Abstract
An important task facing both researchers and animal core facilities is producing sufficient mice for a given project. The inherent biologic variability of mouse reproduction and litter size further challenges effective research planning. A lack of precision in project planning contributes to the high cost of animal research, overproduction (thus waste) of animals, and inappropriate allocation of facility resources. To examine the extent daily prepartum maternal weight gain predicts litter size in 2 commonly used mouse strains (BALB/cJ and C57BL/6J) and one mouse stock (Swiss Webster), we weighed ≥ 25 pregnant dams of each strain or stock daily from the morning on which a vaginal plug (day 0) was present. On the morning when dams delivered their pups, we recorded the weight of the dam, the weight of the litter itself, and the number of pups. Litter sizes ranged from 1 to 7 pups for BALB/cJ, 2 to 13 for Swiss Webster, and 5 to 11 for C57BL/6J mice. Linear regression models (based on weight change from day 0) demonstrated that maternal weight gain at day 9 (BALB/cJ), day 11 (Swiss Webster), or day 14 (C57BL/6J) was a significant predictor of litter size. When tested prospectively, the linear regression model for each strain or stock was found to be accurate. These data indicate that the number of pups that will be born can be estimated accurately by using maternal weight gain at specific or stock-specific time points.
Abbreviations: B6, C57BL/6J; MWG, maternal weight gain; MSE, mean squared error; SW, Swiss Webster
Successful research projects are often determined by the validity of the research data collected and meaningfulness of the results. Unfortunately, the reduction of the number of animals used may not be a priority for some investigators.15 Furthermore, the total number of animals produced for a particular study can be significantly larger than the number of animals actually used in the gathering of the study's data.22 These observations are contrary to the teachings of the Three R's of the use of animals in biomedical research, specifically the notion of reducing the number of animals used.16,20
Researchers are encouraged to limit the number of animals used during a study, and this reduction should also be applied to the number of animals produced to meet the goals of the project. Frequently, the number of animals produced for an experiment exceeds the number needed. Although this situation is unavoidable in some cases (for example, offspring do not meet genetic requirements, only one sex is needed), in many cases overproduction can be avoided through more precise planning.
Overproduction is primarily a rodent specific issue due to their small size, small space requirements, and low cost of maintenance.3 Another factor that contributes to the overproduction of rodents is the inability to plan the number of pups that will be delivered from a given pregnancy. Concerns regarding underproduction (and thus missed experimental deadlines and goals) often lead to reproductive overcompensation and thus possible waste of animals, funding resources, and human effort. A method that can be applied early in gestation and determine the number of pups to be born would facilitate the effort to minimize overproduction. Current approaches to early litter-size prediction include laparotomy, high-frequency ultrasound,9,18 and palpation.1,21 Although these methods are somewhat reliable, they require expensive equipment or extensive knowledge of palpation technique and, in some circumstances, familiarity with a specific mouse strain or stock.
The main purpose of this study was to evaluate the effectiveness of a single measurement (weight) for predicting the number of pups that would be born in 2 commonly used mouse strains and one mouse stock. Maternal weight gain (MWG) was selected as the parameter because it is an objective measurement that is easily obtained, requires little to no training, and induces minimal stress on the pregnant female. Furthermore, weight has been shown to be a more reliable indicator of pregnancy when compared with other methods, such as vaginal plug detection.13 A secondary objective of the current project was to design, implement, and test a method that could be used as a predictor of litter size at other institutions.
Here we report that MWG during pregnancy is an easily obtained and reliable predictor of litter size in BALB/cJ, Swiss Webster, and C57BL/6J mice. Our results show that although weight gain during pregnancy is not a perfect predictor of litter size in these strains and stock, it is a significantly predictive variable that is easily and objectively measured and that can serve as a refinement to reduce the likelihood of overproduction of mice for a specific experiment.
Materials and Methods
All animal procedures were performed in an AAALAC-accredited facility and approved by the IACUC of City of Hope Beckman Research Institute.
Animals.
