Abstract
Agonistic behavior in group-housed male mice is a recurring problem in many animal research facilities. Common management procedures, such as the removal of aggressors, are moderately successful but often fail, owing to recurrence of aggressive behavior among cagemates. Studies have incorporated enrichment devices to attenuate aggression, but such devices have had mixed results. However, these studies did not include research manipulations when assessing the benefits of various enrichment devices. We obtained 100 male athymic nude mice and studied the efficacy of various enrichment devices, including cotton squares, paper rolls, shredded paper, nylon bones, and a mouse house and wheel combination in the reduction of fighting during an ongoing study that involved randomization followed by prostate and intratibial injections. Groups were evaluated according to a numerical grading system for wound assessment. Examination of the data revealed that the enrichment devices had no effect on the presence of wounds, thus none of the devices tested affected fighting in nude mice. However, when mice began experimental use, fight wounds increased significantly at cage change and after randomization, reflecting a disruption of existing social hierarchies. Therefore, in the context of an actual research study that involves common manipulations, the specific enrichment device had less effect on aggression in male nude mice than did the destruction and reconstruction of social structures within each group.
Much is known about the normal behavior of mice in captivity. Mice prefer the company of conspecifics and are highly social.6,29 Singly housed mice may exhibit signs of isolation syndrome that include stereotypies, anxiety, restlessness, and aggression; this syndrome can result in physiologic changes that alter the immune system and result in increased pathology.15,53,63,64 Therefore, mice should be group-housed unless there is a strong scientific justification for doing otherwise.
Mice are a territorial species.56 In the wild, groups generally live within a territory and consist of social units composed of a dominant male, several females, their progeny, and, occasionally, subordinate males.17,26,35,36,58 A single dominant mouse often controls a self-identified territory, which often has a range of several hundred square meters.19 Other male mice respond to olfactory cues, primarily urine marking within and around the borders of the territory, that are dispersed by the dominant mouse and therefore avoid the territory and the risk of attack from its possessor.26 Nevertheless, even when living in a small, limited space, such as a cage, male mice prefer the companionship of a dominant male over a solitary existence.58 This behavior further supports the importance of social housing for the wellbeing and social structure development of mice.
The importance of social housing for the wellbeing and social structure development of mice has been well documented.5,15,29,53,58,63,64 The social organization of mice in the wild varies between 2 types: dominance hierarchy and territorial organization.13 When balanced, dominance hierarchies mitigate additional aggression and allow for priority access for the most-dominant animals to limited resources such as food, territory, and mates.3 In addition, dominance hierarchies serve to prevent high levels of intermale aggression, which may be detrimental to the social group in the form of reduced foraging and reproduction and increased injury and death.3
Therefore, in the laboratory setting, housing in groups meets the needs of the mice; however, the typical housing of multiple males or multiple females within the confines of a cage may frustrate their natural preference for small family groups and their attempts to develop the requisite social structure.4,5,29,56 This situation results in agonistic behavior, one component in the repertoire of normal mouse behaviors that is demonstrated in the wild but that has become amplified and extreme.
Agonistic behavior is any social behavior related to aggression, including fighting, defending oneself, reconciliation, and avoidance.11,12,22,47 Aggression has several functions: the development and maintenance of dominance hierarchies, acquisition of limited resources, and competition for mates. The primary causes of direct physical conflict in mice are defense of territory by males and defense of pups by females.40 Perhaps most importantly, physical aggression is an essential means of ascension to and preservation of male dominance status in a territorial group.11,12,22,47 Clearly, the limited territory within the cage environment impedes access to the typical channels for the resolution of aggression. As a result of the limited living space, a dominant mouse has easy access to the subordinate, and the subordinate has no means of escape from the odor of marking scents deposited by aggressors or from direct attack by an aggressor. Furthermore, subordinates are prevented from demonstrating typical behaviors of retreat during conflicts with conspecific cagemates.5,26,41 The inability to control the social and physical environment can lead to an escalation in aggressive behavior.58
A mouse's proclivity for aggression is affected by past experiences. Past combative experiences result in learning, which influences future aggression; for example, aggressive behavior decreases after the experience of defeat.44 Perhaps reduced experience with aggression limits the learning of aggressive behaviors. Consequently, if all studies could use siblings and mice that are familiar with each other and have established dominance hierarchies, aggression would likely be reduced. However, in many research studies, mice are assigned randomly to new groups, causing them to be removed from a cage and group of familiar mice and placed into a cage of unfamiliar mice.
Ultimately, to meet the highest standards of animal welfare and produce reliable scientific data, a solution must be found to minimize aggression in mice within the confines of the research environment. Currently, the routine response to the observation of wounded mice in a cage is to remove the aggressor. This practice temporarily resolves the existing issue, but aggression often recurs as social hierarchies are reestablished. In addition, relocating mice involves an investment of resources, including time, effort, and increased housing costs for each additional cage.
