Abstract
To determine how housing density and ambient temperature interact to influence the physiology and behavior of mice, we systematically varied housing density (1 to 5 mice per cage) and ambient temperature (22, 26, or 30 °C) and measured effects on body weight, food intake, diurnal patterns of locomotor activity and core temperature, fecal corticosterone, and serum cytokine and adipokine panels. Temperatures inside cages housing 5 mice were 1 to 2 °C higher than the ambient temperature. As the housing density decreased, in-cage temperatures began to fall at a density of 2 or 3 mice per cage and did not differ from ambient temperature at 1 mouse per cage. Ambient temperature, but not housing density, significantly affected food intake. Although neither ambient temperature nor housing density affected core temperature or activity, hyperthermia and behavioral activation occurred during the 12-h period after cage change. Fecal concentrations of corticosterone metabolites and serum cytokines, chemokines, insulin, and leptin were not influenced by cage density and were only sporadically influenced by ambient temperature. Our data document that the number of mice housed per cage influences the intracage environmental conditions and that ambient temperature influences food intake even when temperatures are within or near recommended or thermoneutral ranges. We conclude that investigators should be cautious when changing the number of mice housed in a cage over the course of a study, because doing so significantly alters the cage environment to which remaining mice are exposed.
Numerous studies have investigated the effects of cage size and housing density on various physiologic and behavioral parameters in mice, with little resultant consensus on whether any generally adequate housing arrangement offers particular advantages or disadvantages with regard to animal use or wellbeing (see reference 29 for a review of this literature). However, a variant that is generally not systematically varied or even measured in such studies, yet is likely to influence the impact of housing density, is ambient temperature within the cage. The mouse thermoneutral zone is generally considered to range between 29 and 31 °C, although wider ranges have been reported, and many factors influence the thermoneutral range.2-4,17 Moreover, animals (including humans) may prefer a cooler environment than thermoneutral temperatures in some situations.4,12,23 In mice, the preferred ambient temperature is likely to be influenced by factors such as the presence of cagemates, the type and amount of bedding, and whether the cage is open-topped, closed and static, or closed and ventilated.
Because of the high surface-to-mass ratio of mice, variations in ambient temperature are likely to significantly influence the allostatic load they experience, with resultant changes in the basal state of their homeostatic coping responses. For example, serum corticosterone concentrations may change in response to conditions the animal encounters in its external environment and its internal metabolic state.16,18,24,26 In performing research using group-housed mice, the need may arise to remove an animal from its group for reasons including illness, aggression, and experimental use, which in turn changes both group size and housing density. Consequently, a change in housing density could alter environmental conditions within the cage, perhaps in ways that might influence sensitive measures of physiologic experimental outcomes.
We hypothesized that reducing the group size within a cage would alter environmental conditions within a cage and in turn influence physiologic measures in the remaining mice housed in that cage. We tested this hypothesis by comparing cage environmental conditions and physiologic measures in mice that were stably housed at either 1 or 5 animals per cage or that were housed 5 per cage with 1 mouse removed each week. This design was applied at 3 ambient temperatures: 22, 26, and 30 °C. Dependent variables were mouse core temperature, locomotor activity, fecal corticosterone, and various serum analytes reflecting metabolism or immune function. The goal of the study was to provide information regarding whether 1) changes in housing density alter behavioral and physiologic measures in individual animals within the group as the group size changes, 2) different ambient temperatures differentially influence these measures, and 3) housing density and ambient temperature interact to influence patterns of behavior and physiology. The data show that reductions in housing density are associated with changes in the cage environment and with some changes in serum concentrations of leptin and some cytokine. The findings indicate that the removal of mice from a stable group influences cage environment and perhaps physiologic measures in the mice that remain.
Materials and Methods
Animals.
This study used 198 adult female C57BL/6J mice (age, 4 wk; Jackson Laboratory, Bar Harbor, ME) housed in groups of 5 under a 12:12-h light:dark cycle in static filter-topped cages (12 in. × 7.5 in. × 5 in.; 90 in.2 of floor space), with the exception of one group that was housed individually for the duration of the study. At the start of data collection, mice weighed between 19 and 21 g. Cages were maintained in environmental chambers that were programmed to maintain ambient temperatures of 22, 26, or 30 °C. Within each cage and chamber, ambient temperature, relative humidity, and dewpoint were monitored continuously by placing a data logger (USB-502-PLUS; Measurement Computing, Norton, MA) within each cage and centrally within each chamber. All cages, regardless of census, contained 4 oz. of birch wood chips (Beta-Chips, Gateway Supply, St Louis, MO). This amount provided a bedding depth of approximately 1/3 in., which is standard in our facility. Cages were changed and all other manipulations were performed at weekly intervals immediately after light onset. Mice had unrestricted access to food (Purina Lab Diet 5008, distributed by Gateway Labs) and municipal tap water. Mice were not monitored for estrous cycling, which could contribute to variation in some dependent variables among animals. All animal use was approved by the Laboratory Animal Care and Use Committee of the Southern Illinois University School of Medicine prior to animal procurement and use.
Experimental design.
All individually housed mice and one mouse per cage of 5 underwent surgery at 5 wk of age for implantation of an abdominal transmitter (model TA-F10 [1.6 g], Data Sciences, St Paul, MN) that allowed telemetric measurement of locomotor activity and core temperature, as previously described.27 Transmitters were gas-sterilized prior to implantation. Mice were anesthetized by subcutaneous injection of a mixture of ketamine (50 mg/kg) and xylazine (50 mg/kg) and were supplemented with additional anesthetic during surgery when needed. All surgery was conducted by using standard aseptic techniques. The abdomen was shaved with a no. 40 clipper blade, and the skin was disinfected with alternating scrubs of povidone–iodine and alcohol. A midline incision of approximately 3 cm was made in the abdominal skin. The linea alba was identified and incised, exposing the abdominal cavity. The transmitter was implanted with the rounded end directed cranially. Sterile saline (1 mL) was added to the abdominal cavity to lubricate the transmitter and support hydration in the mouse. The abdominal muscles were closed in a simple continuous pattern with 4-0 polyglycolic acid suture. The skin was closed with no fewer than 3 simple interrupted sutures with 4-0 polyglycolic acid suture. The mice recovered from anesthesia in a cage placed on a heating pad. When present, sutures were removed at 10 d after surgery. Mice received the analgesic ibuprofen in the drinking water (1 mg/mL) beginning on the day before surgery and continuing for 5 d after surgery.