Female BALB/cJ, C57BL/6J (B6), and Swiss Webster (SW) mice that were 6 to 18 wk at the start of the study were used. A relatively wide range of breeding ages was used to increase the likelihood that the results could be applied broadly rather than restricted to a narrow age group. The BALB/cJ mice were purchased shortly before initiation of this study from Jackson Laboratories (Bar Harbor, ME) for a breeding colony used by various investigators on campus. The SW (Charles River Laboratories, Wilmington, MA) and B6 (Jackson Laboratories) mice were part of our institution's regular breeding program, and offspring produced during this study were later used in other research projects. Mice in our inhouse breeding program are bred year round, with new breeding males purchased from their respective companies every 6 mo. All mice were housed in the same breeding room for the entire experiment and thus parameters such as temperature, humidity and light intensity/duration were consistent among all groups (with a 14:10 h light:dark cycle). Mice were designated as SPF for: mouse rotavirus, Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus, Theiler murine enchephalomyelitis virus, mouse reovirus type 3, mouse norovirus, lymphocytic choriomeningitis virus, mouse thymic virus, mouse adenovirus types 1 and 2, mouse cytomegalovirus, polyoma virus, K virus, ectromelia virus, Hantavirus, Prospect Hill virus, cilia-associated respiratory bacillus, Encephalitozoon cuniculi, and Mycoplasma pulmonis. Mice also were free of Helicobacter spp., Clostridium piliforme, and endo- and ectoparasites.
The initial parity of the dams ranged from 0 to 3 litters for all mice. The differences in parity allowed us to draw from a large pool of possible breeders. Initial weight ranges for the dams were 20 to 22.9 g (BALB/cJ), 24 to 32.9 g (SW), and 17.8 to 22.6 g (B6). Male mice of all strains or stock were experienced breeders. Mice were housed in individually ventilated cages (Allentown, Allentown, NJ) with free-choice access to reverse-osmosis–purified water and rodent chow (LabDiet 5013, Purina Mills International, St Louis, MO). The bedding was Bed-o'Cobs 1/8 in. (The Andersons, Maumee, OH), and nesting squares and PVC tubes were provided for environmental enrichment. Prior to breeding, females were housed 4 to a cage. During breeding, 2 females were with one male mouse. After confirmed copulation, dams were housed 1 to 4 mice per cage until day 13 to 15 of their pregnancy, after which they were singly housed.
Study design.
This study was broken into 2 breeding components. The first component (phase 1) was used to establish the linear regression equations for the earliest day of gestation that were significantly predictive of litter size in each strain or stock. The second phase (phase 2) was a prospective study to evaluate the viability of the linear equations obtained in phase 1.
Phase 1.
On Monday mornings, 2 female mice were placed in the cage of a single male mouse. On the following Tuesday through Friday, the female mice were examined for the presence of a copulatory plug. Those found with a plug (day 0; pregnant) immediately were weighed to the nearest 0.1 g (model CS200, Ohaus, Parsippany, NJ) and placed in a new cage, either alone or with other dams found to be pregnant on the same day. Pregnant dams were group housed until day 13 to 15, at which point they were transferred to individual housing. Each pregnant dam was weighed daily until she delivered her pups. Any mice that were in active labor at the time of weighing were left alone and subsequently were excluded from the final data analysis. All plug checking and weights were performed between 0730 and 1000. After parturition, the combined litter weight and total number of pups in the litter were recorded when the dams were weighed (Figure 1). When pups were found dead (infanticide or otherwise), the total number of pups was recorded, but the combined weight of all pups was not measured. All breeding for phase 1 was completed between December 2012 and May 2013.
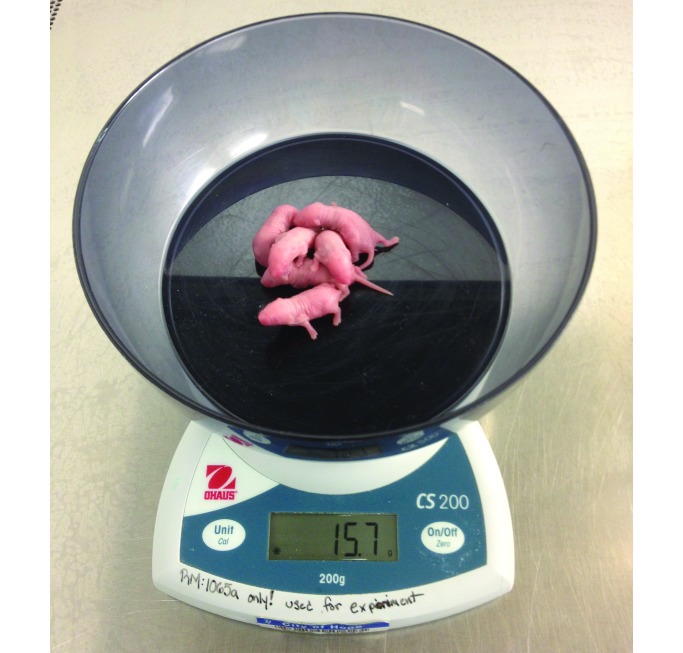
Figure 1.
