibacterial characteristics of the silvered CVC films
The ability of the DCVC-Ag samples to prevent viable bacteria colonization was verified by Zol test and fluorescence staining. The Zol test is based on the leaching of silver ions from the surface, the inhibition of the bacterial growth depends on a sufficient concentration of silver ions in the surrounding aqueous environment.23 Figure 5 shows the Zol test results by DCVC-Ag1, DCVC-Ag2, and DCVC-Ag3 on S. aureus. All the DCVC-Ag samples showed a clear inhibition zone, which indicated the efficient antibacterial ability of these samples. The diameter of the bacteria inhibition zone ranged from 0.2 to 0.35 cm against S. aureus. Based on the Ag concentrations in Table 1, the antibacterial effect could be modulated by the density of coated AgNPs.
Figure 5.
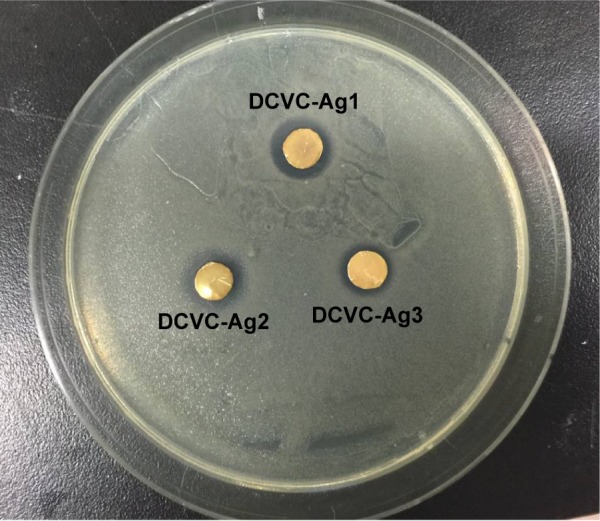
Photographs showing the zone of inhibition assay for DCVC-Ag samples.
Abbreviation: DCVC, central venous catheters coated with polydopamine films.
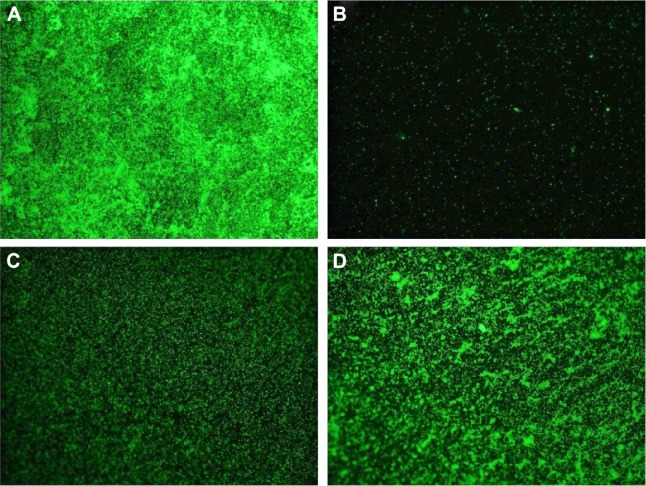
The antimicrobial effect of DCVC-Ag samples was also confirmed by bacteria viability after 6 hours of incubation. As shown in Figure 6, large amounts of viable bacteria were observed on the surface of DCVC (Figure 6A). There were a relatively small number of viable bacteria on the DCVC-Ag3 (Figure 6D). Due to the increased Ag concentrations in DCVC-Ag2 and DCVC-Ag1, much fewer bacteria can be detected on DCVC-Ag2 and nearly no viable bacteria on DCVC-Ag1.
Figure 6.
Fluorescent microscope images of Staphylococcus aureus stained on (A) DCVC, (B) DCVC-Ag1, (C) DCVC-Ag2, and (D) DCVC-Ag3 after 6 hours of culture.
Abbreviation: DCVC, central venous catheters coated with polydopamine films.
Cytocompatibility assays
The WST-1 assay was used to evaluate the cytotoxicity of the substrates on MC3T3-E1 cells, and the optical density (OD) value was considered to indicate cell growth on different surfaces. As shown in Figure 7, the OD value of both CVC and DCVC increased obviously with the culture time, and the OD value of DCVC was slightly higher than CVC. On day 1, there was no appreciable difference on the OD value among all the samples (P>0.05). On day 3, proliferation of MC3T3-E1 cells in DCVC-Ag1 and DCVC-Ag2 group was markedly inhibited as compared to DCVC-Ag3 (P<0.05), and the tendencies were even more obvious after 7 days incubation. The cytotoxicity caused by the DCVC-Ag samples is due to the Ag ions released from the coated AgNPs. In the DCVC-Ag3 group, the OD value increased with the culture time. Though the OD values were slightly lower than that of CVC and DCVC, the difference is statistically insignificant. Hence, DCVC-Ag3 exhibited good cytocompatibility compared with DCVC-Ag1 and DCVC-Ag2.
