Abstract
Few studies have evaluated the long-term effects of providing environmental resources to mice. This consideration is important given that mice are often maintained in vivaria for months. We evaluated the effects of providing simple cage resources (wood wool, cotton nesting material, a plastic tunnel, and oat cereal) compared with standard housing (solid-bottom cage with hardwood chips) to group-housed adult male and female C57BL/6 and BALB/c mice (n = 20/sex/strain/group) over 6 mo to determine whether these resources had a lasting effect on animal physiology, anatomy, and behavior. Body weights increased in all groups over time but were proportionately higher in male and female BALB/c mice housed in resource-supplemented environments. Throughout the study, adding environmental resources had no effect on hematology and lymphocyte subsets, fecal corticoid metabolite levels, response to LPS injection, or dendritic spine length or density. Strain- or sex×environment-specific changes occurred in dark–light activity and thermal nociceptive responses. Dominant agonistic behaviors, abnormal conspecific sexual behaviors, and social nonagonistic behaviors demonstrated sex and strain×environment interactions such that fewer maladaptive social behaviors were noted in mice that were provided with environmental resources. This association was particularly evident in male mice of both strains in resource-supplemented environments. A small but significant increase in brain weight:body weight ratios occurred in mice in resource-supplemented environments. Under the conditions evaluated here, consistent use of simple environmental resources had a positive long-term effect on the behavioral wellbeing of male and female BALB/c and C57BL/6 mice yet minimally affected other aspects of murine physiology and neuroanatomy.
Discussions within the laboratory animal science community continue regarding tangible benefits to animals and science when providing environmental enrichments to laboratory rodents, and recent systematic evaluations have demonstrated that environmental enrichment is not used consistently by all facilities housing mice.42,55 In times of economic restraint, institutional commitment to providing high-quality environments for these species may waiver, particularly when the benefits of environmental enhancement are unclear and when underlying concerns regarding the effect of these measures on the outcome of the experiments are present.41,44 Furthermore, husbandry personnel may need to be convinced of the benefit of adding items to rodent cages, to make a consistent effort to do so. Because mice are the most commonly used mammal in biomedical research,22 the decision of whether and how to enhance murine environments has a profound effect on overall laboratory animal wellbeing, potentially affecting tens of millions of animals worldwide.
Both social and physical factors contribute to the quality of the environment experienced by mice. Mice are highly social species and should be housed in small groups whenever possible.62 Female and juvenile male mice typically are housed in small groups, but single housing of adult male mice is common in research facilities.42 Although agonistic behavior (aggression) is reported as the most common reason for single housing of male mice, strength-of-preference tests have demonstrated that male mice will work to have access to a conspecific, independent of the level of aggression experienced or their social status.64 Although agonistic interactions, food competition, and decreased allogrooming can occur in some social situations of mice, heat transfer and dispersion, discovery of food resources, and an ingrained sense of protection from predators are considered to be more important, beneficial parameters contributing to the need for social living in mice.6 This stance implies that social housing is a crucial factor contributing to murine wellbeing; however, provision of social groupings alone does not appear to be sufficient to address all rodent needs in laboratory environments.58,63
Many resources and cage additions, including running wheels, shelters, cage size, and nesting material, have been evaluated in various ways for their effects on laboratory mice.10,19,30,48,53,57,67 Of these, nesting material, particularly that made of paper or wood wool,61 and the provision of some type of shelter have consistently been demonstrated to be beneficial in studies as long as 8 wk by using environmental preference testing as the experimental outcome.46,53 Because mice are commonly housed in production and research facilities for 6 mo or longer, an important consideration is to ascertain whether the consistent use of simple cage resources, such as nesting material and shelters, continues to enhance the environment over time or whether mice rapidly become habituated to their presence, such that no benefit is accrued.
Precisely how environmental improvement achieves beneficial effects in mice is unknown. Some authors have proposed that the addition of items that promote species-typical behavior reduces anxiety and social stress and, by doing, so may also improve immune function.6 Standard assays for measuring these outcomes include indirect assessment of food consumption through alterations in body weight;59 evaluation of anxiety in the dark–light test;2,5 changes in response latency during thermal nociceptive testing (that is, hotplate latency);2 noninvasive monitoring of the hypothalamic–pituitary–adrenal axis by evaluation of fecal corticosterone metabolite levels;10 direct observation of behavior in open-field or other tests;59 and evaluation of changes in bone marrow function and ratios of lymphocyte subsets,3,6 particularly in the face of immune challenge, such as potency testing or LPS injection.43,59,66 Others authors have demonstrated that provision of complex environments to rodents, in which objects and food items are rotated on a daily basis, enhances cognitive development and sociability, which can be assessed by monitoring increases in brain weight;20,57 alterations in learning acquisition and memory retention;38 and changes in hippocampal neurogenesis and dendritic interconnections.19,45 Because the effect of minor changes in the environment likely is incremental and can be measured in many different ways, studies attempting to discern the effect of minor changes in cage environment need to use multiple measures.23 This multipronged approach also is important for predicting whether specific changes in routine mouse husbandry are likely to affect specific areas of research.
The literature is replete with reports of studies examining the effect of environmental enrichment on mice and their wellbeing. However, the term ‘environmental enrichment’ is confusing, poorly defined, and in connection with rodents, has been used to indicate anything from a standard solid-bottom cage with bedding substrate to large multidimensional holding units with cage furniture and food foraging items changed several times weekly. To avoid confusion in terminology, the term ‘resource-supplemented’ has been used to describe the simple food and environmental resources that were added to cages of study mice.
The aims of the current study were to use multiple, diverse assays to characterize the physiologic and behavioral effects of consistently providing simple and readily implemented environmental resources to male and female BALB/c and C57BL/6 mice for 6 mo, to determine the potential effect of these housing conditions on various types of research, and to enhance our understanding of how such environmental modifications affect animal wellbeing. These 2 mouse strains were selected because they both are commonly used in research but have different immune responses and emotional reactivity;29,65 these traits might yield different outcomes in a study examining alterations in behavior or physiology.
Materials and Methods
Animals.
BALB/cAnNCrl (that is, BALB/c) and C57BL/6NCrl (that is, C57BL/6) male and female mice were purchased from Charles River Laboratories (St Constant, Quebec, Canada) at 5 wk of age. Vendor health surveillance reports indicated that the mice were free from mouse adenovirus, mouse hepatitis virus, mouse parvoviruses, mouse rotavirus, mouse norovirus, Theiler murine encephalomyelitis virus, Bordetella bronchiseptica, Citrobacter rodentium, Corynebacterium kutscheri, Mycoplasma pulmonis, Salmonella spp., Helicobacter spp., Klebsiella spp., Pasteurella spp., Staphylococcus aureus, Streptococcus spp., ectoparasites, endoparasites, and enteric protozoa. A total of 160 mice (20 mice per strain per sex per housing paradigm). For the week prior to study initiation, mice were acclimated to a 12:12-h reversed light cycle (lights off, 0700). Mice were randomized on arrival and housed in same-sex groups of 5 animals per in standard polycarbonate cages (16.5 × 29.85 × 12.7 cm [6.5 × 11.75 × 5 in.]) with wire lids and corncob bedding (no. 7092, Harlan, Mississauga, Ontario, Canada) but without additional items.
After photoperiod acclimation, cages of mice were randomly assigned to 1 of 2 environments: cages containing contact hardwood chip bedding only (that is, the standard environment) or cages containing contact hardwood chip bedding, cotton nesting material (Nestlet, Ancare, Belmore, NY), a clear amber tube (BioServ, Flemington, NJ) and 10 g of aspen wood wool (Tapvei, Kiili, Estonia; that is, the resource-supplemented environment). Individual mice were numbered from 1 to 5 in each cage, and identified by ear punch. Cage size for this study was 1098 cm2 (67 in.2), which provided 213 cm2 (13 in.2) per mouse. Food (Teklad Global 14% Protein Rodent Maintenance Diet, Harlan) and water were provided free choice. On a weekly basis, cages were changed, amber tubes were autoclaved, and new squares of cotton nesting material were provided. New wood wool was provided every 3 wk during cage changing, to minimize weekly pheromone disruption. The University of Guelph Animal Care Committee approved all experiments and procedures prior to study initiation. Animal research and facilities at the University of Guelph are in compliance with the Animals for Research Act of Ontario and adhere to the guidelines of the Canadian Council on Animal Care.11,47
Clinical observations and body weight.
