Abstract
Compassion, professional ethics, and public sensitivity require that animals are euthanized humanely and appropriately under both planned and emergent situations. According to the 2013 AVMA Guidelines for the Euthanasia of Animals, intraperitoneal injection of ethanol is “acceptable with conditions” for use in mice. Because only limited information regarding this technique is available, we sought to evaluate ethanol by using ECG and high-definition video recording. Mice (n = 85) and rats (n = 16) were treated with intraperitoneal ethanol (70% or 100%), a positive-control agent (pentobarbital–phenytoin combination [Pe/Ph]), or a negative-control agent (saline solution). After injection, animals were assessed for behavioral and physiologic responses. Pain-assessment techniques in mice demonstrated that intraperitoneal injection of ethanol was not more painful than was intraperitoneal Pe/Ph. Median time to loss of consciousness for all mice that received ethanol or Pe/Ph was 45 s. Median time to respiratory arrest was 2.75, 2.25, and 2.63 min, and time (mean ± SE) to cardiac arrest was 6.04 ± 1.3, 2.96 ± 0.6, and 4.03 ± 0.5 min for 70% ethanol, 100% ethanol, and Pe/Ph, respectively. No mouse that received ethanol or Pe/Ph regained consciousness. Although successful in mice, intraperitoneal ethanol at the doses tested (9.2 to 20.1 g/kg) was unsuitable for euthanasia of rats (age, 7 to 8 wk) because of the volume needed and prolonged time to respiratory effects. For mice, intraperitoneal injection of 70% or 100% ethanol induced rapid and irreversible loss of consciousness, followed by death, and should be considered as “acceptable with conditions.”
Abbreviations: GABA, γ-aminobutyric acid; MGS, mouse grimace scale; Pe/Ph, a pentobarbital–phenytoin combination product; Q1, first quartile; Q3, third quartile; r s, Spearman correlation coefficient
The care and treatment of research animals is of critical importance to researchers, veterinarians, and the general public, particularly during euthanasia. Compassion, professional ethics, and personal sensitivity require that euthanasia be performed in an appropriate, approved, and compliant method. Expert guidance, like that provided in the AVMA Guidelines for the Euthanasia of Animals,2 is essential when selecting or implementing euthanasia methods. Furthermore, the sensitive nature of this topic emphasizes the need for rigorous scientific study to evaluate euthanasia methods and identify refinements to current practice. Due to the numbers of laboratory mice (Mus musculus) and rats (Rattus norvegicus) enrolled in active protocols for research initiatives worldwide, their treatment at the time of terminal sample collection (experimental endpoint) or for welfare-related reasons (humane endpoint) is a key area of interest when exploring refinements to the practice of euthanasia.
The Office of Laboratory Animal Welfare and the USDA Animal and Plant Health Inspection Service endorse a heightened emphasis on contingency planning46,26,55 for emergencies involving animal facilities. There have been numerous recent examples of emergency circumstances under which the euthanasia of research animals might be necessary.16,17,20,23,29,41,53 Currently the most widely used euthanasia method for laboratory rodents is CO2 inhalation; however, because of debate in the scientific literature,13,34,56 the AVMA Guidelines were updated to provide very specific ‘conditions’ for the use of this inhalant.1,2 Having an available alternative to CO2 (contingent to unplanned disruption of supply or unforeseen shortage) would benefit the entire laboratory animal community, not only during emergencies but also for situations in which the conditions for use of CO2 cannot be met. For these reasons, alternatives to CO2 euthanasia continue to be explored.7 Ethanol, if humane and efficacious, would be advantageous for the research community as an alternative to other agents.
Ethanol is not controlled by federal agencies, is readily available in most research facilities, is relatively inexpensive, and requires no specialized anesthetic equipment to administer. It is available in pharmaceutical grade, does not readily support bacterial growth, has a long shelf-life, and can be stored at room temperature. Furthermore, the technique of intraperitoneal injection is commonly used in research animals and carries no greater risk to personnel than do other injections that induce only momentary discomfort in animals. For these reasons, ethanol euthanasia may be appealing for more frequent implementation in animal care programs.