Figure 7.
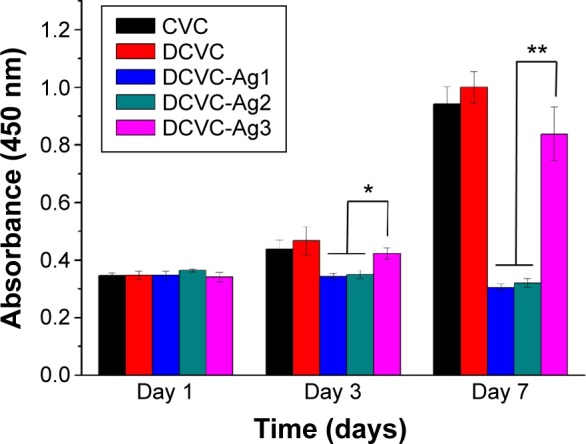
WST-1 assay for proliferation of MC3T3-E1 cell on different samples at various incubation periods.
Note: Error bars represent mean ± SD (n=5), *P<0.05, **P<0.01.
Abbreviations: CVC, central venous catheter; DCVC, central venous catheters coated with polydopamine films; SD, standard deviation; WST-1, water soluble tetrazolium salts.
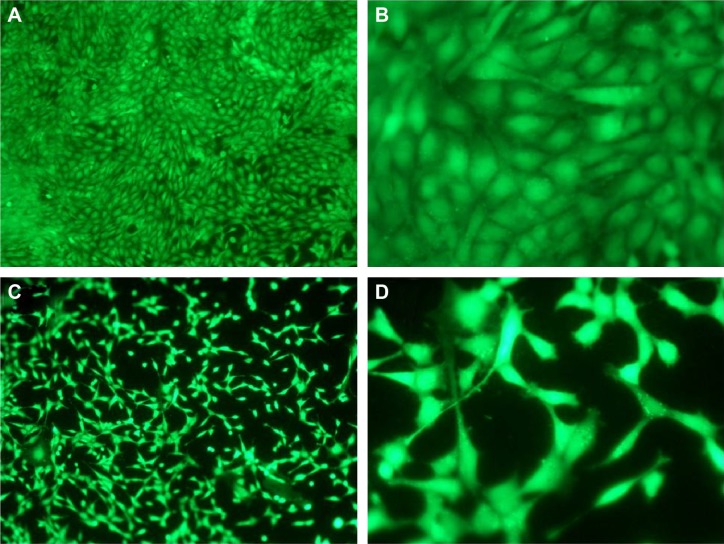
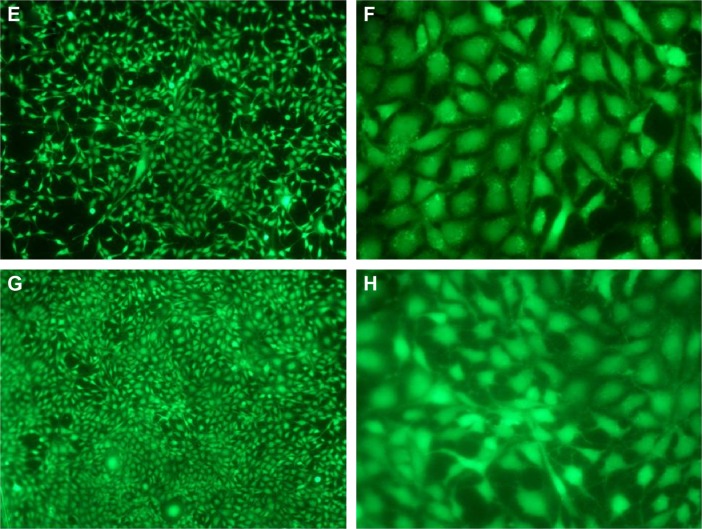
Fluorescence microscopy and SEM were used to characterize adhesion and spreading of cells cultured on different substrates after 3-day incubation. As shown in Figure 8, well adhesion and growth of cells were seen on the surface of DCVC. After co-incubated with different DCVC-Ag sample for 3 days, cells showed an obvious dose-dependent decrease in cell viability compared with that of the DCVC group. Figure 9 shows the cell morphology on different substrates, which assisted to understand the cell behavior. The spread of cells was remarkably regulated by the amount of AgNPs. After 3 days of culture, cells on DCVC and DCVC-Ag3 displayed a polygonal shape with prominent filopodia protrusions. On the contrary, DCVC-Ag1 and DCVC-Ag2 are unfavorable to cell growth. It is noted that the filopodia protrusions vanished on DCVC-Ag1. After 7 days, a great quantity of cells adhered to the surface of DCVC and DCVC-Ag3, and numerous pseudopodia spread around the cell body. The number of adherence cells on DCVC-Ag1 and DCVC-Ag2 surfaces were significantly less than on DCVC-Ag3, and no filopodia protrusions could be found. The cell morphology again confirmed the best compatibility of DCVC-Ag3. These results were consistent with the WST-1 assay.