Pups were weighed (to the nearest 0.1 g) as a group on the morning that they were delivered.
Our preexperimental power analysis indicated that 25 litters per strain or stock was sufficient to provide statistical significance. However, more than 25 pregnancies occurred per strain or stock, because breeding continued while we waited to ascertain whether other females were truly pregnant. Excess mice were used for other IACUC-approved projects within the institution or were euthanized by CO2 according to the American Veterinary Medical Association's Guidelines for the Euthanasia of Animals, 2013 edition.
Phase 2.
All aspects of breeding for this phase were identical to those for phase 1, with a few important exceptions. Most importantly was the number of times that the dams were weighed. Phase 1 was designed to help identify the earliest day of gestation on which MWG was predictive of litter size, whereas phase 2 was designed to evaluate the results obtained from phase 1. Therefore, dams used in phase 2 were weighed only on the morning of their positive plug check and on the earliest day predictive of little size (determined in phase 1) rather than daily, as was done in phase 1. Breeding for phase 2 occurred between August and December 2014.
Statistics.
The average weights of pups for each mouse strain or stock were described by mean, SD, median, and range. The Shapiro–Wilk normality test was used to check whether the average weights of pups followed a normal distribution. The Mann–Whitney test was done to examine whether the distributions of the means of the average weights of pups differed between 2 mouse types (for example, SW compared with B). Average gestation length was calculated, and significant differences among the strains/stock were determined by using a 2-sample t test and the nonparametric Wilcoxon rank sum test.
To determine the earliest day on which MWG predicted the size of the litter for each mouse strain or stock (phase 1), a linear regression model was fitted for daily weight gain pregnancy data. In the model, the weight gain was the predictor and the number of pups was the response. When the slope estimate from the linear model is significantly different from 0, the MWG on that particular day can be used to predict the number of pups to be born. Among the linear models with significant slope estimates, the one for the earliest gestational date was taken as the final model.
The mean squared error (MSE) was used to assess the performance of the established linear regression equation in terms of prediction accuracy (phase 2). The MSE is defined as the average of the squares of the difference between the actual observed number of pups and the predicted number of pups using the linear equations. A smaller MSE value indicates a higher degree of similarity between the predicted number of pups and the actual number born. The R software (http://www.r-progject.org/) was used to perform all statistical analyses. An effect was considered to be significant when the P value was less than 0.05. All P values are 2-sided unless otherwise noted.
Results
Phase 1.
A total of 142 positive plug checks (SW, 41; B6, 38; BALB/cJ, 63) were obtained, with a total of 92 litters born (SW, 34; B6, 27; BALB/cJ, 31). Therefore, the overall efficiency of copulatory plug detection for predicting pregnancy was 64.8% (92 pregnancies / 142 plugs) for these strains and stock of mice (SW, 82.9%; B6, 71.1%; BALB/cJ, 49.2%). No cannibalism was noted for any of the SW pregnancies; there were 6 instances of infanticide for the B6 mice and 7 for the BALB/cJ pregnancies. Weights from these litters were not included in the final data.
Litter size varied greatly among the strains and stock evaluated (SW, 2 to 13 pups; B6, 5 to 11 pups; BALB/cJ, 1 to 7 pups), but very large or very small litters were rare. The SW mice had the largest average litter size (10.7 pups; SD, 2.0 pups), whereas the litter sizes of the B6 dams (mean, 7.5 pups; SD, 1.3) and BALB/cJ mice (5.9 pups; SD, 2.7) were much smaller. Gestation length (day 0, the morning of the positive plug check; last day, the morning the litter was discovered) in our study animals (SW, 18.8 ± 0.7 d; B6, 19.0 ± 0.2 d; BALB/cJ, 19.6 ± 0.6 d) did not differ from those seen previously in our breeding colony. For gestation length, both the 2-sample t test and the Wilcoxon rank sum test demonstrated no significant difference between the SW and B6 mice, however significant difference were present between B6 and BALB/cJ and SW and BALB/cJ (P <0.0001 for both).
Table 1 presents a summary of the statistics for the average weight per pup and the results of the Shapiro–Wilk normality test. Because the BALB/cJ data were determined to be nonnormal (P < 0.001), the Mann–Whitney test was done to examine the significance of any difference between the distributions of the means of the average weights of pups between mouse types. Such analysis indicated a significant difference between the B6 and SW pups (P < 0.00001), between SW and BALB/cJ pups (P = 0.0003), and between B6 and BALB/cJ pups (P < 0.00004).
