Abstract
Recent efforts have focused on mitigating anesthetic gas emissions during laboratory animal experiments. A recently developed, digitally controlled, integrated digital vaporizer (IDV) using a syringe pump has been designed to use and administer anesthetic gas to mice and rats more efficiently. The entire IDV system can be placed on a laboratory bench, requires fewer charcoal filters to act as passive scavengers when used at a low gas flow rate, and does not need compressed gas to operate, a requirement for traditional passive systems. The objective of this study was to compare isoflurane usage between a traditional vaporizer (TdV) and an IDV system at both the same settings and those recommended by the manufacturer. We used 10 C57BL/6 male mice and administered isoflurane through either nose cones or tracheal tubes connected to a pulsatile ventilator. The results showed that isoflurane usage is highly dependent on the flow rate of the carrier gas, but the IDV system was more precise and handled low flow rates (150 mL/min) better than did the TdV system. We observed only slight differences in heart rate, respiration rate, core body temperature, time to loss of the righting reflex, and recovery time between group averages for both systems when set to manufacturer-recommended settings. Although observed decreased levels of waste anesthetic gas at low flow rates are expected from the IDV system, further work is needed to assess environmental anesthetic gas levels and exposure to laboratory personnel.
Abbreviations: IDV, integrated digital vaporizer; TdV, traditional vaporizer
Isoflurane, a methyl ethyl ether, has been used as a gas anesthetic since the 1960s.28 Previous work has shown that isoflurane is physically stable, has low blood solubility, and facilitates easy control of anesthetic depth.2 Isoflurane has been used extensively for laboratory animal research since the 1970s.3,11,25 Because of isoflurane's volatility, traditional vaporizers (TdV) have been used since the 1950s to carefully control the percentage of anesthetic in breathable gas.18,19 Vaporizer technology has stayed relatively constant during the past few decades.23 Exposure to excess anesthetic gas can lead to genetic and neurologic damage, including increased sister chromatid exchange,12,13 neurodegeneration,15 and long-term deficits in learning and memory.20 Therefore, any technology that limits the amount of waste anesthetic gas will benefit those working with isoflurane and other anesthetic gasses.
A tabletop anesthesia system that combines an integrated digital vaporizer (IDV), electronic gas flow sensor, and syringe pump in a single system that does not require periodic manual calibration has recently been developed. While a TdV depends on gas flow and atmospheric pressure for passive anesthetic vaporization, the IDV anesthetic system uses a direct injection method for vaporizing anesthetic into the gas stream. Furthermore, TdV often have flow meters that are not calibrated to low flow rates of carrier gas. These vaporizers therefore become inaccurate when used at rates below 500 mL/min, which is commonly regarded as the minimal flow rate in the veterinary field.17 The electronic gas flow sensor of the IDV system constantly measures the gas flow going to the animal. Using the ideal gas law, a microcontroller unit within the system calculates the number of moles of anesthetic gas being used. The microcontroller unit then uses the molar mass of the liquid anesthetic to determine the number of moles needed to reach the desired concentration of anesthetic in the carrier gas stream, which is equivalent to the dial setting on the vaporizer. The microcontroller unit continually sends instructions to the syringe pump to run at the required flow rate, depending on the syringe size and anesthetic percentage setting, to deliver the desired amount of anesthetic within the gas stream. The IDV system was designed for use with mice and rats up to 500 g in weight; can accommodate 2-, 5-, and 10-mL syringes, and can be used with isoflurane and sevoflurane.24 Furthermore, the system can use compressed gas up to 10 psi or room air with an internal pump, an option that eliminates the need for compressed O2 or other gases. Although the IDV is commercially available, data quantifying anesthetic usage levels with this system have not yet been reported.
The purpose of the current study was to compare a TdV with the IDV at similar and at manufacturer-recommended settings. To this end, we used standard laboratory mice and recorded multiple physiologic parameters to assess the depth of anesthesia during a 2-h study. Although the vast majority of researchers using small-animal gas anesthesia use a TdV for isoflurane,6-10 several groups have begun to use this commercially available IDV.1,5,14,16,27 Therefore, it would be helpful to quantify the amounts of isoflurane, carrier gas, and charcoal filters22 that are used during a typical anesthetic session for both systems. This information might be useful for groups conducting noninvasive imaging or surgical procedures that cannot easily obtain compressed gas, have limited space, and want to minimize the need for scavenging excess waste anesthetic.
Materials and Methods
Vaporizer analysis of isoflurane levels.
Both systems were analyzed by using a portable anesthetic gas indicator (model FI-21, RKI Instruments, Union City, CA). Compressed O2 at 0.5 L/min was used to calibrate the gas-indicator system. The analyzer system then was used to measure isoflurane levels with a sample time of at least 60 s. Both vaporizer systems were set to levels between 1% and 4% in increments of 0.5%. The room temperature for all experiments was 24 °C.
Animals and anesthetic systems.
