First described in the 1980s by pulmonologist David Flenley, “overlap” syndromes refer to the coexistence of chronic lung disease and obstructive sleep apnea (OSA).1 Although it could refer to any of the lung diseases and OSA, “the” Overlap Syndrome (OVS) is usually reserved for the coexistence of OSA and chronic obstructive pulmonary disease (COPD), which Flenley believed to have unique adverse health consequences distinct from either condition alone. Given the high prevalence of each disorder alone, Overlap syndrome is also likely to be common and clinically relevant. However, although Overlap syndrome has been described in the literature for nearly 30 years, the lack of standard diagnostic criteria for the syndrome has limited rigorous discussion of diagnosis, prevalence, pathophysiology, treatment, and outcomes. These challenges are explored in more detail below, and throughout this review. Importantly, several recent studies suggest that Overlap Syndrome does, as Flenley believed, have worse outcomes than either disease in isolation. These findings have highlighted the urgent need for further study of both “the” Overlap Syndrome and all overlaps between OSA and chronic lung disease.
Clinical and research challenges of the Overlap Syndromes
Overlap syndromes are poorly understood for many reasons. Using “the” Overlap syndrome as a prototype:
The diagnosis of Overlap syndrome is nebulous, as both OSA and COPD are heterogeneous disorders. COPD and OSA both have wide ranges of severity, in terms of both objective measurements of disease (e.g. forced expiratory volume in 1 second, FEV1, and apnea-hypopnea index, AHI) and patient-reported symptoms (e.g. dyspnea and daytime tiredness). Overlap syndrome is defined by the presence of both conditions regardless of the relative burden of one or the other. Therefore patients with overlap syndrome may represent a very heterogeneous population, falling into one of many potential categories: mild COPD with mild OSA, mild COPD with severe OSA, severe COPD with mild OSA, severe COPD with severe OSA, etc. Prognosis and treatment, therefore, could be considerably different depending on the relative impact of each condition. A minor point, but there is not a single International Statistical Classification of Diseases (ICD – 9) code for Overlap syndrome, which impedes even epidemiological research.
The diagnosis of OSA in the setting of hypoxemic lung disease is uncertain. The definition of OSA includes hypopneas, reductions in airflow with associated desaturation, which is more likely to occur in those with chronic lung disease. The apnea-hypopnea index (AHI), used to grade OSA severity, does not differentiate between apneas and hypopneas. Thus, a patient with severe COPD might have the same AHI consistent with severe OSA (based on a large number of hypopneas) as another patient with a very collapsible upper airway without lung disease (who has predominantly apneas). In addition, a 10 minute prolonged desaturation due to hypoventilation may be scored as a single hypopnea since event duration has minimal effect on the definitions used. More rigorous definitions of OSA might be useful, such as the apnea index (AI), or scoring based on airflow alone and arousals independent of oxygen desaturation.
The interactions of COPD and OSA are not understood. Thus, it is unknown at a pathophysiological level whether each disorder might predispose to the other disease. As above, the baseline hypoxemia of COPD likely predisposes to a diagnosis of OSA. But other links are possible, for example, the changes in lung volumes that occur with COPD might impact upper airway collapsibility. How COPD and OSA interact to cause the increased morbidity and mortality attributable to OVS is not known. Is it simply from more prolonged hypoxemia or hypercapnia than either disorder alone? Or are poor outcomes due to the indirect effects of the disorders, such as cardiovascular disease?
Thus, the goals of therapy in OVS are poorly defined. For a patient with severe OSA with many apneas, the goal of therapy may be to support patency of the upper airway and eliminate apneic events. For a patient with evidence of hypoventilation, the goal may be to improve nocturnal gas exchange and hypercarbia. Maybe the best approach would be intensive modification of cardiovascular risk factors (e.g. blood pressure, cholesterol modification). These uncertainties contribute to the confusion as to the ideal therapy to employ.
The optimal treatment of overlap syndrome is unknown. Few large clinical trials have been undertaken, and no large studies have compared long-term outcomes between randomized therapies. Although continuous positive airway pressure (CPAP) is the most commonly applied therapy, some groups have used Bilevel positive airway pressure, which provides a higher pressure during inspiration than during expiration. Bi-level may have benefits over CPAP for some patients, particularly among severe COPD patients where it may aid with nocturnal ventilation and resting of respiratory muscles. Finally, the role of oxygen therapy—another treatment used clinically—has not been fully explored in this population. The role of medical therapy aimed at limiting cardiovascular events has also not been explored.
An additional, under-recognized consideration is that sleep is poor in chronic lung diseases, independent of upper airway collapse. Many studies have highlighted the high prevalence of sleep complaints among patients with chronic lung diseases. There are many reasons behind this finding, ranging from cough interfering with sleep, increased anxiety and insomnia, side effects of medications (e.g. chronic glucocorticoids, beta agonists) and frequent arousals. Although treatment of overlap syndrome with CPAP may improve upper airway patency, CPAP will not addresses many of the non-respiratory problems that plague sleep in this population. Thus, CPAP adherence may be challenged in ways that are unique compared to those without chronic lung disease.
These points will be illustrated as we discuss what is known about OVS, and OSA and idiopathic pulmonary fibrosis (IPF), perhaps the most common of the interstitial lung diseases.
COPD and OSA
Throughout this section overlap syndrome (OVS) will refer exclusively to those with COPD and OSA.
Chronic obstructive pulmonary disease (COPD)
COPD is a progressive lung disease characterized by irreversible airway obstruction (forced expiratory volume in 1 second/forced vital capacity, FEV1/FVC <70%). This disease can involve the small airways, pulmonary parenchyma, or both. COPD results from an inflammatory response that can result in chronic sputum production (chronic bronchitis) as well as the destruction of alveolar walls distal to the terminal bronchioles, leading to enlargement of the airspaces (emphysema). Although tobacco use is strongly associated with development of COPD, it is not the only risk factor. In developing countries, exposure to indoor air pollution plays a critical role, in particular as a result of fuels burned for cooking and heating. Occupational causes are also well described, such as irritants and fumes. Estimates are now that the majority of COPD worldwide is non-smoking related, emphasizing caution about labeling the disease as self-inflicted. COPD may present as dyspnea, wheezing, cough, sputum production, poor exercise tolerance, hypoxic and/or hypercarbic respiratory failure, and right heart failure (cor pulmonale). There are GOLD (Global initiative for chronic Obstructive Lung Disease)-defined stages of disease severity, based on pulmonary function testing (the FEV1) and symptoms. In the United States, over 5% of the population—at least 13.7 million people—is burdened by COPD,2,3 which is a leading cause of morbidity. Worldwide, about 10% of the population is affected.4 Though medications may improve symptomatic control of the disease and slow progression, the health-related consequences of COPD remain high.3 As of 2011, COPD was the third leading cause of death in the United States.5 Annual expenditures for the disease are approaching $40 billion when direct and indirect costs are considered.6
Though COPD is often considered a respiratory condition, the impact on other organ systems and overall health is increasingly well recognized. The most recent GOLD definition of the disease highlights COPD as a systemic process with “significant extrapulmonary effects that may contribute to the severity in individual patients.”7 Indeed, depression, skeletal-muscle myopathy, anemia, and osteoporosis are all common in COPD. Similarly, as will be discussed below, sleep disturbance and its consequences could be thought of as one of these extrapulmonary manifestations. COPD is associated with adverse cardiac outcomes as well, which may be of particularly importance when thinking about the overlap with OSA, which also has cardiovascular consequences.8–12 Even after consideration of shared risk factors, such as cigarette smoking, COPD is associated with higher rates of coronary artery disease, congestive heart failure, and arrhythmias.13,14 Additional mechanisms by which COPD may play a role in cardiovascular disease include increased oxidative stress, inflammation, and increased platelet activation.
Of particular interest in the current discussion, COPD is a heterogeneous disorder, with variable amounts of airway and parenchymal disease. Most patients have a predominance of one phenotype, though there is usually some overlap. In the past, patients with chronic bronchitis were described as “blue bloaters,” referring to hypoxemia, polycythemia and cor pulmonale that often accompanies patients with this form of COPD. “Pink puffers” were those with an emphysematous phenotype of COPD, often with muscle wasting and hyperinflation but without oxygen desaturation. The GOLD criteria are designed to be inclusive to maximize disease recognition and prompt treatment, and therefore do not highlight these distinctions. However, there may be critical differences in pathophysiology among different phenotypes that are important when considering OVS.
Sleep and COPD
More than three-quarters of patients with COPD report bothersome nocturnal symptoms such as dyspnea.15,16 Patients who report cough and wheeze during the day are more likely to have sleep disturbances than those who do not.17 Patients report trouble falling asleep, frequent awakening, difficultly returning to sleep, and non-restorative sleep. In a survey of COPD patients, over 60% had experienced at least one sleep symptom in the preceding 28 days.15 Rates of clinical insomnia are high among COPD patients, present in over one-fourth.18 As compared to controls, COPD confers an increased risk of insomnia nearly twice that of non-COPD patients.19 These sleep disturbances are chronic, persisting over many years.20
Sleep complaints increase with more severe disease. While mild obstructive lung disease is associated with preserved sleep quality,21 more severe obstructive disease is associated with increased sleep complaints.22 Severe disease negatively impacts a number of objectively sleep parameters such as total sleep time, sleep efficiency, REM sleep,23 as well as sleep onset latency, arousals, and sleep stage transitions.24–27
The mechanisms behind the sleep disturbances are likely multi-factorial. Symptoms such as cough and wheezing may play a role as noted above.28,29 Recent work has also highlighted non-respiratory factors that also perturb sleep among COPD patients.30 For example, restless leg syndrome (RLS) has been found in up to a third of COPD patients, and periodic limb movements are associated with worse insomnia.31 As a result of all of the above, the use of medications to aid sleep is common, especially sedative hypnotics, which are used by 25% of COPD patients.17 Although data are sparse, these medications could theoretically worsen hypoxemia/hypercapnia, though this may not be true for all patients.32–35
Changes in respiration during sleep with COPD and NOD
Nocturnal oxygen desaturation (NOD) in chronic lung disease is the result of the normal changes that occur in ventilation with sleep. Put another way, sleep is a stress test for those with chronic lung disease that leads to nocturnal hypoxemia and hypercapnia. Understanding the normal changes in respiration that occur with sleep is key to understanding NOD.