Data collection began at 8 wk of age, thus allowing mice to recover from surgery, grow to adult size, achieve sexual maturity, and acclimate to their environment and their cagemates. The mice with transmitters were weighed weekly at the time of cage change, and fecal pellets were collected from those mice for measurement of fecal corticosterone. Body weights were not corrected for transmitter weight (1.6 g) because all mice from which weights were collected had been implanted with transmitters. Food intake was measured weekly for each cage according to the remaining weight of premeasured feed, with no attempt to correct for spillage; daily food intake was then calculated per mouse. Locomotor activity was collected continuously from each implanted mouse; core temperature was sampled and stored every 10 min.
Three environmental chambers (model 352602, 28 in. × 23 in. × 53 in., 19 ft3; Hotpack, Philadelphia, PA) were used in this study (1 per environmental temperature). Each chamber held 4 cages with 5 mice per cage and 2 cages with 1 mouse per cage. Cages containing only 1 mouse included a small plastic weigh boat as an enrichment device. Cage placements were randomized within each chamber. Two of the group cages in each chamber were designated as test cages, from which 1 mouse was removed each week. This design was repeated 3 times for a total of 198 mice in 54 cages. In addition, chamber temperatures were changed so that each chamber was used at each of the 3 of the temperatures over the course of the 3 experimental runs.
Every week at the time of cage change, a nonimplanted mouse from the test cage was selected at random for euthanasia. Thus, the number of mice in each test cage dropped to 4, 3, 2, and eventually 1 over the course of the 5-wk study. Average food intake, body weights, and patterns of temperature and activity from cages that continually contained either 1 mouse or 5 mice were compared with those of mice in cages with weekly reductions in population. This design allowed us to determine whether 1) changes in housing density alter dependent measures in remaining mice in the group, 2) different ambient temperatures differentially influence these measures, and 3) housing density and ambient temperature interact to influence patterns of behavior and physiology.
Fecal boli were collected weekly from mice implanted with transmitters. This design allowed us to repeatedly monitor individually identified mice that would remain in the cage for the duration of the experiment. Fecal boli were obtained by placing individual mice implanted with transmitters into a clean cage lined with a paper towel and collecting fecal pellets that were excreted within a 10-min period (typically 2 to 4). Boli were picked up from the paper towel by using a sterile 20-gauge syringe needle. Care was taken to avoid contamination of the feces with urine. Fecal boli from each mouse were immediately placed in a 1.5-mL microfuge tube. Tubes were then snap-frozen in liquid nitrogen and stored at –80 °C until analyzed.
Each week, the mouse removed from the test cage was euthanized for tissue collection. All mice with transmitters were euthanized for tissue collection on the last day of the study. At the time of euthanasia, mice were weighed and fecal pellets were collected as described. Mice were then anesthetized with isoflurane. Blood was collected by cardiocentesis into a sterile 1-mL syringe with a 23-gauge needle. Euthanasia was then performed by cervical dislocation without recovery from anesthesia. Immediately after collection, whole blood was placed in a standard 1.5-mL microcentrifuge tube and allowed to clot at room temperature for 20 to 30 min. The tube was centrifuged at 1000 to 2000 × g for 10 min at 8 °C. The serum was immediately removed, transferred into clean polypropylene screw-top tubes in 0.5-mL aliquots, and stored at –80 °C.
Fecal and serum analysis.
Corticosterone in the fecal pellets was measured as an index of metabolic homeostatic challenge by using a method described previously.30 Samples were thawed and prepared for analysis at room temperature. Fecal pellets from each mouse were weighed and transferred to a 2-mL microfuge tube containing 1 mL of 90% methanol. Samples were homogenized for 20 to 30 s by using a handheld homogenizer and then vortexed at 22 °C and 1400 rpm for 30 s. The homogenates were then centrifuged at 2500 × g; the supernatant was removed and dried in a vacuum drier for 2 h at 45 °C. The dried sample was resuspended in 1 mL PBS, vortexed for 2 to 3 min, and then assayed by using a Corticosterone HS Enzyme ImmunoAssay kit (ImmunoDiagnostic Systems, Fountain Hill, AZ) according to the manufacturer's instructions.
Serum insulin and leptin and a panel of cytokines and chemokines were measured by using multiplex bead-based assays (MPXMCYTO-70K and MADKMAG-71K, Millipore, Bedford, MA) as described by the manufacturer and analyzed on a system (model 100IS , Luminex, Austin, TX) with Bio-Plex Manager 5.0 software (BioRad, Hercules, CA). All samples for insulin and leptin were run on the same plate. Similarly, all samples for cytokines and chemokine were run on one plate. The minimal detectable concentrations (pg/mL) for individual analytes were 13.0 for insulin, 4.2 for leptin, 2.0 for IL1β, 1.8 for IL6, 5.3 for CCL2 (MCP1), 5.6 for GCSF, 0.6 for CXCL10 (IP10), 1.4 for CXCL1 (KC), and 8.7 for CCL3 (MIP1α).
Statistical analysis.
A one-factor ANOVA with Tukey follow-up at each time point was used to compare cage temperature, relative humidity and dew point at each ambient temperature (22, 26 and 30 °C).