We used 10 adult male C57BL/6 mice for this study (age, 10 to 14 wk; weight, 24.1 ± 1.6 g; Harlan Laboratories, Dublin, VA). All mice were SPF as determined by routine testing by the vendor for viruses, bacteria, mycoplasma, fungi, and parasites of the colony before arrival to our facility. The 10 mice were housed in 2 polycarbonate microisolation ventilated cages (Allentown Caging Equipment, Allentown, PA) with 1/8-in. porous processed corncob bedding (Bed-o'Cobs Combo, The Andersons, Maumee, OH). Mice had free-choice access to rodent chow (2018 Teklad Global 18% Protein Rodent Diet, Harlan Laboratories) and reverse-osmosis–purified water supplied by bottle.
Each mouse was rendered unconscious and kept on a moderate anesthetic plane by using isoflurane (Piramal Healthcare, Mumbai, India) from either a TdV (VAD Compact, Vetamac, Rossville, IN) or an IDV (SomnoSuite, Kent Scientific, Torrington, CT; Figure 1). A glass syringe filled with isoflurane was used with the IDV system. The manufacturer-recommended carrier-gas flow rates for these systems differed substantially (TdV, 500 to 10,000 mL/min; IDV, 10 to 800 mL/min). All mice initially were anesthetized in a 0.5-L induction chamber. For the first part of this study, the same settings were used for both the TdV and IDV systems (0.5 L/min compressed O2 and 1.5% isoflurane; abbreviated as TdV1 and IDV1). For this part of the study, we used a 10-mL syringe with the IDV system to ensure that there would be enough liquid isoflurane for initial induction followed by 2 h of anesthetic administration. Each mouse was anesthetized with both systems, with at least 48 h of recovery between sessions (n = 5).
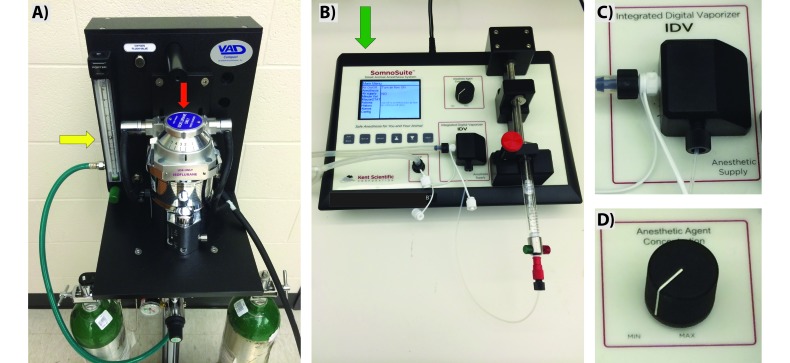
Figure 1.
Anesthetic vaporizer units used for animal studies. (A) Traditional vaporizer with portable oxygen tanks, flow meter (yellow arrow), and isoflurane reservoir (red arrow). (B) Tabletop integrated digital vaporizer with isoflurane-filled syringe, integrated digital vaporizer with digital dial control of anesthetic concentration, and room-air intake from the back of the system (green arrow). Zoomed images highlight (C) the integrated digital vaporizer with heated block and (D) the dial that can be used to adjust the speed of the syringe pump and flow of anesthetic.
For the second part of this study, the systems were operated at industry-standard settings (n = 5), including 3% isoflurane during initial induction (TdV, 1.5 L/min of compressed O2; IDV, 150 mL/min of room air with a 2-mL glass syringe). Mice then remained anesthetized for 3 different 2-h sessions, at intervals of at least 2 d, with the following anesthesia profiles: 1) nose cone with the TdV at 1.5% isoflurane in 1.5 L/min compressed O2 (TdV2); 2) nose cone with the IDV at 1.5% isoflurane in 150 mL/min room air (IDV2); and 3) tracheal tube for ventilation with the IDV at 1.5% isoflurane in a tidal volume of roughly 0.45 mL of room air (peak pressure: 15 cmH2O; mean minute volume: 62 mL/min; MouseVent, Kent Scientific; IDV3).
The nose cones used were part of a coaxial nonrebreathing system designed for small rodents (extra small, model SOMNO-0304, Kent Scientific Corporation). To ventilate these mice, we placed a 1-in. intravenous catheter (20 gauge, B Braun Medical, Bethlehem, PA) into the trachea, by using illumination from a fiberoptic cable attached to a standard flashlight. We set the integrated digital vaporizer temperature to 47.9 °C, although heating to this temperature is not required for use. The controlled heating element eliminates the problems associated with the temperature decrease due to prolonged evaporation in a passive system. In addition, the heating element delivers warm air to the mouse, assisting in maintaining the core body temperature. Isoflurane usage was determined by measuring the amount of liquid pumped from the syringe or by filling the traditional system to the same level as at the beginning of the session. The weight of each vertically positioned charcoal canister (VaporGuard, VetEquip, Pleasanton, CA) used to scavenge excess isoflurane was measured before and after each session. We also recorded the time needed for each mouse 1) to lose the righting reflex in the induction chamber and 2) to become fully ambulatory after the procedure. Specifically, ambulatory or recovery time was measured as the time between the mouse's removal from anesthetic until it was able to walk around the recovery cage. Trained personnel performed all studies in well-ventilated laboratories. The Purdue Animal Care and Use Committee approved all studies.
Monitored physiologic parameters.