Normal changes in respiration during sleep
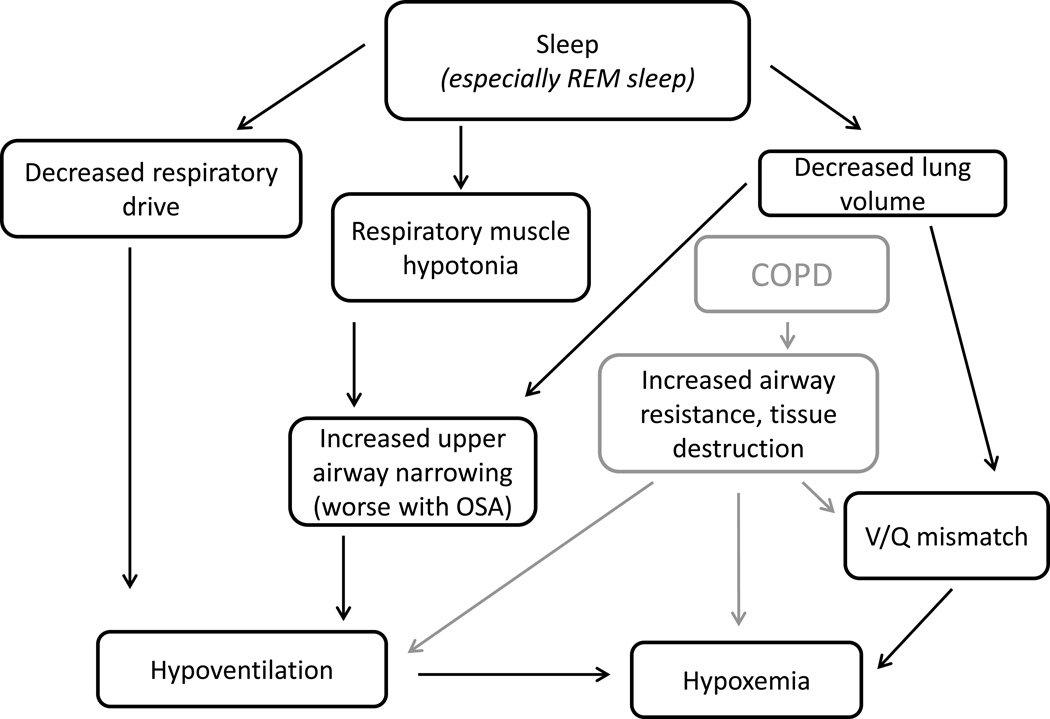
Sleep is divided into different stages based on electroencephalopgraphy (EEG) waveforms, muscle tone, eye movement, and breathing pattern, with the main distinction being between non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. The main respiratory changes that occur during sleep are: a drop in ventilation (largely a decrease in tidal volume without a compensatory increase in respiratory rate) and decreased accessory muscle activity. The changes in respiration are most pronounced in REM sleep, which is notable for skeletal muscle atonia (with the exception of certain muscles including the diaphragm) and, in addition to decreased ventilation, the breathing pattern becomes very irregular (especially during bursts of rapid eye movements). The drop in respiratory drive reflects both a drop in metabolic rate, which results in less CO2 production and thus requirement for elimination, and an increase in the CO2 set point.36 The reduction in minute ventilation is further pronounced during REM, when ventilation may be 40% less compared to wakefulness.37,38 In addition to the decrease in respiratory setpoint, there is decreased responsiveness to hypercapnia and hypoxia compared to wakefulness.39–41 Finally, upper airway resistance increases during sleep, even in those without obstructive sleep apnea.42 An overview of the changes is outlined in FIGURE 1.
Figure 1.
The normal physiological changes that occur with sleep. With sleep onset, respiratory drive is decreased, there is respiratory muscle hypotonia and a drop in lung volumes. Even without OSA, the result is hypoventilation compared to wakefulness. Particularly with OSA and COPD, there are further pathophysiological changes that lead to greater hypoventilation and hypoxemia.
Sleep and breathing with COPD
All of the above changes are physiologic changes that occur from wakefulness to sleep. However, in the presence of lung disease the consequences may be dramatic and lead to oxygen desaturation. First, these patients may already have borderline hypoxemia which puts them on the steep part of the oxygen hemoglobin binding curve – that is, small changes in PaO2 lead to a decrease in oxygen saturation. Second, patients with COPD have increased minute ventilation for a variety of reasons, and frequently rely on accessory muscles to aid ventilation. As a result, ventilation can fall dramatically during sleep and particularly in REM sleep when muscle activity decreases. Furthermore, COPD patients may have chest hyperinflation which stretches the diaphragm and impairs contractile function.43
Nocturnal oxygen desaturation is perhaps the most common sleep abnormality attributed to COPD, occurring in anywhere from one- to three-quarters of patients with an awake oxygen saturation >90–95%.44–46 During sleep, desaturations are frequent among patients with FEV1/FVC <65%,21 and increasing severity of obstructive disease is associated with more severe desaturations during sleep. Among those with severe obstructive lung disease (FEV1 <30%), a 20% drop of oxygen saturation can be seen during non-REM sleep and an impressive decline of 40% during REM.37 There is substantial variation in reported nocturnal oxygen desaturation rates, in part due to heterogeneous nature of COPD as well as definition of nocturnal oxygen desaturation, which may be based either on nadir levels or duration of low oxygen tension.
COPD and OSA: The Overlap Syndrome
Obstructive sleep apnea (OSA) is a common disorder, characterized by partial or complete collapse of the upper airway during sleep, resulting in intermittent hypoxia and arousals. The repetitive nature of these breathing events results in fragmented and non-restorative sleep. Among middle aged (50–70 years old) men, the prevalence of moderate to severe OSA is predicted to be as high as 17%, and slightly lower but still concerningly high 9% among middle aged women.47 OSA is associated with an increased risk of serious neurocognitive and cardiovascular consequences, including hypertension, congestive heart failure, and stroke.48–51 Continuous positive airway pressure (CPAP) is the gold standard treatment for OSA, and consists of a mask worn during sleep connected to a machine which delivers pressurized air, thereby splinting open the airway during sleep. Though CPAP is efficacious in treating OSA in almost all cases, its effectiveness is limited by patient adherence. Though adherence rates may be improved through intensive support and - behavioral therapy, the “real world” non-adherence rates may approach 50%. In the context of Overlap syndrome these facts illustrate the potentially large number of OSA patients at risk for Overlap syndrome, that both OSA and COPD have substantial cardiovascular morbidity and mortality, and that positive airway pressure is unlikely to be accepted by many patients.
Diagnosing OSA among those with COPD
OSA is diagnosed through polysomnography, with apneas and hypopneas recorded during sleep. The tendency toward oxygen desaturation described above in those with chronic lung disease impacts the diagnosis of OSA. Although the designation of apneas is straightforward and independent of oxygen desaturation, hypopneas are based on flow limitation of at least 30% and require either an accompanying ≥3% oxygen desaturation or an arousal. Based on the sigmoidal shape of the oxygen-Hb desaturation curve, any small change in PaO2 that occurs during sleep will be reflected as a larger (scorable) change in oxygen saturation. Put another way, two patients with the same upper airway tendency to collapse, but one healthy and the other with chronic lung disease might have very different apnea-hypopnea indices. A similar observation that makes the same point is that the AHI improves with descent from altitude, largely because of a decrease in the number of hypopneas.52 Nevertheless, there are no current alternative scoring criteria or guidelines for OSA diagnosis in the setting of chronic lung disease.
Among COPD patients, there are clues to suggest OSA beyond the classic symptoms of snoring, witnessed apneas, and daytime sleepiness. For example, headaches with the initiation of nocturnal supplemental oxygen suggest co-existent OSA (due to increased hyercapnia). Hypercapnia, despite relatively preserved pulmonary function tests, may also signal presence of sleep disordered breathing and prompt evaluation. Indeed, based on findings from one cohort, FEV1 was severely decreased among COPD-only patients with hypercapnia, but only moderately reduced in patients who had both COPD and OSA. Despite this difference in pulmonary function tests, daytime PaCO2 was higher among those with overlap syndrome compared to COPD-only.53,54 Additionally, obesity is more common among hypercarbic COPD patients that have OSA as compared to COPD-only.54 For comparison, the characteristics of COPD-alone, OSA-alone, and Overlap syndrome from one cohort are outlined in TABLE 1.
Table 1.