Intermale aggression is affected by modifications in the cage environment,20 and therefore, one possible solution for agonistic behavior in mice—and more specifically, fighting—is environmental enrichment. Environmental enrichment is the modification of the microenvironment of the mouse through the addition of social groupings and physical structures to reduce undesirable behavior and stress while promoting and facilitating normal, positive species-typical behaviors with the goal of improving animal wellbeing.8,42,52,64 Enrichment must address physiologic needs as well as behavioral needs of mice, which include social behavior, foraging, burrowing, nest construction, and exploration.8 Ideally, appropriate devices should satisfy other common behaviors also, including exercising, burrowing, foraging, digging, gnawing, climbing, and nesting.5,29 Commonly used devices include manipulanda, nesting materials, shelters, tubes, platforms, running wheels, and enlarged cage space.8,42 However, the efficacy of enrichment to attenuate agonistic behavior in mice remains unclear.
Environmental enrichment studies have had varied outcomes regarding the effect of enrichment on aggression in mice. Many studies have demonstrated an increase in aggression resulting from enrichment.7,10,20,21,23,27,38,53,65 In contrast, multiple other studies have found that environmental enrichment reduces aggression in mice.2,4,14,45,55,57-59 Still other studies have demonstrated no effect of enrichment on aggression.21,38,55,57,58
Numerous explanations can be provided for the different outcomes in environmental enrichment studies. Variations are likely due to differences in mouse strain, age, or sex; population density in the experimental cages; variety of enrichment device or duration of enrichment; assessment criteria for aggression; and experimental design.37,58 For instance, enrichment has a greater effect on males and variable effect on different strains, such that the choice of sex and strain in a study could have a significant bearing on results.15,38,53,61,64,65 One thing that studies of the effects of enrichment on aggression in male mice have in common is that they have not taken into account the use of strategies to ameliorate aggression during actual research studies and mouse manipulation, particularly after randomization of mice.
We sought to fill this gap in knowledge by examining the effect of various enrichment devices on nude mice that proved to be aggressive during a research study involving randomization and surgery at our institution. To this end, we compared the efficacy of various enrichment devices with cotton squares, the standard enrichment device used in our facility. Previous research has indicated that, compared with other enrichment items, nesting material is most preferred by mice and effectual, but whether this preference and efficacy remains after the stressors experienced during a research study is unknown currently.42,50,54 We hypothesized that alternative enrichment devices would prove more beneficial to reduce aggression in nude mice before and after manipulation.
Materials and Methods
Animals.
We obtained 100 male athymic NCrnu/nu mice (weight, 25 to 30 g; age, 8 wk) from the National Cancer Institute (Frederick Cancer Research Facility, Frederick, MD). To fulfill the ideals of reduction, refinement, and replacement, we performed this study concurrently with an ongoing study involving randomization, a surgical procedure, and imaging.
All animals were part of an IACUC-approved study at The University of Texas MD Anderson Cancer Center (Houston, TX), were housed in accordance with the Guide for the Care and Use of Laboratory Animals, and were maintained in an AAALAC-accredited facility.28 As part of the health surveillance program, sentinel mice were assessed quarterly and annually for mouse parvoviruses, mouse hepatitis virus, Theiler murine encephalomyelitis virus, epizootic diarrhea of infant mice, Sendai virus, pneumonia virus, reovirus, lymphocytic choriomeningitis virus, mouse adenovirus, ectromelia virus, K virus, polyoma virus, and Mycoplasma pulmonis and found to be SPF. In addition, testing excluded mice with pathogenic endo- or ectoparasites.
Husbandry.
Mice were group-housed, 5 per cage, in individually ventilated polycarbonate cages (365 × 207 × 130 mm; floor area, 542 cm2) containing 0.5-cm corncob bedding (The Andersons, Maumee, OH). The temperature, humidity, and lighting were maintained at 72 ± 2 °F (22.2 ± 1.1 °C), 50% ± 5%, and 12:12-h light:dark, respectively. Mice were provided free-choice irradiated rodent chow (LabDiet 5053, PMI, St Louis, MO) and reverse-osmosis–purified, chlorinated, water by automated delivery. Cages were cleaned and the bedding replaced once every 2 wk.
Enrichment.
The study used 5 enrichment devices: 2-inch squares of cotton fiber (Nestlets, Ancare, Bellmore, NY); small paper rolls that can be unfurled (Enrich-n-Nests, The Andersons, Maumee, OH); 1/8-in. shredded paper strips used by rodents to build nests (Enviro-Dri, Shepherd Specialty Papers, Milford, NJ); natural flavor, petite nylon bones for gnawing (Nylabones, Bio-Serve, Frenchtown, NJ); and polycarbonate enclosures and wheels that provide shelter and exercise (Mouse Igloos with Fast-Trac, Bio-Serve, Frenchtown, NJ). At each biweekly cage cleaning, cotton squares and paper rolls were renewed, and shredded paper, nylon bones, and houses with running wheels were gently removed and reused. Each enrichment group included 20 mice (4 cages with 5 mice per cage).
Wound grading system.
Environmental enrichment devices and aggression have been assessed in several ways in previous studies.1,22,25,39,58 We sought to measure a single component of agonistic behavior: fighting. Wounds inflicted in nude mice while fighting may be the most readily quantified element of agonistic behavior because the lesions are easily visualized during and after a physical encounter.