Table 1.
Summary statistics regarding pup weight
| Pup weight (g) | P (Shapiro–Wilk) | ||||
| Mean | SD | Median | Range | ||
| SW | 1.6 | 0.1 | 1.6 | 1.4 to 1.9 | 0.8115 |
| BALB/c | 1.5 | 0.2 | 1.4 | 1.3 to 2.3 | 0.0002 |
| B6 | 1.3 | 0.1 | 1.3 | 1.6 to 1.4 | 0.5500 |
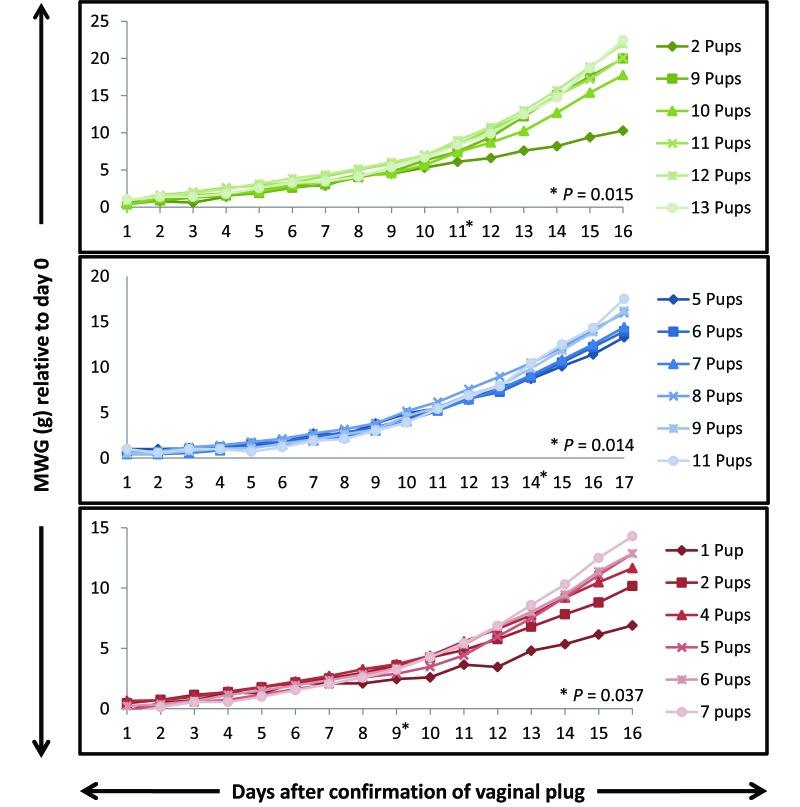
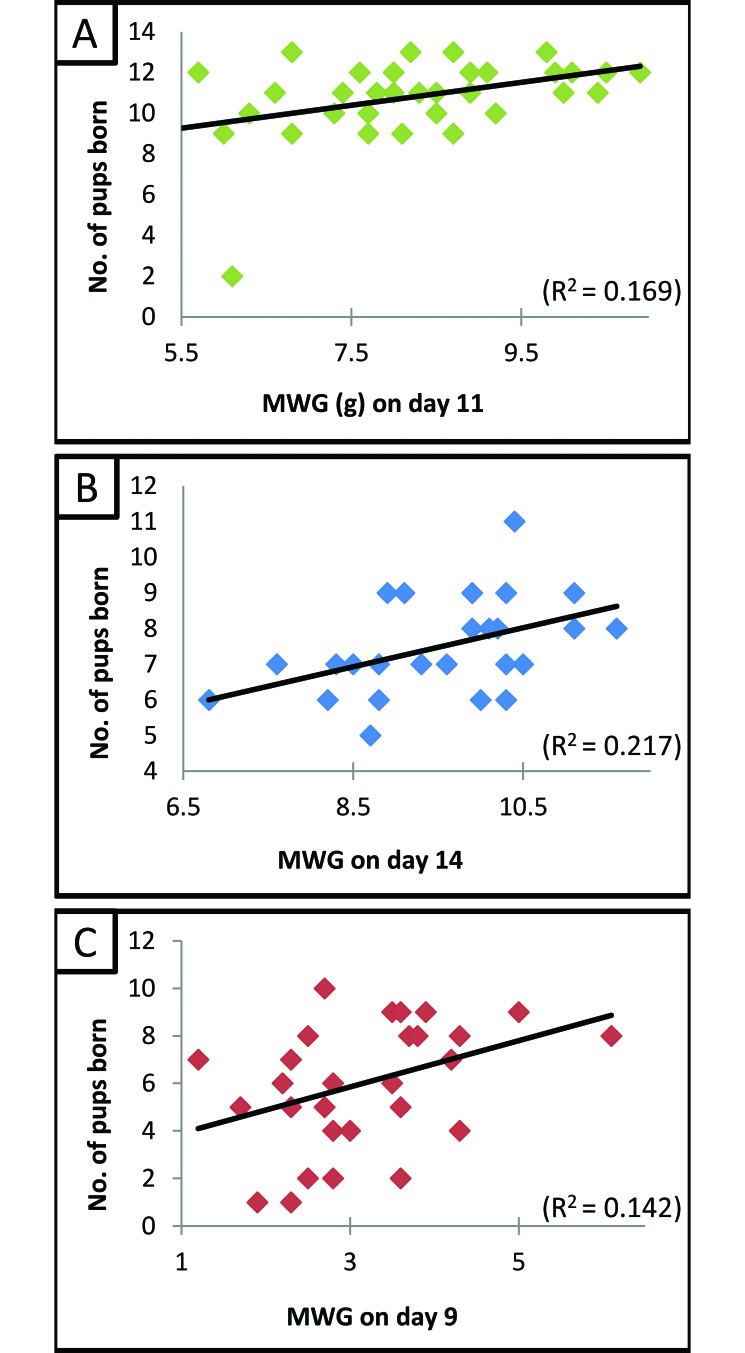
MWG during pregnancy is shown in Figure 2. The earliest day on which MWG was a significant (P < 0.05) predictor for the number of pups to be born varied among the 3 types of mice (SW, day 11; B6, day 14; and BALB/cJ, day 9). Linear regression analysis of the MWG (on their respective earliest predictive days) and number of pups born are shown in Figure 3. From these linear regression models of weight gain and the average numbers of pups that were born, the following equations were derived.
 |
Figure 2.
MWG over time in each strain or stock (Swiss Webster, green; C57BL/6J, blue; BALB/cJ, red). The asterisk indicates the earliest day during gestation at which MWG was a significant (P < 0.05) predictor of litter size.
Figure 3.
MWG compared with the number of pups delivered in (A) SW (n = 34), (B) C57BL6 mice (n = 27), and (C) BALB/cJ (n = 31) mice. The earliest day at which the slope of the regression line differed significantly from 0 was day 11 (P = 0.015) for SW mice (except for the pregnancy with only 2 pups, all of the data points are clustered together), day 14 (P = 0.014) for C57BL6 mice, and day 9 (P = 0.037) for BALB/cJ mice.
Phase 2.