Characteristics and physiologic measures of patients with COPD only, OSA only, and Overlap Syndrome. (Adapted from Resta, 2002).54
| COPD Group (n = 32) |
Overlap Group (n = 29) |
Pure OSA Group (n = 152) |
|
|---|---|---|---|
| Age (y) | 60.1 ± 10.4 | 57.2 ± 9.5 | 48.9 ± 12.9 |
| Weight (kg) | 87.6 ± 17.5 | 102.2 ± 20.6 | 106.8 ± 28.8 |
| BMI (kg/m2) | 31 ± 7 | 36 ± 6 | 39 ± 10 |
| FVC1 (% predicted) | 60 ± 19 | 72 ± 17 | 87 ± 20 |
| FEV1/FVC (%) | 59 ± 9 | 67 ± 5 | 87 ± 9 |
| PaO2 (mm Hg) | 69 ± 10 | 70 ± 11 | 79 ± 12 |
| PaO2 (mm Hg) | 40 ± 5 | 45 ± 5 | 39 ± 4 |
| AHI (events/h) | 6 ± 5 | 40 ± 20 | 42 ± 23 |
| % Time SpO2 < 90% | 16 ± 28 | 48 ± 28 | 30 ± 28 |
Values are mean ± SD.
OSA = obstructive sleep apnea
Overlap = both COPD and OSA
The American Thoracic Society/European Respiratory Society guidelines also highlight the role of referring for overnight testing among those with mild COPD and evidence of pulmonary hypertension. Though only 16% of patients with OSA have been observed to have pulmonary hypertension, this number jumps to 86% for those with overlap syndrome.55 This is an intriguing finding, given that traditional markers of OSA severity and nocturnal hypoxia in COPD are not predictive of pulmonary hypertension. However, time spent with oxygen saturation <90% is high among overlap syndrome patients, even without severe obstructive pattern on spirometry.
Prevalence & Epidemiology of Overlap Syndrome
In general, small studies from the early 1990s suggested that severe COPD was a risk factor for OSA.56 For example, one early study found >80% prevalence of OSA among those with COPD and excessive daytime sleepiness referred for evaluation.12 In certain populations, too, such as Veterans Administration patients, the co-existence of OSA and COPD was high (29%)—among patients who had polysomnogram and spirometry data available.57
More recently, larger epidemiology studies including a more broad range of subjects such as the Sleep Heart Health Study and Multinational Monitoring of Trends and Determinants in Cardiovascular Disease have not demonstrated an increased risk of OSA among those with obstructive lung disease, at least among those with mild obstructive lung disease.21,58 In these large cohorts, the prevalence of OSA was 11–14%, which was similar in those with or without obstructive lung disease.59,60 Thus, it seems likely that there is little connection amongst those with mild COPD; whether more severe COPD can contribute to OSA is not clear.
Although the answer is not yet known, proposed mechanisms of OSA risk in severe COPD include the following: fluid shifts in those with cor pulmonale from lower extremity edema to the neck,61 a generalized myopathy from COPD alone that affects the upper airway muscles,62 or a steroid-induced myopathy from systemic or inhaled corticosteroids. All of these changes would increase upper airway collapsibility.
Clinical Consequences of OSA and COPD
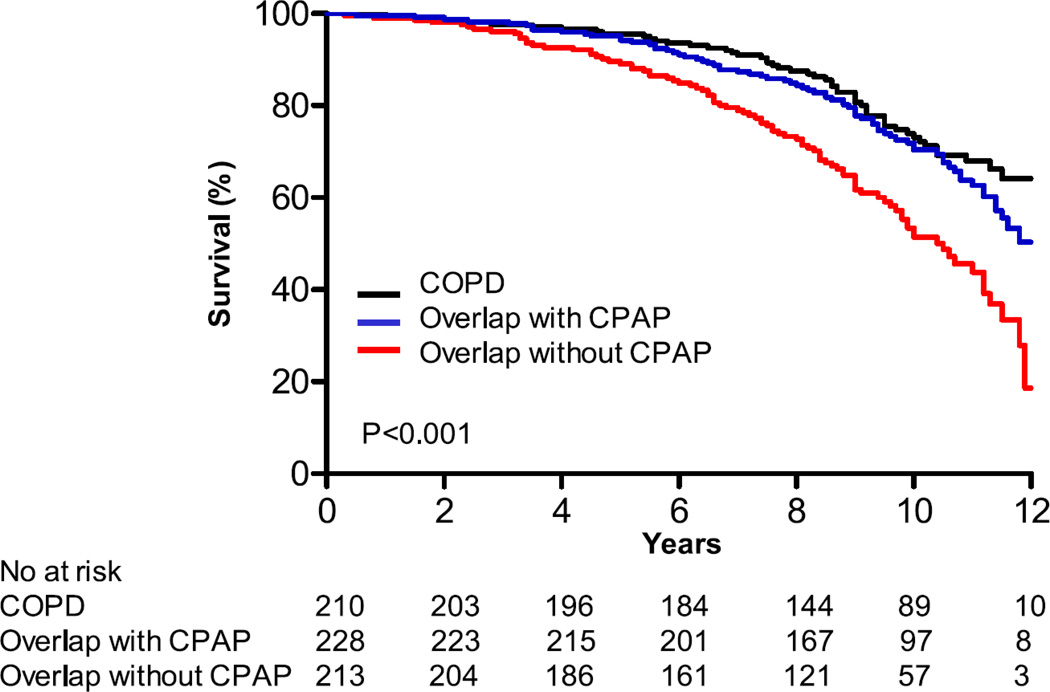
The large cohort studies above did highlight that among those with obstructive lung disease and obstructive sleep apnea, the nocturnal desaturations and sleep disturbances are greater (both oxygen saturation nadir and duration of hypoxemia) than would be expected for either disease alone.21 Whether causal or not, more recent reports have suggested increased mortality in OVS compared to COPD and OSA alone and have increased awareness about overlap syndromes. First, Marin and colleagues found decreased survival among patients with Overlap syndrome compared to either COPD or OSA alone (see FIGURE 2).63 There were differences in death from any cause and cardiovascular causes when Overlap syndrome patients using CPAP were compared to those not on CPAP. No differences were seen between COPD-only and Overlap patients using CPAP.63 That OVS patients using CPAP have reduced mortality compared to OVS without CPAP has now also been reported in other cohorts64,65,66 Jaoude and colleagues found that CPAP only improved outcomes from OVS in those patiens who were also hypercapnic.66 Further exploring the observed therapeutic benefit of CPAP, Stanchina and colleagues found that greater time on CPAP was associated with reduced mortality in OVS patients.65
Figure 2.
Kaplan-Meier survival curves for COPD patients, Overlap patients on CPAP, and Overlap patients not on CPAP. Treatment with CPAP appears to prevent against the excess mortality in Overlap patients. Importantly, these data are observational (Adapted from Marin AJRCCM 2010, Figure 2).63
Although the improvement with CPAP appears dramatic, these are not randomized data: these were cohort studies in which subjects choose to adhere or to abandon CPAP therapy. Patients who do not adhere to CPAP may be those with more COPD/less OSA, have more respiratory symptoms such as dyspnea or sputum that limit CPAP use, or be less likely to adhere to other medication therapy which is also important for limiting poor outcomes (e.g. statin therapy). Nevertheless these findings highlight the need to focus more resource on the care and understanding of these patients.
It is assumed, but not known, that the worse outcomes in OVS are due to excess cardiovascular events. As above, both COPD and OSA increase cardiovascular risk. Some data support this potential mechanism, suggesting that OSA can augment vascular changes among COPD patients such as arterial stiffness.67 Sharma and colleagues found that patients with OSA have more extensive remodeling of the right ventricle seen on cardiac MR compared to those with COPD alone; the extent of RV remodeling was correlated with the oxygen desaturation index.68
Treatment of Overlap Syndrome
Treatment for overlap may be thought of as addressing underlying COPD, OSA, or both. Though the specific goals of treatment remain poorly defined, most clinicians strive to eliminate sleep disordered breathing and eliminate nocturnal oxygenation desaturation. What to target for ideal oxygen saturation, however, remains unclear, as does the impact of normalizing hypercarbia. The most commonly applied therapy is continuous positive airway pressure (CPAP).
Before CPAP is applied, however, it is critical to consider the use of therapies that target the underlying COPD, such as bronchodilators and anti-inflammatories. Therapies aimed at COPD alone can improve nocturnal oxygen saturation, as well as decrease symptoms. Ipratropium and tiotropium, cholinergic bronchodilators, long-acting beta-agonists, and oral steroids all have data to support improvements in oxygen saturation during sleep.69–72 Some of these agents, such as ipratropium, have been shown to improve sleep quality and increase REM and total sleep time as well, although surprisingly, tiotropium did not.69,70,72 Though the mechanism of these improvements has yet to be teased out, these studies suggest that optimizing COPD treatment can play a key role in degree of nocturnal oxygen saturation. The impact on upper airway patency is unknown – some have hypothesized that (inhaled) steroids might predispose the upper airway to myopathy and increased collapsibility. However, at least in asthmatic patients receiving high dose inhaled corticosteroids, there was no increase in collapsibility.73
Nocturnal oxygen is a mainstay of therapy for hypoxemia in COPD with demonstrated mortality benefits.74,75 Among OSA patients, nocturnal oxygen therapy alone may improve hypoxemia, however arousals, sleep architecture, and daytime symptoms such as sleepiness are not impacted,76 pointing to the potential impact of sleep fragmentation due to arousals triggered by airway obstruction, which is not addressed by oxygen therapy. Thus, supplemental oxygen alone for OSA seems unlikely to be of benefit.