For research, technical, and veterinary staff to select euthanasia methods appropriate for laboratory animals, federal regulations require the application of the AVMA Guidelines1,2 under most circumstances. The 2013 update to this guidance document introduced intraperitoneal ethanol as a method of euthanasia for laboratory mice.2 This method is listed under the category of “acceptable with conditions.” To date, only a few publications cite this method,31–33 and it was experimentally evaluated as a method of euthanasia in only one of these.31 That study31 found that the intraperitoneal injection of 0.5 mL of 70% ethanol induced death in 2 min 41 s (± 52 s) and that “no discomfort was observable in these mice.”31 Control groups were not included in the study, and specific descriptive methods for assessment of discomfort and distress in mice were not reported.31
Our current study aimed to reevaluate the physiologic and behavioral effects of intraperitoneal injection of ethanol with the benefit of advanced monitoring equipment and pain evaluation techniques. In particular, our goal was to determine whether intraperitoneal ethanol injection reliably and irreversibly resulted in loss of consciousness (defined by loss of righting reflex) followed by death (cessation of heartbeat determined by noninvasive electrocardiography). We also explored whether an intraperitoneal injection of ethanol (at 70% or 100%) results in adverse behavioral reactions including licking or chewing at the abdomen, hunched posture, abdominal constriction or writhing, pressing the abdomen against the floor, or an increased score on the Mouse Grimace Scale (MGS) 11,12,19,28 as compared with intraperitoneal injection of a pentobarbital–phenytoin combination product (Pe/Ph) or saline (0.9% NaCl) solution. Finally, after confirmation of death, samples of organs within the peritoneal cavity were collected for microscopic evaluation to assess any postinjection tissue changes.
Materials and Methods
Mice.
Mice were donated from existing rodent colonies and included animals that were not the appropriate genotype for other studies, were retired breeders, or were at the end of their experimental need. For inclusion in the current study, mice were required to be at least 8 wk of age, not pregnant or nursing, and clinically and phenotypically normal with no prior history of intraperitoneal injection. Enrolled mice (n = 91; 50 female and 41 male) ranged in age from 8 to 30 wk (mean, 17.35 wk), weighed between 18 and 36 g (mean, 27.1g), and were of mixed genetic background representing the following stocks or strains: Swiss Webster (n = 30), C57BL/6 (n = 14), and crosses between C57BL/6 and FVB/N (n = 39) or 129 (n = 8). To control for background strain and sex, a randomized block design was used. Mice first were grouped by sex and strain and then randomly assigned to treatment group. We used 6 of these 91 mice in a pilot study to verify that electrocardiography could be monitored after injection and to determine whether behavioral indices could be measured prior to loss of consciousness. All activities involving mice were approved by the IACUC of the University of Pennsylvania.
Mice were housed in accordance with the Guide for the Care and Use of Laboratory Animals.26 Briefly, mice were maintained on a 12:12-h light cycle within individual static isolation cages (Max 75, Alternative Design, Siloam Springs, AR) that were autoclaved with bedding (0.12-in. Bed-O-Cobs, Animal Specialties and Provisions, Quakertown, PA) prior to use. Mice received autoclaved, acidified water from autoclaved water bottles and were provided unrestricted access to autoclaved chow (LabDiet 5010, Animal Specialties and Provisions). Cotton squares (Ancare, Bellmore, NY) or paper shelters (Shepherd Specialty Papers, Milford, NJ) were provided to all cages for enrichment. Mice were housed in same-sex groups of 5 or fewer animals per cage.
For 3 quarters of each year sentinel mice at our institution were tested inhouse and were found to be free from fur mites and pinworms (Syphacia spp. and Aspiculuris spp., by anal tape test and cecal exam). In addition, sentinel mice were tested serologically and were negative for antibodies to mouse hepatitis virus, mouse parvovirus, minute virus of mice, rotavirus, and Theiler murine encephalomyelitis virus. For one quarter each year, live sentinels from the housing facility were shipped to Charles River Laboratories (Wilmington, MA) for testing (HM Plus Panel, Charles River Laboratories) and found to be free from all evaluated pathogens; in addition, their mesenteric lymph nodes were tested by PCR assay for and were free of mouse parvoviral DNA.