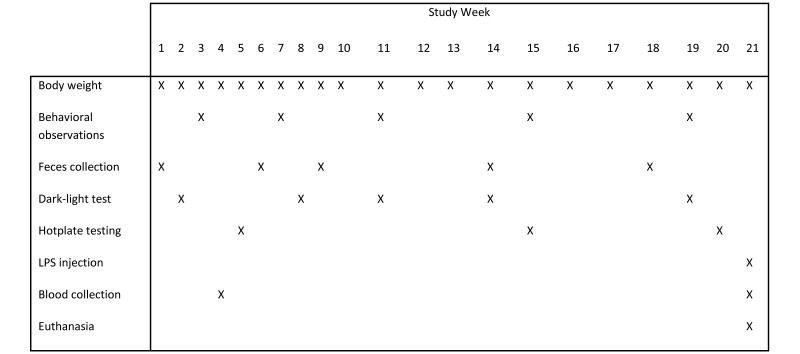
Individual body weights were collected weekly and clinical observations recorded daily (Figure 1). Because abundant nesting material can make daily observation of mice difficult, a food reward in the form of a single piece of toasted oat cereal (Cheerios, General Mills, Mississauga, Ontario, Canada) was offered individually to each mouse in the resource-supplemented housing condition 3 times weekly to enhance clinical observation.
Figure 1.
Study timeline.
Behavioral observations.
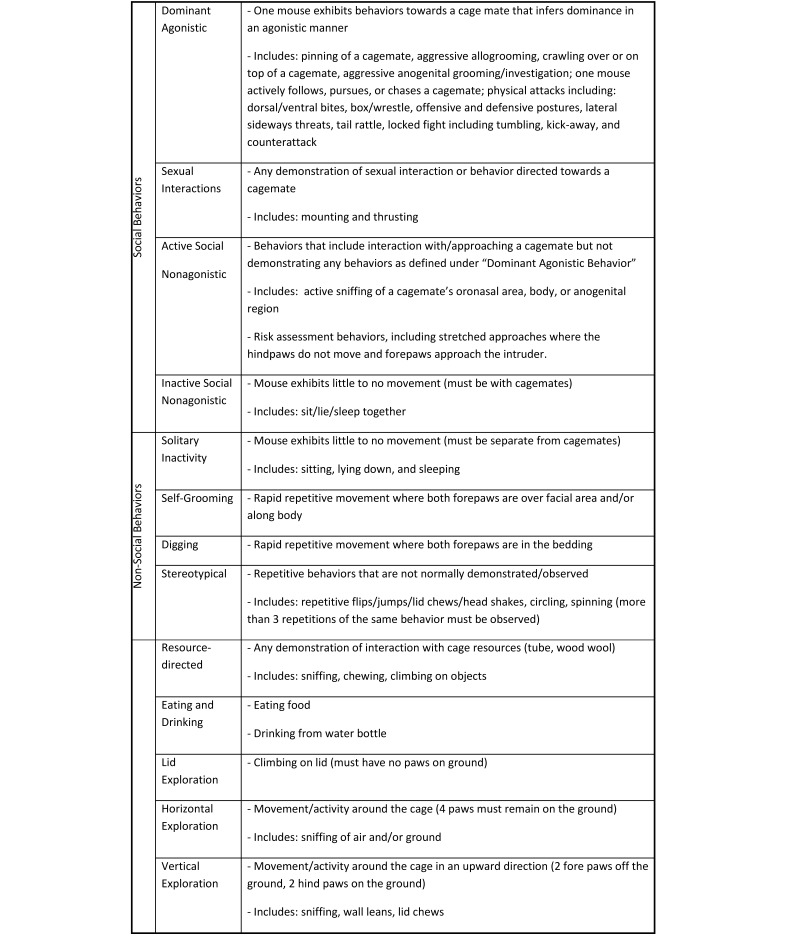
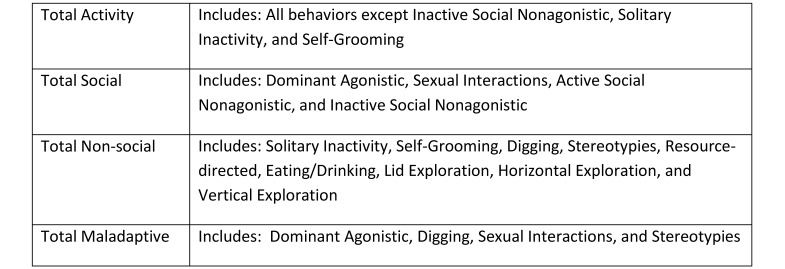
Monthly, mice were videotaped in their home cages for 30 min, beginning 1 h into the dark phase. Videotapes were assessed individually and scored through continuous observation by a trained observer using Observer Video Analysis Software (Noldus Information Technology, Wageningen, the Netherlands) and an ethogram28 (modified from those in references 12 and 14; Figure 2). No a priori assumptions were made about various behaviors when ethogram data were collected, because this process was intended to yield a general understanding of how and whether mouse behavior was altered by environment. Overlapping grouped behaviors (total activity, total social, total nonsocial, and total maladaptive) were calculated (Figure 3; modified from references 12 and 13) to assess potential overall nonspecific effects of housing paradigm. For example, digging was categorized as an active, nonsocial, and potentially maladaptive54 behavior. Sexual interactions were categorized as both a social and potentially maladaptive behavior, in light of the clinical signs that were noted in BALB/c male mice during the study. Fighting (dominant agonistic) was classified as both social and maladaptive behavior, whereas stereotypies were considered to be both nonsocial and maladaptive behaviors. This technique was used to assess the relative contribution of each component behavior to the overall observations within each category to determine whether any significant changes were due to a single, multiple, or all component behaviors.
Figure 2.
Definition of behaviors for scoring.
Figure 3.
Definitions of grouped behaviors.
Dark:light test.
Dark:light tests were conducted at weeks 2, 8, 11, 14, and 19 during the first 5 h of the dark phase. Facing away from the opening, individual mice were placed on the dark-covered half of a 40 × 40 × 30.5 cm acrylic open-field apparatus (AccuScan Instruments, Columbus, OH) and given 5 min to explore the entire arena. All sides of the apparatus are fitted with 16 infrared light beams 1 cm from the floor, and 2 opposite sides are fitted with a second level of beams 7 cm above the floor level. Vertical and horizontal activity were measured when a moving animal interrupted the beams. Latency to enter the light compartment, total activity, and both the duration of time spent and the distance traveled in both the light and dark compartments were measured by using Versamax Analyzer software (AccuScan Instruments).
Thermal nociception testing.
Nociceptive responses were evaluated prestudy and during weeks 5, 15, and 20 by using a 50 °C hotplate test (model no. LE7406, Letica Scientific Instruments, Barcelona, Spain). This hotplate temperature is not associated with thermal injury in mice.32 This evaluation was run as a 2-d test, and reaction times were measured 30 min after administering saline (1 mL/kg SC) on day 1 or morphine (10 mg/kg SC; morphine sulfate, Sandoz, Boucherville, Quebec, Canada) on day 2. Testing occurred during the first 4 h of the dark phase. The endpoint used was licking or shaking a paw or jumping. An arbitrary cutoff time of 80 s was adopted. If no endpoint was achieved, a latency of 80 s was assigned, at which time the mouse was removed from the hotplate.
Fecal corticoid metabolites.
For fecal corticoid determination, at weeks 1, 6, 9, 14, and 18, all feces produced during a light or dark period over 12 h were collected and weighed. Samples were frozen at –20 °C until extracted. Extraction followed a published technique.18 Briefly, samples were dried for 2 h at 30 °C, weighed, and pulverized, and a 0.2-g sample was removed for extraction. To the fecal sample, 0.8 mL water and 5 mL of dichloromethane were added; and samples were vortexed for 30 s total in 5-s pulses. Samples then were centrifuged for 15 min at 1690 × g. The bottom dichloromethane fraction was transferred and washed with 1 mL of 0.1 M NaOH by vortexing for 10s, followed by centrifugation for 10 min at 1690 × g. The dichloromethane fraction was transferred and washed twice with water, centrifuged, and again transferred to a fresh tube. Of the final dichloromethane fraction, 1 mL was transferred and evaporated to dryness under N2 for approximately 15 min and then stored at –20 °C until analyzed. Samples were resuspended in 1 mL of 95% ethanol, vortexed, and diluted 1:25 with assay buffer (Correlate EIA Kit, Assay Designs, Ann Arbor, MI). Concentrations of fecal corticoids was determined according to manufacturer's instructions. ELISA plates were read at 405 nm (PowerWave XS, BioTek). Concentration was determined as % bound by using a standard curve ranging from 32 to 20,000 pg/mL (kit sensitivity, 27 pg/mL). Values were expressed relative to the total feces collected over a time period and as ng corticosterone per g of feces. The assay kit has 28.6% crossreactivity with deoxycorticosterone or desoxycorticosterone, metabolites of corticosterone. Therefore, the values measured and reported largely represent corticosterone and these metabolites. Although the term ‘fecal corticoid metabolites’ more accurately reflects the assay outcome, the term ‘total corticosterone’ is used in figures for the sake of brevity. All samples were run in duplicate, and samples from different test periods were randomized to ELISA plates.
The intraassay coefficient of variation was 3.3%, and the interassay coefficient of variation was 5.8%. Linear regression performed on the standard concentration to percentage of corticoid bound demonstrated excellent linearity (R2 ≥ 0.99 with an S.D. of 0.003%).
Hematology and lymphocyte subset analyses.