CPAP and lung function in COPD
There are a few studies that have assessed treatment with CPAP in overlap syndrome patients. Small studies have demonstrated improvements in daytime oxygen saturation and degree of hypercarbia with nocturnal CPAP use.77,78 Improvements in FEV1, echocardiogram estimates of mean pulmonary artery pressure, PaO2, and PaCO2 have been documented.77,79,80 Other studies have found a decline in lung function among overlap syndrome patients who were adherent to CPAP therapy.81 Given differences in study design, other factors such as weight loss, progression of underlying disease, and selection bias of among those who choose to use CPAP are all important considerations. The reason behind these improvements in gas exchange may reflect improvement in daytime lung function, though the mechanism remains unclear and controversial. Prevention of repetitive upper airway collapse in an animal model appeared to improve lower airway resistance.82 Off-loading of respiratory muscles during sleep through CPAP may also be important, contributing to decreased oxygen consumption, carbon dioxide production, and reducing sleep hypoventilation. After CPAP initiation, fewer COPD-related hospital admissions are seen in some populations.65,83
As above, recent papers suggest that treatment of OVS with CPAP is associated with reduced mortality. First, in the Brazilian cohort, 5-year survival with CPAP was 71%, as compared to 26% among patients using oxygen alone.64 This cohort included over 600 patients who required long-term oxygen therapy for hypoxemic COPD, and had at least moderate OSA. Patients with OSA were prescribed CPAP, and those who were non-adherent to CPAP continued to use oxygen for COPD. Similarly, findings from a Spanish cohort of overlap syndrome patients also suggested benefit, lowering the mortality risk to that of COPD alone.63 The striking improvement in both cohorts supports a beneficial role of CPAP, as well as highlighting the very poor outcomes in those with OVS. Again, patients in both studies were not randomized, but were self-selected based on adherence to CPAP (or were not able to afford CPAP therapy). That is, these are observational studies comparing patients with OVS who are and are non-adherent to CPAP. Though these studies do not elicit the mechanism for the reduced mortality, if due to CPAP, this may be through reduction in cardiac risk factors. Indeed, CRP levels, a non-specific marker of inflammation, were significantly reduced in overlap syndrome patients using CPAP as compared to pre-treatment.84
Non-invasive ventilation (NIV), such as bi-level positive airway pressure, is an attractive treatment modality in this population. Even in the absence of OSA, nocturnal NIV is often applied for patients with more severe COPD to off-load respiratory muscles, supplement ventilation, decrease hypercapnia, and reduce hypoxemia. Studies in this area have generally been small and non-randomized and in patients with stable disease. Taken together, these studies did not demonstrate any improvements in lung function, gas, exchange, sleep efficiency or mortality according to a 2003 meta-analysis.85 Since that time, however, two areas of investigation deserve treatment. Among overlap patients with stable hypercapnic COPD (patients with OSA were excluded), one moderately large randomized trial demonstrated a mortality benefit with NIV use, though NIV was accompanied by a decrease in quality of life.86 A mortality benefit compared to historical controls has also been seen using very high ventilation settings.87 These authors argue that so-called high-intensity non-invasive positive pressure ventilation (HI-NPPV)—with very high driving pressures, for example inspiratory pressures of 28 cm of H2O and respiratory rate of over 20 breaths per minute—among COPD patients does not appear to impact sleep quality and may have some benefits such as improvement in gas exchange and lung function.88–90
Weight loss is beneficial among patients with OSA and obesity.91 Among patients with COPD alone, however, weight loss is often a concerning finding, stemming from pulmonary cachexia, infection, malignancy, or deconditioning. The role of weight loss among overlap patients has not been examined, however, it is probably safe for obese patients with overlap syndrome to target weight loss.92 Although purely speculative, given the high rates of cardiovascular disease in OSA and COPD, it may also make sense to consider cardioprotective therapies (e.g. aspirin, statin) as primary prevention in OVS patients.
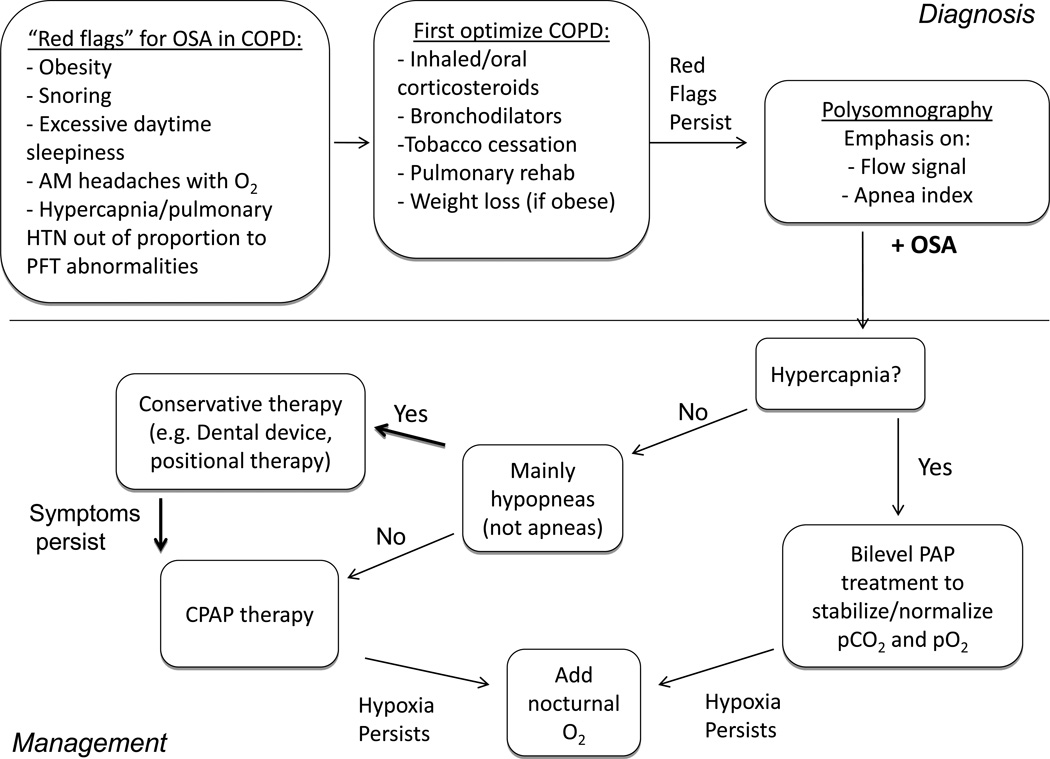
Based on all of the above, we propose the a diagnostic and treatment algorithm in FIGURE 3.
Figure 3. Management algorithm for patients with COPD.
Patients with COPD should be assessed for any “red flags” that might suggest the presence of concurrent OSA. If present, COPD should be optimized prior to undergoing polysomnography. Attention should be paid to the flow signal and apnea index when assessing the severity of OSA. If hypercapnia is present, the patient can begin on BiLevel positive airway pressure. If flow limitation is present without significant apneas, conservative therapy such as a mandibular advancement device, weight loss, and positional therapy should be considered. If apneas predominate, CPAP should be started. Supplemental oxygen should be added if hypoxemia persists.
OSA and ILD, with emphasis on Idiopathic Pulmonary Fibrosis (IPF)
ILD/IPF Background
Interstitial lung disease (ILD) may refer to a number of heterogeneous conditions, such as idiopathic pulmonary fibrosis, sarcoidosis, auto-immune related pulmonary disorders such as systemic sclerosis, hypersensitivity pneumonitis, or secondary to an environmental or drug exposure such as amiodarone. The common features of the ILDs are: 1) that these are distinct from obstructive lung diseases (such as COPD), and demonstrate restrictive physiology, 2) that the anatomical basis of disease is usually the interstitium: the alveolar epithelium, pulmonary capillary endothelium, basement membrane, perivascular and perilymphatic tissues. The focus of our discussion will be primarily among patients with idiopathic pulmonary fibrosis (IPF).
Idiopathic pulmonary fibrosis (IPF) is a restrictive lung disease of unknown etiology. It is characterized by chronic, progressive, lung fibrosis of unknown cause.93 It is an irreversible process, with an unpredictable course. Progression can vary markedly on an individual basis, from slow chronic decline to a rapid acceleration of disease; acute exacerbations may also punctuate the disease course. Prognosis is generally very poor, and there are no known effective medical treatments. Despite ongoing research, the etiology of the disease remains poorly understood. Histologically, IPF correlates with the pattern of usual interstitial pneumonitis (UIP) – the terms are sometimes used synonymously.
As compared to COPD, IPF is a rare condition, affecting approximately 14–28 per 100,000 people in the United States.94 It is more common in older individuals and males.95 The relatively low prevalence of IPF means that, as compared to COPD, the prevalence of co-existing OSA and IPF (or any ILD) is presumably also rare. However, OSA and IPF might be worth studying if: 1) IPF predisposes to OSA, 2) symptoms traditionally ascribed to IPF (e.g. fatigue) are actually due to OSA and can be successfully treated with OSA treatment, and 3) treatment of OSA in these patients improves outcomes.
Sleep in IPF
Poor sleep is common among patients with IPF. Global measures of sleep quality and excessive daytime sleepiness are significantly different as compared to controls, with IPF patients complaining of poor sleep and excessive daytime sleepiness.96 Insomnia is also a frequent occurrence, found in almost one-half of IPF patients, which may contribute to the high rates of daytime symptoms.97 When sleep is objectively assessed by polysomnography, as compared to controls, IPF patients have increased sleep fragmentation and stage I sleep.98–100 Total sleep time, sleep efficiency, and REM sleep are all reduced.98–100
The mechanisms for these sleep abnormalities remain incompletely understood, though are likely to be multifactorial. Disruption from cough has been frequently cited as one factor that contributes to sleep disturbance and inability to sleep.97,101–103 The effect of medications such as corticosteroids—which are still used empirically given the lack of other treatment options—may further contribute to some of the sleep abnormalities reported by patients with IPF. Nearly two-thirds of patients in one series where on prednisone,96 which when used at high doses may interfere with sleep. IPF patients often are additionally burdened by depression and other mood disorders, which are often characterized by sleep disturbances and often changes in energy level; medications used to treat these disorders may also impact sleep and daytime function.