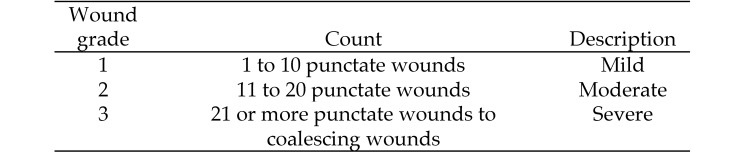
On arrival, mice were placed into standard cages (5 mice per cage) according to the original groupings from the vendor. During the 10-d acclimation period, they were housed in standard cages containing corncob bedding and cotton squares. After the acclimation period, wounds were assessed according to a numeric grading system (Table 1) based on the number of wounds counted. Wounds were defined as a complete break in the continuity of the epithelium. Wound grades were assessed daily during the same 3-h interval (11:00 to 14:00) by 4 observers trained to count wounds and follow the grading system. Wound assessments occurred for all mice on days 1 through 50. Mice were removed from the cage when grade 3 wounding occurred.
Table 1.
Summary of wound rates over time by type of enrichment
| Day | Cotton squares | Nylon bone | Mouse house and wheel | Paper rolls | Shredded paper |
| Before randomization | |||||
| 1 | 0.25 | 0.07 | 0.25 | 0.08 | 0.22 |
| 7 | 0.30 | 0.28 | 0.29 | 0.08 | 0.28 |
| 14 | 0.28 | 0.18 | 0.49 | 0.05 | 0.17 |
| 19 | 0.42 | 0.12 | 0.25 | 0.07 | 0.07 |
| After randomization | |||||
| 21 | 0.63 | 0.50 | 0.49 | 0.57 | 0.69 |
| 23 | 0.68 | 0.44 | 0.42 | 0.47 | 0.67 |
| 26 | 0.53 | 0.30 | 0.26 | 0.25 | 0.40 |
| 38 | 0.27 | 0.17 | 0.29 | 0.05 | 0.23 |
| 41 | 0.20 | 0.07 | 0.23 | 0.04 | 0.10 |
| 50 | 0.02 | 0.04 | 0.03 | 0.04 | 0.09 |
To visually compare the enrichment groups during the evaluation period, we adjusted for the variation in onset of wound rates. Therefore, wound rates were centralized by subtracting the rate of each group on day 1. After adjustment, the difference in wound rates started at 0 for all groups.
Manipulations.
Because this study was conducted concurrently with another study, common research variables were incorporated, which allowed us to assess the effect of manipulations on aggression and enrichment in the mice. These manipulations included randomization on day 20, surgery (prostate and tibial injections) on days 24 and 25, anesthesia with imaging on day 40, and intraperitoneal injections 3 times weekly from day 26 through day 49.32
Randomization.
On day 20, mice were randomized intentionally such that 3 mice were removed from each cage; each group of 3 mice was separated further, and each of these 3 mice was moved to a new cage containing 2 mice original to that cage, thus returning the cage population to 5 mice. When complete, each cage retained 2 original mice and 3 new mice. Randomization of mice occurred within the same enrichment-device group.
Statistical methods.
The means of the wound grades were used to summarize fighting among mice on each day according to the type of enrichment. The mean was calculated as a ratio of the total wound grade divided by the maximal wound grade for each enrichment group on each day. For example, the maximal wound grade on a given day is 60 for 20 mice and 57 for 19 mice. If a mouse was removed from the study due to death or separation, the mouse was excluded from the calculation of the maximal wound grade on that day and thereafter. The difference in grade between 2 consecutive days for each mouse was used to represent new wounds. When the difference was greater than 0, we determined that the mouse received new wounds on that day. If the difference was less than or equal to 0, we determined that the mouse's existing wounds were healing, and it did not have new wounds. The rate of new wounds was calculated as a ratio of the total new-wound grade divided by the maximal wound grade for each enrichment group on each day. The proportion of mouse-days that a mouse received new wounds was calculated across all days for all mice in each enrichment group. Pearson χ2 tests were used to determine the significance of differences in proportions between the cotton-squares group (control group) and the other 4 enrichment groups (a pairwise comparison) and among all 5 enrichment groups (an overall comparison). P values less than 0.05 were considered significant.
Any adverse event experienced by a mouse, such as separation, that resulted in removal of the mouse prior to the end of the study was recorded, and the sum of mouse-days for each event was calculated for each enrichment group. If a mouse experienced more than 1 event during the period of observation, the event and corresponding mouse-days were assigned to the mouse in the following hierarchy: death > separation > other events. All analyses were conducted in SAS 9.3 (SAS Institute, Cary, NC).