Blood (0.1 mL) was collected from the saphenous vein during week 4 and by cardiocentesis immediately after euthanasia at study end and into tubes containing EDTA anticoagulant. PCV, Hgb, WBC absolute and differential counts, RBC count, and platelet count were determined by using EDTA-treated blood diluted 1:1 in physiologic saline in an automated hematology analyzer using preset parameters for mouse blood (ADVIA 2120, Siemens, Erlangen, Germany). Immediately after euthanasia, the femoral bone marrow cavity was flushed with 0.4 mL of EDTA in saline and collected into 0.5-mL tubes. Bone marrow suspensions were analyzed in an automated hematology analyzer as described earlier, and smears were prepared and stained (Wright stain), from which 500 nucleated hematopoietic cells were counted and differentiated. Erythrocytes in the remaining blood and bone marrow samples were lysed by treatment with ammonium chloride buffer, and leukocytes were labeled with fluorochrome-conjugated antibodies against CD4/CD8 (clones YTS191.1/KT15, Serotec, Cedarlane, Hornby, Ontario, Canada) and CD3/CD19 (clones 6D5/KT3, Serotec). Control samples were labeled with an isotype-matched antibody to an irrelevant epitope (IgG antibody, clone DC037, Serotec). All samples were analyzed in a flow cytometer (FACScan, BD Biosciences, Mississauga, Ontario, Canada) using CellQuest software (BD Biosciences); flow cytometry buffer comprised PBS containing 1m M EDTA, 1% horse serum, and 0.1% Na azide, pH 7.35 (all chemicals from Sigma, St Louis, MO).
LPS injection.
At the end of week 20, half of the mice from each paradigm were randomly selected and injected I.P. with 10 μg LPS (Sigma) in 0.5 mL of sterile water. The remainder of the mice received 0.5 mL of sterile water intraperitoneally. The LPS dose was selected to induce neutrophil and monocyte migration without inducing significant clinical signs.36
Dendritic spine detection and counts.
At 24 h after injection of LPS or water, all mice were euthanized by CO2 inhalation. After euthanasia, brains were removed, weighed for brain:body ratio determination, and placed in Golgi–Cox solution (1% potassium dichromate, 0.8% potassium monochromate, 1% mercuric chloride; Sigma) for 72 h in the dark. Tissue processing followed a previously published protocol.26 Briefly, tissues were placed in 20% sucrose solution for 48 h in the dark at 4 °C, sectioned (200 μm) by using a vibrating microtome (VT1000s, Leica Microsystems, Richmond Hill, Ontario, Canada), and stored in 6% sucrose at 4 °C. Sections of fixed brains were mounted on 3% gelatin-coated slides, and slides were air-dried at room temperature for approximately 3 h. Slides were developed in double-distilled water for 1 min, followed by 1% NH4OH in double-distilled H2O for 15min, double-distilled H2O for 1min,1% Kodak Rapid Fix (Part A; catalog no. P7542-IGA, Sigma) for 15 min, and finally double-distilled H2O for 1min. Tissues were dehydrated sequentially through 70% ethanol, 95% ethanol, 100% ethanol twice, and xylene, each for 30 s. Slides were coverslipped, allowed to air dry overnight, and coded prior to evaluation. The density and average length of dendritic spines on pyramidal neurons within the hippocampal CA1 region were evaluated. For neuronal analysis, neurons had to be parallel to the rostral–caudal plane between –2.155 and –3.08 mm from bregma and were relatively isolated from other impregnated cells; in addition, the dendritic arbor had to demonstrate consistent and dark stain impregnation along its entire extent. From each mouse, 3 to 5 dendrites from each of the right and left hemispheres were selected for analysis.
Statistical analyses.
Because the effect of environment was the primary factor of interest and because mice were grouped within environments, statistical evaluations were conducted at the cage level. Data initially were evaluated for normality and homogeneity of variance (sphericity). For all data, planned comparisons were achieved by evaluating the effects of environmental resources in each sex–strain group.50 ANOVA was used to assess the effects of environmental resources, sex, strain, and time on body weight, dark–light testing, thermal nociception, fecal corticoid metabolites, hematology, and dendritic spine parameters. Holm–Bonferroni correction was applied to comparison series to account for multiple comparisons.1 Post hoc Tukey tests were used when significant interactions were found. Significance was set at a P value less than 0.05. Data were analyzed by using SPSS 19.0 software (IBM, Chicago, IL).
Results
Body weight.
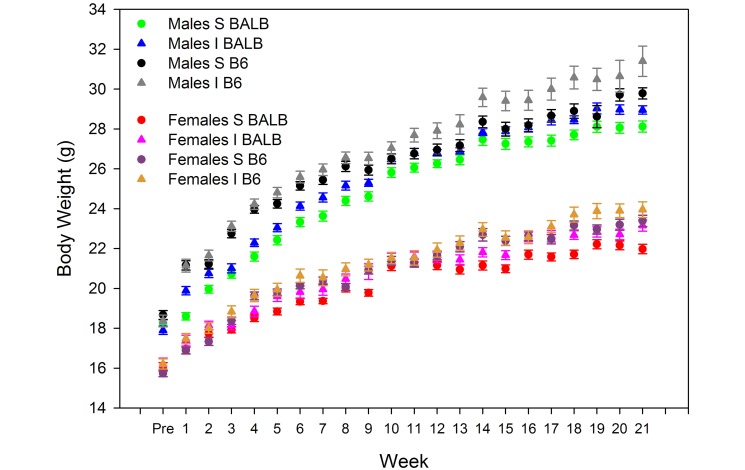
Planned comparisons indicated that animals in the resource-supplemented housing weighed more than did those maintained in standard housing for both female BALB/c mice (F1,6 = 7.42, P = 0.034) and male (F1,6 = 16.49, P = 0.007), but no significant effects of environment were seen for body weights of C57BL/6 mice of either sex. In addition, ANOVA showed significant main effects of sex (F1,24 = 780.66, P < 0.001) and strain (F1,24 = 30.98, P < 0.001), with higher body weights noted in male mice and in C57BL/6 mice (Figure 4). Body weight increased over time in all mice (F22,528 = 861.55, P < 0.001).
Figure 4.
Effect of housing environment on mean (± SE) body weight (g) over time in male (M) and female (F) BALB/c and C57BL/6 mice housed in improved (I) or standard (S) environments.
Clinical observations.
Mice in resource-supplemented housing readily adapted to the food resource, and 100% of mice accepted the oat cereal each time it was offered throughout the study period. Almost all mice survived to study end (that is, 3 mice were found dead, and 1 mouse was euthanized), and no mice in either housing paradigm required separation from cagemates because of aggression. Stereotypic behavior (circling, back flips, or ritualistic pacing) was noted in a single standard-housed male BALB/c mouse in month 4, a single standard-housed female C57BL/6 mouse in month 4, and a single resource-supplemented female C57BL/6 mice in month 4, suggesting no particular consistent trend or effect of environment. Unusual sexual behavior, consisting of multiple attempts at mounting or intromission exceeding 30 to 50 per 10-min period was noted during month 3 in 2 cages of standard-housed male BALB/c mice, during month 5 in 2 cages of standard-housed female C57BL/6 mice, during month 4 in 2 cages of resource-supplement-housed male BALB/c mice, and during month 3 in a single cage of resource-supplement-housed female BALB/c mouse, again suggesting no particular trend by housing group. The mouse initiating the unusual sexual activity within a particular cage changed over time, such that only one animal demonstrated the behavior in a cage at a given time. In the cages in which this behavior occurred, the rectums of recipient male mice were noted to be moderately to markedly erythematous and edematous, but overt rectal prolapses did not occur. Agonistic behaviors were noted in one cage of standard-housed male BALB/c mouse during month 1, in 3 cages of standard-housed male C57BL/6 mice during month 3, in 2 resource-supplemented cages of male C57BL/6 mice during month 1, and in one cage of standard-housed female C57BL/6 mice during month 1. None of these findings required separation of animals.
Minor bite wounds and other minor lacerations interpreted to arise secondary to agonistic interactions were noted in a single standard-housed male BALB/c mice during months 3, 4, and 5 (different cages) and a single resource-supplemented male BALB/c mouse in month 5. Focal to multifocal areas of dorsal alopecia (barbering) were noted in a single cage of resource-supplemented male C57BL/6 mice during month 3, in 4 resource-supplemented female C57BL/6 mice in month 3 (separate cages), and in a single resource-supplemented female C57BL/6 mice in month 5. All mice remained in their original groupings and did not require separation (that is, because of serious wounds and lacerations).
A single female BALB/c mouse in the resource-supplemented group appeared mildly ataxic and thin at the beginning of the study. During the first month, that mouse received intraperitoneal saline on several occasions, as well as moistened rodent chow. This mouse exhibited repetitive circling activity during month 4, was subsequently euthanized and the data was excluded from from the study.
Throughout the study, 3 mice were found dead (necropsy did not reveal a specific cause of death), and one male BALB/c mouse was euthanized because of a nonhealing skin wound.
Observation of homecage behavior.