We monitored and recorded each mouse's heart rate, respiration rate, and body temperature every 15 min by using a commercially available monitoring and gating system (model 1030, Small Animal Instruments, Stony Brook, NY). In accordance with the manufacturer's recommendations, heart rate was recorded by using 3 subcutaneous electrodes placed across the chest and medial to the left hindpaw. Respiration was monitored by using a passive pneumatic respiration sensor placed underneath each mouse. Mice were placed on top of a 50-W heating pad warmed to roughly 40 °C (model E12107-828B, Sunbeam, Boca Raton, FL), and lubricated fiberoptic rectal temperature probes were used to monitor core body temperature (Figure 2). We also directly assessed each animal's interdigital pinch withdrawal reflex every 15 min. The same 2 laboratory personnel recorded all of the physiologic parameters, weights, and times.
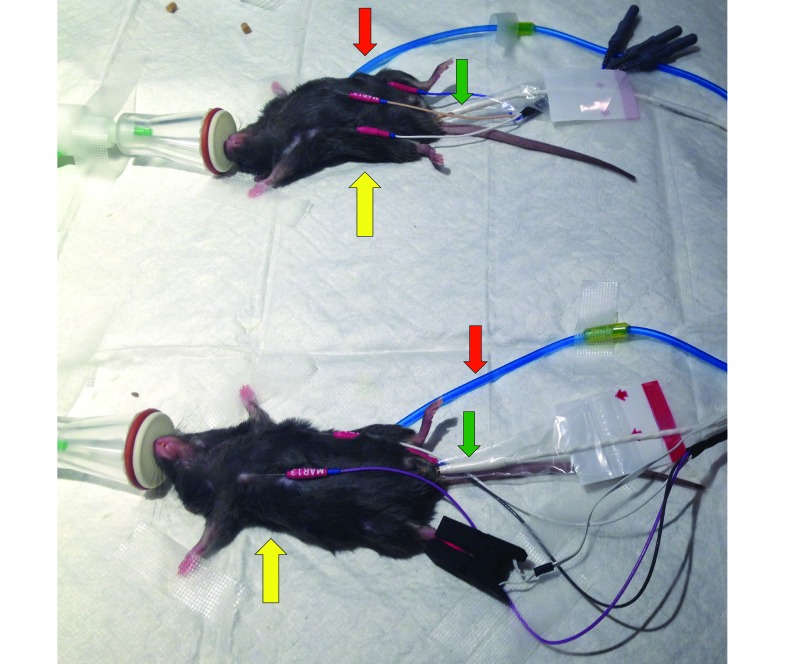
Figure 2.
Anesthetized mice undergoing physiologic monitoring. Mice breathed 1.5% isoflurane through nose cones from a traditional vaporizer (top) and integrated syringe pump–heated vaporizer (bottom) systems. Heart rate was recorded from 3 subcutaneous electrodes (yellow arrow), respiration rate was monitored by using a pneumatic pillow (red arrow), and body temperature was measured with a rectal probe (green arrow).
Statistical analysis.
JMP statistical software (version 10.0.0, SAS Institute, Cary, NC) was used for all analysis. Linear regression of the calibration data was used to quantify the slope, y intercept, R2, and 95% confidence intervals. One-way ANOVA and Tukey–Kramer post hoc tests were run to determine significance across multiple groups. We compared mean isoflurane usage, heart rate, respiration rate, body temperature, time to loss of righting reflex, and recovery time for each vaporizer system. For all tests, a P value less than 0.05 was considered significant.
Results
Anesthetic vapor production.
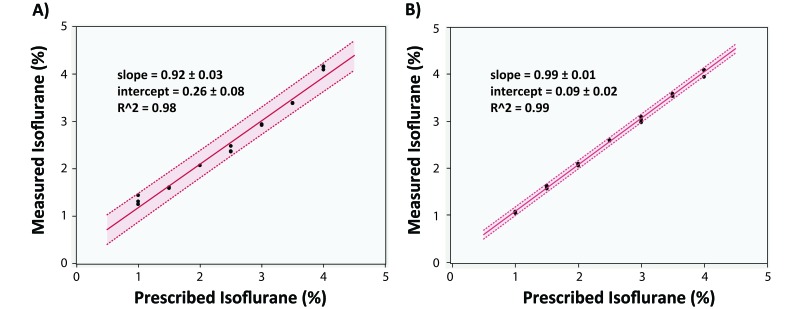
The high R2 values and slopes approximately equal to 1 for both systems suggest that these vaporizers produced fairly accurate isoflurane levels at a carrier-gas flow rate of 0.5 L/min (Figure 3). The larger 95% confidence intervals with the TdV system suggest that the IDV system was more precise when set to a specific level.
Figure 3.
Vaporizer analysis from both systems. Measured and prescribed isoflurane levels with both the (A) TdV and (B) IDV systems. Linear regression with high R2 values show that both systems are accurate, but the 95% confidence intervals suggest that the IDV is more precise when trying to set the systems to a single, specific value.
Isoflurane, charcoal filter, and carrier-gas usage.