Results
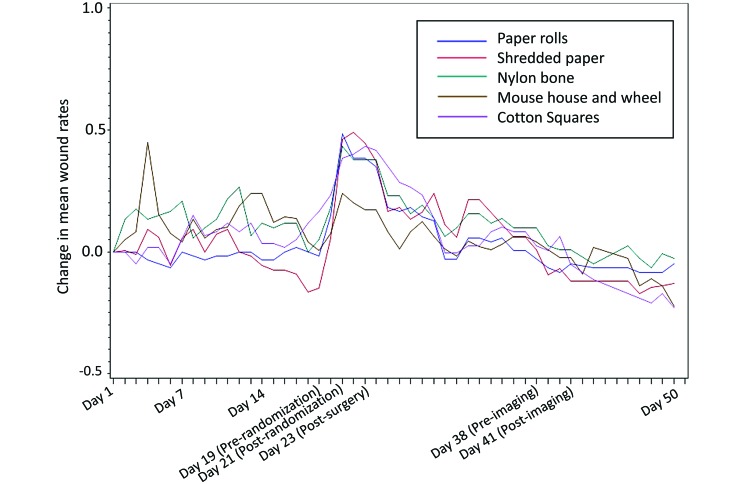
During the first week of wound assessments (Figure 1), mean wound rates increased rapidly for a short duration when the mice experienced a cage change-out. Wound counts were highest at the time of randomization and manipulation, days 20 through 26 (Figure 2); the mean wound rates decreased gradually thereafter. During the first week, the enrichment group with the mouse house and wheel had the highest mean wound rate, with a spike at day 4; after 50 d, their mean wound rate had decreased the most.
Figure 1.
Wound assessment metric.
Figure 2.
Differences in wound rates over time according to type of enrichment.
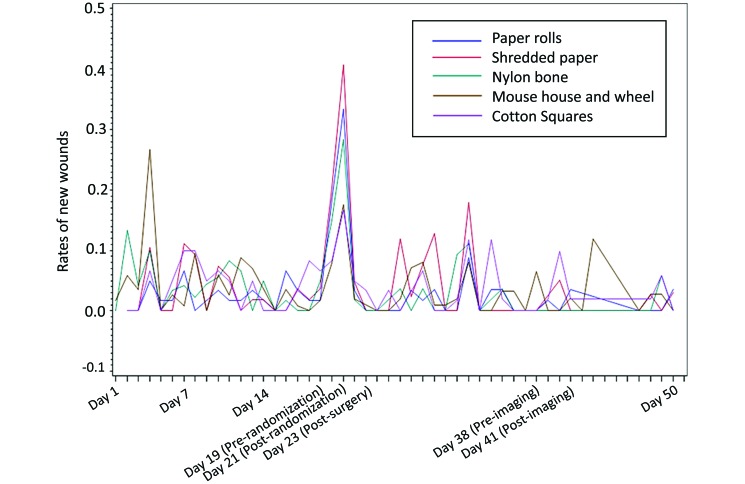
Similarly, wound rates (Table 1) in the group exposed to the mouse house and wheel were increased during the first week. In addition, wound rates between days 20 and 21 were increased in all enrichment groups. When we evaluated the rates of new wounds over time by type of enrichment (Figure 3), we noted several consistent patterns. During the first week, the mice given the mouse house and wheel had a marked spike in new wounds, whereas the rates of new wounds for this group subsequently were consistent with those of the other groups. In addition, all groups showed a spike in new wounds at days 19 and 20.
Figure 3.
The rate of new wounds over time according to type of enrichment.
During the course of the study, 5 mice were removed from the study due to fighting. In addition, 3 mice died secondary to manipulation, and 1 died due to fighting (most likely).
Overall, the proportions of mouse-days with new wounds (Table 2) were not significantly different among the 5 enrichment groups (P = 0.24). The proportion of mouse-days with new wounds in the cotton-squares group did not differ significantly from that of any other group.
Table 2.
Proportion of mouse–days with new wounds by type of enrichment
| No. of mouse-days with new wounds / total no. of mouse-days | Proportion (95% CI) | Pairwise Pa | |
| Cotton squares (control) | 89 / 835 | 0.107 (0.086–0.128) | — |
| Nylon bones | 89 / 948 | 0.094 (0.075–0.112) | 0.372 |
| Mouse house and wheel | 140 / 1480 | 0.095 (0.080–0.110) | 0.353 |
| Paper rolls | 69 / 862 | 0.080 (0.062–0.098) | 0.060 |
| Shredded paper | 75 / 674 | 0.111 (0.088–0.135) | 0.771 |
The number of mouse-days was defined as the sum of the follow-up days for all mice in a group.
From χ2 test for association. Overall P = 0.2412.
Discussion
In animal facilities, devices are chosen for enrichment not only for their benefit to the animal but also in light of economic, ergonomic, and facility standardization factors.31,58 The cage environment must meet the physiologic and behavioral requirements of multiple strains of mice within a single facility. Therefore, we elected to use devices and materials commonly used in laboratory animal facilities. Each device chosen for this study was intended to functionally mimic the animals’ natural environment in some way and provide the opportunity to express a particular positive, species-typical behavior. Cotton squares, which served as the control, and shredded paper are commonly used nesting materials. Nesting material has been demonstrated to be preferred by mice over other enrichment devices.42,48-50,54,55 Nesting material is manipulated by both male and female mice and thus provides increased activity, thermoregulation, and a place to hide, breed, and avoid light or other conspecifics.16,20,33,34,54,60 Cotton squares facilitate many typical behaviors, particularly nesting, and also confers control over their environment.18 Paper rolls were chosen for their properties as a manipulable device and as an alternative nesting material. Nylon bones satisfy the needs to gnaw and manipulate. Finally, the mouse house and wheel was used because it has properties that fulfill the needs of hiding, climbing, sheltering, and exercising.