To determine the effects of cage change on locomotor activity and core body temperature, data were summarized for analysis as values obtained during light and dark phases on the day before cage change and the day after cage change, with cage change performed immediately after light onset.
Core temperature and locomotor activity data were analyzed by using a 4-factor ANOVA design with housing density (5 mice per cage, 1 mouse per cage, and 5 mice decreasing to 1 mouse per cage), ambient temperature (22, 26, or 30 °C), and phase (light and dark on the day before and day after cage change) as between-subjects factors and time (in weeks) as the repeated measure. Other than food intake, which was calculated per mouse per week for each cage, all measures were taken each week from the same mouse within each cage (that is, the implanted mouse). Subject was treated as a random factor in analyses of body weight, locomotor activity, and core temperature. A 3-factor ANOVA design with housing density and ambient temperature as between-subjects factors and time (in weeks) as the repeated measure was used for the analysis of food intake.
Cytokine, chemokine and adipokine data were log-transformed due to the nonnormal distribution of values.20 Cytokine concentrations that were below the assay limits of detection were assigned the minimal detectable concentration, for purposes of statistical analysis. A 2-factor ANOVA (housing density and ambient temperature) was used to test the overall model. When significant effects of housing density or ambient temperature or significant interactions were detected, specific comparisons were performed by using one-factor ANOVA with Tukey follow-up to identify significant differences between groups. On the basis of analysis of all values measured for a given parameter, some samples were excluded from the analysis as outliers (that is, GCSF, IL5, KC, MIP1α, and fecal corticosterone, 1 sample each; IP-10, 2 samples), all of which were 3 to 7 standard deviations above the population mean.
For analysis of fecal corticosterone, a 3-factor ANOVA design with housing density and ambient temperature as between-subjects factors was used to test the overall model for the repeated measure of fecal corticosterone over time.
All values are presented as mean ± SEM. An α level of a P value less than 0.05 was considered to indicate a statistically significant effect. SPSS Statistics (version 22, IBM, Armonk, NY) was used for all data analysis.
Results
Influence of ambient temperature and housing density on cage environment.
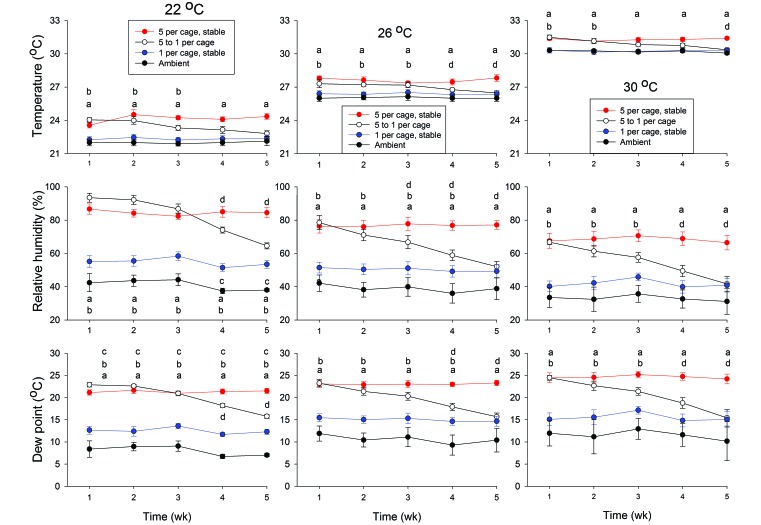
Ambient (that is, chamber) temperature and housing density interacted to produce clear effects on the internal cage environment (Figure 1). In cages that housed 5 mice, values measured in cages were consistently higher (P < 0.05) than those measured in the chamber, whereas in cages containing 1 mouse, significant differences between intracage and ambient values were relatively uncommon (Figure 1). As the number of mice was reduced from 5 to 1 per cage (the 5-to-1 group), intracage temperatures fell, eventually becoming significantly different from cages containing 5 mice (Figure 1). As expected, ambient temperature did not affect the dewpoint, whereas relative humidity fell as the ambient temperature increased.
Figure 1.
Environmental conditions for mice housed at 1 to 5 per group under different ambient temperatures. Female C57BL/6J mice were housed as stable populations of 1 or 5 per group or as a population that was initially 5 (at week 1) and was decreased by 1 mouse each week, resulting in 1 mouse in the cage at week 5 (5-to-1 group). Devices that logged temperature, relative humidity, and dew point at 1-h intervals were placed in the bedding of each cage and centrally within each environmental chamber. Ambient chamber temperature was maintained at 22, 26, or 30 °C. Data were downloaded from the data loggers weekly and summarized across weeks of the study. Lowercase letters indicate significant differences between intracage and chamber values (a, 5 mice per cage compared with chamber; b, 5-to-1 group compared with chamber; c, 1 mouse per cage compared with chamber; d, 5 mice per cage compared with 5-to-1 group).
Body weight and food intake.