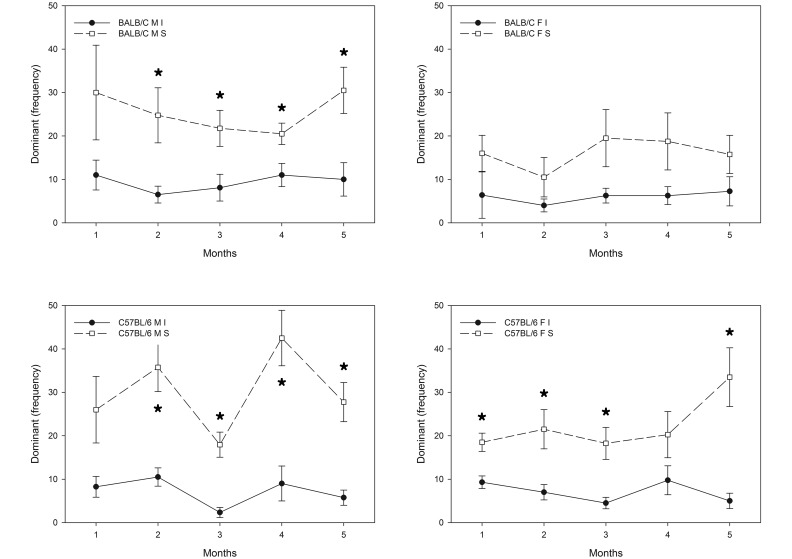
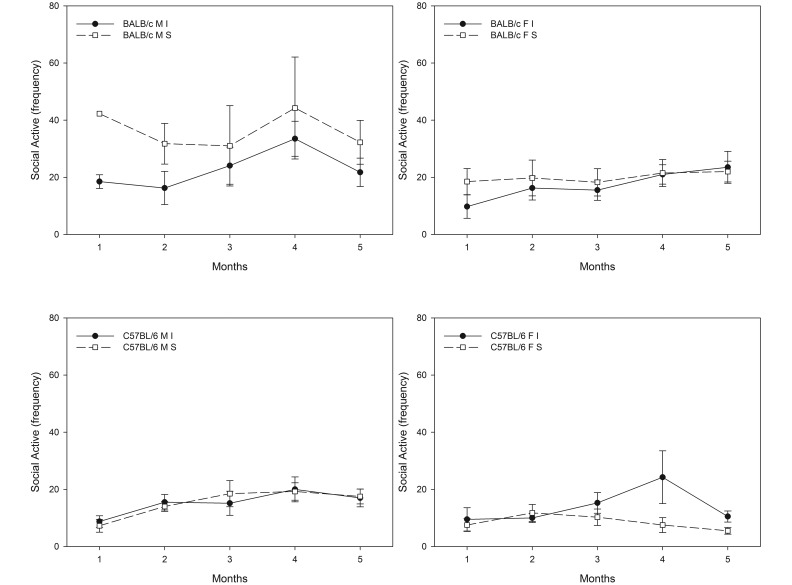
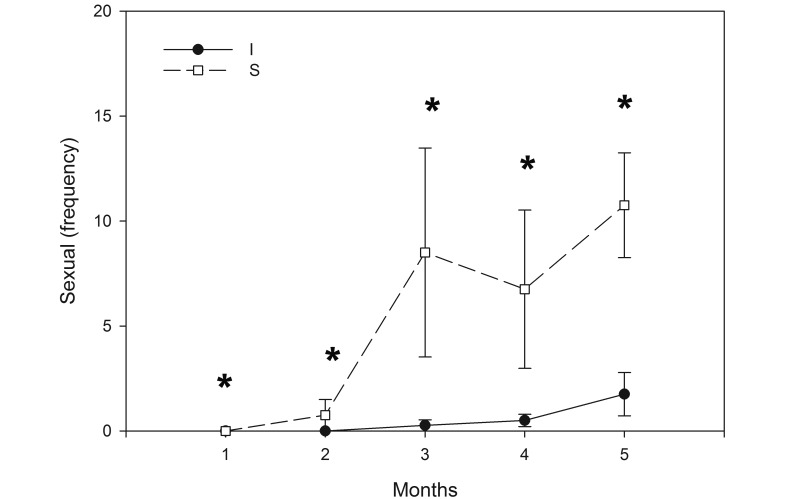
Of the social behaviors evaluated, dominant agonistic, sexual interactions, and active social nonagonistic behaviors demonstrated significant sex×environment (F1,22 = 11.701, P = 0.002; F1,22 = 20.549, P < 0.001; F1,22 = 5.166, P = 0.033, respectively] and strain×environment (F1,22 = 19.880, P < 0.001; F1,22 = 2.998, P = 0.004) interactions (Figures 5 through 7). Specifically, provision of a resource-supplemented environment reduced dominant agonistic and active social nonagonistic behaviors (Figures 5 and 7) in male (F1,15 = 62.430, P < 0.001; F1,15 = 24.505, P < 0.001, respectively), female mice (F1,15 = 44.660, P < 0.001; F1,15 = 13.912, P = 0.002, respectively], and in male and female BALB/c mice (F1,15 = 25.559, P < 0.001; F1,15 = 12.973, P = 0.003) and C57BL/6 mice (F1,15 = 95.838, P < 0.001; F1,15 = 15.716, P = 0.001; Figures 5 and 7). BALB/c males housed in resource-supplemented environments showed fewer conspecific repetitive sexual interactions (Figure 6) than did standard-housed BALB/c male mice (F1,4 = 10.955, P = 0.030).
Figure 5.
Effect of environment on dominant agonistic behaviors over time. (A) Frequency of dominant agonistic behaviors between improved- and standard-housed male BALB/c mice over time (improved [I] < standard [S]; F1,7 = 15.822, P = 0.007, months 1 through 5). (B) Frequency of dominant agonistic behaviors between improved- and standard-housed male C57BL/6 mice over time (I < S; F1,7 = 166.593, P < 0.001, months 1 through 5). (C) Frequency of dominant agonistic behaviors between improved- and standard-housed female BALB/c mice over time (I < S; F1,7 =19.996, P = 0.004, months 1 through 5). (D) Frequency of dominant agonistic behaviors between improved- (I) and standard-housed (S) female C57BL/6 mice over time (I < S; F1,7 = 47.019, P < 0.001, months 1 through 5). *, P < 0.01 between groups.
Figure 7.
Effect of environment (I, improved; S, standard) on active social nonagonistic behavior over time. (A) Frequency of social active nonagonistic behaviors between improved- and standard-housed (S) male BALB/c mice over time (I < S; F1,7 = 41.317, P = 0.001, months 1 through 5). (B) Frequency of social active nonagonistic behaviors between improved- and standard-housed male C57BL/6 mice over time (I > S; F1,7 = 8.042, P = 0.030, months 1 through 5).(C) Frequency of social active nonagonistic behaviors between improved- and standard-housed female BALB/c mice over time (I < S; F1,7 = 10.651, P = 0.017, months 1 through 5). (D) Frequency of social active nonagonistic behaviors between improved- and standard-housed female C57BL/6 mice over time (I < S; F1,7 = 7.308, P = 0.035, months 1 through 5).
Figure 6.
Effect of environment (I, improved; S, standard) on sexual behaviors in male BALB/c mice over time. (I < S, F = 9.640, P = 0.021, months 1 through 5). *, P < 0.02 between environments.
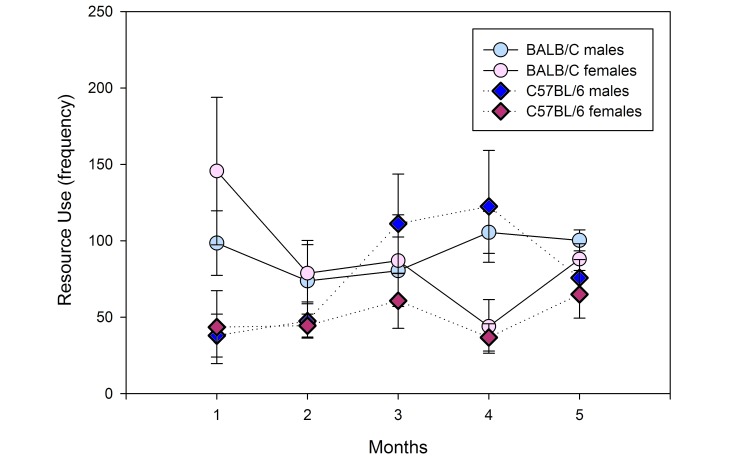
Of the nonsocial behaviors, both self-groom and eat–drink demonstrated significant strain×environment interaction (F1,22 = 12.325, P = 0.002; F1,22 = 6.319, P = 0.020). Provision of a resource-supplemented environment led to increased self-grooming in both sexes of BALB/c (F1,15 = 23.348, P < 0.001) and C57BL/6 (F1,15 = 15.697, P = 0.001) mice, whereas housing in the resource-supplemented environment significantly increased eat–drink behavior in female C57BL/6 mice only (F1,15 = 12.786, P = 0.003). Although no significant sex- or strain-associated differences in item use were noted over time, all mice in resource-supplemented housing continued to interact with cage resources at the same level of intensity throughout the 6-mo study (Figure 8). Compared with standard-housed C57BL/6 male mice, male C57BL/6 mice housed in the resource-supplemented environment showed more stereotypic behavior and they also were more active. The significant increase in stereotypic behavior remained after ANCOVA was used to control for total activity, indicating that this increase was not dependent on the increase in total activity (F1,8 = 8.55, P = 0.033).