Intraperitoneal injection of mice.
Intraperitoneal injection42 was performed by a single researcher (KAW). Briefly, mice were restrained by firmly grasping the skin over the dorsal neck by using the thumb and forefinger of the handler's nondominant hand, with the tail held between the palm and ring finger of the same hand. The mouse's head was tilted downward and the needle inserted at an angle of approximately 30° to the abdominal wall, on the left of midline in the caudal left abdominal quadrant. A fresh 25-gauge, 3/4-in. needle was used for each mouse, and the needle was inserted no more than 0.5 cm into the abdomen. To minimize the risk of local tissue irritation, the needle was wiped with a dry piece of nonsterile gauze (Kendall Versalon nonsterile 4 × 4, Covidien, Mansfield, MA) prior to insertion in the abdomen, as described.31 The plunger was retracted to verify negative pressure within the peritoneal space and the absence of ingesta. The injected volume was standardized at 0.5 mL, regardless of treatment group. Pure, USP grade, 100% ethanol (Deacon Industrial Supply, King of Prussia, PA) either was diluted to 70% (v/v) in 0.9% sterile saline (Baxter Healthcare, Deerfield, IL) by using aseptic technique to transfer ethanol and saline to an empty sterile vial for mixing (10-mL sterile empty vial, Hospira, Lake Forest, IL) prior to injection at an average dose of 10.2 g/kg or used without dilution (that is, 100%) at an average dose of 15.3 g/kg. Unused diluted ethanol was stored at room temperature and discarded after 48 h. A Pe/Ph combination product (390 mg pentobarbital sodium and 50 mg phenytoin sodium per mL, Euthasol, Virbac Animal Health, Fort Worth, TX) was used (average dose, 5.4 g pentobarbital per kg of body weight) as the positive control for euthanasia. Sterile saline (0.5 mL of 0.9% saline) was used as a negative control for euthanasia. Mice that received saline were euthanized 5 min after injection by carbon dioxide inhalation, followed by cervical dislocation as a secondary method, in accordance with the AVMA Guidelines for the Euthanasia of Animals.2
ECG recordings in mice.
Heart rate and time to cardiac arrest were determined by using noninvasive ECG recording (ECGenie, Mouse Specifics, Quincy, MA) as previously described.6 Briefly, mice were introduced to the elevated recording platform and allowed to freely explore the recording stage. A disposable pad containing 3 lead-electrode plates (ECGenie, Mouse Specifics, Quincy, MA) covered the surface of the recording stage beneath the mouse. ECG recording was possible when at least 2 of the mouse's paws were in contact with 2 of the electrode plates. Mice remained on the recording platform for 5 min, during which short interpretable segments of ECG were recorded when the mouse was in contact with the electrode plates and not actively moving. After this 5-min period of baseline recording mice, were removed from the platform to receive an intraperitoneal injection. At the time of injection, all mice were observed for signs of discomfort, including kicking at the needle or vocalization in the range of human hearing (no specialized audio recording devices were used to record ultrasonic vocalizations). The noninvasive ECG recording study included 40 mice total: 10 mice received Pe/Ph, 16 received 70% ethanol, and 14 received 100% ethanol. No saline-treated mice were included in the ECG recording group, because these animals were not expected to experience cardiac arrest as a result of their intraperitoneal injection.
Immediately after injection, each mouse was returned to the recording platform, and ECG recording resumed as previously. When QRS complexes were absent, the unconscious mouse was carefully repositioned to verify that a loss of contact with the electrodes was not the cause of signal loss. ECG recording continued until cardiac arrest (absence of recognizable QRS complexes for 60 s or longer). The time at which the last recognizable QRS complex was recorded was taken as the time of cardiac arrest. After cardiac arrest, the mouse was removed from the recording platform and placed at room temperature on a padded surface in dorsal recumbency. No secondary method of euthanasia (for example, cervical dislocation) was performed; instead the mouse was monitored for recovery or the development of rigor mortis. For the purpose of this study, a mouse was determined to be in rigor mortis when the abdominal muscles became taut and the legs could not easily be flexed or extended with manual pressure.