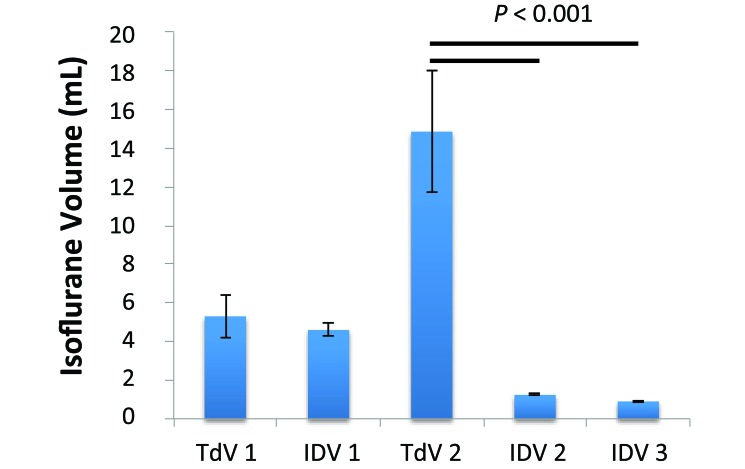
The usages of isoflurane, charcoal filters, and carrier-gas were highly dependent on the carrier-gas setting (Figure 4). When the same flow rate was used (TdV1 compared with IDV1), the TdV system used roughly 15% more isoflurane, accounting for charcoal filter weight increase of 23% more, but neither of these increases was significant (Table 1). When industry-standard flow rates were used (TdV2, IDV2, and IDV3),17 the IDV consumed significantly (P < 0.001) less isoflurane than did the TdV (Table 1, Figure 4). Charcoal canister weights increased during all sessions, but this gain was significantly (P < 0.01) more for TdV2 than IDV2 and IDV3 (Table 1). Furthermore, the TdV2 settings used roughly 180 L of compressed O2 gas, whereas the IDV 2 and IDV3 groups each used 1.8 L of room air during the same 2-h period.
Figure 4.
Isoflurane usage (mean ± 1 SD) over 2 h. Isoflurane usage is highly dependent on the flow rate of the carrier gas. Although no significant difference was observed between TD1 and IDV1, TdV2 used significantly (P < 0.001) more than the IDV during both steady flow through a nose cone and ventilated studies through a tracheal tube.
Table 1.
Anesthetic and filter usage (mean ± 1 SD)
| Amount of isoflurane vaporized (mL) | Increase in weight of charcoal filter (g) | |
| TdV1 | 5.3 ± 1.1 | 3.2 ± 2.8 |
| IDV1 | 4.6 ± 0.3 | 2.6 ± 1.5 |
| TdV2 | 14.9 ± 3.1a | 5.0 ± 2.2b |
| IDV2 | 1.24 ± 0.05 | 0.32 ± 0.50 |
| IDV3 | 0.90 ± 0.04 | 1.1 ± 0.13 |
The amount of isoflurane vaporized is highly dependent on the flow rate of the carrier gas. The initial settings with the same flow rate are similar between the 2 systems (TdV 1 and IDV 1). However, when used at manufacturer-recommended settings, significantly more isoflurane and charcoal filters were consumed with the traditional vaporizer (TdV) compared with the integrated digital system (IDV; a, P < 0.001 compared with both IDV2 and IDV3). Likewise, the weight (and therefore use) of the charcoal filter increased more with the traditional vaporizer (b, P < 0.01 compared with IDV2 and IDV3).
Physiologic parameters.
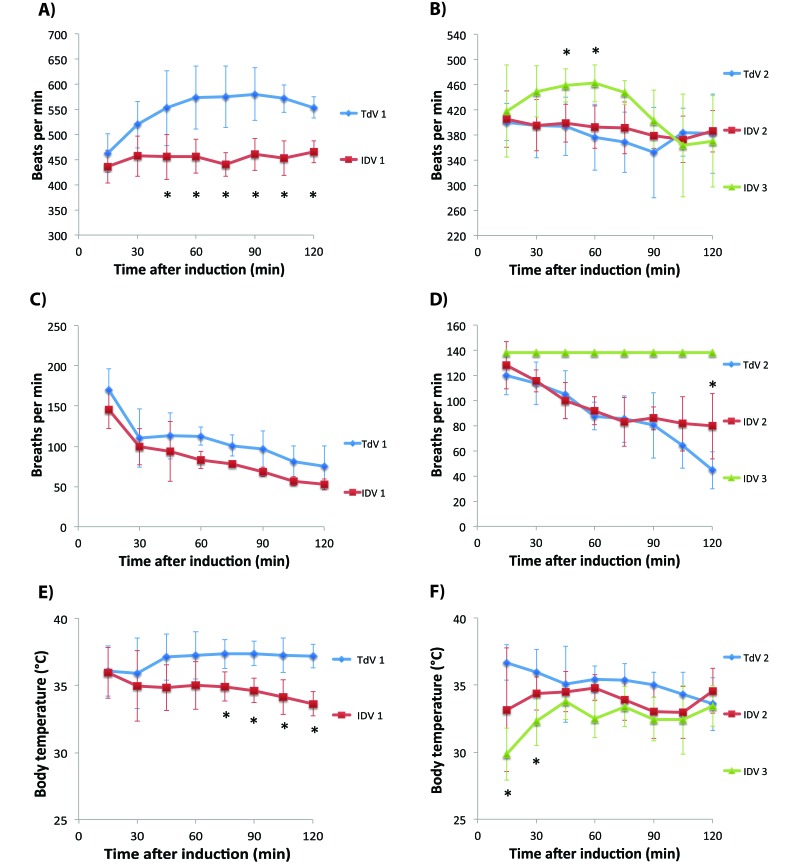
Once anesthetized, none of the mice displayed any visible withdrawal reflex from a firm interdigital pinch. The mean heart rate and body temperature were higher for the TdV1 group compared with the IDV1 group (P < 0.01; Table 2). Similar trends were seen with the instantaneous data that showed lower heart rate, respiration rate, and body temperature for some of the time points (P < 0.01; Figure 5 A, C, and E). For the second half of this study, the mean heart rate did not differ significantly between TdV2, IDV2, and IDV3 (Table 2), nor did the heart rates at the 15-min intervals differ, except at the 45- and 60-min time