Figure 8.
Frequency of interactions of mice in improved housing with nesting material and tunnels over time. No significant sex×time or strain×time interactions were noted, and all mice continued to use resources at the same frequency over time.
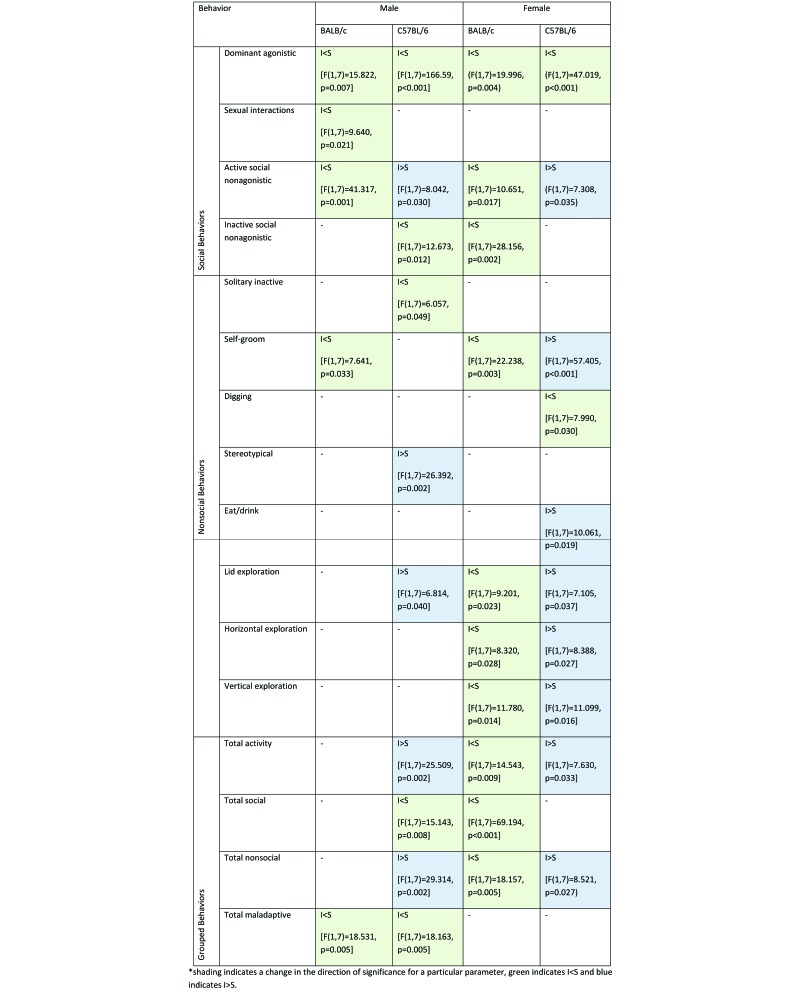
Significant overall effects of environment on frequency of observed behaviors are summarized in Figure 9. Except for dominant agonistic behaviors, which were significantly decreased across time in all groups of mice in resource-supplemented environments, there were no consistent changes in overall patterns of behaviors for male and female mice of both strains. This result perhaps occurred because some behaviors were noted only in specific groups of mice (for example, repetitive sexual activity was noted in male BALB/c mice only and was lower in resource-supplemented environments) or because some behaviors are known to be specific to mouse strain or sex (for example, basal locomotion and exploration are more common in C57BL/6 mice than BALB/c mice).7,14
Figure 9.
Significant effects of environment (I, improved; S, standard) on behaviors.
Dark–light test.
Planned comparisons demonstrated that male C57BL/6 mice traveled significantly farther in the light compartment when housed in resource-supplemented environment (F1,6 = 7.77, P = 0.032). No other significant differences were noted between the resource-supplemented- and standard-housed mice for each sex–strain combination in either the dark or light compartment. Male mice spent more time (F1,24 = 38.70, P < 0.001) and traveled further in both the light (F1,24 = 25.19, P < 0.001) and dark (F1,24 = 12.75, P = 0.002) compartments than did female mice. BALB/c mice spent more time in the dark than did C57BL/6 mice (F1,24 = 28.59, P < 0.001). In addition, sex×strain interactions indicated that male C57BL/6 mice spent more time (F1,24 = 5.764, P = 0.024) and traveled further in the light compartment (F1,24 = 22.517, P < 0.001) than did any other sex–strain combination. There was a main effect of month on all parameters (all F > 6.61, all P < 0.001), with more time spent in the dark compartment for all groups over time, as well as month×sex interactions for duration in light and dark (F4,96 = 7.78, P < 0.001) and distance traveled in light (F4,96 = 7.22, P < 0.001). Overall, male and C57BL/6 mice traveled farther in the light compartment and spent less time in the dark compartment than did female and BALB/c mice, respectively.
Fecal corticoid metabolites.
Housing environment had no effect on fecal corticoid metabolite levels for either sex or strain and in either phase of the photoperiod. A significant main effect of sex (F1,24 = 7.196, P = 0.013) was found; female mice had higher fecal corticoid metabolite levels in the light phase than did male mice (F1,24 = 7.196, P = 0.013). In addition, a significant sex×strain interaction (F1,24 = 4.446, P = 0.046) indicated that BALB/c mice had higher fecal corticoid metabolite levels than did C57BL/6 mice in both the light (F1,24 = 72.176, P < 0.001) and dark (F1,24 = 12.862, P = 0.001) phases. Fecal corticoid metabolite levels varied by month (F4,96 = 2.773, P < 0.031), peaking at month 2 and then fell steadily until study end. Concentrations were consistently higher in the dark phase compared with the light phase (F1,24 = 133.021, P < 0.001).
Thermal nociceptive testing.
The difference in hotplate latency between saline and morphine treatment was significantly greater in male (F1,6 = 19.65, P = 0.004) and female (F1,6 = 6.57, P = 0.043) BALB/c mice in resource-supplemented environments but not in C57BL/6 mice. In addition, BALB/c mice demonstrated greater between-treatment differences in latency than did C57BL/6 mice (F1,24 = 188.66, P < 0.001). Environment×sex (F1,24 = 6.51, P = 0.017), sex×strain (F1,24 = 18.40, P < 0.001), sex×strain×environment (F1,24 = 24.25, P < 0.001), and month×sex (F2,48 = 3.55, P = 0.037) were also detected. Male C57BL/6 mice had an greater hotplate latency in response to morphine than did female C57BL/6 mice (F1,14 = 13.23, P = 0.003). Female BALB/c mice in both standard (F1,6 = 152.24, P < 0.001) and resource-supplemented (F1,6 = 134.90, P < 0.001) housing showed a longer response latency after morphine than did female C57BL/6 mice, whereas only male BALB/c mice in standard housing had longer latencies after morphine compared with their C57BL/6 counterparts (F1,6 = 38.32, P = 0.001).
Brain weight:body weight ratio.
Because LPS did not exert significant main effects on ratios of brain weight to body weight, data from LPS-treated mice were included in the overall statistical analyses of brain weight to body weight ratios. Increased brain weight:body weight ratios occurred in resource-supplemented mice across all mice (F1,24 = 224.90, P < 0.001) and in each sex–strain group (all F > 5.80, all P < 0.002). In addition, brain weight:body weight ratios were higher in BALB/c mice than in C57BL/6 mice (F1,24 = 5.61, P = 0.026).
Hematology.
No significant main effects of LPS were seen on neutrophil or lymphocyte counts in any group, so data from LPS-treated mice were included in the overall statistical analyses of hematologic measures. Planned comparisons demonstrated that housing environment had no significant effect on neutrophil or lymphocyte counts in any group. Male mice had higher neutrophil (F1,24 = 9.68, P = 0.005) and lower lymphocyte (F1,24 = 10.29, P = 0.004) counts than did female mice, and BALB/c mice had higher neutrophil (F1,24 = 6.23, P = 0.020) and lymphocyte (F1,24 = 28.84, P < 0.001) counts than did C57BL/6 mice. In addition, both neutrophil and lymphocyte counts increased between the first and second samples (F1,24 = 54.217, P < 0.001; F1,24 = 107.29, P < 0.001, respectively).
Lymphocyte subset markers.
Mice that received LPS displayed a ruffled hair coat within 2 h of injection but appeared clinically normal by 24 h after treatment. The administration of LPS had no significant main effect on CD4:CD8 or CD19:CD3 ratios, and mice given LPS were included in subsequent analyses of lymphocyte subsets. Planned comparisons found no significant effect of housing conditions in any sex–strain combination. However, BALB/c mice had higher CD19:CD3 ratios than did C57BL/6 mice (F1,24 = 5.81, P = 0.024), and these ratios decreased between the first and second samples (F1,24 = 67.80, P < 0.001).