High-definition video recording of mice.
Mice were gently placed in a clear acrylic chamber and allowed to acclimate to the chamber for at least 5 min. Video recording began when the mouse was introduced to the chamber (preinjection control) and continued through injection until respiratory arrest (absence of spontaneous respiration for 60 s or longer). A high-definition camcorder (HDR-CX 380, Sony Electronics, Tokyo, Japan) was used at a rate of 60 interlaced frames per second and a resolution of 1920 × 1080. Because the noninvasive ECG recording equipment interfered with behavioral observations and video recording, the mice used in this experiment were distinct from those described earlier. The video monitoring population comprised 45 mice: 10 mice received Pe/Ph, 10 received saline, 13 received 70% ethanol, and 12 mice received 100% ethanol.
At the time of injection, all mice were observed for signs of discomfort, including kicking at the needle and vocalization, as described for the ECG mice. In addition, after injection, but prior to loss of consciousness, video recorded mice were monitored for potential signs of abdominal pain, including hunched posture, abdominal constriction or writhing, licking or chewing at the abdomen, and pressing the abdomen to the floor. Every 15 s after injection, the mouse was gently manipulated to determine when the righting reflex was lost. After loss of the righting reflex the mouse was observed for evidence of respiration and the time of respiratory arrest (absence of spontaneous respiration or agonal breaths for 60 seconds or longer) was noted. The time at which the mouse took its last overt breath was recorded as the time of respiratory arrest. At 3 min after respiratory arrest, mice were tested for a withdrawal reflex by firmly pinching the toes on a hindpaw; mice that lacked a withdrawal reflex were confirmed dead by cervical dislocation. A postmortem exam was performed and tissues collected for histopathologic evaluation.
MGS scoring.
Images were obtained for evaluation by using a modified version of the MGS, as described previously.28 Action units of the MGS were used except for whisker position, which was deemed too difficult to observe and score in the current study. Briefly, still images (3 before injection, 3 after injection [1 each from 0 to 15 s, 15 to 30 s, and 30 to 45 s]) of the mouse's face in which the ears, nose, and eyes were in focus were captured from the video file. No images for MGS analysis were obtained following loss of righting reflex. Images were cropped so that the mouse's body posture was not visible. Cropped images were added to a PowerPoint (Microsoft, Redmond, WA) presentation, and the order of images randomized by using an available macro (http://www.tushar-mehta.com/powerpoint/randomslideshow/index.htm).28,35 Images in the randomized slide show were numbered sequentially and scored by 3 observers trained to use the modified MGS. Observers were blinded regarding treatment group and whether the image was taken during the pre- or postinjection interval. Action units were scored on a scale of 0 (not present), 1 (moderately visible), or 2 (severe or obviously visible). Scores from each observer were averaged to obtain a mean preinjection and mean postinjection score for each mouse. The difference between these scores was calculated to determine the change in MGS for each animal. Indicating the degree of agreement in MGS scores among the 3 observers, the intraclass correlation coefficient (95% confidence interval) was: preinjection, 0.32 (0.13, 0.52); postinjection, 0.41 (0.23, 0.59); and mean score change, 0.40 (0.21, 0.58).
Histologic preparation and scoring of mouse tissues.