Figure 8.
Fluorescence microscopy images of Calcein-AM stained MC3T3-E1 cell attached on (A and B) DCVC, (C and D) DCVC-Ag1, (E and F) DCVC-Ag2, and (G and H) DCVC-Ag3 for 3 days.
Abbreviation: DCVC, central venous catheters coated with polydopamine films.
Figure 9.
The SEM images of MC3T3-E1 cell attachment on DCVC, DCVC-Ag1, DCVC-Ag2, and DCVC-Ag3 samples for days 3 and 7.
Abbreviations: DCVC, central venous catheters coated with polydopamine films; SEM, scanning electron microscopy.
Discussion
Based on the earlier studies, dopamine could self-polymerize to form nanometers thickness of multifunctional adhesive film on various surfaces. It is known that polydopamine can reduce the silver ions to form AgNPs. The catechol groups in the polydopamine film could chelated with Ag, and then in situ reduced the Ag+ to Ag0. And the Ag0 could be bonded on the N-site and O-site in polydopamine film and formed seed precursor. The seed Ag0 could grow to nanoparticles through the atom by atom growth with the reduction of Ag+.17 In this study, the particle coverage of the surface increased with silver nitrate solution concentration obviously. The huge difference in the particle coverage of surface is mainly due to the silver precursor concentration.
As an antibacterial agent, AgNPs coating has been applied in several medical devices, among which catheters,24 drains,25 and wound dressings26 are the most prominent representatives. When it enters the body, metallic silver will react and result in the formation and release of silver ions, which are highly potent as an antibacterial agent. In this study, the antimicrobial effects of AgNPs coated CVCs were investigated. The CVCs coated with spherical AgNPs with a size of 30–50 nm showed strong antimicrobial ability. As shown in Figure 5, all DCVC-Ag samples show obvious antibacterial activity and the killing efficiency is dependent on the AgNPs dose. When incubated with S. aureus, the DCVC-Ag samples are highly effective against bacteria and the antibacterial ability is positively correlated with AgNPs density as shown in Figure 6. It is worth mentioning that intensive bacteria (108 CFUs/mL) were used in our antibacterial assay, and the condition is much harsher than the real in vivo environments.
However, one problem of AgNPs is their biotoxicity, thus, AgNPs coatings must be well tolerated. The biosafety of AgNPs has attracted widespread attention for the disappointing results of metallic silver in contact with blood and tissues.6,27 Generally, it is accepted that a high dose of silver can induce cytotoxicity. It is also reported that the cytotoxicity of AgNPs can be attributed to continuous leaching of Ag+ and its accumulation in the culture medium.28 Ag+ release from AgNPs was detected from many aspects (such as size, shape, surface properties, and degree of aggregation), especially the particle size.29 In light of an earlier report, the cytotoxicity of AgNPs was primarily mediated by a size-dependent release of Ag+, and the smaller AgNPs (with a diameter of 50 nm) could facilitate fast dissolution and release of Ag+.30 There is little literature discussed the toxicity of the dopamine reduced AgNPs coatings. Sureshkumar et al showed that the AgNPs deposited on polydopamine coated polyethylene possessed effective biocidal properties and did not inhibit the growth of L-929 cells mouse fibroblasts after 24-hour co-incubation.31 But long-term toxicity should be detected for such AgNPs coated materials. It is reported that the biocompatibility and toxicity of AgNPs were highly dependent on the density of AgNPs.32 The nature of the silver particles as well as how these are incorporated in the coating will determine the efficacy of such modified medical devices.6 In general, it has been suggested that Ag ions are considered to be biocompatible only at relatively lower concentrations.33–35 The cytocompatibility property of the AgNPs coated CVCs was also studied. All the DCVC-Ag samples showed no cytotoxicity on day 1. The results are consistent with that from Sureshkumar et al.31 However, obvious cytotoxicity were observed on DCVC-Ag1 and DCVC-Ag2 on day 3 and day 7, which may due to the relative high density of AgNPs. It is possible that the bacteria could be efficiently killed without cell cytotoxicity at specific AgNPs density. DCVC-Ag3 demonstrated both obvious antibacterial property and good biocompatibility in this study. Thus, the CVCs with proper AgNPs coatings via this green preparation method are promising in clinical application. Extended future works including in vivo test are compulsory for these CVCs with AgNPs coatings.