points, when IDV3 mice had a faster heart rate (P < 0.05; Figure 5 B). Mean respiration rate did not differ between the TdV and IDV (Table 2). Neither did the instantaneous measurements differ, except at 120 min when the IDV2 with a nosecone had a significantly (P < 0.05) higher rate than did the IDV3 group (Figure 5 D). When IDV3 mice were ventilated, the respiration rate was set to 138 breaths per minute, which was significantly (P < 0.05) faster than the natural respiration rate observed when the mice were breathing through nosecones. Finally, mean core body temperature for IDV3 mice was significantly (P < 0.01) lower than that when using the TdV (Table 2). This difference was predominantly due to the early time points of 15 and 30 min, when the ventilated mice were still in the process of achieving a sufficient anesthetic level (P < 0.05, Figure 5 F).
Table 2.
Physiologic parameters (mean ± 1 SD) over 2 h
| Heart rate (bpm) | Respiration rate (breaths per min) | Body temperature (°C) | |
| TdV1 | 548.9 ± 39.9b | 107.4 ± 29.0 | 37.0 ± 0.6b |
| IDV1 | 453.3 ± 10.1 | 84.7 ± 29.5 | 34.8 ± 0.7 |
| TdV2 | 381.4 ± 39.0 | 87.6 ± 12.8 | 35.2 ± 1.2 |
| IDV2 | 390.4 ± 24.2 | 96.0 ± 12.6 | 33.9 ± 1.1 |
| IDV3 | 421.0 ± 25.8 | 138a | 32.6 ± 1.2b |
When the same flow rate was used, the average heart rate and body temperature were higher with the TdV compared with the IDV. When manufacturer-recommended settings were used, the average heart rate did not differ significantly between the groups. The ventilated respiration rate was set to 138 breaths per minute (a, P < 0.001 compared with either the TdV or IDV). Finally, the mean body temperature for the ventilated mice was lower than that of mice using the traditional system (b, P < 0.01). This difference is likely due to the time needed to place the tracheal tube, given that mice were anesthetized but not warmed during this period.
Figure 5.
Heart rate (A, B), respiration rate (C, D), and body temperature (E, F) (mean ± 1 SD). Mice were anesthetized for 2 h, and the physiologic parameters were recorded every 15 min. Heart rates, respiration rates, and body temperatures were higher in the TdV1 group compared with IDV1 at several time points (A, C, E; *, P < 0.05). Ventilated mice had significantly faster heart rates at 45 and 60 min than did those anesthetized with a nose cone (B; *, P < 0.05), and lower body temperatures at 15 and 30 min than did mice anesthetized by using the traditional vaporizer (F; *, P < 0.05). The respiration rate was faster for mice that breathed through a nose cone from the IDV than from the TdV at 120 min (D; *, P < 0.05). The respiration rate for the ventilated mice was set by the system to 138 breaths per minute.
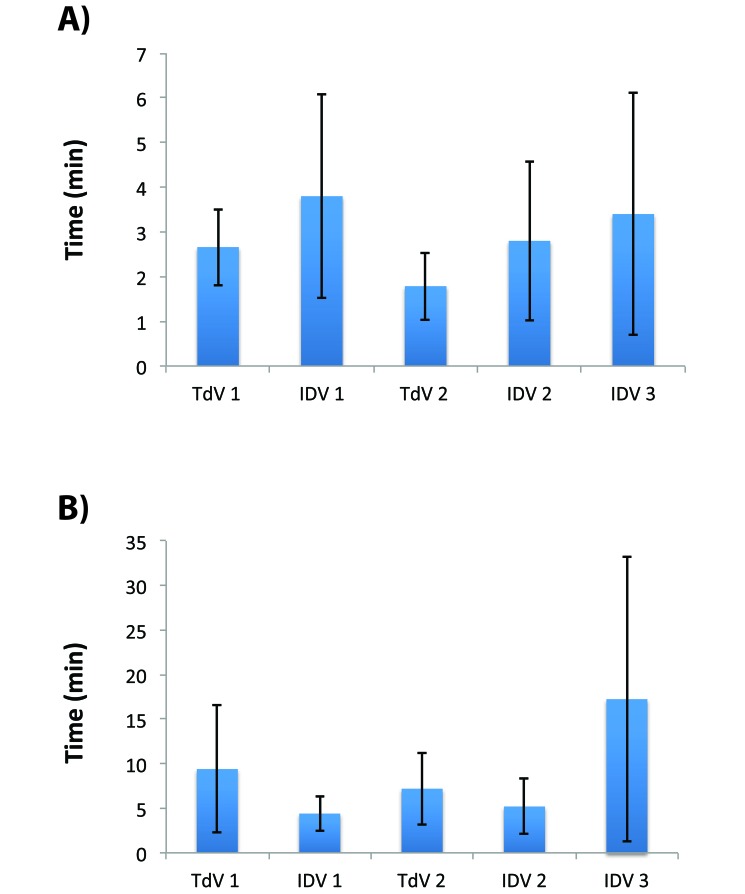
Times until loss of righting reflex and recovery.