After high-definition video recording, each mouse was necropsied. During necropsy, the observer noted the presence of erythema, bruising, intraabdominal fluid, hemorrhage, edema, and distention of organs or subcutaneous or intramuscular space. The presence of segmental bruising and enlargement or distention of a viscus or structure, absence of free fluid in the abdomen, and a prolonged time to loss of righting reflex or respiratory or cardiac arrest was considered indicative of incorrect intraperitoneal injection technique. No dye was added to the injected agents to avoid interference with subsequent histologic staining and evaluation. Samples of the trachea, esophagus, heart, lungs, salivary glands, liver, spleen, pancreas, kidneys, adrenal glands, urinary bladder, gonads, uterus or seminal vesicles, intestines, cecum, stomach, and left and right abdominal body wall including peritoneum were collected and fixed in 10% neutral buffered formalin (Thermo Fisher Scientific, Waltham, MA). All collected organs were fixed for a minimum of 3 d prior to trimming. Tissues were systematically trimmed and arranged into cassettes. Formalin-fixed tissues were processed and stained with hematoxylin and eosin by the Histology Laboratory of the Veterinary Hospital of the University of Pennsylvania (Philadelphia, PA) according to standard operating procedure. The slides were evaluated by a board-certified veterinary pathologist (AKB) blinded to treatment group, age, and strain of mouse. Individual organs were assigned a score of 0 to 3 for observed changes: 0, minimal to no change; 1, mild change; 2, moderate change; and 3, severe change. Tissues were scored according to 3 categories of change: hemorrhage or edema, inflammation or inflammatory cells, and loss of architectural detail and tinctoral quality (differential affinity for hematoxylin and eosin was absent or reversed; cell boundaries or nuclei were indistinct).
Statistical analysis of mouse studies.
For the data that were normally distributed, ANOVA was used to compare means among treatment groups (70% and 100% ethanol, Pe/Ph, and saline). For the data that were not normally distributed (that is, time to loss of righting reflex and time to respiratory arrest), the Wilcoxon rank-sum test was used to compare medians across treatment groups. The χ2 test was used to compare proportions of mice exhibiting reactions to injection across treatment groups. The Fisher exact test was used to compare the frequency distribution of histology scores and pooled postinjection behavioral signs of pain, due to the small number of observations in some score categories. Wilcoxon rank-sum and Fisher exact tests were used to compare the outcomes between incorrectly and correctly injected mice.
The correlations between dose and age with the time to cardiac arrest and time to rigor mortis were evaluated by using Spearman correlation coefficients (rs). Repeated-measures ANOVA was used to compare the pre- and postinjection MGS scores between treatment groups. All statistical analyses were performed in SAS version 9.2 (SAS Institute, Cary, NC). Statistical significance was set at a P value of less than 0.05. For the statistical power, because these studies involved various outcome measurements and because the magnitude of means and standard deviations differed across measurements, we used the effective size (defined as the mean difference divided by the standard deviation) for calculating the statistical power.
Rats.
Rats were donated from existing colonies and included those animals that were not the appropriate genotype for other studies or were at the end of their experimental need. For inclusion in the current study, rats were required to be at least 7 wk old, not pregnant or nursing, and clinically and phenotypically normal, with no prior history of intraperitoneal injection. Enrolled rats (n = 16; 6 female and 10 male) ranged in age from 7 to 8 wk (mean, 7.5 wk), weighed between 178 and 289 g (mean, 236.5 g), and were all Long–Evans outbred stock (Charles River, Wilmington, MA). All activities involving rats were approved by the IACUC of the University of Pennsylvania.
Rats were housed in accordance with the Guide for the Care and Use of Laboratory Animals.26 Briefly, rats were maintained on a 12:12-h light cycle in individual static isolation cages (141 in.2, polycarbonate, Alternative Design) that were autoclaved with bedding (0.25-in. Bed-O-Cobs, Animal Specialties and Provisions) prior to use. Rats received autoclaved, acidified water through autoclaved water bottles and were provided ad libitum access to autoclaved chow (LabDiet 5012, Animal Specialties and Provisions). Red polycarbonate tubes (3 × 6 in., Animal Specialties and Provisions) were provided to all cages for enrichment. Rats were housed in same-sex groups of 3 to 6 animals per cage, depending on body weight.
Sentinel rats at our institution were tested quarterly and were found to be free from fur mites and pinworms (by anal tape and cecal exams). Sera from sentinel rats were tested serologically and were negative for antibody to rat coronaviruses, rat parvoviruses, rat theilovirus, and Pneumocystis carinii (Prevalent Panel, Charles River Laboratories).
Intraperitoneal injection of rats.