Dendritic spine length and density.
LPS injection had no significant effect on dendritic spine length or density, and data from mice given LPS were included in subsequent analyses of these parameters. No significant effects of environment or strain were observed for this measure. Standard-housed male mice had longer dendritic spines (F1,24 = 12.517, P = 0.001 but no change in density as compared with resource-supplemented male mice. Female mice demonstrated greater dendritic spine density than did male mice (F1,24 = 14.322, P < 0.001).
Discussion
Behavioral observations proved to be of greatest value for determining the long-term effect on animal wellbeing of minor changes to the intracage environment. The provision of cage resources to mice had a significant effect on the frequency of several observed behaviors of social interaction, although these effects were not always consistent across strains. For example, active social nonagonistic behaviors decreased in male and female BALB/c mice housed in resource-supplemented environments but increased in C57BL/6 mice housed in the same environment. An overall decrease in agonistic behaviors was seen across both sexes and strains of mice held in resource-supplemented environments. Other studies have demonstrated increased agonistic interactions in male mice that were given highly valued but limited resources, such as running wheels.31,44 In the current study, items were provided in sufficient quantity to be used by all animals in a group, such that no or minimal resource restriction was perceived.
Cage resource supplementation induced several strain- or sex-specific behavioral differences as well. For example, resource-supplemented environments resulted in a decrease in total activity for female BALB/c mice, whereas the opposite occurred for female C57BL/6 mice. Male and female C57BL/6 mice housed in resource-supplemented environments generally displayed more exploratory behaviors throughout the course of the study, although this pattern included an increase in the frequency of stereotypic behaviors for male C57BL/6 mice. These results may reflect basal strain behavioral characteristics, given that C57BL/6 mice generally display higher basal levels of locomotion than do BALB/c mice.8,15,20 Although the frequency of social behaviors increased in both male and female BALB/c mice housed in resource-supplemented environments, the provision of environmental resources did not have the same overall effect for the C57BL/6 mice, which are a more social strain than are BALB/c mice.4,20,21 In addition, male BALB/c mice in resource-supplemented environments demonstrated fewer repetitive sexual behaviors than did standard-housed mice, a finding that correlated clinically to less rectal irritation. Individual sex- and strain-associated differences in behaviors have been reported for several strains of inbred mice and were not unexpected.2,4,7,9,15,21 Overall, these findings indicate that neither male nor female mice nor mice of different genotypes appear to habituate to the presence of simple environmental resources in the form of nesting materials, a tunnel, and a periodic food reward, and these objects seem to continue to exert reduce agonistic and abnormal repetitive sexual behaviors even after months of continuous supplementation.
Studies examining the short-term effects of environmental resources on mouse physiology have reported variable effects on the body weights of mice housed in standard compared with resource-supplemented cage conditions that depended on the nature of the item provided.46,59 For example, consistent with the effects noted in BALB/c mice in the current study, provision of increased nesting material has been associated with increased body weights in male and female BALB/c mice.60 These changes have been attributed to increased thermal comfort and decreased metabolic energy expenditure required to maintain the core body temperature.24 Changes in body weight in response to provision of nesting material have not been observed consistently across mouse strains24 and were not seen in C57BL/6 mice in the current study. The finding that body weight did not consistently increase between standard and resource-supplemented housing across strains in the current study also indicated that the use of toasted oat cereal to enhance clinical observations of mice during this study (or potentially when used for positive reinforcement in other studies) likely had no effect on overall body weight or the growth of mice receiving the food treat. Although female C57BL/6 mice in resource-supplemented environments were observed eating more frequently than were other mice, the significance of this finding is unknown because they did not weigh more at study end than did standard-housed female C57BL/6 mice. Food consumption and wastage were not recorded during the current study, so whether more or less food was required to maintain the body weight of mice in either housing condition or whether animals in one housing condition or the other wasted more food is unknown.
Measures of anxiety or adverse stress used in this study included the dark–light test, thermal nociceptive latency, and fecal corticoid metabolite levels. The dark–light test is based on the concept that mice instinctively avoid bright lights and open spaces and is a measure of mouse behavior and activity in a modified environment, thus allowing the observer to deduce conclusions regarding the anxiety level of mice.5 In this study, housing in a resource-supplemented environment increased the distance traveled in the light compartment only in male C57BL/6 mice. Although this finding may suggest decreased anxiety in this group, innate differences in emotionality exist between both sexes and strains of mice, with female mice typically showing more anxiety than male mice and with BALB/c mice recognized as being more emotional than C57BL/6 mice.5,7,40 Our findings conflict with the results of a 5-wk study that examined the effect of housing on dark–light activity, in which no overall effect of housing was seen in C57BL/6 or BALB/c mice.5 The types of cage resources provided and the sources of mice differed between the current and previous study,5 which also had smaller group sizes, making it difficult to compare the results directly.
Although thermal nociceptive latency is not typically used as a measure of anxiety in rodents, more anxious rodents are known to demonstrate mild hyperalgesia, which is expressed as a longer baseline hotplate latency in response to saline but smaller prolongation of latency in response to an efficacious dose of analgesic.34,51 In the current study, both sexes of BALB/c mice in resource-supplemented environments demonstrated increased responsiveness to morphine, leading to prolonged thermal nociceptive latencies as compared with standard-housed male and female BALB/c mice. This result suggests that BALB/c mice may show less anxiety when housed in resource-supplemented environments, but confirmation will require additional work. Known sex- and genotype-associated differences in hotplate latencies2,27,35 were replicated in the current study, with male and BALB/c mice exhibiting longer thermal nociceptive latencies in response to opioids than did female and C57BL/6 mice, respectively; environment did not affect thermal nociception and response to opioid treatment.
Fecal corticoid metabolite concentrations measured in the current study are consistent with those of previous studies, in that normal diurnal–nocturnal variation in metabolite levels and higher overall levels of metabolites were detected in male compared with female mice.56 Strain affected fecal corticoid metabolite levels, with BALB/c mice demonstrating higher levels than C57BL/6 mice, regardless of housing environment. A formal comparison of the excretion of fecal corticoid metabolites between BALB/c and C57BL/6 mice has not been conducted to date, but the current findings are reasonable, given the greater and characteristic emotionality of BALB/c mice seen both clinically and experimentally.8 We hypothesized that cage resource supplementation would result in an overall reduction in stress, resulting in lower serum corticosteroid levels, with subsequent lower measurements of fecal corticoid metabolites. This outcome was not observed, and in retrospect, because the resources added may represent only a modest change in environment, they would be unlikely to have a significant and lasting effect on basal metabolism and thus hypothalamic–pituitary–adrenal axis activity. These findings also indicate that the environmental resources used in the current study did not interfere with or mask basic behavioral or emotional characteristics of either mouse strain.
The effect of environmental resource supplements on immune parameters was assessed by evaluating baseline levels of and changes in WBC counts over time as well as changes in lymphocyte subsets between mice housed in standard compared with resource-supplemented environments before and after LPS challenge. In dogs, exogenously administered glucocorticoids downregulate surface markers of lymphocyte phenotype.3 To date, a similar study had not been conducted in mice; however, mice housed in highly enriched environments from weaning demonstrate a more robust immune response to viral challenge.16 In addition, chronically stressed mice have alterations in lymphoid function, morphology, and circulating subsets, with no change in B cells (CD19 positive) and a mild increase in immature T cells (CD3 positive).17 LPS, a component of the outer cell membrane of gram-negative bacteria, is used as a reliable and potent means of inducing an acute inflammatory response.36 Therefore, the bone marrow response to acute nonlethal LPS challenge can be used to evaluate immune responsiveness in the face of chronic stress. The CD4, CD8, CD19, and CD3 markers were quantified in the current study, and CD4:CD8 (Thelper:Tcytotoxic) and CD19:CD3 (B cell:T cell) ratios were calculated to determine whether chronic housing under standard compared with environmental resource-supplemented conditions led to altered immune responsiveness, potentially as a response to stress-induced increases in endogenous glucocorticoids. Housing environment had no effect on neutrophil or lymphocyte counts or on lymphocyte subsets or ratios in either sex or strain of mice. Sex- and strain-associated differences occurred and were expected, because hematology variations exist for both parameters.37 In all cases, WBC counts and absolute ratios of cells remained within standard reference intervals. In summary, the environmental resource supplements used in the current study had no significant effects on baseline immune parameters or on the immune response induced by LPS injection.