Respiration during sleep in IPF
The pathological changes in pulmonary fibrosis are decreased lung compliance and increased ventilation/perfusion mismatch. These changes will increase minute ventilation and work of breathing. As a result, patients with IPF exhibit rapid, shallow breathing during wakefulness.100 During sleep, the tachypnea persists, and as compared to normal controls, there is no drop in respiratory rate, although tidal volume decreases.100 Thus, similar to COPD above, among patients with interstitial lung disease, sleep may serve as a stressor to the respiratory system. Oxygen desaturation is frequently more profound than during wakefulness. The importance of evaluating nighttime respiratory patterns has been recently highlighted, as it may have prognostic value in assessing mortality in interstitial lung disease.104 Specifically, among newly diagnosed idiopathic pulmonary fibrosis (IPF) patients, the degree of nocturnal desaturation was greater than seen during exercise and was predictive of survival,105,106,107 possibly mediated through worsening pulmonary artery hypertension.105
Interstitial Lung Disease and Sleep Disordered Breathing
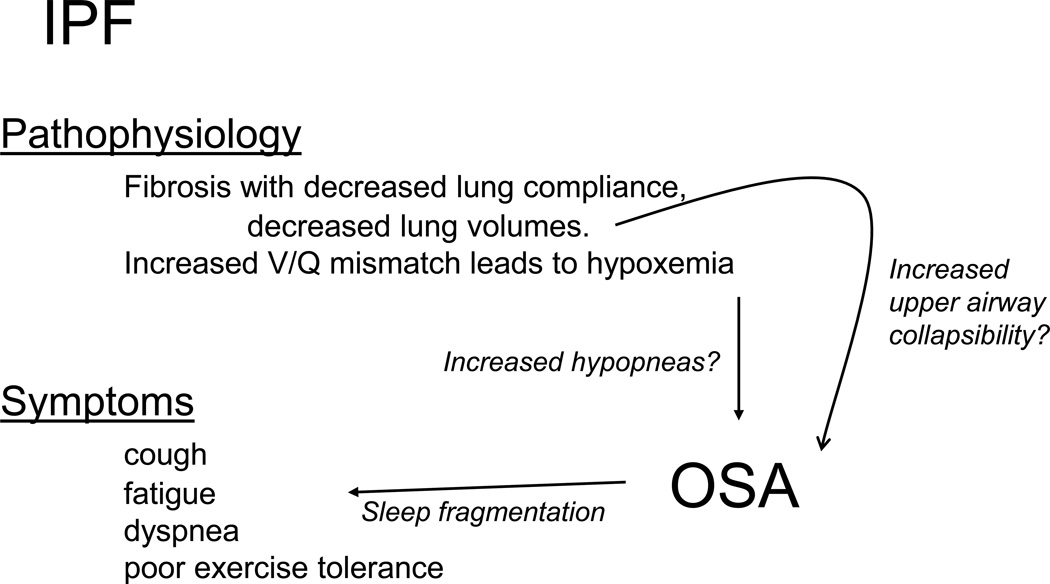
The prevalence of sleep-disordered breathing is reported to be extraordinarily high among patients with interstitial lung disease. Symptoms such as fatigue, commonly reported in IPF patients, may be attributable to this.100 In published series, the incidence of OSA ranges from over two-thirds to nearly 90%.108–110 FIGURE 4 outlines the symptoms that are commonly reported in IPF and how they may overlap with OSA. The nature of OSA in these populations remains incompletely characterized, such as whether events are due to airway collapse and flow limitation, or oxygen desaturations. Among IPF patients, AHI is not strongly correlated with body mass index (BMI), again suggesting that other mechanisms—aside from obesity—may be contributing to the diagnosis OSA in this population.110 Indeed, as compared to controls, patient with IPF spend more time with an oxygen saturation <90%, even when apnea-hypopnea index (AHI) is similar. These observations raise the possibility the lower baseline oxygen saturation and increased tendency towards desaturation are over-estimating the collapsibility of the upper airway The 2009 study by Lancaster is helpful in this regard. First, their subjects with mild OSA had a mean AHI of 10.7 events per hour, of which less than 1/hour were apneas.110 Additionally, approximately half of the hypopneas were scored based on a 3% oxygen desaturation (rather than arousal). In those with moderate-to-severe OSA the average AHI was 39.4 events per hour, but again, the apnea index was only 7.1/hour and nearly half of all hypopneas were scored based on oxygen desaturation.
Figure 4.
Links between IPF and OSA. Many symptoms and findings among by patients with IPF overlap with those of OSA, including daytime fatigue, poor sleep, and nocturnal hypoxia. Similarly, the pathophysiological changes of IPF may contribute to OSA.
In support of a mechanistic link between IPF and OSA, some authors have invoked so-called “tracheal traction” – the link between lung volumes and the upper airway.111,112 Briefly, in patients without lung disease, a decrease in lung volumes leads to increased upper airway resistance, increased collapsibility, and worse OSA severity.113,114 However, whether this relationship still holds when compliance of the lung is altered in not clear, and no formal measurement of airway resistance or collapsibility has been made in IPF patients to test this hypothesis. Again, in the study by Lancaster and colleagues, total lung capacity did not seem to predict OSA.
Treatment
There are no proven therapies that target the underlying disease process in IPF. Oxygen therapy is widely used as supportive care. Studies suggest that oxygen therapy can be associated with improvements in exercise performance.115,116 However no studies have demonstrated a mortality benefit117 or improvement in exertional dyspnea,118 as rapid shallow breathing persists despite addressing hypoxia.
Treatment with CPAP among IPF patients with at least moderate OSA results in gains in sleep-related quality of life measures, though adherence to CPAP may be challenging in light of chronic cough and other barriers.119 There are no studies that have explored the impact of CPAP on outcomes in IPF such disease progression or mortality. Taken together, OSA may be common in IPF, and treatment with CPAP may improve OSA symptoms.
Other overlap syndromes: Beyond COPD and IPF
From above, it is clear that there are many research and clinical questions that remain for overlap syndromes, even for “the” Overlap syndrome which is relatively common. Even less is known about the prevalence, consequences, and best management of OSA among other chronic lung diseases. However, there are some pearls that the sleep physician should know regarding other lung diseases.
Sarcoidosis is a chronic condition of unknown etiology characterized by formation of granulomas in many organs, most commonly the lung. Lung disease may range from mild to severe and fibrosing in nature. Steroids are often given in more severe disease. Fatigue and excessive daytime sleepiness are more common among patients with sarcoid as compared to controls.120–122 Consideration of OSA among these patients is therefore important, particularly among those with abnormal lung function.120 There remains a population of patients, however, with hypersomnolence unrelated to OSA. Relevant for Sleep medicine physicians, fatigue improves with stimulant therapy (armodafinil).123 This improvement may serve as a paradigm for patients with chronic lung disease and fatigue to receive empiric therapy.
Though the majority of data is in pediatric populations, OSA appears to be more common among patients with sickle cell anemia as compared to controls.124–126 OSA among sickle cell disease patients is accompanied by more severe nocturnal desaturations and hypercarbia.125 Though larger studies are needed to better describe the relationship, OSA, through nocturnal hypoxia, may serve as a trigger for vaso-occlusive sickle events.127 This highlights the potential importance of recognizing and treating OSA among those with sickle cell disease.
Cystic fibrosis (CF) is a systemic disease characterized by abnormal chloride channel function. Obstructive lung disease, including bronchiectasis and repeated pulmonary infections due to tenacious sputum, are common in among CF patients. Sleep apnea is common, in up to 70% of children with CF.128 OSA presents at an early age as compared to controls, as young as preschool-aged.128 Nocturnal oxygen desaturation is also common among CF patients, particularly those with awake oxygen saturation <94%.129
Conclusions
The combination of chronic lung disease and obstructive sleep apnea in a single patient are still, as yet, poorly understood. Many research and clinical questions remain, including how best to quantify upper airway collapsibility and sleep fragmentation in patients already at risk for hypoxemia due to chronic lung disease. These questions must be answered given the high prevalence of “the” overlap syndrome – COPD and OSA – and observational cohort studies that show very high mortality without OSA treatment.
Other chronic lung diseases such as idiopathic pulmonary fibrosis are much less common, yet diagnosis and treatment of OSA may be important. Within these patient populations, there are few or no therapies available to target the underlying disease and its consequences. Recognition and treatment of OSA, therefore, could offer key benefits such as improvements in quality of life or fatigue level.
Key Points.
Overlap syndrome refers to the coexistence of chronic lung dung disease and obstructive sleep apnea (OSA) in the same patient. To date, overlap syndromes have been poorly studied for a variety of reasons.
Nevertheless, recent data in COPD and OSA overlap patients highlight the increased morbidity and mortality of overlap syndromes compared to either underlying disorder alone.
The underlying disorders in overlap syndrome (OSA and chronic lung disease) may be of very different severity. Thus, there is a great amount of patient heterogeneity and goals of therapy may differ in different patients.
Unrecognized OSA may contribute to symptoms of sleepiness and fatigue in those with chronic lung disease. Thus, clinicians should be mindful of the overlap syndromes in these patients.