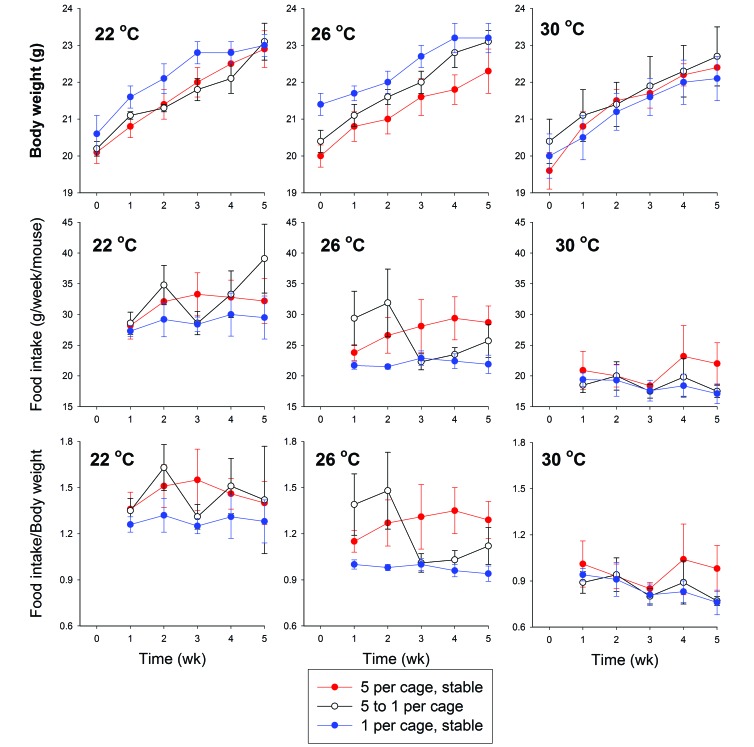
With regard to body weight, time was a significant (P < 0.001) factor at all 3 ambient temperatures, consistent with growth of the mice (Figure 2). However, housing density was not a significant factor at any of the 3 ambient temperatures, nor was ambient temperature a significant factor at any of the housing densities.
Figure 2.
Body weight, food intake, and intake:weight ratio for mice housed at 5, 1, or 5-to-1 mice per cage under different ambient temperatures. The transmitter-implanted mouse in each cage was weighed weekly. Food intake was measured for all mice in each cage and expressed as weekly food intake per mouse. Ratios were calculated by dividing the daily average food intake per mouse in each cage during the preceding week by the body weight of the implanted mouse in that cage. Body weight showed a significant effect of time, whereas food intake and the intake:weight ratio were significantly affected by ambient temperature.
With regard to food intake, time was a significant factor only for mice housed at 22 °C (P = 0.032), with the 5-to-1 group significantly different from the other 2 groups. Housing density was not associated with a significant effect on food intake (P > 0.05) Ambient temperature was a significant factor for food intake regardless of housing density, with significantly (P < 0.001) greater food intake in mice housed at 22 °C as compared with 30 °C. Mice housed at 26 °C had intermediate food intakes that were not significantly different from those of mice housed at either of the other 2 temperatures.
Core body temperature and locomotor activity.
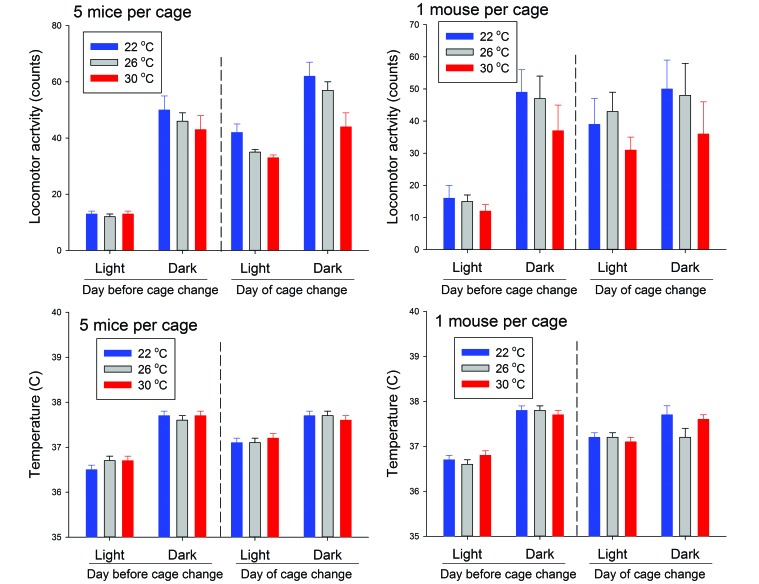
Temperature and locomotor activity data that were collected during the 24-h period before and after the weekly cage change were analyzed. Among mice housed stably at either 1 or 5 per cage (Figure 3), ambient temperature had no significant effect on mouse core temperature. On the day before cage change, mice showed significant circadian increases in temperature and activity during the dark phase at all 3 ambient temperatures (P ≤ 0.024 for all comparisons). During the light phase on the day after cage change, locomotor activity and core temperatures were higher (P ≤ 0.027) than those during the light phase on the previous day, regardless of ambient temperature, whereas dark-phase values before cage change did not differ before and after cage change. Thus, hyperthermia and behavioral activation occurred during the 12-h period after cage change and were not influenced by either ambient temperature or housing density.
Figure 3.
Locomotor activity and body temperature in mice housed at 1 or 5 per cage under different ambient temperatures. Temperature and locomotor activity from the implanted mouse in each cage were analyzed for 24 h before and after the weekly cage change. Data were summarized over 12-h intervals and analyzed as values obtained on the day before cage change and those obtained at light onset on the day after cage change. Neither ambient temperature nor housing density had a significant effect on mouse core temperature, although mice showed significant circadian increases in temperature and activity during the dark phase at all 3 ambient temperatures (P ≤ 0.024 for all comparisons).