The mean time until loss of the righting reflex did not differ significantly between the IDV and TdV systems (Figure 6 A). Likewise, the recovery time was similar between the traditional and IDV systems with steady flow from a nose cone (Figure 6 B). The ventilated mice (IDV3) appeared to take longer to recover, but this increase was not significant (P > 0.15) and was largely due to a single outlier, which took 41 min to become fully ambulatory. When this mouse was excluded from analysis, the mean recovery time for ventilated mice was reduced to 9.3 ± 3.1 min.
Figure 6.
Times to loss of righting reflex and recovery (mean ± 1 SD). (A) Mice appeared to take longer to lose consciousness in a 0.5-L chamber with the integrated digital system, but these differences were not statistically significant. (B) Likewise, recovery time did not differ between groups.
Discussion
The recent development of an anesthetic vaporizer that combines a syringe pump with a heating element is an intriguing development in vaporizer technology. As the harmful effects of exposure to excess anesthetic gas have become clearer,12,13,15,20 efforts to minimize this exposure to laboratory personnel working with laboratory animals have increased. For example, passive sampling badges can be used to collect anesthetic gas vapors over a specified period of time,21 and infrared spectroscopy measurements can be used to quantify waste anesthetic gas levels near and around anesthetized animals.22,26 These previous studies showed that both anesthetic scavenging and purging the induction chamber before opening help to improve air quality.22,26
The results of the current study showed that the IDV can be used at carrier-gas flow rates much lower than those typically used with TdV systems. Whereas the IDV certifiable carrier-gas rate range is 10 to 500 mL/min with the internal pump and 10 to 800 mL/min with compressed gas, the recommended minimal flow rate for mice with a coaxial, non-rebreathing circuit is 2 to 2.2 times the animal's minute volume.4 This recommendation means the minimal carrier gas flow rate is approximately 50 mL/min for a 25-g mouse, suggesting that all of the flow rates we used in this study should have delivered sufficient anesthetic to the mice. In addition, a flow rate of 150 mL/min is substantially lower than typical TdV ranges and most commonly used flow rates in the field of small animal research. This difference in flow rate is important, because the flow rate of the carrier gas is directly correlated to the amount of isoflurane used and scavenged.
The IDV system has several advantages over traditional systems. First, the smaller size (21.5 × 29.5 × 8 cm3) might be ideal for small spaces and research groups with few laboratory benches. Second, the ability to use room air instead of compressed gasses is beneficial for groups in facilities with limited access to gas tanks (typically containing O2 or medical-grade air). Third, the IDV system uses a microcontroller unit to actively measure the anesthetic concentration and comes precalibrated, meaning yearly calibration or maintenance typically is unnecessary. This feature reduces the cost of maintaining the vaporizer system. Fourth, the integration of ventilation and monitoring modules into the anesthetic system might be beneficial for lengthy procedures. Finally, we showed in the current study that the IDV system can be used at a low carrier-gas flow rate, thus decreasing the amount of carrier gas, number of scavenger charcoal filters, and volume of liquid anesthetic needed.
At manufacturer-recommended settings for typical usage, compared with the TdV, the IDV used roughly 1/12 the isoflurane needed for steady flow through a nose cone and 1/16 when animals were ventilated. Furthermore, the increase in charcoal filter weight with the IDV was 1/15 the weight added with the TdV when a nose cone was used (IDV2) and 1/4 that of the TdV system when the mice were ventilated (IDV3). Although waste gas levels were not quantified in this study, these data suggest that environmental exposure to waste gas is reduced with the IDV system when low flow rates are used. Additional work regarding monitoring of environmental isoflurane levels is needed to determine the actual personnel exposure with both systems. It is worth noting that the percentage of anesthetic was kept at a constant rate throughout testing, but during an actual study, isoflurane percentage likely would be decreased periodically. This scenario is especially true when an animal has to remain unconscious for several hours. Finally, the times for loss of righting reflex and recovery are somewhat subjective metrics that are not always repeatable, leading to large variability. This outcome is not unexpected and agrees with our prior experience when using isoflurane to anesthetize mice.