A total of 34 pregnancies (SW, 12; B6, 10; BALB/cJ, 12) were used in phase 2 to assess the accuracy of the linear equations determined in phase 1. The MSE for each strain or stock was used to assess prediction accuracy (an MSE of 0 indicates perfect prediction accuracy). In general, the smaller the MSE value, the more predictive the linear model of the prospective data. The MSE values were; SW, 1.269; B6, 0.3; and BALB/cJ, 0.925. In addition, the corresponding correlation coefficients were: SW, 0.793; B6, 0.892; and BALB/cJ, 0.721. The coefficients of determination (R2) for these litters were: SW, 0.63; B6, 0.80; and BALB/cJ, 0.52.
Discussion
Participants in the field of biomedical research have a responsibility to limit the number of animals used in experiments.7,8,20,23 Reduction of animal numbers is important for ethical reasons and is fiscally responsible, which is a concern for many investigators.6,12 There are various ways in which researchers managing their own murine breeding needs can be proactive in reducing animal numbers, often with the added benefit of minimizing overall costs as they obtain reliable data. These methods include robust preexperimental statistical power analysis, serial imaging modalities, sequential sampling, efficient production of genetically engineered mice, and avoiding overproduction of animals. Producing the appropriate number of mice for a particular experiment relies on familiarity with the potential litter size of the murine strain or stock being used,5 although this knowledge provides only a crude estimation. Here we demonstrate that a simple and easily obtained metric (MWG) can be used to predict the litter size of an individual murine pregnancy more precisely.