Within the hippocampus, the CA1 pyramidal neurons comprise basal and apical dendritic trees, and each neuron is covered by thousands of dendritic spines that terminate in postsynaptic membranes and participate in neurotransmission at the synapse.52 Increased hippocampal neurogenesis occurs in mice housed with a running wheel or in highly enriched environments in which cage contents are changed several times weekly.9 Increases in dendritic spine density have also been noted in mice housed in ‘super-enriched’ environments, in which environmental resources, cage furnishings, and food foraging treats are changed daily.49 Changes in neuronal morphology and dendritic spine complexity in these other studies were associated with improved learning and memory.25,39,68,69 In the current study, environmental resource supplements did not alter dendritic spine density, possibly because the resources that were provided were relatively simple. The brain weight:body weight ratios of all groups of mice housed in resource-supplemented environments were significantly increased relative to standard-housed mice. Increased brain weight:body weight ratios have been correlated with increased sociability in mice;20 however, no consistent change in nonagonistic social behavior was noted in the current study for mice housed in resource-supplemented environments. Although the increases in brain weight:body weight ratios were statistically significant, the changes were small in magnitude and remained within published reference ranges, suggesting that the changes may not have been biologically relevant.33
In summary, providing minor additions to the environments of mice in the form of nesting materials and a shelter, as well as giving each mouse a single piece of toasted oat cereal 3 times weekly, significantly reduced undesirable agonistic interactions between group-housed male and female mice of 2 strains. These effects persisted for the duration of the 6-mo study and occurred without inducing detectable alterations in murine hypothalamic–pituitary–adrenal axis activity, immune responsiveness to acute challenge, or hippocampal neuronal complexity. How such minor changes in the environment can induce a beneficial change in mouse behavior is unknown but may be related to meeting an important biologic drive (that is, nest building), providing mice with a variety of optional activities (that is, weekly nest building and ongoing modifications, ability to move through or around the tunnel or nest), and providing a wider range of environmental opportunities (that is, location in cage, conspecifics to be near, escape from agonistic interactions, degree of thermoregulation). We made no attempt to separate the effect of the food reward from the other environmental resources but considered only the overall effect of improving the home cage environment. Although no single study can examine the potential effect of environmental changes on all types of research with all strains of mice, the current findings indicate that the influence of the consistent use of nesting material and shelters in mouse cages on physiology and behavior is minimal yet positive and that the use of these resources is unlikely to affect the results of many types of behavioral, immunologic, physiologic, or other studies as it significantly improves aspects of mouse wellbeing.
Acknowledgment
We thank Megan Melillo and Anna Phan for technical support during the study and Gianni Chiappetta for assistance with figures. Funding for this work was provided by the ACLAM Foundation and Abbott Laboratories. Laura Ruggiero received partial stipend support from an ASLAP Fellowship.
References
- 1.Abdi H. 2010. Holm's sequential Bonferroni procedure. p 574–578. In Salkind NJ. Encyclopedia of research design. Thousand Oaks (CA): Sage. [Google Scholar]
- 2.Abramov U, Puussaar T, Raud S, Kurrikoff K, Vasar E. 2008. Behavioral differences between C57BL/6 and 129S6/SvEv strains are reinforced by environmental enrichment. Neurosci Lett 443:223–227. [DOI] [PubMed] [Google Scholar]
- 3.Ammersbach MA, Kruth SA, Sears W, Bienzle D. 2006. The effect of glucocorticoids on canine lymphocyte marker expression and apoptosis. J Vet Intern Med 20:1166–1171. [DOI] [PubMed] [Google Scholar]
- 4.An XL, Zou JX, Wu RY, Yang Y, Tai FD, Zeng SY, Jia R, Zhang X, Liu EQ, Broders H. 2011. Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and ALB/cJ mice. Exp Anim 60:111–123. [DOI] [PubMed] [Google Scholar]
- 5.Augustsson H, van de Weerd HA, Kruitwagen CL, Baumans V. 2003. Effect of enrichment on variation and results in the light–dark test. Lab Anim 37:328–340. [DOI] [PubMed] [Google Scholar]
- 6.Bartolomucci A. 2007. Social stress, immune functions and disease in rodents. Front Neuroendocrinol 28:28–49. [DOI] [PubMed] [Google Scholar]
- 7.Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. 2005. Behavioral differences among 14 inbred mouse strains commonly used as disease models. Comp Med 55:326–334. [PubMed] [Google Scholar]
- 8.Brinks V, van der Mark M, de Kloet R, Oitzl M. 2007. Emotion and cognition in high and low stress-sensitive mouse strains: a combined neuroendocrine and behavioral study in BALB/c and C57BL/6J mice. Front Behav Neurosci 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. 2003. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci 17:2042–2046. [DOI] [PubMed] [Google Scholar]
- 10.Bundgaard CJ, Kalliokoski O, Abelson KS, Hau J. 2012. Acclimatization of mice to different cage types and social groupings with respect to fecal secretion of IgA and corticosterone metabolites. In Vivo 26:883–888. [PubMed] [Google Scholar]
- 11.Canadian Council on Animal Care. [Internet] 1993. CCAC guide to care and use of experimental animals, vol 1. [March 29, 2015]. Available at: http://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf
- 12.Clipperton AE, Spinato JM, Chernets C, Pfaff DW, Choleris E. 2007. Differential effects of estrogen receptor α- and β-specific agonists on social learning of food preferences in female mice. Neuropsychopharmacoly. 33:2362–2375. [DOI] [PubMed] [Google Scholar]
- 13.Clipperton-Allen AE, Almey A, Melichercik A, Allen CP, Choleris E. 2011. Effects of an estrogen receptor α agonist on agonistic behavior in intact and gonadectomized male and female mice. Psychoneuroendocrinology. 36:981–995. [DOI] [PubMed] [Google Scholar]
- 14.Clipperton-Allen AE, Cragg CL, Wood AJ, Pfaff DW, Choleris E. 2010. Agonistic behavior in males and females: effects of an estrogen receptor β agonist in gonadectomized and gonadally intact mice. Psychoneuroendocrinology. 35:1008–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. 1997. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132:107–124. [DOI] [PubMed] [Google Scholar]
- 16.de Sousa AA, Reis R, Bento-Torres J, Trévia N, Lins NA, Passos A, Santos Z, Diniz JA, Vasconcelos PF, Cunningham C, Perry VH, Diniz CW. 2011. Influence of enriched environment on viral encephalitis outcomes: behavioral and neuropathological changes in albino Swiss mice. PLOS ONE 6:e15597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domínguez-Gerpe L, Rey-Méndez M. 2001. Alterations induced by chronic stress in lymphocyte subsets of blood and primary and secondary immune organs of mice. BMC Immunol 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksson E, Royo F, Lyberg K, Carlsson HE, Hau J. 2004. Effect of metabolic cage housing on immunoglobulin A and corticosterone excretion in faeces and urine of young male rats. Exp Physiol 89:427–433. [DOI] [PubMed] [Google Scholar]
- 19.Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. 2009. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci 3:50 doi.org/10.3389/ neuro.22.002.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairless AH, Dow HC, Kreibich AS, Torre M, Kuruvilla M, Gordon E, Morton EA, Tan J, Berrettini WH, Li H, Abel T, Brodkin ES. 2012. Sociability and brain development in BALB/cJ and C57BL/6J mice. Behav Brain Res 228:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairless AH, Katz JM, Vijayvargiya N, Dow HC, Kreibich AS, Berrettini WH, Abel T, Brodkin ES. 2013. Development of home-cage social behaviors in BALB/cJ vs C57BL/6J mice. Behav Brain Res 237:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Festing MFW, Lovell DP. 1981. Domestication and development of the mouse as a laboratory animal, p 43–62. In: Berry RJ. Symposium of the Zoological Society of London. Volume 47: the biology of the house mouse. London (United Kingdom): Academic Press. [Google Scholar]
- 23.Foltz C, Carbone L, DeLong D, Rollin BE, Van Loo P, Whitaker J, Wolff A. 2007. Considerations for determining optimal mouse caging density. Lab Anim (NY) 36:40–49. [DOI] [PubMed] [Google Scholar]
- 24.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. 2013. Impact of nesting material on mouse body temperature and physiology. Physiol Behav 110–111:87–95. [DOI] [PubMed] [Google Scholar]
- 25.Geinisman Y. 2000. Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cereb Cortex 10:952–962. [DOI] [PubMed] [Google Scholar]
- 26.Gibb R, Kolb B. 1998. A method for vibratome sectioning of Golgi–Cox-stained whole rat brain. J Neurosci Methods 79:1–4. [DOI] [PubMed] [Google Scholar]
- 27.Gioiosa L, Chen X, Watkins R, Umeda EA, Arnold AP. 2008. Sex chromosome complement affects nociception and analgesia in newborn mice. J Pain 9:962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant EC, Makintosh JH. 1963. A comparison of the social postures of some common laboratory rodents. Behaviour. 21:246–259. [Google Scholar]
- 29.Griebel G, Belzung C, Perrault G, Sanger DJ. 2000. Differences in anxiety-related behaviors and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 148:164–170. [DOI] [PubMed] [Google Scholar]
- 30.Hess SE, Rohr S, Dufour BD, Gaskill BN, Pajor EA, Garner JP. 2008. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J Am Assoc Lab Anim Sci 47:25–31. [PMC free article] [PubMed] [Google Scholar]
- 31.Howerton CL, Garner JP, Mench JA. 2008. Effects of a running wheel–igloo enrichment on aggression, hierarchy linearity, and stereotypy in group-housed male CD1 (ICR) mice. Appl Anim Behav Sci 115:90–103. [Google Scholar]
- 32.Hunskaar S, Berge OG, Hole K. 1986. A modified hot-plate test sensitive to mild analgesics. Behav Brain Res 21:101–108. [DOI] [PubMed] [Google Scholar]
- 33.The Jackson Laboratory Morphometric (organ weight) survey of 11 inbred strains of mice. MPD:22704. Mouse phenome database. [Cited 29 March 2015] http://phenome.jax.org.