We can extrapolate these data from a 2-h session with typical usage settings (TdV 2, IDV 2, and IDV 3) to approximate usage for 1 y. A research group using a vaporizer for 2 h, 5 d a week, for 52 wk each year would use 3.8 L of isoflurane for a TdV, or 12 bottles (250 mL each). In comparison, the IDV system would use less than 0.32 L, or just 2 bottles. A similar yearly reduction in charcoal filter usage likely would occur as well. If we assume each filter can hold 50 g of scavenged anesthetic, then the traditional system uses roughly 21 filters, whereas the IDV system uses 6 or fewer. Finally, using compressed gas from of a tank with a storage capacity of 9500 L corresponds to almost 5 large gas cylinders used every year, compared with only one cylinder with the IDV system. However, if room air were sufficient as a carrier gas, the IDV system would not require any compressed gas tanks. We have not observed any differences when anesthetizing mice with room air compared with compressed O2 for short surgeries and procedures less than 60 min (data not shown), but the IDV manufacturer recommends the use of compressed O2 for longer surgeries. Taken as a whole, the reduction in anesthetic, charcoal filters, and compressed gas usage suggests substantial yearly savings with the IDV. Although we expect a lower operating cost with the IDV system than the TdV, the exact savings will depend on the overall isoflurane usage, frequency of use, available vendors, and scavenging procedures.
Despite its several advantages, the IDV has several limitations as well. First, once the system is powered on, the heating element requires several minutes to achieve a working temperature. Although the IDV system can be used immediately without waiting for the element to warm, environmental temperature differences will not stabilize until the element reaches a constant level. Second, the maximal flow rate with the IDV system is 0.8 L/min (or 0.5 L/min with the internal pump), where most TdV can accommodate flow rates of as high as 10 L/min. In addition, most TdV systems include a flush valve that can be used to purge induction chambers of vaporized anesthetic gas before opening. Because the IDV system lacks a flush valve, the anesthetic can be eliminated from the induction chamber only by stopping the flow of isoflurane and increasing the gas flow rate. This process can take 1 min or longer, depending on the size of the chamber, and thus increasing the chance that the animal might regain consciousness. Finally, situations may arise during which the 2-mL glass syringe requires refilling during an anesthetic session. Although refilling can typically be done quickly, filling a glass syringe and replacing it into the pump takes more time than does filling a TdV. This delay means that an animal has to come off anesthetic gas for a prolonged period of time (something that should be avoided when performing surgical procedures). Using 5-, 10-, or 25-mL syringes or having a ‘backup’ 2-mL syringe might prevent many of the complications associated with this limited volume.
Future work is needed to characterize other aspects of the IDV's overall performance. Specifically, additional physiologic parameters (including end-tidal CO2 levels, oxygen saturation, and mean blood pressure) could be monitored during an experiment that requires anesthesia. Other anesthetic types could be evaluated, given that the IDV system has also been designed to work with sevoflurane. Finally, infrared spectroscopy22,26 and personal anesthetic dosimeter badges21 could be used to quantify isoflurane waste gas levels near the animal, near laboratory personnel, and in the environment. All of these measures might further differentiate the IDV from a TdV system.
In summary, the results we presented here show that a digital IDV can adequately anesthetize laboratory mice by using a low flow rate, thus consuming less isoflurane than does a traditional system. Although several disadvantages exist with the IDV, the decreased need for liquid anesthetic, compressed gas, and charcoal filters are a benefit. In addition, the IDV system is smaller than is a TdV and might reduce the exposure of laboratory personnel to waste anesthetic gas. The IDV system might be advantageous to research groups that perform rodent procedures requiring large amounts of anesthetic or those with limited laboratory space.
Acknowledgments
This project was supported with equipment and funding by Kent Scientific Corporation, the American Heart Association (SDG18220010), and Purdue University. We gratefully acknowledge the help of Corey Berlant, A Nicole Blaize, Sriram Boppana, Shu-Chi Chang, Sydney Gorman, Ali Sacopulos, and Alexa Yrineo for their assistance recording data.