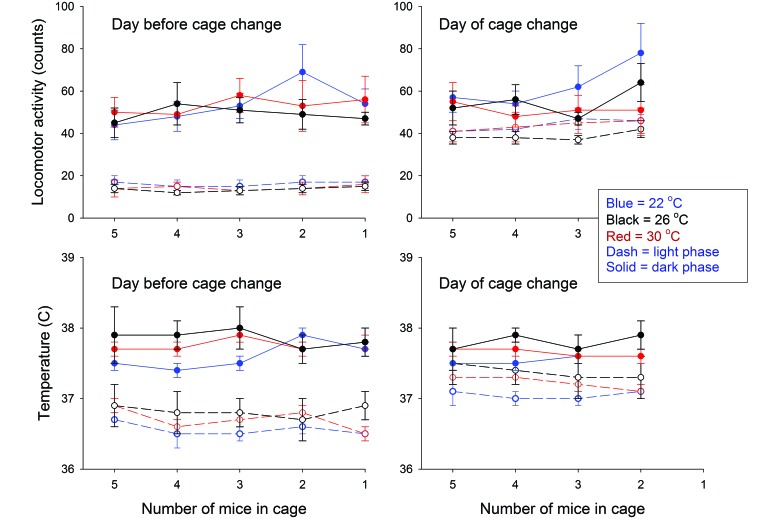
Similar effects occurred in the mice in the 5-to-1 group, according to data collected from the index (that is, implanted) mouse in each cage (Figure 4). Significant (P ≤ 0.011) differences between dark- and light-phase values were present on both the day of and the day after cage change, regardless of ambient temperature and housing density. At all ambient temperatures, activity and core temperature values were higher (P ≤ 0.001) during the second light phase as compared with the first but were equivalent during the 2 dark phases.
Figure 4.
Locomotor activity and body temperature in mice housed under declining densities from 5 to 1 mouse per cage under different ambient temperatures. Temperature and locomotor activity from the implanted mouse in each cage were analyzed for 24 h before and after the weekly cage change. Data were summarized over 12-h intervals and analyzed as values obtained on the day before cage change (left panels) and those obtained on the day after cage change at light onset (right panels). Neither ambient temperature nor housing density had a significant effect on mouse core temperature, although mice showed significant circadian increases in temperature and activity during the dark phase at all 3 ambient temperatures (P ≤ 0.011 for all comparisons).
Blood analytes and fecal corticosterone metabolites.
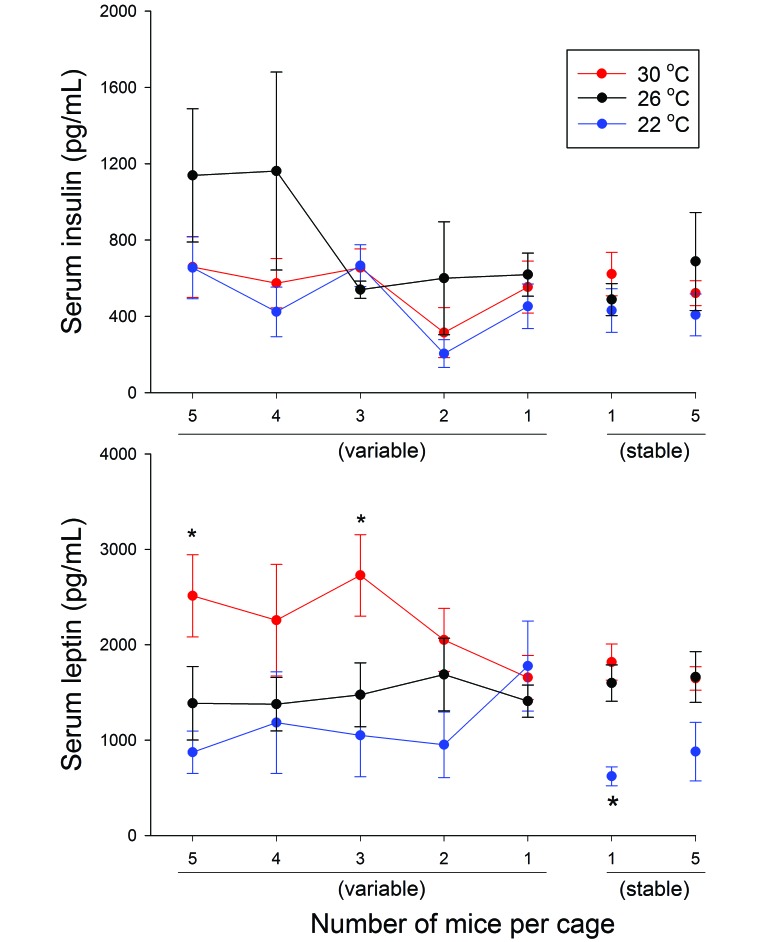
Neither housing density nor ambient temperature significantly influenced serum insulin concentrations. Within each temperature condition, serum leptin levels were not affected by housing density. However, in the 5-to-1 cages, leptin concentrations were higher at 30 °C as compared with 22 °C when the cage contained 5 mice (P = 0.013) and 3 mice (P = 0.032; Figure 5). Among mice housed stably at 1 per cage, those at 22 °C had significantly (P < 0.001) lower leptin concentrations than did those at 26 or 30 °C.
Figure 5.
Serum insulin and leptin concentrations in mice housed at 1 to 5 per group under different ambient temperatures. Serum was collected at euthanasia for measurement of insulin and leptin concentrations. Neither housing density nor ambient temperature significantly influenced insulin concentrations. Significant (*, P < 0.05) intergroup differences in leptin concentrations were detected sporadically.