Although it might seem obvious that MWG forecasts litter size, variations in individual pup weight could confound the ability to make accurate predictions. This caveat is especially true for specific lines of genetically engineered mice (for example, leptin knockout mice4), mice fed calorie-rich diets,25 and older female breeders. Although the prospective portion of our project (phase 2) demonstrated that the linear regression models determined in phase 1 had a fairly high level of prediction accuracy, these models likely are valuable only for the specific mouse strains and stock we tested. For this reason, the data presented here cannot be applied directly to other strains and stocks of mice. We recommend that animal users who are interested in predicting litter sizes follow our methodology of determining the earliest day for which MWG can reliably be used for this purpose. It seems reasonable to assume that this methodology could be applied to other rodent species as well. We do not discount the initial effort that will be required to obtain equations for the predictions of litter size. However, once these equations have been determined, only 2 weights (those for day 0 and the earliest predictive day, as done in phase 2) are needed to begin predicting litter size more effectively.
MWG has been shown to be a reliable indicator of pregnancy in mice.13,17 Although not the focus of the current study, we were able to identify by maternal weight alone those female mice that had a copulatory plug but did not become pregnant. A lack of weight gain at 7 to 9 d after plug detection allowed us to return nonpregnant female mice to the breeding pool with 100% accuracy (for example, no pregnant dams were reexposed to a male mouse inadvertently; data not shown). ‘Recycling’ female mice for breeding purposes ultimately results in fewer mice needed to meet investigator needs.
The main focus of our research was to determine the earliest day at which MWG was a significant predictor of the eventual litter size. Other factors indicative of litter size include age and parity of the dam, genetic background, diet, light intensity and duration, temperature, noise, handling, and experimental conditions. 2,10,19 All of these factors are known to have substantial effects on the size of a litter and should not be ignored when electing to use MWG as a predictor of litter size.
Our research demonstrates that MWG can serve as an effective predictor for determining litter size at as early as day 9 of gestation (BALB/cJ). This early detection represents approximately 10 d of advanced notice to both animal breeding staff and researchers regarding the approximate number of pups to expect from a pregnancy. This early information enables staff and investigators to determine whether breeding intensity should be increased, decreased, or maintained. Better planning directly affects a facility's ability to better use its space, budget, and workforce.
In addition to prediction of litter size, we were able to use MWG as a determinate of pregnancy itself (as has been described previously).13,17 The plugged pregnant rate for our B6 mice (71.1%) was similar to the rates for GFP mice on a B6 background (69%).13 The presence (or absence) of a copulatory plug does not guarantee that a dam will (or will not) become pregnant. Plugs may never form, can be very small, or can fall out prior to being observed, and thus their presence may not be a reliable indicator of successful copulation. The efficacy at which vaginal plugs indicate pregnancy varies dramatically, and their detection is dependent on trained personnel. For this reason, it is worth considering using MWG as a replacement for plug checking or as an additional confirmatory method.
In the current study, the litter sizes of the B6 (7.5 ± 1.3 pups) and BALB/cJ (5.9 ± 2.7 pups) mice were smaller than that of SW (10.7 ± 2.0 pups). Information regarding average litter size for SW mice was unavailable from the vendor, but it did not differ from what has historically been seen in our institution. The Mouse Phenome Database contains average litter size data for B6 and BALB/cJ mice (entry14983), although it does not provide the standard deviation for these strains. However, the range of average litter sizes for our B6 and BALB/cJ mice and the respective standard deviations includes the average value reported in the Mouse Phenome Database. We find it interesting to note that the average individual pup weight for the strains and stock we used in this research all differed significantly from one another (Table 1.) It seems reasonable to assume that the strain or stock with the highest average pup weight would have the earliest day at which MWG predicts litter size, but this was not true for the strains and stock that we examined. For example, although the BALB/cJ mice had a lower average pup weight than did SW mice, the earliest predictive day for BALB/cJ mice was 2 d earlier than that of the SW mice. This finding indicates that aspects of MWG other than fetal weight (for example, volume of amniotic fluid, placental size, amount of adipose tissue) can vary among strains and stocks or that the period of greatest fetal growth perhaps happens at different times for different strains or stocks of mice.