References
- 1.Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6(4):651–661. [PubMed] [Google Scholar]
- 2.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance--united states, 1999–2011. Chest. 2013;144(1):284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD study): A population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 5.Hoyert DLXJ. Natl Vital Stat Rep. 6. Vol. 61. Hyattsville, MD: National Center for Health Statistics; 2012. Deaths: Preliminary data for 2011; pp. 1–65. 21012. [PubMed] [Google Scholar]
- 6.Foster TS, Miller JD, Marton JP, Caloyeras JP, Russell MW, Menzin J. Assessment of the economic burden of COPD in the U.S.: A review and synthesis of the literature. COPD. 2006;3(4):211–218. doi: 10.1080/15412550601009396. [DOI] [PubMed] [Google Scholar]
- 7.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 8.Shepard JW, Jr, Schweitzer PK, Keller CA, Chun DS, Dolan GF. Myocardial stress. exercise versus sleep in patients with COPD. Chest. 1984;86(3):366–374. doi: 10.1378/chest.86.3.366. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher EC, Luckett RA, Miller T, Fletcher JG. Exercise hemodynamics and gas exchange in patients with chronic obstruction pulmonary disease, sleep desaturation, and a daytime PaO2 above 60 mm hg. Am Rev Respir Dis. 1989;140(5):1237–1245. doi: 10.1164/ajrccm/140.5.1237. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher EC, Luckett RA, Miller T, Costarangos C, Kutka N, Fletcher JG. Pulmonary vascular hemodynamics in chronic lung disease patients with and without oxyhemoglobin desaturation during sleep. Chest. 1989;95(4):757–764. doi: 10.1378/chest.95.4.757. [DOI] [PubMed] [Google Scholar]
- 11.Boysen PG, Block AJ, Wynne JW, Hunt LA, Flick MR. Nocturnal pulmonary hypertension in patients with chronic obstructive pulmonary disease. Chest. 1979;76(5):536–542. doi: 10.1378/chest.76.5.536. [DOI] [PubMed] [Google Scholar]
- 12.Guilleminault C, Cummiskey J, Motta J. Chronic obstructive airflow disease and sleep studies. Am Rev Respir Dis. 1980;122(3):397–406. doi: 10.1164/arrd.1980.122.3.397. [DOI] [PubMed] [Google Scholar]
- 13.Cheong TH, Magder S, Shapiro S, Martin JG, Levy RD. Cardiac arrhythmias during exercise in severe chronic obstructive pulmonary disease. Chest. 1990;97(4):793–797. doi: 10.1378/chest.97.4.793. [DOI] [PubMed] [Google Scholar]
- 14.Slutsky R, Hooper W, Ackerman W, et al. Evaluation of left ventricular function in chronic pulmonary disease by exercise gated equilibrium radionuclide angiography. Am Heart J. 1981;101(4):414–420. doi: 10.1016/0002-8703(81)90130-7. [DOI] [PubMed] [Google Scholar]
- 15.Price D, Small M, Milligan G, Higgins V, Gil EG, Estruch J. Impact of night-time symptoms in COPD: A real-world study in five european countries. Int J Chron Obstruct Pulmon Dis. 2013;8:595–603. doi: 10.2147/COPD.S48570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agusti A, Hedner J, Marin JM, Barbe F, Cazzola M, Rennard S. Night-time symptoms: A forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183–194. doi: 10.1183/09059180.00004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klink ME, Dodge R, Quan SF. The relation of sleep complaints to respiratory symptoms in a general population. Chest. 1994;105(1):151–154. doi: 10.1378/chest.105.1.151. [DOI] [PubMed] [Google Scholar]
- 18.Budhiraja P, Budhiraja R, Goodwin JL, et al. Incidence of restless legs syndrome and its correlates. J Clin Sleep Med. 2012;8(2):119–124. doi: 10.5664/jcsm.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34(7):859–867. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodge R, Cline MG, Quan SF. The natural history of insomnia and its relationship to respiratory symptoms. Arch Intern Med. 1995;155(16):1797–1800. [PubMed] [Google Scholar]
- 21.Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167(1):7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 22.Krachman S, Minai OA, Scharf SM. Sleep abnormalities and treatment in emphysema. Proc Am Thorac Soc. 2008;5(4):536–542. doi: 10.1513/pats.200708-134ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valipour A, Lavie P, Lothaller H, Mikulic I, Burghuber OC. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med. 2011;12(4):367–372. doi: 10.1016/j.sleep.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 24.McSharry DG, Ryan S, Calverley P, Edwards JC, McNicholas WT. Sleep quality in chronic obstructive pulmonary disease. Respirology. 2012;17(7):1119–1124. doi: 10.1111/j.1440-1843.2012.02217.x. [DOI] [PubMed] [Google Scholar]
- 25.Manni R, Cerveri I, Bruschi C, Zoia C, Tartara A. Sleep and oxyhemoglobin desaturation patterns in chronic obstructive pulmonary diseases. Eur Neurol. 1988;28(5):275–278. doi: 10.1159/000116283. [DOI] [PubMed] [Google Scholar]
- 26.Fleetham J, West P, Mezon B, Conway W, Roth T, Kryger M. Sleep, arousals, and oxygen desaturation in chronic obstructive pulmonary disease. the effect of oxygen therapy. Am Rev Respir Dis. 1982;126(3):429–433. doi: 10.1164/arrd.1982.126.3.429. [DOI] [PubMed] [Google Scholar]
- 27.Brezinova V, Catterall JR, Douglas NJ, Calverley PM, Flenley DC. Night sleep of patients with chronic ventilatory failure and age matched controls: Number and duration of the EEG episodes of intervening wakefulness and drowsiness. Sleep. 1982;5(2):123–130. doi: 10.1093/sleep/5.2.123. [DOI] [PubMed] [Google Scholar]
- 28.Kwon JS, Wolfe LF, Lu BS, Kalhan R. Hyperinflation is associated with lower sleep efficiency in COPD with co-existent obstructive sleep apnea. COPD. 2009;6(6):441–445. doi: 10.3109/15412550903433000. [DOI] [PubMed] [Google Scholar]
- 29.Krachman SL, Chatila W, Martin UJ, et al. Physiologic correlates of sleep quality in severe emphysema. COPD. 2011;8(3):182–188. doi: 10.3109/15412555.2011.560583. [DOI] [PubMed] [Google Scholar]
- 30.Hynninen MJ, Pallesen S, Hardie J, et al. Insomnia symptoms, objectively measured sleep, and disease severity in chronic obstructive pulmonary disease outpatients. Sleep Med. 2013;14(12):1328–1333. doi: 10.1016/j.sleep.2013.08.785. [DOI] [PubMed] [Google Scholar]
- 31.Lo Coco D, Mattaliano A, Lo Coco A, Randisi B. Increased frequency of restless legs syndrome in chronic obstructive pulmonary disease patients. Sleep Med. 2009;10(5):572–576. doi: 10.1016/j.sleep.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Timms RM, Dawson A, Hajdukovic RM, Mitler MM. Effect of triazolam on sleep and arterial oxygen saturation in patients with chronic obstructive pulmonary disease. Arch Intern Med. 1988;148(10):2159–2163. [PubMed] [Google Scholar]
- 33.Steens RD, Pouliot Z, Millar TW, Kryger MH, George CF. Effects of zolpidem and triazolam on sleep and respiration in mild to moderate chronic obstructive pulmonary disease. Sleep. 1993;16(4):318–326. doi: 10.1093/sleep/16.4.318. [DOI] [PubMed] [Google Scholar]
- 34.Stege G, Heijdra YF, van den Elshout FJ, et al. Temazepam 10mg does not affect breathing and gas exchange in patients with severe normocapnic COPD. Respir Med. 2010;104(4):518–524. doi: 10.1016/j.rmed.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Ekstrom MP, Bornefalk-Hermansson A, Abernethy AP, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease: National prospective study. BMJ. 2014;348:g445. doi: 10.1136/bmj.g445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douglas NJ, White DP, Pickett CK, Weil JV, Zwillich CW. Respiration during sleep in normal man. Thorax. 1982;37(11):840–844. doi: 10.1136/thx.37.11.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker HF, Piper AJ, Flynn WE, et al. Breathing during sleep in patients with nocturnal desaturation. Am J Respir Crit Care Med. 1999;159(1):112–118. doi: 10.1164/ajrccm.159.1.9803037. [DOI] [PubMed] [Google Scholar]
- 38.Catterall JR, Douglas NJ, Calverley PM, et al. Transient hypoxemia during sleep in chronic obstructive pulmonary disease is not a sleep apnea syndrome. Am Rev Respir Dis. 1983;128(1):24–29. doi: 10.1164/arrd.1983.128.1.24. [DOI] [PubMed] [Google Scholar]
- 39.Douglas NJ, White DP, Weil JV, et al. Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis. 1982;125(3):286–289. doi: 10.1164/arrd.1982.125.3.286. [DOI] [PubMed] [Google Scholar]
- 40.Douglas NJ, White DP, Weil JV, Pickett CK, Zwillich CW. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis. 1982;126(5):758–762. doi: 10.1164/arrd.1982.126.5.758. [DOI] [PubMed] [Google Scholar]
- 41.Douglas NJ. Control of ventilation during sleep. Clin Chest Med. 1985;6(4):563–575. [PubMed] [Google Scholar]
- 42.Hudgel DW, Martin RJ, Johnson B, Hill P. Mechanics of the respiratory system and breathing pattern during sleep in normal humans. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(1):133–137. doi: 10.1152/jappl.1984.56.1.133. [DOI] [PubMed] [Google Scholar]
- 43.Ottenheijm CA, Heunks LM, Sieck GC, et al. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):200–205. doi: 10.1164/rccm.200502-262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaouat A, Weitzenblum E, Kessler R, et al. Sleep-related O2 desaturation and daytime pulmonary haemodynamics in COPD patients with mild hypoxaemia. Eur Respir J. 1997;10(8):1730–1735. doi: 10.1183/09031936.97.10081730. [DOI] [PubMed] [Google Scholar]