- 34.Kavaliers M. 1988. Evolutionary and comparative aspects of nociception. Brain Res Bull 21:923–931. [DOI] [PubMed] [Google Scholar]
- 35.Kavaliers M, Choleris E. 1997. Sex differences in NMDA involvement in κ opiate and nonopioid predator-induced analgesia in mice. Brain Res 768:30–36. [DOI] [PubMed] [Google Scholar]
- 36.Kesteman N, Vansanten G, Pajak B, Goyert SM, Moser M. 2007. Injection of lipopolysaccharide induces the migration of splenic neutrophils to the T-cell area of the white pulp: role of CD14 and CXC chemokines. J Leukoc Biol 83:640–647. [DOI] [PubMed] [Google Scholar]
- 37.Kile BT, Mason-Garrison CL, Justice MJ. 2003. Sex- and strain-related differences in the peripheral blood cell values of inbred mouse strains. Mamm Genome 14:81–85. [DOI] [PubMed] [Google Scholar]
- 38.Kim JJ, Lee HJ, Welday AC, Song E, Cho J, Sharp PE, Jung MW, Blair HT. 2007. Stress-induced alterations in hippocampal plasticity, place cells, and spatial memory. Proc Natl Acad Sci USA 104:18297–18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitanishi T, Ikegaya Y, Matsuki N, Yamada MK. 2009. Experience-dependent, rapid structural changes in hippocampal pyramidal cell spines. Cereb Cortex 19:2572–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopp C. 2001. Locomotor activity rhythm in inbred strains of mice: implications for behavioral studies. Behav Brain Res 125:93–96. [DOI] [PubMed] [Google Scholar]
- 41.Kulesskaya N, Rauvala H, Voikar V. 2011. Evaluation of social and physical enrichment in modulation of behavioral phenotype in C57BL/6J female mice. PLoS One 6:e24755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leach MC, Main DCJ. 2008. An assessment of laboratory mouse welfare in UK animal units. Anim Welf 17:171–187. [Google Scholar]
- 43.Merlot E, Moze E, Bartolomucci A, Dantzer R, Neveu PJ. 2004. The rank assessed in a food- competition test influences subsequent reactivity to immune and social challenges in mice. Brain Behav Immun 18:468–475. [DOI] [PubMed] [Google Scholar]
- 44.Mesa-Gresa P, Pérez-Martinez A, Redolat R. 2013. Environmental enrichment improves novel object recognition and enhances agonistic behavior in male mice. Aggress Behav 39:269–279. [DOI] [PubMed] [Google Scholar]
- 45.Nithianantharajah J, Hannan AJ. 2006. Enriched environments, experience-dependent plasticity, and disorders of the nervous system. Nat Rev Neurosci 7:697–709. [DOI] [PubMed] [Google Scholar]
- 46.Olsson IA, Dahlborn K. 2002. Improving housing conditions for laboratory mice: a review of ‘environmental enrichment.’ Lab Anim 36:243–270. [DOI] [PubMed] [Google Scholar]
- 47.Ontario Ministry of Agriculture and Food 1990. Animals for Research Act, as amended. [Internet]. Regulation 24, research facilities and supply facilities. [Cited 29, March, 2015] Available at: http://www.e-laws.gov.on.ca/html/regs/english/elaws_regs_900024_e.htm
- 48.Pham TM, Brené S, Baumans V. 2005. Behavioral assessment of intermittent wheel running and individual housing in mice in the laboratory. J Appl Anim Welf Sci 8:157–173. [DOI] [PubMed] [Google Scholar]
- 49.Rojas JJ, Deniz BF, Miguel PM, Diaz R, Hermel Edo E, Achaval M, Netto CA, Pereira LO. 2013. Effects of daily environmental enrichment on behavior and dendritic spine density in hippocampus following neonatal hypoxia–ischemia in the rat. Exp Neurol 241:25–33. [DOI] [PubMed] [Google Scholar]
- 50.Ruxton GD, Beachamp G. 2008. Time for some a priori thinking about post hoc testing. Behav Ecol 19:690–693. [Google Scholar]
- 51.Siegfried B, Netto CA, Izquierdo I. 1987. Exposure to novelty induces naltrexone-reversible analgesia in rats. Behav Neurosci 101:436–438. [DOI] [PubMed] [Google Scholar]
- 52.Spruston N. 2008. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9:206–221. [DOI] [PubMed] [Google Scholar]
- 53.Swetter BJ, Karpiak CP, Cannon JT. 2011. Separating the effects of shelter from additional cage enhancements for group-housed BALB/cJ mice. Neurosci Lett 495:205–209. [DOI] [PubMed] [Google Scholar]
- 54.Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. 2009. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 204:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toth LA, Kregel K, Leon L, Musch TI. 2011. Environmental enrichment of laboratory rodents: the answer depends on the question. Comp Med 61:314–321. [PMC free article] [PubMed] [Google Scholar]
- 56.Touma C, Sachser N, Mostl E, Palme R. 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278. [DOI] [PubMed] [Google Scholar]
- 57.Tsai PP, Pachowsky U, Stelzer HD, Hackbarth H. 2002. Impact of environmental enrichment in mice. Part 1: effect of housing conditions on body weight, organ weights, and haematology in different strains. Lab Anim 36:411–419. [DOI] [PubMed] [Google Scholar]
- 58.Turner PV, Sunohara-Neilson J, Ovari J, Healy A, Leri F. 2014. Effects of single compared with pair housing on hypothalamic–pituitary–adrenal axis activity and low-dose heroin place conditioning in adult male Sprague–Dawley rats. J Am Assoc Lab Anim Sci 53:161–167. [PMC free article] [PubMed] [Google Scholar]
- 59.Van de Weerd HA, Aarsen EL, Mulder A, Kruitwagen CL, Hendriksen CF, Baumans V. 2002. Effects of environmental enrichment for mice: variation in experimental results. J Appl Anim Welf Sci 5:87–109. [DOI] [PubMed] [Google Scholar]
- 60.Van de Weerd HA, Van Loo PL, Van Zutphen LF, Koolhaas JM, Baumans V. 1997. Nesting material as environmental enrichment has no adverse effects on behavior and physiology of laboratory mice. Physiol Behav 62:1019–1028. [DOI] [PubMed] [Google Scholar]
- 61.Van de Weerd HA, Van Loo PL, Van Zutphen LF, Koolhaas JM, Baumans V. 1997. Preferences for nesting material as environmental enrichment for laboratory mice. Lab Anim 31:133–143. [DOI] [PubMed] [Google Scholar]
- 62.Van Loo PL, Mol JA, Koolhaas JM, Van Zutphen BF, Baumans V. 2001. Modulation of aggression in male mice: influence of group size and cage size. Physiol Behav 72:675–683. [DOI] [PubMed] [Google Scholar]
- 63.Van Loo PL, Van der Meer E, Kruitwagen CL, Koolhaas JM, Van Zutphen LF, Baumans V. 2004. Long-term effects of husbandry procedures on stress-related parameters in male mice of 2 strains. Lab Anim 38:169–177. [DOI] [PubMed] [Google Scholar]
- 64.Van Loo PL, Van Zutphen LF, Baumans V. 2003. Male management: coping with aggression problems in male laboratory mice. Lab Anim 37:300–313. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. 2004. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22:460–466. [DOI] [PubMed] [Google Scholar]
- 66.Welsh CJ, Bustamante L, Nayak M, Welsh TH, Dean DD, Meagher MW. 2004. The effects of restraint stress on the neuropathogenesis of Theiler's virus infection. Part II: NK cell function and cytokine levels in acute disease. Brain Behav Immun 18:166–174. [DOI] [PubMed] [Google Scholar]
- 67.Whitaker J, Moy SS, Saville BR, Godfrey V, Nielsen J, Bellinger D, Bradfield J. 2007. The effect of cage size on reproductive performance and behavior of C57BL/6 mice. Lab Anim (NY) 36:32–39. [DOI] [PubMed] [Google Scholar]
- 68.Workman JL, Bowers SL, Nelson RJ. 2009. Enrichment and photoperiod interact to affect spatial learning and hippocampal dendritic morphology in white-footed mice (Peromyscus leucopus). Eur J Neurosci 29:161–170. [DOI] [PubMed] [Google Scholar]
- 69.Yang G, Pan F, Gan WB. 2009. Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]