References
- 1.Butt MU, Sailhamer EA, Li Y, Liu B, Shuja F, Velmahos GC, DeMoya M, King DR, Alam HB. 2009. Pharmacologic resuscitation: cell-protective mechanisms of histone deacetylase inhibition in lethal hemorrhagic shock. J Surg Res 156:290–296. [DOI] [PubMed] [Google Scholar]
- 2.Eger EI., 2nd 1981. Isoflurane: a review. Anesthesiology 55:559–576. [DOI] [PubMed] [Google Scholar]
- 3.Eger EI, 2nd, White AE, Brown CL, Biava CG, Corbett TH, Stevens WC. 1978. A test of the carcinogenicity of enflurane, isoflurane, halothane, methoxyflurane, and nitrous oxide in mice. Anesth Analg 57:678–694. [PubMed] [Google Scholar]
- 4.Flecknell P. 2009. Laboratory animal anaesthesia. Academic Press. [Google Scholar]
- 5.Fukudome EY, Li Y, Kochanek AR, Lu J, Smith EJ, Liu B, Kim K, Velmahos GC, deMoya MA, Alam HB. 2012. Pharmacologic resuscitation decreases circulating cytokine-induced neutrophil chemoattractant 1 levels and attenuates hemorrhage-induced acute lung injury. Surgery 152:254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goergen CJ, Azuma J, Barr KN, Magdefessel L, Kallop DY, Gogineni A, Grewall A, Weimer RM, Connolly AJ, Dalman RL, Taylor CA, Tsao PS, Greve JM. 2011. Influences of aortic motion and curvature on vessel expansion in murine experimental aneurysms. Arterioscler Thromb Vasc Biol 31:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goergen CJ, Barr KN, Huynh DT, Eastham-Anderson JR, Choi G, Hedehus M, Dalman RL, Connolly AJ, Taylor CA, Tsao PS, Greve JM. 2010. In vivo quantification of murine aortic cyclic strain, motion, and curvature: implications for abdominal aortic aneurysm growth. J Magn Reson Imaging 32:847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goergen CJ, Chen HH, Bogdanov A, Sosnovik DE, Kumar AT. 2012. In vivo fluorescence lifetime detection of an activatable probe in infarcted myocardium. J Biomed Opt 17:056001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goergen CJ, Johnson BL, Greve JM, Taylor CA, Zarins CK. 2007. Increased anterior abdominal aortic wall motion: possible role in aneurysm pathogenesis and design of endovascular devices. J Endovasc Ther 14:574–584. [DOI] [PubMed] [Google Scholar]
- 10.Goergen CJ, Li HH, Francke U, Taylor CA. 2011. Induced chromosome deletion in a Williams–Beuren syndrome mouse model causes cardiovascular abnormalities. J Vasc Res 48:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hitt BA, Mazze RI, Cook TL, Beppu WJ, Kosek JC. 1977. Thermoregulatory defect in rats during anesthesia. Anesth Analg 56:9–15. [DOI] [PubMed] [Google Scholar]
- 12.Hoerauf K, Lierz M, Wiesner G, Schroegendorfer K, Lierz P, Spacek A, Brunnberg L, Nusse M. 1999. Genetic damage in operating room personnel exposed to isoflurane and nitrous oxide. Occup Environ Med 56:433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoerauf KH, Wiesner G, Schroegendorfer KF, Jobst BP, Spacek A, Harth M, Sator-Katzenschlager S, Rudiger HW. 1999. Waste anaesthetic gases induce sister chromatid exchanges in lymphocytes of operating room personnel. Br J Anaesth 82:764–766. [DOI] [PubMed] [Google Scholar]
- 14.Hu S, Ma L, Luo HM, Lin ZL, Wang XQ, Jia YH, Bai XD, Zhou FQ, Sheng ZY. 2014. Pyruvate is superior to reverse visceral hypoperfusion in peritoneal resuscitation from hemorrhagic shock in rats. Shock 41:355–361. [DOI] [PubMed] [Google Scholar]
- 15.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. 2003. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manousiouthakis E, Mendez M, Garner MC, Exertier P, Makita T. 2014. Venous endothelin guides sympathetic innervation of the developing mouse heart. Nat Commun 5:3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKelvey D, Hollingshead KW. 2003. Veterinary anesthesia and analgesia. St Louis (MO): Mosby. [Google Scholar]
- 18.Morris LE. 1952. A new vaporizer for liquid anesthetic agents. Anesthesiology 13:587–593. [DOI] [PubMed] [Google Scholar]
- 19.Morris LE, Feldman SA. 1958. Considerations in the design and function of anesthetic vaporizers. Anesthesiology 19:642–649. [DOI] [PubMed] [Google Scholar]
- 20.Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. 2002. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol 12:488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JC. 2008. Waste anesthetic gas safety. p. 183–193. Anesthesia and analgesia in laboratory animals, 2nd ed. London (United Kingdom): Academic Press. [Google Scholar]
- 22.Smith JC, Bolon B. 2002. Atmospheric waste isoflurane concentrations using conventional equipment and rat anesthesia protocols. Contemp Top Lab Anim Sci 41:10–17. [PubMed] [Google Scholar]
- 23.Sonner JM, Gong D, Li J, Eger EI, 2nd, Laster MJ. 1999. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg 89:1030–1034. [DOI] [PubMed] [Google Scholar]
- 24.Stachnik J. 2006. Inhaled anesthetic agents. Am J HealthSyst Pharm 63:623–634. [DOI] [PubMed] [Google Scholar]
- 25.Stevens WC, Eger EI, 2nd, White A, Halsey MJ, Munger W, Gibbons RD, Dolan W, Shargel R. 1975. Comparative toxicities of halothane, isoflurane, and diethyl ether at subanesthetic concentrations in laboratory animals. Anesthesiology 42:408–419. [DOI] [PubMed] [Google Scholar]
- 26.Todd TE, Morse JM, Casagni TJ, Engelman RW. 2013. Monitoring and mitigating isoflurane emissions during inhalational anesthesia of mice. Lab Anim (NY) 42:371–379. [DOI] [PubMed] [Google Scholar]
- 27.Tsukamoto A, Serizawa K, Sato R, Yamazaki J, Inomata T. 2015. Vital signs monitoring during injectable and inhalant anesthesia in mice. Exp Anim 64: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitcha JF. 1971. A history of forane. Anesthesiology 35:4–7. [DOI] [PubMed] [Google Scholar]