Interestingly, the one device that resulted in a spike of aggression during the first week was the mouse house and wheel. Shelters such as the mouse house are known to affect strains differently, as do different forms of shelters.30,51,58 Shelters have been found to both increase and reduce aggression.24,30,58 However, the addition of a running wheel has consistently been shown to increase fighting, likely because it is a coveted object that has limited access.24,30,51 Unlike nesting material, which alters only the quality of the space, shelters (and ostensibly the running wheel) also increase the quantity of space.56 Increasing the quantity of space allows territory to expand, causing the roles within a social group to become unstable.56 Therefore, the introduction of this highly valued territorial item likely led to an initial shifting of social structure in our mice. However, early wound rates were consistent among the other devices.
In the framework of an actual research study that involved common manipulations, the specific enrichment device had less of an effect on aggression in male nude mice than did the destruction and reconstruction of social structures within each group. Although the difference was not statistically significant, the wound rate of the paper-rolls group was lower consistently than that of the other enrichment device groups. Even in the peak period between randomization and surgery, the wound rate remained lower than those of the cotton squares and shredded paper groups. We deduce that during increased stress, a paper device distributed over the cage floor for manipulation may be slightly more beneficial than devices promoting behaviors involving gnawing, nesting, exercise, and hiding. More specifically, this enrichment may have fewer of the negative effects that arise with other devices involving territoriality and social hierarchy destabilization, owing to the generally uniform availability of the paper roll enrichment to all mice within a cage. Because this device is easily distributed throughout the entire cage, all mice within a cage are able to access it without having to be in close proximity to each other; moreover, the paper rolls provide the opportunity for mice to work. In contrast, even cotton squares and shredded paper, demonstrated to be preferred by mice, may not be widely distributed or accessible by all within a cage at any given time.42,50,54In addition, the group sizes we used in the current study may have reduced the potential effects of enrichment. Behavioral assays have shown that the optimal size for a stable dominance hierarchy in the cage environment is 3 mice.58 Therefore, our group size of 5, which is common in most animal facilities, exceeded ideal conditions and contributed to social instability. Overall, the effect of social hierarchy likely muted the benefit of the enrichment devices.
In the 20 d preceding randomization, there was mild fluctuation in fighting within all enrichment groups. However, wound rates and new wounds increased significantly in all enrichment groups at the time of manipulations, particularly from the onset of randomization through the completion of surgery. Therefore, although no enrichment device was effective at reducing fighting among the mice during this period, much was revealed about aggression. The data demonstrate that stressors placed on each group of male mice resulted in increased fight wounds, which indicates increased aggression. Both wound rates and new wound rates increased after various stressors, whether environmental in the form of a cage change early in the study, physical in the form of a research manipulation, or social in the form of randomization.
In particular, randomization of the mice on day 20 was a key feature of this study. After randomization, established hierarchies within a cage were disrupted or destroyed, resulting in the need to reestablish roles. Aggression is necessary for the development of stable dominance hierarchies. However, the aggression that occurs during the shifting of social roles in the development of a group's social hierarchy is a significant source of negative stress; moreover, stress has been shown to further increase territorial aggression.43 Stressors and aggression may influence each other, resulting in a self-reinforcing feedback loop that can be demonstrated through elevated physiologic stress indicators.20
Another interesting finding in our study was that by day 50, both wound rates and new wound rates across all enrichment groups declined to levels less than those observed at any time between days 1 and 49 and below the baseline level (days 1 to 20). This finding implies that stabilization of social hierarchy may take as long as 30 d to resolve after a significant shift in group composition. There were several possible reasons for this decline.
The initial decline, which occurred after surgical manipulation, was likely due to a reduction in activity while the mice received analgesics and as they advanced through the process of healing.46 The continued declines in both fight wound rates and new wound rates may have been due to the successful reestablishment of stable dominance hierarchies.
Prior to randomization, we were able to assess the effects of enrichment on male mice that were familiar with each other and that existed in stable, established dominance hierarchies. After randomization, we were able to assess the effects of enrichment on male mice housed with unfamiliar males. Essentially, at the point of randomization, we were able to remove the potentially confounding factor of assessing animals that were familiar with each other; thus, it allowed us to focus on the more typical research situation in which unfamiliar animals are housed together and which is a key cause of aggressive behaviors.
From this experimental design, we can infer that randomization likely resulted in increased fighting due to the disruption of existing dominance hierarchies. Reduction in fight wounds after randomization therefore reflects the reestablishment of a dominant male and subordinates and renewed social stability. Therefore, if randomization is required as part of the study design in studies that may be affected by high levels of manipulation, such as immunologic studies, it may be advisable to delay any manipulations or data collection until 30 d after randomization. As demonstrated in this study, the stress associated with the shift in social hierarchy likely will have subsided by this point, thus eliminating it as a potentially confounding factor.