Another interesting aspect of the data we collected for this project are the comparisons that can be drawn with a previous mouse breeding study.14 In our study, the gestation time of BALB/cJ mice (19.6 d) was significantly longer than that of either B6 (19.0 d) or SW (18.8 d) mice. These gestation times are in line with those seen by others,14 although the previous study used BALB/cByJ mice rather than our BALB/cJ and did not include SW mice. The slight discrepancy between our gestation times and those in the previous work14 might be explained by their more precise determination of actual parturition time through the use of infrared cameras and their use of BALB/cByJ mice. In our current study, the gestation lengths of SW and B6 mice were similar, but their average pup weights differed significantly. Furthermore, when examining SW, B6 and BALB/cJ pregnancies, we found the same inverse correlation between litter size and gestation length that was noted in the earlier study.14 In addition, the cited work14 examined only a small component of MWG (captured only at embryonic day 14.5), such that the data cannot be compared directly between the 2 studies. However, because the best linear regression model for our B6 mice was determined to be day 14, we can infer some information. Specifically, given the MWG averages for B6 provided in the earlier study,14 our regression model yields an average litter size range of 6.4 to 6.9 pups, whereas the actual average litter sizes were 7.8 to 8.2 pups. These ranges do not overlap, and their differences might be explained by genetic drift, differences in housing conditions, or seasonal variation.
Despite the controlled environment of an animal facility, seasonality can have a significant effect on several phenotypic characteristics (for example, gestation length) in some mice.14 The 2 phases of our study did overlap in the time of year, but only during the month of December. Although we did not appreciate any significant differences in gestation length or litter size between the 2 phases, we cannot rule out the possibility that the predictive potential of MWG might vary during other seasons.
In an effort to broaden the applicability of our findings, the breeding strategies mimicked those already practiced in the facility. This approach necessitated the use of dams of different ages, weights, and parities. Therefore normal mouse growth could contribute to the MWG and might influence the accuracy of the linear equation that was calculated. Although normal growth might represent an uncontrolled variable, the low MSE values of phase 2 indicate that its effect was minimal. In addition, phase 1 demonstrated a great deal of variability in individual litter sizes for a given MWG, especially for B6 and BALB/cJ pregnancies. This finding indicates a lack of accuracy for predicting future litter sizes. Although this factor might be true for any specific litter, as a whole the linear regression models are helpful for predicting litter size (as seen in the results of the phase 2 prospective study). Certainly having more litters in phase 1 would have been helpful in determining the linear regression models, but the numbers that were used (along with the results of phase 2) appear to be sufficient to demonstrate that MWG can act as a predictor of litter size in SW, B6, and BALB/cJ mice.
Litter size is known to vary with the parity of the dam,11 as is the occurrence of filial cannibalism.24 Although both of these phenomena can affect the total number of pups that survive to weaning, neither should have an influence on the linear equations used to predict litter size. If a strain or stock of mouse has a small first litter, our data suggest that a smaller MWG would be observed. Similarly, cannibalism does not affect the number of pups born, only the number of those that survive. For this reason, when filial cannibalism occurred (as it did in some B6 and BALB/cJ pregnancies), we counted the number of pups born (according to the pup heads present) rather than the number of viable pups. However, when the strain or stock of interest is known to have a high rate of infanticide, this characteristic needs to be accommodated in the breeding practices to obtain the number of mice actually desired for any given study.
Our current study shows that a refinement of breeding practices can lead to a reduction in the number of animals that are produced unnecessarily in biomedical research. MWG is a simple and practical way to assess the eventual outcome of a murine pregnancy and has many advantages over other methodologies. Facilities that regularly host large-scale breeding projects might benefit from implementing the strategies described here. Additional studies involving rats and other strains or stocks of mice would be beneficial to determine whether the same methodology might be used ubiquitously throughout animal care and use programs.
Acknowledgments
This work was supported by Grants for Laboratory Animal Science (GLAS) from the American Association for Laboratory Animal Science. We thank Dr Stephanie Murphy for providing unpublished maternal weight gain data to allow us to do our power analysis in the study-design phase. We also thank Stacey Sparkes, Danielle Kim, and Jeremy LaDou for their efforts in helping with copulatory plug verification and recording of body weights.