Conclusion
In summary, we developed a facile biomimetic strategy to decorate uniformly small and well dispersed AgNPs on CVC surface. The SEM results indicated that the particle size was within the range of 30–50 nm, and the content of the nanoparticles could be controlled by the concentration of silver nitrate solution. The XPS and Raman analysis confirmed the presence of metallic silver particles on the CVC films. The AgNPs coated CVC samples showed excellent antibacterial efficacy, and the antibacterial ability was associated with AgNPs density. The cytotoxicity was also associated with the content of coated AgNPs. The DCVC-Ag3 sample showed both obvious antibacterial ability and good biocompatibility. The results suggested that antibacterial and biocompatible AgNPs coating could be formed by carefully controlling the size and the content of nanoparticles, which has a promising prospect in clinical application.
Acknowledgments
The authors acknowledge financial support from the Young Foundation of the Affiliated Dongnan Hospital of Xiamen University (12Y001), Medical Scientific Youth Program Supported by Nanjing Command (18FBQN2014008) and National Natural Science Foundation of China (Grant No.51571169).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pongruangporn M, Ajenjo MC, Russo AJ, et al. Patient- and device-specific risk factors for peripherally inserted central venous catheter-related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189. doi: 10.1086/669083. [DOI] [PubMed] [Google Scholar]
- 2.LeMaster CH, Schuur JD, Pandya D, et al. Infection and natural history of emergency department–placed central venous catheters. Annf Emerg Med. 2010;56(5):492–497.e491. doi: 10.1016/j.annemergmed.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 3.Sousa C, Henriques M, Oliveira R. Mini-review: antimicrobial central venous catheters – recent advances and strategies. Biofouling. 2011;27(6):609–620. doi: 10.1080/08927014.2011.593261. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert RE, Harden M. Effectiveness of impregnated central venous catheters for catheter related blood stream infection: a systematic review. Curr Opin Infect Dis. 2008;21(3):235–245. doi: 10.1097/QCO.0b013e3282ffd6e0. [DOI] [PubMed] [Google Scholar]
- 5.Bong JJ, Kite P, Wilco MH, McMahon MJ. Prevention of catheter related bloodstream infection by silver iontophoretic central venous catheters: a randomised controlled trial. J Clin Pathol. 2003;56(10):731–735. doi: 10.1136/jcp.56.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knetsch ML, Koole LH. New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles. Polymers. 2011;3(1):340–366. [Google Scholar]
- 7.Macocinschi D, Filip D, Paslaru E, et al. Polyurethane – extracellular matrix/silver bionanocomposites for urinary catheters. J Bioact Compat Polym: Biomed Appl. 2014 0883911514560661. [Google Scholar]
- 8.Filip D, Macocinschi D, Paslaru E, et al. Polyurethane biocompatible silver bionanocomposites for biomedical applications. J Nanopart Res. 2014;16(11):17. [Google Scholar]
- 9.Agarwala M, Barman T, Gogoi D, Choudhury B, Pal AR, Yadav RNS. Highly effective antibiofilm coating of silver-polymer nanocomposite on polymeric medical devices deposited by one step plasma process. J Biomed Mater Res Part B. 2014;102(6):1223–1235. doi: 10.1002/jbm.b.33106. [DOI] [PubMed] [Google Scholar]
- 10.Saez S, Fasciani C, Stamplecoskie KG, et al. Photochemical synthesis of biocompatible and antibacterial silver nanoparticles embedded within polyurethane polymers. Photochem Photobiol Sci. 2015;14(4):661–664. doi: 10.1039/c4pp00404c. [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H, Lee BP, Messersmith PB. A reversible wet/dry adhesive inspired by mussels and geckos. Nature. 2007;448(7151):338–334. doi: 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Jiang Y, Liao Y, Tian M, Zou H, Zhang L. Fabrication of silver-coated silica microspheres through mussel-inspired surface functionalization. J Colloid Interface Sci. 2011;358(2):567–574. doi: 10.1016/j.jcis.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Cheng W, Tian M, Zou H, Li L, Zhang L. Preparation of PET/Ag hybrid fibers via a biomimetic surface functionalization method. Electrochimica Acta. 2012;79:37–45. [Google Scholar]