In conclusion, fight wound trends between the 5 enrichment groups were consistent with a similar pattern of rise and fall in fight wound rates and new wound rates over time that occurred regardless of the enrichment device used. Changes in wound rate were correlated with events of negative stress and were minimally influenced by enrichment devices. The proportions of mouse-days with new wounds did not differ among the enrichment groups or between the mice given cotton squares (controls) and other enrichment groups. On the basis of these results, we believe that cotton squares are as effective as any other standard enrichment device. Despite the lack of statistically significant differences among the 5 environmental enrichment devices, many valuable observations emerged in regard to mouse behavior after randomization and manipulation. Finally, we found that use of the various enrichment devices had no effect on study outcomes, including tumor development, progression and incidence.9,62
Although the current study was performed concurrently with another study involving animal manipulations, and therefore afforded the gathering of data relevant to the day-to-day use of animals in research, there were limitations to this approach. First are the inherent introduction of variables to an enrichment study that arises from another involving manipulations. In addition, because handling of the mice was limited, observations of fight wounds were the main source of data collection, and as such were somewhat restricted in scope.
Ultimately, it is in the best interest of the research community to seek out ways to attenuate aggressive behavior among unfamiliar mice to improve animal wellbeing and research outcomes. Further research may be needed to clarify the effect of randomization on studies that may be affected by high stress levels, such as those involving the immune system. In addition, more study is needed to understand the effect of randomization on the social structure of mice. Additional study of the influence of mouse age on fighting in response to randomization and enrichment is also warranted. Moreover, improved knowledge of the time required for renewal of dominance hierarchies in mice, and among different strains, is required. Finally, future studies using a housing density of less than 3 male mice per cage may be needed to determine the efficacy of environmental enrichment devices in mitigating unfamiliar intermale aggression.
Acknowledgment
We thank Kristin Pegram, Anicia Roberson, Kimberly Scott, David Worth, and Diana Tran.
References
- 1.Abou-Ismail UA. 2011. The effects of cage enrichment on agonistic behaviour and dominance in male laboratory rats (Rattus norvegicus). Res Vet Sci 90:346–351. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose N, Morton DB. 2000. The use of cage enrichment to reduce male mouse aggression. J Appl Anim Welf Sci 3:117–125. [Google Scholar]
- 3.Anholt RR, Mackay TF. 2012. Genetics of aggression. Annu Rev Genet 46:145–164. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong KR, Clark TR, Peterson MR. 1998. Use of corn-husk nesting material to reduce aggression in caged mice. Contemp Top Lab Anim Sci 37:64–66. [PubMed] [Google Scholar]
- 5.Balcombe J. 2010. Laboratory rodent welfare: thinking outside the cage. J Appl Anim Welf Sci 13:77–88. [DOI] [PubMed] [Google Scholar]
- 6.Balcombe JP. 2006. Laboratory environments and rodents’ behavioural needs: a review. Lab Anim 40:217–235. [DOI] [PubMed] [Google Scholar]
- 7.Barnard CJ, Behnke JM, Sewell J. 1996. Environmental enrichment, immunocompetence, and resistance to Babesia microti in male mice. Physiol Behav 60:1223–1231. [DOI] [PubMed] [Google Scholar]
- 8.Baumans V. 2005. Environmental enrichment for laboratory rodents and rabbits: requirements of rodents, rabbits, and research. ILAR J 46:162–170. [DOI] [PubMed] [Google Scholar]
- 9.Bayne K, Würbel H. 2014. The impact of environmental enrichment on the outcome variability and scientific validity of laboratory animal studies. Rev Sci Tech 33:273–280. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann P, Militzer K, Buttner D. 1994. Environmental enrichment and aggressive behaviour: influence on body weight and body fat in male inbred HLG mice. J Exp Anim Sci 37:59–78. [Google Scholar]
- 11.Bisazza A. 1980. Social organization and territorial behaviour in 3 strains of mice. Bollentino di zoologia 48:157–167. [Google Scholar]
- 12.Brain P. 1975. What does individual housing mean to a mouse? Life Sci 16:187–200. [DOI] [PubMed] [Google Scholar]
- 13.Butler RG. 1980. Population size, social behaviour, and dispersal in house mice: a quantitative investigation. Anim Behav 28:78–85. [Google Scholar]
- 14.Chamove AS. 1989. Cage design reduces emotionality in mice. Lab Anim 23:215–219. [DOI] [PubMed] [Google Scholar]
- 15.Chapillon P, Manneche C, Belzung C, Caston J. 1999. Rearing environmental enrichment in 2 inbred strains of mice. 1. Effects on emotional reactivity. Behav Genet 29:41–46. [DOI] [PubMed] [Google Scholar]