References
- 1.Brown SD, Zurakowski D, Rodriguez DP, Dunning PS, Hurley RJ, Taylor GA. 2006. Ultrasound diagnosis of mouse pregnancy and gestational staging. Comp Med 56:262–271. [PubMed] [Google Scholar]
- 2.Colston MJ, Levy L. 1987. Mouse breeding and husbandry. Int J Leprosy 55:819–822. [PubMed] [Google Scholar]
- 3.Danneman P, Suckow MA, Brayton C, Suckow MA. 2013. Important biological features, p 1–17. In: Hedrich H. The laboratory mouse, 2nd ed. Boca Raton (FL): Taylor and Francis. [Google Scholar]
- 4.Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, Obrosova IG. 2006. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes 55:3335–3343. [DOI] [PubMed] [Google Scholar]
- 5.Falconer DS. 1960. The genetics of litter size in mice. J Cell Comp Physiol 56 Suppl 1:153–167. [DOI] [PubMed] [Google Scholar]
- 6.Fenwick N, Danielson P, Griffin G. 2011. Survey of Canadian animal-based researchers’ views on the 3 Rs: replacement, reduction, and refinement. PLOS ONE 6:e22478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Festing MF. 1992. The scope for improving the design of laboratory animal experiments. Lab Anim 26:256–268. [DOI] [PubMed] [Google Scholar]
- 8.Festing MF. 1994. Reduction of animal use: experimental design and quality of experiments. Lab Anim 28:212–221. [DOI] [PubMed] [Google Scholar]
- 9.Greco A, Ragucci M, Coda AR, Rosa A, Gargiulo S, Liuzzi R, Gramanzini M, Albanese S, Pappata S, Mancini M, Brunetti A, Salvatore M. 2013. High-frequency ultrasound for in vivo pregnancy diagnosis and staging of placental and fetal development in mice. PLOS ONE 8:e77205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampshire V, Davis JA. 2005. The role of the veterinary staff in mouse breeding colony management. Lab Anim (NY) 34:45–49. [DOI] [PubMed] [Google Scholar]
- 11.Krackow S, Gruber F. 1990. Sex ratio and litter size in relation to parity and mode of conception in 3 inbred strains of mice. Lab Anim 24:345–352. [DOI] [PubMed] [Google Scholar]
- 12.Lake JP, Haines D, Linder C, Davisson M. 1999. Dollars and sense: time and cost factors critical to establishing genetically engineered mouse colonies. Lab Anim (NY) 28 Suppl:8–14. [Google Scholar]
- 13.Mader SL, Libal NL, Pritchett-Corning K, Yang R, Murphy SJ. 2009. Refining timed pregnancies in 2 strains of genetically engineered mice. Lab Anim (NY) 38:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, Donahue LR. 2010. Mouse gestation length is genetically determined. PLOS ONE 5:e12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nevalainen T. 2004. Training for reduction in laboratory animal use. Altern Lab Anim 32 Suppl 2:65–67. [DOI] [PubMed] [Google Scholar]
- 16.Ohno Y. 2002. ICH guidelines—implementation of the 3Rs (refinement, reduction, and replacement): incorporating best scientific practices into the regulatory process. ILAR J 43 Suppl:S95–S98. [DOI] [PubMed] [Google Scholar]
- 17.Onojafe FI. 2005. Vaginal mucous plug and weight gain in mice. Tech Talk 10:4. [Google Scholar]
- 18.Pallares P, Gonzalez-Bulnes A. 2009. Use of ultrasound imaging for early diagnosis of pregnancy and determination of litter size in the mouse. Lab Anim 43:91–95. [DOI] [PubMed] [Google Scholar]
- 19.Reimer JD, Petras ML. 1967. Breeding structure of the house mouse, Mus musculus, in a population cage. J Mammal 48:88–99. [PubMed] [Google Scholar]
- 20.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London (United Kingdom): Methuen. [Google Scholar]
- 21.Russo M, Meomartino L, Greco A, Catone G, Cocchia N, Tortora G, Brunetti A. 2007. Pregnancy detection in mice using ultrasound. Vet Rec 160:446–447. [DOI] [PubMed] [Google Scholar]
- 22.Schiffelers MJ, Blaauboer BJ, Fentener van Vlissingen JM, Kuil J, Remie R, Thuring JW, Vaal MA, Hendriksen CF. 2007. Factors stimulating or obstructing the implementation of the 3Rs in the regulatory process. ALTEX 24:271–278. [DOI] [PubMed] [Google Scholar]
- 23.Sparrow SS, Robinson S, Bolam S, Bruce C, Danks A, Everett D, Fulcher S, Hill RE, Palmer H, Scott EW, Chapman KL. 2011. Opportunities to minimise animal use in pharmaceutical regulatory general toxicology: a cross-company review. Regul Toxicol Pharmagol 61:222–229. [DOI] [PubMed] [Google Scholar]
- 24.Weber EM, Algers B, Wurbel H, Hultgren J, Olsson IA. 2013. Influence of strain and parity on the risk of litter loss in laboratory mice. Reprod Domest Anim 48:292–296. [DOI] [PubMed] [Google Scholar]
- 25.West DB, Boozer CN, Moody DL, Atkinson RL. 1992. Dietary obesity in 9 inbred mouse strains. Am J Physiol 262:R1025–R1032. [DOI] [PubMed] [Google Scholar]