For intraperitoneal injection, rats were restrained within a towel wrapped around the rat's head and body. The rat was held, within the towel, with its back along the handler's forearm with one hind leg exposed and held in the user's nondominant hand to prevent the rat from rotating within the towel. The needle was inserted at an angle of approximately 30° from the abdominal wall to the left of midline at the level of the prepuce in male rats or between the most caudal 2 nipples in the mammary chain in female rats. A fresh 22-gauge, 1-in. needle was used, and the needle was inserted no more than 0.5 cm into the abdomen. To minimize the risk of local tissue irritation, the needle was wiped with a dry piece of nonsterile gauze (Kendall Versalon nonsterile 4 × 4, Covidien) prior to insertion in the abdomen.31 The plunger was retracted to verify negative pressure within the peritoneal space and the absence of ingesta. Pure, USP grade, 100% ethanol (Deacon Industrial Supply) was either diluted to 70% (v/v) in 0.9% sterile saline (Baxter Healthcare) by using aseptic technique to transfer ethanol and saline to an empty sterile vial (10-mL sterile empty vial, Hospira) for mixing prior to injection (average dose, 9.2 g/kg) or used without dilution (that is, 100%) at an average dose of 13.4, 17.6, or 20.1 g/kg. Unused diluted ethanol was stored at room temperature and discarded after 48 h. A Pe/Ph combination (Euthasol, Virbac Animal Health) was used as the positive control for euthanasia, and 0.9% sterile saline was used as a negative control. Of the 16 rats assessed, the majority (n = 12) were used to determine a dose range for intraperitoneal injection of ethanol to achieve euthanasia. Two rats received only saline (Baxter Healthcare, Deerfield, IL) at a volume corresponding to the volume associated with the highest tested dose of 100% ethanol (7 mL) to determine whether adverse behavioral signs were associated with the volume of injected fluid. Two rats received Pe/Ph at 200 mg pentobarbital per kg of body weight as a positive control for euthanasia. Rats that received saline or ethanol but failed to lose consciousness were euthanized 5 min after injection by carbon dioxide inhalation in accordance with the AVMA Guidelines for the Euthanasia of Animals.2 At the time of injection, all rats were observed for signs of discomfort, including kicking at the needle and vocalization, and for potential signs of abdominal pain. including hunched posture, abdominal constriction or writhing, licking or chewing at the abdomen, and pressing the abdomen to the floor. Data from rats were not analyzed statistically.
During testing of intraperitoneal ethanol injection for euthanasia of rats, we found that it was unsuitable for this purpose; thus work on rats was halted. For this reason, the rat studies lack statistical power, and only descriptive analysis of rat data was performed. These studies involved various outcome measurements, and the magnitudes of the means and standard deviations differed across measurements; therefore we used the effective size (defined as the mean difference divided by the standard deviation) for calculating the statistical power. The power calculation indicated that the rat study provided approximately 66% power to detect an effective size of 1.0, 84% power to detect an effective size of 1.25, and 95% power to detect an effective size of 1.50 at the α level of 0.05.
Results
ECG of mice.
After intraperitoneal injection the time (mean ± SE) to cardiac arrest was 4.6 ± 0.5 min for the Pe/Ph group (n = 10), 6.0 ± 1.3 min for the 70% ethanol group (n = 12), and 3.0 ± 0.6 min for the 100% ethanol group (n = 11). No statistical differences were found between Pe/Ph and either 70% or 100% ethanol in the time to induce cardiac arrest. After cardiac arrest, mice were observed for the development of rigor mortis; cervical dislocation was not performed. All mice that received ethanol or Pe/Ph progressed to rigor mortis, with none of the animals recovering consciousness after loss of righting reflex. Time from cardiac arrest to onset of rigor mortis was 49.8 ± 2.3 min for the Pe/Ph group, 53.2 ± 4.3 min for the 70% ethanol group, and 49.2 ± 2.8 min for the 100% ethanol group. Once again, no statistical differences were found in the time to achieve rigor mortis between the Pe/Ph and either the 70% or 100% ethanol groups. There were no significant correlations identified between the dose of Pe/Ph or ethanol given and time to cardiac arrest for any of the treatment groups. Similarly, there were no significant correlations identified between age of mouse and time to cardiac arrest for any of the treatment groups (data not shown).
Loss of righting reflex and respiratory arrest.