- 16.Conour LA, Murray KA, Brown MJ. 2006. Preparation of animals for research—issues to consider for rodents and rabbits. ILAR J 47:283–293. [DOI] [PubMed] [Google Scholar]
- 17.Crowcroft P. 1966. Mice all over. London (Great Britain): GT Foulis. [Google Scholar]
- 18.Gonder JC, Laber K. 2007. A renewed look at laboratory rodent housing and management. ILAR J 48:29–36. [DOI] [PubMed] [Google Scholar]
- 19.Gray SJ, Hurst JL. 1998. Competitive behaviour in an island population of house mice, Mus domesticus. Anim Behav 56:1291–1299. [DOI] [PubMed] [Google Scholar]
- 20.Haemisch A, Gartner K. 1994. The cage design affects intermale aggression in small groups of male laboratory mice: strain-specific consequences on social organization and endocrine activations in 2 inbred strains (DBA/2J and CBA/J). J Exp Anim Sci 36:101–116. [PubMed] [Google Scholar]
- 21.Haemisch A, Gartner K. 1997. Effects of cage enrichment on territorial aggression and stress physiology in male laboratory mice. Acta Physiol Scand Suppl 640:73–76. [PubMed] [Google Scholar]
- 22.Haemisch A, Voss T, Gartner K. 1994. Effects of environmental enrichment on aggressive behavior, dominance hierarchies, and endocrine states in male DBA/2J mice. Physiol Behav 56:1041–1048. [DOI] [PubMed] [Google Scholar]
- 23.Henderson ND. 1976. Short exposures to enriched environments can increase genetic variability of behaviour in mice. Dev Psychol 9:549–553. [DOI] [PubMed] [Google Scholar]
- 24.Howerton DL, Garner JP, Mench JA. 2008. Effects of a running wheel–igloo enrichment on aggression, hierarchy linearity, and stereotypy in group-housed male CD1 (ICR) mice. Appl Anim Behav Sci 115:90–103. [Google Scholar]
- 25.Hunt C, Hambly C. 2006. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group-housed males. Physiol Behav 87:519–526. [DOI] [PubMed] [Google Scholar]
- 26.Hurst JL, Fang J, Barnard CJ. 1993. The role of substrate odours in maintaining social tolerance between male house mice, Mus musculus domesticus. Anim Behav 45:997–1006. [Google Scholar]
- 27.Hutchinson E, Avery A, Vandewoude S. 2005. Environmental enrichment for laboratory rodents. ILAR J 46:148–161. [DOI] [PubMed] [Google Scholar]
- 28.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 29.Jennings M, Batchelor GR, Brain PF, Dick A, Elliott H, Francis RJ, Hubrecht RC, Hurst JL, Morton DB, Peters AG, Raymond R, Sales GD, Sherwin CM, West C. 1998. Refining rodent husbandry: the mouse. Report of the Rodent Refinement Working Party. Lab Anim 32:233–259. [DOI] [PubMed] [Google Scholar]
- 30.Kaliste EK, Mering SM, Huuskonen HK. 2006. Environmental modification and agonistic behavior in NIH/S male mice: nesting material enhances fighting but shelters prevent it. Comp Med 56:202–208. [PubMed] [Google Scholar]
- 31.Kostomitsopoulos NG, Paronis E, Alexakos P, Balafas E, van Loo P, Baumans V. 2007. The influence of the location of a nest box in an individually ventilated cage on the preference of mice to use it. J Appl Anim Welf Sci 10:111–121. [DOI] [PubMed] [Google Scholar]
- 32.Lee HJ, Yu HK, Papadopoulos JN, Kim SW, He J, Park YK, Yoon Y, Kim JS, Kim SJ. 2012. Targeted antivascular therapy with the apolipoprotein(a) kringle V, rhLK8, inhibits the growth and metastasis of human prostate cancer in an orthotopic nude mouse model. Neoplasia 14:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lisk RD, Pretlow RA, 3rd, Friedman SM. 1969. Hormonal stimulation necessary for elicitation of maternal nest-building in the mouse (Mus musculus). Anim Behav 17:730–737. [DOI] [PubMed] [Google Scholar]
- 34.Lynch CB, Hegmann JP. 1972. Genetic differences influencing behavioral temperature regulation in small mammals. I. Nesting by Mus musculus. Behav Genet 2:43–53. [DOI] [PubMed] [Google Scholar]
- 35.Mackintosh JH. 1970. Territory formation by laboratory mice. Anim Behav 18:177–183. [Google Scholar]
- 36.Mackintosh JH. 1973. Factors affecting the recognition of territory boundaries by mice (Mus musculus). Anim Behav 21:464–470. [Google Scholar]
- 37.Macri S, Ceci C, Altabella L, Canese R, Laviola G. 2013. The Directive 2010/63/EU on animal experimentation may skew the conclusions of pharmacological and behavioural studies. Sci Rep 3:2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marashi V, Barnekow A, Ossendorf E, Sachser N. 2003. Effects of different forms of environmental enrichment on behavioral, endocrinological, and immunological parameters in male mice. Horm Behav 43:281–292. [DOI] [PubMed] [Google Scholar]
- 39.McGregor PK, Ayling SJ. 1990. Varied cages result in more aggression in male CFLP mice. Appl Anim Behav Sci 26:277–281. [Google Scholar]
- 40.Miczek KA, Maxson SC, Fish EW, Faccidomo S. 2001. Aggressive behavioral phenotypes in mice. Behav Brain Res 125:167–181. [DOI] [PubMed] [Google Scholar]