- 15.Liao Y, Wang Y, Feng X, Wang W, Xu F, Zhang L. Antibacterial surfaces through dopamine functionalization and silver nanoparticle immobilization. Mater Chem Phys. 2010;121(3):534–540. [Google Scholar]
- 16.Sileika TS, Kim H-D, Maniak P, Messersmith PB. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl Mater Interfaces. 2011;3(12):4602–4610. doi: 10.1021/am200978h. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Shi X, Ma H, Lv Y, Zhang L, Mao Z. The preparation and antibacterial effects of dopa-cotton/AgNPs. Appl Surf Sci. 2011;257(15):6799–6803. [Google Scholar]
- 18.Sureshkumar M, Lee P-N, Lee C-K. Stepwise assembly of multimetallic nanoparticles via self-polymerized polydopamine. J Mater Chem. 2011;21(33):12316–12320. [Google Scholar]
- 19.Tan KL, Woon LL, Wong HK, Kang ET, Neoh KG. Surface modification of plasma-pretreated poly(tetrafluoroethylene) films by graft-copolymerization. Macromolecules. 1993;26(11):2832–2836. [Google Scholar]
- 20.Lai Y, Zhuang H, Xie K, et al. Fabrication of uniform Ag/TiO2 nanotube array structures with enhanced photoelectrochemical performance. New J Chem. 2010;34(7):1335–1340. [Google Scholar]
- 21.Li J, Qiao Y, Zhu H, Meng F, Liu X. Existence, release, and antibacterial actions of silver nanoparticles on Ag-PIII TiO2 films with different nanotopographies. Int J Nanomed. 2014;9:3389–3402. doi: 10.2147/IJN.S63807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito Y, Wang JJ, Smith DA, Batchelder DN. A simple chemical method for the preparation of silver surfaces for efficient SERS. Langmuir. 2002;18(8):2959–2961. [Google Scholar]
- 23.Juan L, Zhimin Z, Anchun M, Lei L, Jingchao Z. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int J Nanomed. 2010;5:261–267. doi: 10.2147/ijn.s8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leuck A-M, Johnson JR, Hunt MA, et al. Safety and efficacy of a novel silver-impregnated urinary catheter system for preventing catheter-associated bacteriuria: a pilot randomized clinical trial. Am J Infect Control. 2015;43(3):260–265. doi: 10.1016/j.ajic.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lajcak M, Heidecke V, Haude KH, Rainov NG. Infection rates of external ventricular drains are reduced by the use of silver-impregnated catheters. Acta Neurochir. 2013;155(5):875–881. doi: 10.1007/s00701-013-1637-9. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Cheng F, Gao J, Wang L. Antibacterial wound dressing from chitosan/polyethylene oxide nanofibers mats embedded with silver nanoparticles. J Biomater Appl. 2015;29(8):1086–1095. doi: 10.1177/0885328214554665. [DOI] [PubMed] [Google Scholar]
- 27.Park EJ, Yi J, Kim Y, Choi K, Park K. Silver nanoparticles induce cytotoxicity by a trojan-horse type mechanism. Toxicol In Vitro. 2010;24(3):872–878. doi: 10.1016/j.tiv.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Wang H, Huo K, et al. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011;32(24):5706–5716. doi: 10.1016/j.biomaterials.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 29.Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit Rev Toxicol. 2010;40(4):328–346. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- 30.Albers CE, Hofstetter W, Siebenrock KA, Landmann R, Klenke FM. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology. 2013;7(1):30–36. doi: 10.3109/17435390.2011.626538. [DOI] [PubMed] [Google Scholar]
- 31.Sureshkumar M, Siswanto DY, Chen Y-C, Lee C-K, Wang M-J. Antibacterial and biocompatible surfaces based on dopamine autooxidized silver nanoparticles. J Polym Sci B Polym Phys. 2013;51(4):303–310. [Google Scholar]
- 32.Politano AD, Campbell KT, Rosenberger LH, Sawyer RG. Use of silver in the prevention and treatment of infections: silver review. Surg Infect. 2013;14(1):8–20. doi: 10.1089/sur.2011.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Xing Q, Janjanam J, et al. Facile electrochemical synthesis of antimicrobial TiO2 nanotube arrays. Int J Nanomed. 2014;9:5177–5187. doi: 10.2147/IJN.S65386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu X-HN. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. Acs Nano. 2007;1(2):133–143. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Travan A, Pelillo C, Donati I, et al. Non-cytotoxic silver nanoparticle-polysaccharide nanocomposites with antimicrobial activity. Biomacromolecules. 2009;10(6):1429–1435. doi: 10.1021/bm900039x. [DOI] [PubMed] [Google Scholar]