Mice that received saline did not lose righting reflex or experience respiratory arrest as a result of their injection; therefore, these mice were not compared with mice receiving Pe/Ph or ethanol for these parameters. The median time (first [Q1] and third [Q3] quartiles) to loss of righting reflex was 45 s (Q1, 45; Q3, 60) for the Pe/Ph group (n = 10), 45 s (Q1, 38; Q3 = 60) for the 70% ethanol group (n = 12), and 45 s (Q1, 45; Q3, 45) for the 100% ethanol group (n = 11); there were no differences among treatment groups. After the righting reflex was lost mice were observed for respiratory arrest. The median time from intraperitoneal injection to respiratory arrest was 2.6 min (Q1, 2.5; Q3, 3.0) for the Pe/Ph group (n = 10), 2.8 min (Q1, 2.0; Q3, 4.25) for the 70% ethanol group (n = 12), and 2.3 min (Q1, 2.0; Q3, 3.0) for the 100% ethanol group (n = 11); none of these differences were statistically significant. There were no correlations identified between the dose of Pe/Ph or ethanol and time to loss of righting reflex for any of the treatment groups. Similarly, correlations between dose and time to respiratory arrest were not identified in any of the treatment groups. However, a statistically significant positive correlation was identified between the age of mouse and the time to loss of righting reflex both for the Pe/Ph group alone (rs= 0.72; P = 0.02) and for all groups combined (rs = 0.40; P = 0.02). A similar trend of positive correlation (rs = 0.34; P = 0.051) was seen between age of mouse and time to respiratory arrest when all groups were taken together.
Pain assessments.
The proportion of mice that vocalized on injection differed significantly (χ2; P = 0.03) between the 4 treatment groups, with the saline group having the greatest proportion of mice which vocalized during injection (6 of 10 mice). There were no statistical differences among the proportions of mice that vocalized in the 70% ethanol (6 of 24 mice) and Pe/Ph (3 of 20 mice) groups, but there was a trend (P = 0.050) toward more mice in the 100% ethanol group (11 or 24 mice) vocalizing than those in the Pe/Ph group. There were no significant differences in the proportion of mice that kicked at the needle during injection among the Pe/Ph (7 of 20 mice), saline (4 of 10 mice), 70% ethanol (10 of 24 mice), and 100% ethanol (10 of 24 mice) groups. Because very few mice showed any behavioral signs of pain after injection, all signs were pooled for analysis. The Fisher exact test revealed a significant (P = 0.039) difference in the proportion of mice showing postinjection behavioral signs of pain among the 4 treatment groups. More saline-treated mice (3 of 10) exhibited at least one sign of abdominal pain (abdominal constriction, hunched posture) than did mice in the other treatment groups. When compared with the Pe/Ph treatment group (2 of 20 mice; hunched posture, licking/chewing at abdomen), there were no statistical differences for mice treated with 100% ethanol (2 of 24; hunched posture, licking/chewing at abdomen) or 70% ethanol (0 of 24) in the proportion of mice exhibiting behavioral signs of pain. MGS scores for preinjection, postinjection, and mean score changes from before to after injection (Table 1) revealed no differences in the preinjection MGS scores among groups. Relative to preinjection scores, the average postinjection MGS scores increased for both 100% ethanol (P = 0.02) and Pe/Ph (P = 0.01) mice. There were no differences in the postinjection MGS scores between the saline, 70% ethanol, and Pe/Ph groups or between the postinjection MGS scores of the Pe/Ph and 100% ethanol groups. Postinjection MGS scores were significantly (P = 0.01) higher for the 100% ethanol group when compared with saline group. The mean MGS score change was significantly higher (P < 0.02) for both the Pe/Ph and 100% ethanol groups than for the saline group.
Table 1.