- 41.Nevison CM, Hurst JL, Barnard CJ. 1999. Strain-specific effects of cage enrichment in male laboratory mice (Mus musculus). Anim Welf 8:361–379. [Google Scholar]
- 42.Olsson IA, Dahlborn K. 2002. Improving housing conditions for laboratory mice: a review of “environmental enrichment”. Lab Anim 36:243–270. [DOI] [PubMed] [Google Scholar]
- 43.Peng X, Lang CM, Drozdowicz CK, Ohlsson-Wilhelm BM. 1989. Effect of cage population density on plasma corticosterone and peripheral lymphocyte populations of laboratory mice. Lab Anim 23:302–306. [DOI] [PubMed] [Google Scholar]
- 44.Penn JK, Zito MF, Kravitz EA. 2010. A single social defeat reduces aggression in a highly aggressive strain of Drosophila. Proc Natl Acad Sci USA 107:12682–12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietropaolo S, Branchi I, Cirulli F, Chiarotti F, Aloe L, Alleva E. 2004. Long-term effects of the periadolescent environment on exploratory activity and aggressive behaviour in mice: social versus physical enrichment. Physiol Behav 81:443–453. [DOI] [PubMed] [Google Scholar]
- 46.Rock ML, Karas AZ, Rodriguez KB, Gallo MS, Pritchett-Corning K, Karas RH, Aronovitz M, Gaskill BN. 2014. The time-to-integrate-to-nest test as an indicator of wellbeing in laboratory mice. J Am Assoc Lab Anim Sci 53:24–28. [PMC free article] [PubMed] [Google Scholar]
- 47.Scott JP, Fredericson E. 1951. The causes of fighting in mice and rats. Physiol Zool 24:273–309. [Google Scholar]
- 48.Sherwin CM, Nichol CJ. 1997. Behavioural demand functions of caged laboratory mice for additional space. Anim Behav 53:67–74. [Google Scholar]
- 49.Sluyter F, Bult A, Lynch CB, van Oortmerssen GA, Koolhaas JM. 1995. A comparison between house mouse lines selected for attack latency or nest building: evidence for a genetic basis of alternative behavioral strategies. Behav Genet 25:247–252. [DOI] [PubMed] [Google Scholar]
- 50.Smith AL, Corrow DJ. 2005. Modifications to husbandry and housing conditions of laboratory rodents for improved wellbeing. ILAR J 46:140–147. [DOI] [PubMed] [Google Scholar]
- 51.Swetter BJ, Karpiak CP, Cannon JT. 2011. Separating the effects of shelter from additional cage enhancements for group-housed BALB/cJ mice. Neurosci Lett 495:205–209. [DOI] [PubMed] [Google Scholar]
- 52.Toth LA, Kregel K, Leon L, Musch TI. 2011. Environmental enrichment of laboratory rodents: the answer depends on the question. Comp Med 61:314–321. [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai PP, Pachowsky U, Stelzer HD, Hackbarth H. 2002. Impact of environmental enrichment in mice. 1: effect of housing conditions on body weight, organ weights and haematology in different strains. Lab Anim 36:411–419. [DOI] [PubMed] [Google Scholar]
- 54.van de Weerd HA. 1997. Evaluation of environmental enrichment for laboratory mice. Vet Q 19:59. [DOI] [PubMed] [Google Scholar]
- 55.Van Loo PLP, Kruitwagen CLJJ, Koolhaas JM, Van de Weerd HA, Van Zutphen LFM, Baumans V. 2002. Influence of cage enrichment on aggressive behaviour and physiological parameters in male mice. Appl Anim Behav Sci 76:65–81. [Google Scholar]
- 56.Van Loo PL, Mol JA, Koolhaas JM, Van Zutphen BF, Baumans V. 2001. Modulation of aggression in male mice: influence of group size and cage size. Physiol Behav 72:675–683. [DOI] [PubMed] [Google Scholar]
- 57.Van Loo PL, Van de Weerd HA, Van Zutphen LF, Baumans V. 2004. Preference for social contact versus environmental enrichment in male laboratory mice. Lab Anim 38:178–188. [DOI] [PubMed] [Google Scholar]
- 58.Van Loo PL, Van Zutphen LF, Baumans V. 2003. Male management: coping with aggression problems in male laboratory mice. Lab Anim 37:300–313. [DOI] [PubMed] [Google Scholar]
- 59.Vestal BM, Schnell GD. 1986. Influence of environmental complexity and space on social interactions of mice (Mus musculus and Peromyscus leucopus). J Comp Psychol 100:143–154. [Google Scholar]
- 60.Watson DS. 1993. Evaluation of inanimate objects on commonly monitored variables in preclinical safety studies for mice and rats. Lab Anim Sci 43:378–380. [PubMed] [Google Scholar]
- 61.Whitaker J, Moy SS, Godfrey V, Nielsen J, Bellinger D, Bradfield J. 2009. Effects of cage size and enrichment on reproductive performance and behavior in C57BL/6Tac mice. Lab Anim (NY) 38:24–34. [DOI] [PubMed] [Google Scholar]
- 62.Wolfer DP, Litvin O, Morf S, Nitsch RM, Lipp HP, Wurbel H. 2004. Laboratory animal welfare: cage enrichment and mouse behaviour. Nature 432:821–822. [DOI] [PubMed] [Google Scholar]
- 63.Würbel H. 2001. Ideal homes? Housing effects on rodent brain and behaviour. Trends Neurosci 24:207–211. [DOI] [PubMed] [Google Scholar]
- 64.Würbel H. 2007. Environmental enrichment does not disrupt standardisation of animal experiments. ALTEX 24:70–73. [PubMed] [Google Scholar]
- 65.Würbel H, Garner JP. 2007. Refinement of rodent research through environmental enrichment and systematic randomization. London (Great Britain): National Centre for the Replacement, Refinement, and Reduction of Animals in Research [Google Scholar]