- 45.Fletcher EC, Miller J, Divine GW, Fletcher JG, Miller T. Nocturnal oxyhemoglobin desaturation in COPD patients with arterial oxygen tensions above 60 mm hg. Chest. 1987;92(4):604–608. doi: 10.1378/chest.92.4.604. [DOI] [PubMed] [Google Scholar]
- 46.Lewis CA, Fergusson W, Eaton T, Zeng I, Kolbe J. Isolated nocturnal desaturation in COPD: Prevalence and impact on quality of life and sleep. Thorax. 2009;64(2):133–138. doi: 10.1136/thx.2007.088930. [DOI] [PubMed] [Google Scholar]
- 47.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 49.Kendzerska T, Mollayeva T, Gershon AS, Leung RS, Hawker G, Tomlinson G. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: A systematic review. Sleep Med Rev. 2014;18(1):49–59. doi: 10.1016/j.smrv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 51.Findley LJ, Barth JT, Powers DC, Wilhoit SC, Boyd DG, Suratt PM. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest. 1986;90(5):686–690. doi: 10.1378/chest.90.5.686. [DOI] [PubMed] [Google Scholar]
- 52.Patz D, Spoon M, Corbin R, et al. The effect of altitude descent on obstructive sleep apnea. Chest. 2006;130(6):1744–1750. doi: 10.1378/chest.130.6.1744. [DOI] [PubMed] [Google Scholar]
- 53.Sharma B, Feinsilver S, Owens RL, Malhotra A, McSharry D, Karbowitz S. Obstructive airway disease and obstructive sleep apnea: Effect of pulmonary function. Lung. 2011;189(1):37–41. doi: 10.1007/s00408-010-9270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Resta O, Foschino Barbaro MP, Brindicci C, Nocerino MC, Caratozzolo G, Carbonara M. Hypercapnia in overlap syndrome: Possible determinant factors. Sleep Breath. 2002;6(1):11–18. doi: 10.1007/s11325-002-0011-6. [DOI] [PubMed] [Google Scholar]
- 55.Hawrylkiewicz I, Palasiewicz G, Plywaczewski R, Zielinski J. Pulmonary hypertension in patients with pure obstructive sleep apnea. Pol Arch Med Wewn. 2004;111(4):449–454. [PubMed] [Google Scholar]
- 56.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151(1):82–86. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Acevedo MN, Torres-Palacios A, Elena Ocasio-Tascon M, Campos-Santiago Z, Rodriguez-Cintron W. Overlap syndrome: An indication for sleep studies? : A pilot study. Sleep Breath. 2009;13(4):409–413. doi: 10.1007/s11325-009-0263-5. [DOI] [PubMed] [Google Scholar]
- 58.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: A population study. Respiration. 2005;72(2):142–149. doi: 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 59.Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167(1):7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 60.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: A population study. Respiration. 2005;72(2):142–149. doi: 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 61.White LH, Motwani S, Kasai T, Yumino D, Amirthalingam V, Bradley TD. Effect of rostral fluid shift on pharyngeal resistance in men with and without obstructive sleep apnea. Respir Physiol Neurobiol. 2014;192:17–22. doi: 10.1016/j.resp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Agusti AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 63.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: The overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 64.Machado MC, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J. 2010;35(1):132–137. doi: 10.1183/09031936.00192008. [DOI] [PubMed] [Google Scholar]
- 65.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: The overlap syndrome. J Clin Sleep Med. 2013;9(8):767–772. doi: 10.5664/jcsm.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaoude P, Kufel T, El-Solh AA. Survival benefit of CPAP favors hypercapnic patients with the overlap syndrome. Lung. 2014 doi: 10.1007/s00408-014-9555-z. [DOI] [PubMed] [Google Scholar]
- 67.Shiina K, Tomiyama H, Takata Y, et al. Overlap syndrome: Additive effects of COPD on the cardiovascular damages in patients with OSA. Respir Med. 2012;106(9):1335–1341. doi: 10.1016/j.rmed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Sharma B, Neilan TG, Kwong RY, et al. Evaluation of right ventricular remodeling using cardiac magnetic resonance imaging in co-existent chronic obstructive pulmonary disease and obstructive sleep apnea. COPD. 2013;10(1):4–10. doi: 10.3109/15412555.2012.719050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin RJ, Bartelson BL, Smith P, et al. Effect of ipratropium bromide treatment on oxygen saturation and sleep quality in COPD. Chest. 1999;115(5):1338–1345. doi: 10.1378/chest.115.5.1338. [DOI] [PubMed] [Google Scholar]
- 70.McNicholas WT, Calverley PM, Lee A, Edwards JC Tiotropium Sleep Study in COPD Investigators. Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J. 2004;23(6):825–831. doi: 10.1183/09031936.04.00085804. [DOI] [PubMed] [Google Scholar]
- 71.Ryan S, Doherty LS, Rock C, Nolan GM, McNicholas WT. Effects of salmeterol on sleeping oxygen saturation in chronic obstructive pulmonary disease. Respiration. 2010;79(6):475–481. doi: 10.1159/000235619. [DOI] [PubMed] [Google Scholar]
- 72.Sposato B, Mariotta S, Palmiero G, Ricci A, Gencarelli G, Franco C. Oral corticosteroids can improve nocturnal isolated hypoxemia in stable COPD patients with diurnal PaO2 >60 mmHg. Eur Rev Med Pharmacol Sci. 2007;11(6):365–372. [PubMed] [Google Scholar]
- 73.Teodorescu M, Xie A, Sorkness CA, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: A pilot study. J Clin Sleep Med. 2014;10(2):183–193. doi: 10.5664/jcsm.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. nocturnal oxygen therapy trial group. Ann Intern Med. 1980;93(3):391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 75.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. report of the medical research council working party. Lancet. 1981;1(8222):681–686. [PubMed] [Google Scholar]
- 76.Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: A placebo-CPAP-controlled study. Sleep. 2006;29(4):564–571. doi: 10.1093/sleep/29.4.564. [DOI] [PubMed] [Google Scholar]
- 77.Mansfield D, Naughton MT. Effects of continuous positive airway pressure on lung function in patients with chronic obstructive pulmonary disease and sleep disordered breathing. Respirology. 1999;4(4):365–370. doi: 10.1046/j.1440-1843.1999.00206.x. [DOI] [PubMed] [Google Scholar]
- 78.Sforza E, Krieger J, Weitzenblum E, Apprill M, Lampert E, Ratamaharo J. Long-term effects of treatment with nasal continuous positive airway pressure on daytime lung function and pulmonary hemodynamics in patients with obstructive sleep apnea. Am Rev Respir Dis. 1990;141(4 Pt 1):866–870. doi: 10.1164/ajrccm/141.4_Pt_1.866. [DOI] [PubMed] [Google Scholar]
- 79.de Miguel J, Cabello J, Sanchez-Alarcos JM, Alvarez-Sala R, Espinos D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath. 2002;6(1):3–10. doi: 10.1007/s11325-002-0003-6. [DOI] [PubMed] [Google Scholar]
- 80.Toraldo DM, De Nuccio F, Nicolardi G. Fixed-pressure nCPAP in patients with obstructive sleep apnea (OSA) syndrome and chronic obstructive pulmonary disease (COPD): A 24-month follow-up study. Sleep Breath. 2010;14(2):115–123. doi: 10.1007/s11325-009-0291-1. [DOI] [PubMed] [Google Scholar]
- 81.O'Brien A, Whitman K. Lack of benefit of continuous positive airway pressure on lung function in patients with overlap syndrome. Lung. 2005;183(6):389–404. doi: 10.1007/s00408-005-2551-6. [DOI] [PubMed] [Google Scholar]
- 82.Nadel JA, Widdicombe JG. Reflex effects of upper airway irritation on total lung resistance and blood pressure. J Appl Physiol. 1962;17:861–865. doi: 10.1152/jappl.1962.17.6.861. [DOI] [PubMed] [Google Scholar]
- 83.Peker Y, Hedner J, Johansson A, Bende M. Reduced hospitalization with cardiovascular and pulmonary disease in obstructive sleep apnea patients on nasal CPAP treatment. Sleep. 1997;20(8):645–653. doi: 10.1093/sleep/20.8.645. [DOI] [PubMed] [Google Scholar]
- 84.Nural S, Gunay E, Halici B, Celik S, Unlu M. Inflammatory processes and effects of continuous positive airway pressure (CPAP) in overlap syndrome. Inflammation. 2013;36(1):66–74. doi: 10.1007/s10753-012-9520-z. [DOI] [PubMed] [Google Scholar]
- 85.Wijkstra PJ, Lacasse Y, Guyatt GH, et al. A meta-analysis of nocturnal noninvasive positive pressure ventilation in patients with stable COPD. Chest. 2003;124(1):337–343. doi: 10.1378/chest.124.1.337. [DOI] [PubMed] [Google Scholar]
- 86.McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: A randomised controlled trial. Thorax. 2009;64(7):561–566. doi: 10.1136/thx.2008.108274. [DOI] [PubMed] [Google Scholar]
- 87.Windisch W, Haenel M, Storre JH, Dreher M. High-intensity non-invasive positive pressure ventilation for stable hypercapnic COPD. Int J Med Sci. 2009;6(2):72–76. doi: 10.7150/ijms.6.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dreher M, Ekkernkamp E, Walterspacher S, et al. Noninvasive ventilation in COPD: Impact of inspiratory pressure levels on sleep quality. Chest. 2011;140(4):939–945. doi: 10.1378/chest.11-0253. [DOI] [PubMed] [Google Scholar]