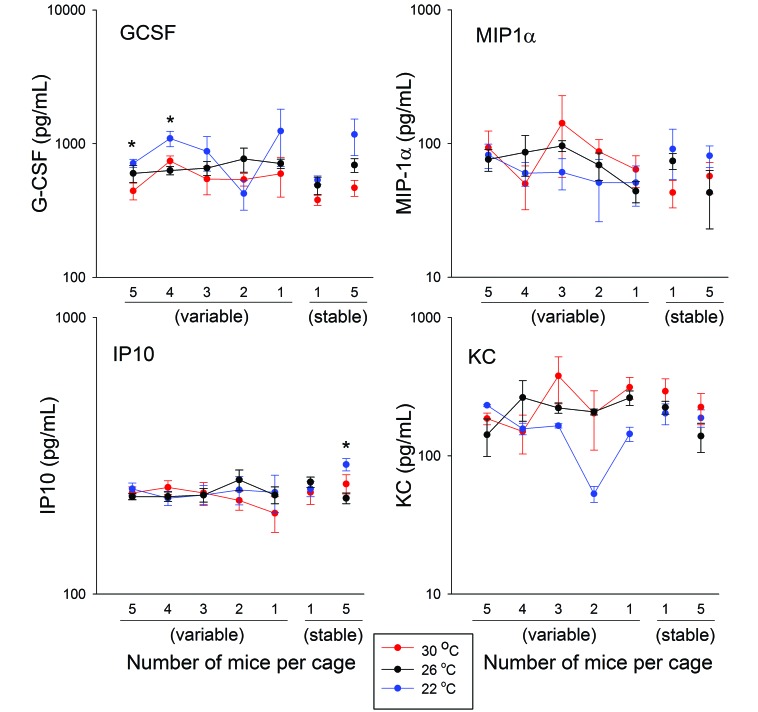
In general, serum cytokines and chemokines were not influenced by cage density at any of the 3 ambient temperatures. In the 5-to-1 cage, GCSF concentrations were significantly higher at 22 °C as compared with 30 °C when the cage contained 5 mice (P = 0.043) and at 22 °C as compared with 26 °C when the cage contained 4 mice (P = 0.032; Figure 6). Among mice house stably at 5 per cage, those at 22 °C had significantly (P = 0.016) higher IP10 concentrations than did those at 26 °C. Other analytes tested showed no significant effects.
Figure 6.
Serum cytokine and chemokine concentrations in mice housed at 1 to 5 per group under different ambient temperatures. Serum was collected at euthanasia for measurement of a panel of cytokines and chemokines. Concentrations of these substances were not significantly influenced by cage density at any of the 3 ambient temperatures. With regard to temperature, significant (*, P < 0.05) intergroup differences were detected sporadically.
Neither housing density nor ambient temperature significantly influenced fecal concentrations of corticosterone metabolites (data not shown).
Discussion
This study evaluated the interactive effects of 2 independent variables—ambient temperature and housing density—on the cage environment and facets of behavior and physiology in mice. Both of these variables both exerted significant influences on temperature, relative humidity, and dewpoint within the cage. With regard to the mice, food intake was significantly influenced by ambient temperature but not by housing density. Neither ambient temperature nor housing density affected core body temperature, locomotor activity, or concentrations of fecal corticosterone metabolites and most serum analytes. Under all test conditions, mice showed clear circadian rhythms of core temperature and locomotor activity on the day before cage change, with significant relative hyperthermia and behavioral activation during the light phase after cage change.
This work provides new data that document interactions between ambient temperature and mouse housing density with regard to altering both the internal cage environment and mouse physiology. Most notably, changing the number of mice in the cage significantly altered the cage environment at all 3 ambient temperatures tested. In addition, different ambient temperatures significantly influenced mouse metabolism, as reflected by food intake. Note that although we generally refer to effects related to housing density, our study as designed cannot distinguish between effects related to group size compared with housing density; with a fixed cage size, density will necessarily fall as group size is reduced. These 2 parameters have distinct influences on mouse aggression.28
Our current findings have important implications for studies that use group-housed mice but that reduce the number of mice in the cage as the study progresses. Our data show that the mice remaining in the cage may experience different environmental conditions (lower temperatures and dewpoints) than those housed under higher-density conditions. Such changes in the environmental steady-state in turn might influence food intake by requiring metabolic adjustment to a cooler temperature. Our data showed a significant effect of ambient temperature on food intake, but changes in group size, which also caused a decrease in cage temperature, did not alter food intake, perhaps because the temperature change was relatively small (changes of 4 to 8 °C in ambient temperature, as compared with 1 to 2 °C due to group size). In addition, we maintained the mice under the conditions associated with a reduction in group size for only 1 wk before assessment. Failure to detect significant effects of group size on mouse physiology and behavior might indicate that changes in mouse physiology related to either a change in housing density or a reduction in the number of mice in the cage may require longer than 1 wk, and perhaps longer than 5 wk, to develop.
The mice used in our study remained within National Research Council (NRC) recommendations for housing mice (77.4 cm2 [12 in.2] of floor space per mouse for 15- to 25-g mice)10 and received 18 in.2 per mouse when housed at 5 per cage and 90 in.2 per mouse when housed individually. Much of the published work on this topic has evaluated housing densities that exceed NRC recommendations, with few, if any, significant adverse effects. For example, a study of C57BL/6J mice compared 2 housing densities (10.3 and 5.7 in.2 per mouse) and found no differences in hematologic measures, plasma lipids and glucose, growth, bone mineral density, or percentage body fat between the 2 groups; however, the more densely housed mice had significantly smaller adrenal glands and lower heart rates and food intake.21 As in our current study, increased housing density in the previous study21 was associated with higher intracage temperatures. In another study, C57BL/6 mice were allocated at weaning to treatment groups in which cage density was either 5.5 in.2 or varied from 6 to 12 in.2, in accordance with NRC recommendations based on mouse weight.8 Food intake, growth rate, and fecal corticosterone were not consistently influenced by group size.8 Temperatures within the cages were not measured.8 However, our data suggest that housing density can alter the internal cage environment, thus indirectly affecting homeostatic burden. As in our current study, others have reported that the number of mice per cage and the housing density do not influence fecal corticosterone concentrations,8,9 although one group reported higher fecal corticosterone concentrations in mice that were housed in groups of 8.19
Although mice can regulate their core temperature over a relatively wide range of ambient temperatures, a substantial body of work indicates that they prefer ambient temperatures in the range of 26 to 29 °C (reviewed in reference 6). For example, a study of CD1, BALB/c, and C57BL/6 mice that were housed in a set of 2 connected cages, each maintained at a different temperature by using a water bath, indicated that overall both male and female mice prefer temperatures between 26 to 29 °C.2 Similarly, CD1 mice housed individually or in groups of 5 within a temperature gradient displayed circadian preferences in ambient temperatures, selecting temperatures of approximately 29 °C during the light phase and temperatures that were about 4 °C lower during the dark phase, when locomotor activity was relatively high.7 Furthermore, these preferences were influenced by the age of the mice.7 The goal of behavioral thermoregulation by mice is to minimize energy expenditure; their zone of thermal comfort falls between the thresholds for increased heat production and heat loss.6 Basal energy expenditure demands are about 45% lower in mice housed at 30 °C compared with 22 °C because mice housed at thermoneutrality do not allocate extra energy for heat production.22 One study used indirect calorimetry (to measure energy expenditure), thermography (to measure thermogenesis by interscapular brown adipose tissue), and positron emission tomography (to measure glucose uptake in brown adipose tissue) in C57BL/6J and Crl:NU-Foxn1(nu) nude mice at ambient temperatures of 21, 26, and 31 °C.1 As compared with mice housed at the 2 higher temperatures, both C57BL/6J and nude mice housed as 21 °C had significantly higher energy expenditure, a shift in metabolism toward glucose utilization, and significant activation of brown adipose tissue.1 These effects were greater in hairless nude mice than in haired C57BL/6J mice.1 The greater thermogenic demands and higher total energy expenditure of mice housed at 21 to 22 °C may mask or alter physiologically relevant changes in energy expenditure.22 Clearly, ambient temperature can affect the physiology of mice under conditions similar to those we tested. For example, the heart rates of WT and sympathodeficient mice were identical at an ambient temperature of 30 °C, whereas those of vagal-deficient mice were significantly higher.25 However, when the ambient temperature was 23 °C, heart rates in the sympathodeficient mice were lower than those of WT and vagal-deficient mice.25 In addition, behavioral and physiologic thermoregulatory adjustments in mice can be modified by bedding, drugs, chemicals, and pathologic conditions in a manner that interacts with the ambient temperature.5,6
With regard to the internal cage environment in our study, higher ambient temperatures were associated with higher internal cage temperature and lower relative humidity, whereas higher housing density also was associated with higher values for dewpoint. For people, air with a dewpoint of 20 °C is generally considered uncomfortable, and air with a dewpoint above 24 °C is perceived as ‘sticky,’ almost regardless of the actual air temperature and, thus, the relative humidity.15 When viewed from this perspective, our data suggest that the most comfortable conditions for mice, assuming some similarity to the human condition, occurred at an ambient temperature of 26 °C; under this condition, ambient temperatures within the cage ranged from 26 to 28 °C, relative humidity from 50% to 80%, and dewpoint from 15 to 23 °C. At the ambient temperature of 30 °C, the internal cage temperature was uniformly higher than the dewpoint, a situation that might contribute to an environment that felt both warm and humid. In contrast, at an ambient temperature of 22 °C, cage temperatures were lower than the dewpoint, suggesting that the cage felt relatively dry despite the high relative humidity. Under all 3 ambient temperatures, having fewer mice per cage was associated with lower internal cage temperatures, relative humidity, and dewpoint.
Accumulating data indicate significant effects of ambient temperatures on the immune responses of mice. For example, in a study conducted to evaluate the effect of ambient temperature (22, 26, and 30 °C) on influenza infection in C57BL/6J mice, we found that viral titers were equivalent regardless of ambient temperature; however, in that study, mice housed at 30 °C had less leukopenia and less cytokine induction than did mice maintained at 22 and 26 °C, respectively.11 These data are consistent with the increased level of GCSF we measured at 22 °C in the current study and suggest that less inflammation develops at the higher ambient temperature. Other observations indicate that antitumor immunity in mice is significantly influenced by ambient housing temperature.13
Standard housing temperature for laboratory mice in research facilities is mandated to be between 20 to 26 °C; however, these subthermoneutral temperatures cause mild chronic cold stress and require the activation of thermogenesis to maintain normal body temperature.13 Furthermore, the health of mice may influence these interactions. For example, in temperature-preference studies, tumor-bearing mice selected a higher ambient temperature than did nontumor-bearing mice.13 When mice are housed at 30 to 31 °C, as compared with 20 to 26 °C, they show reductions in tumor formation, growth rate, and metastasis in association with a more effective adaptive immune response.13 This immune response is characterized by significantly greater numbers of antigen-specific and activated CD8+ T lymphocytes and fewer myeloid-derived suppressor cells and regulatory T lymphocytes in the tumor microenvironment at the thermoneutral temperature.13 The data suggest that cooler environmental temperatures suppress antitumor immunity in mice.13 In addition, naïve and tumor-bearing mice housed at either a standard housing temperature or at a thermoneutral ambient temperature show significant phenotypic and functional differences among dendritic cell subsets, indicating that the housing temperature of mice can affect fundamental properties and functions of these cells.14
Although we detected some statistically significant changes in serum leptin concentrations, these changes were small in magnitude and may have limited physiologic effect. However, the increased leptin concentrations in the mice housed at 30 °C are consistent with their decreased food intake. Fecal corticosterone concentrations were not significantly influenced by either group size or ambient temperature, suggesting that the group sizes and temperatures experienced by these mice were not overtly stressful over the period of study.
In summary, our data document that the number of mice housed per cage influences the intracage environmental conditions, creating situations that might influence metabolism and the immune response. We conclude that investigators should be cautious when changing the number of mice housed in a cage over the course of a study, because these changes can alter the cage environment to which remaining mice are exposed.
Acknowledgments
We thank Michelle Randle for excellent technical assistance and Dr Steve Verhulst for advice on the statistical analysis. This work was supported in part by AALAS and by the Southern Illinois University School of Medicine.