- 89.Windisch W, Kostic S, Dreher M, Virchow JC, Jr, Sorichter S. Outcome of patients with stable COPD receiving controlled noninvasive positive pressure ventilation aimed at a maximal reduction of pa(CO2) Chest. 2005;128(2):657–662. doi: 10.1378/chest.128.2.657. [DOI] [PubMed] [Google Scholar]
- 90.Windisch W, Dreher M, Storre JH, Sorichter S. Nocturnal non-invasive positive pressure ventilation: Physiological effects on spontaneous breathing. Respir Physiol Neurobiol. 2006;150(2–3):251–260. doi: 10.1016/j.resp.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 91.Poulain M, Doucet M, Major GC, et al. The effect of obesity on chronic respiratory diseases: Pathophysiology and therapeutic strategies. CMAJ. 2006;174(9):1293–1299. doi: 10.1503/cmaj.051299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sood A, Petersen H, Meek P, Tesfaigzi Y. Spirometry and health status worsen with weight gain in obese smokers but improve in normal-weight smokers. Am J Respir Crit Care Med. 2014;189(3):274–281. doi: 10.1164/rccm.201306-1060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raghu G. Idiopathic pulmonary fibrosis: Guidelines for diagnosis and clinical management have advanced from consensus-based in 2000 to evidence-based in 2011. Eur Respir J. 2011;37(4):743–746. doi: 10.1183/09031936.00017711. [DOI] [PubMed] [Google Scholar]
- 94.Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: Review of the literature. Eur Respir Rev. 2012;21(126):355–361. doi: 10.1183/09059180.00002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 96.Krishnan V, McCormack MC, Mathai SC, et al. Sleep quality and health-related quality of life in idiopathic pulmonary fibrosis. Chest. 2008;134(4):693–698. doi: 10.1378/chest.08-0173. [DOI] [PubMed] [Google Scholar]
- 97.Bajwah S, Higginson IJ, Ross JR, et al. The palliative care needs for fibrotic interstitial lung disease: A qualitative study of patients, informal caregivers and health professionals. Palliat Med. 2013;27(9):869–876. doi: 10.1177/0269216313497226. [DOI] [PubMed] [Google Scholar]
- 98.Perez-Padilla R, West P, Lertzman M, Kryger MH. Breathing during sleep in patients with interstitial lung disease. Am Rev Respir Dis. 1985;132(2):224–229. doi: 10.1164/arrd.1985.132.2.224. [DOI] [PubMed] [Google Scholar]
- 99.Bye PT, Issa F, Berthon-Jones M, Sullivan CE. Studies of oxygenation during sleep in patients with interstitial lung disease. Am Rev Respir Dis. 1984;129(1):27–32. [Google Scholar]
- 100.Mermigkis C, Stagaki E, Amfilochiou A, et al. Sleep quality and associated daytime consequences in patients with idiopathic pulmonary fibrosis. Med Princ Pract. 2009;18(1):10–15. doi: 10.1159/000163039. [DOI] [PubMed] [Google Scholar]
- 101.Swigris JJ, Stewart AL, Gould MK, Wilson SR. Patients' perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61. doi: 10.1186/1477-7525-3-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mermigkis C, Mermigkis D, Varouchakis G, Schiza S. CPAP treatment in patients with idiopathic pulmonary fibrosis and obstructive sleep apnea--therapeutic difficulties and dilemmas. Sleep Breath. 2012;16(1):1–3. doi: 10.1007/s11325-010-0476-7. [DOI] [PubMed] [Google Scholar]
- 103.Rasche K, Orth M. Sleep and breathing in idiopathic pulmonary fibrosis. J Physiol Pharmacol. 2009;60(Suppl 5):13–14. [PubMed] [Google Scholar]
- 104.Corte TJ, Wort SJ, Talbot S, et al. Elevated nocturnal desaturation index predicts mortality in interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29(1):41–50. [PubMed] [Google Scholar]
- 105.Kolilekas L, Manali E, Vlami KA, et al. Sleep oxygen desaturation predicts survival in idiopathic pulmonary fibrosis. J Clin Sleep Med. 2013;9(6):593–601. doi: 10.5664/jcsm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lettieri CJ, Nathan SD, Browning RF, Barnett SD, Ahmad S, Shorr AF. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med. 2006;100(10):1734–1741. doi: 10.1016/j.rmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 107.Triantafillidou C, Manali E, Lyberopoulos P, et al. The role of cardiopulmonary exercise test in IPF prognosis. Pulm Med. 2013;2013:514–817. doi: 10.1155/2013/514817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pihtili A, Bingol Z, Kiyan E, Cuhadaroglu C, Issever H, Gulbaran Z. Obstructive sleep apnea is common in patients with interstitial lung disease. Sleep Breath. 2013;17(4):1281–1288. doi: 10.1007/s11325-013-0834-3. [DOI] [PubMed] [Google Scholar]
- 109.Mermigkis C, Stagaki E, Tryfon S, et al. How common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? Sleep Breath. 2010;14(4):387–390. doi: 10.1007/s11325-010-0336-5. [DOI] [PubMed] [Google Scholar]
- 110.Lancaster LH, Mason WR, Parnell JA, et al. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136(3):772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol (1985) 1988;65(5):2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 112.Ruhle KH. Commentary on how common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? mermigkis C. et al. Sleep Breath. 2010;14(4) doi: 10.1007/s11325-010-0336-5. 289-010-0341-8. Epub 2010 Mar 16. [DOI] [PubMed] [Google Scholar]
- 113.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61(5):435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Owens RL, Malhotra A, Eckert DJ, White DP, Jordan AS. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J Appl Physiol (1985) 2010;108(2):445–451. doi: 10.1152/japplphysiol.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harris-Eze AO, Sridhar G, Clemens RE, Gallagher CG, Marciniuk DD. Oxygen improves maximal exercise performance in interstitial lung disease. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1616–1622. doi: 10.1164/ajrccm.150.6.7952624. [DOI] [PubMed] [Google Scholar]
- 116.Harris-Eze AO, Sridhar G, Clemens RE, Zintel TA, Gallagher CG, Marciniuk DD. Role of hypoxemia and pulmonary mechanics in exercise limitation in interstitial lung disease. Am J Respir Crit Care Med. 1996;154(4 Pt 1):994–1001. doi: 10.1164/ajrccm.154.4.8887597. [DOI] [PubMed] [Google Scholar]
- 117.Crockett AJ, Cranston JM, Antic N. Domiciliary oxygen for interstitial lung disease. Cochrane Database Syst Rev. 2001;3(3):CD002883. doi: 10.1002/14651858.CD002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nishiyama O, Miyajima H, Fukai Y, et al. Effect of ambulatory oxygen on exertional dyspnea in IPF patients without resting hypoxemia. Respir Med. 2013;107(8):1241–1246. doi: 10.1016/j.rmed.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 119.Mermigkis C, Bouloukaki I, Antoniou KM, et al. CPAP therapy in patients with idiopathic pulmonary fibrosis and obstructive sleep apnea: Does it offer a better quality of life and sleep? Sleep Breath. 2013;17(4):1137–1143. doi: 10.1007/s11325-013-0813-8. [DOI] [PubMed] [Google Scholar]
- 120.Patterson KC, Huang F, Oldham JM, Bhardwaj N, Hogarth DK, Mokhlesi B. Excessive daytime sleepiness and obstructive sleep apnea in patients with sarcoidosis. Chest. 2013;143(6):1562–1568. doi: 10.1378/chest.12-1524. [DOI] [PubMed] [Google Scholar]
- 121.De Vries J, Rothkrantz-Kos S, van Dieijen-Visser MP, Drent M. The relationship between fatigue and clinical parameters in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(2):127–136. [PubMed] [Google Scholar]
- 122.Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J. 2012;40(1):255–263. doi: 10.1183/09031936.00002512. [DOI] [PubMed] [Google Scholar]
- 123.Lower EE, Malhotra A, Surdulescu V, Baughman RP. Armodafinil for sarcoidosis-associated fatigue: A double-blind, placebo-controlled, crossover trial. J Pain Symptom Manage. 2013;45(2):159–169. doi: 10.1016/j.jpainsymman.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Salles C, Ramos RT, Daltro C, Barral A, Marinho JM, Matos MA. Prevalence of obstructive sleep apnea in children and adolescents with sickle cell anemia. J Bras Pneumol. 2009;35(11):1075–1083. doi: 10.1590/s1806-37132009001100004. [DOI] [PubMed] [Google Scholar]
- 125.Kaleyias J, Mostofi N, Grant M, et al. Severity of obstructive sleep apnea in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30(9):659–665. doi: 10.1097/MPH.0b013e31817eb7ef. [DOI] [PubMed] [Google Scholar]
- 126.Samuels MP, Stebbens VA, Davies SC, Picton-Jones E, Southall DP. Sleep related upper airway obstruction and hypoxaemia in sickle cell disease. Arch Dis Child. 1992;67(7):925–929. doi: 10.1136/adc.67.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okoli K, Irani F, Horvath W. Pathophysiologic considerations for the interactions between obstructive sleep apnea and sickle hemoglobinopathies. Med Hypotheses. 2009;72(5):578–580. doi: 10.1016/j.mehy.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 128.Spicuzza L, Sciuto C, Leonardi S, La Rosa M. Early occurrence of obstructive sleep apnea in infants and children with cystic fibrosis. Arch Pediatr Adolesc Med. 2012;166(12):1165–1169. doi: 10.1001/archpediatrics.2012.1177. [DOI] [PubMed] [Google Scholar]
- 129.Perin C, Fagondes SC, Casarotto FC, Pinotti AF, Menna Barreto SS, Dalcin Pde T. Sleep findings and predictors of sleep desaturation in adult cystic fibrosis patients. Sleep Breath. 2012;16(4):1041–1048. doi: 10.1007/s11325-011-0599-5. [DOI] [PubMed] [Google Scholar]