References
- 1.David JM, Chatziiannou AF, Taschereau R, Wang H, Stout DB. 2013. The hidden cost of housing practices: using noninvasive imaging to quantify the metabolic demands of chronic cold stress of laboratory mice. Comp Med 63:386–391. [PMC free article] [PubMed] [Google Scholar]
- 2.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. 2012. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLOS ONE 7:e32799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaskill BN, Rohr SA, Pajor EA, Lucas JR, Garner JP. 2009. Some like it hot: mouse temperature preferences in laboratory housing. Appl Anim Behav Sci 116:279–285. [Google Scholar]
- 4.Gordon CJ. 1985. Relationship between autonomic and behavioral thermoregulation in the mouse. Physiol Behav 34:687–690. [DOI] [PubMed] [Google Scholar]
- 5.Gordon CJ. 2004. Effect of cage bedding on temperature regulation and metabolism of group-housed female mice. Comp Med 54:63–68. [PubMed] [Google Scholar]
- 6.Gordon CJ. 2012. Thermal physiology of laboratory mice: defining thermoneutrality. J Therm Biol 37:654–685. [Google Scholar]
- 7.Gordon CJ, Becker P, Ali JS. 1998. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol Behav 65:255–262. [DOI] [PubMed] [Google Scholar]
- 8.Horn MJ, Hudson SV, Bostrom LA, Cooper DM. 2012. Effects of cage density, sanitation frequency, and bedding type on animal wellbeing and health and cage environment in mice and rats. J Am Assoc Lab Anim Sci 51:781–788. [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt C, Hambly C. 2006. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group-housed males. Physiol Behav 87:519–526. [DOI] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 11.Jhaveri KA, Trammell RA, Toth LA. 2007. Effect of environmental temperature on sleep, locomotor activity, core body temperature, and immune responses of C57BL/6J mice. Brain Behav Immun 21:975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kingma B, Frijns A, van Marken Lichtenbelt W. 2012. The thermoneutral zone: implications for metabolic studies. Front Biosci (Elite Ed). 4:1975–1985. [DOI] [PubMed] [Google Scholar]
- 13.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, Repasky EA. 2013. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA 110:20176–20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokolus KM, Spangler HM, Povinelli BJ, Farren MR, Lee KP, Repasky EA. 2014. Stressful presentations: mild cold stress in laboratory mice influences phenotype of dendritic cells in naïve and tumor-bearing mice. Front Immunol 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence MG. 2005. The relationship between relative humidity and the dewpoint temperature in moist air. Am Meteorilog Soc 86:225–233. [Google Scholar]
- 16.Lightman SL. 2008. The neuroendocrinology of stress: a never-ending story. J Neuroendocrinol 20:880–884. [DOI] [PubMed] [Google Scholar]
- 17.Lodhi IJ, Sememkovich CF. 2009. Why we should put clothes on mice. Cell Metab 9:111–112. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa H, Okamura N. 2010. Coordinated regulation of circadian rhythms and homeostasis by the suprachiasmatic nucleus. Proc Jpn Acad Ser B Phys Biol Sci 86:391–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholson A, Malcolm RD, Russ PL, Cough K, Touma C, Plame R, Wiles MV. 2009. The response of C57BL/6J and BALB/cJ mice to increased housing density. J Am Assoc Lab Anim Sci 48:740–753. [PMC free article] [PubMed] [Google Scholar]
- 20.Olivier J, Johnson WD, Marshall GD. 2008. The logarithmic transformation and the geometric mean in reporting experimental IgE results: what are they and when and why to use them? Ann Allergy Asthma Immunol 100:333–337. [DOI] [PubMed] [Google Scholar]
- 21.Paigen B, Svenson KL, Von Smith R, Marion MA, Stearns T, Peters LL, Smith AL. 2012. Physiological effects of housing density on C57BL/6J mice over a 9-month period. J Anim Sci 90:5182–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravussin Y, LeDuc CA, Watanabe K, Leibel RL. 2012. Effects of ambient temperature on adaptive thermogenesis during maintenance of reduced body weight in mice. Am J Physiol Regul Integr Comp Physiol 303:R438–R448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. 2005. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 10:2193–2216. [DOI] [PubMed] [Google Scholar]
- 24.Rowland NE. 2007. Food or fluid restriction in common laboratory animals: balancing welfare considerations with scientific inquiry. Comp Med 57:149–160. [PubMed] [Google Scholar]
- 25.Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM. 2008. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol 294:H1581–H1588. [DOI] [PubMed] [Google Scholar]
- 26.Toth LA, Gardiner TW. 2000. Food and water restriction protocols: physiological and behavioral considerations. Contemp Top Lab Anim Sci 39:9–17. [PubMed] [Google Scholar]
- 27.Trammell RA, Verhulst S, Toth LA. 2013. Environmental perturbation, inflammation and behavior in healthy and virus-infected mice. Brain Behav Immun 33:139–152. [DOI] [PubMed] [Google Scholar]
- 28.Van Loo PLP, Mol JA, Koolhaas JM, Van Zutphen BFM, Baumans V. 2001. Modulation of aggression in male mice: influence of group size and cage size. Physiol Behav 72:675–683. [DOI] [PubMed] [Google Scholar]
- 29.Whittaker AL, Howarth GS, Hickman DL. 2012. Effects of space allocation and housing density on measures of wellbeing in laboratory mice: a review. Lab Anim 46:3–13. [DOI] [PubMed] [Google Scholar]
- 30.Wright-Williams SL, Courade JP, Richardson CA, Roughan JV, Flecknell PA. 2007. Effects of vasectomy surgery and meloxicam treatment on faecal corticosterone levels and behaviour in 2 strains of laboratory mouse. Pain 130:108–118. [DOI] [PubMed] [Google Scholar]