Summary of Mouse Grimace Scale (MGS) scores and change (mean [SE]) in MGS score
| Treatment group (n) | Preinjection score | Postinjection score | Change in score |
| Pentobarbital–phenytoin (10) | 0.381 (0.080) | 0.481 (0.093) | 0.100 (0.039)a |
| 0.9% Saline (10) | 0.368 (0.058) | 0.303 (0.051) | −0.065 (0.055) |
| 70% Ethanol (12) | 0.351 (0.036) | 0.355 (0.039) | 0.004 (0.058) |
| 100% Ethanol (11) | 0.363 (0.063) | 0.468 (0.042)b | 0.105 (0.047)c |
Significant (P = 0.01) difference between pre- and postinjection values
Significant (P = 0.01) difference between postinjection scores for 100% ethanol and saline groups
c.Significant (P = 0.02) difference in change in score between 100% ethanol and saline groups
Histologic score.
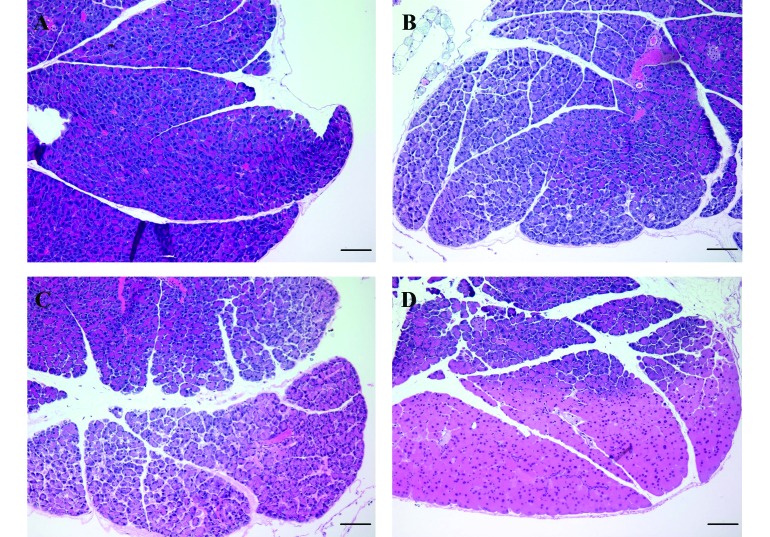
The most common histologic score (that is, the mode) for each tissue and the percentage of mice assigned that score are provided in Table 2. Mild alveolar hemorrhage was a frequent finding in the lungs of mice in this study, regardless of treatment group. A mild, diffuse, lymphoplasmacytic and histiocytic enteritis or enterocolitis was a consistent finding, and affected mice were present in all treatment groups. In addition, mild perivascular hepatitis and injection-site abdominal myositis were identified occasionally, but no significant association with treatment group was present. Mice that received an intraperitoneal injection of Pe/Ph had significantly higher histologic scores for loss of tinctoral quality and architectural detail of the liver, spleen, pancreas, kidneys, and intestine than mice in all other treatment groups (P < 0.03 for all comparisons). Representative images of each score level for loss of tinctoral quality are shown in Figure 1. Similarly, mice that received Pe/Ph had a significantly (P < 0.0001) higher histologic score for splenic hemorrhage than did mice treated with saline, 70% ethanol, or 100% ethanol. In most cases, the loss of tinctoral quality and cellular architectural detail was most apparent in the subcapsular region of the affected organ, with the exception of the pancreas, which was often diffusely affected.
Table 2.
Most frequent histologic score for mice in each treatment group and organ
| Pe/Ph (n = 10) | Saline (n = 10) | 70% Ethanol (n = 12) | 100% Ethanol (n = 11) | ||
| Liver | Loss of tinctoral quality | 3a (90) | 0 (100) | 0 (83) | 0 (72) |
| Edema or hemorrhage | 0 (90) | 0 (100) | 0 (67) | 0 (81) | |
| Inflammation | 0 (90) | 0 (100) | 0 (67) | 0 (81) | |
| Spleen | Loss of tinctoral quality | 3b (80) | 0 (100) | 0 (92) | 0 (91) |
| Edema or hemorrhage | 3c (80) | 0 (100) | 1d (67) | 1d (72) | |
| Inflammation | 0 (100) | 0 (100) | 0 (100) | 0 (100) | |
| Pancreas | Loss of tinctoral quality | 3e (100) | 0 (90) | 1f (50) | 1f (45) |
| Edema or hemorrhage | 0 (100) | 0 (100) | 0 (100) | 0 (100) | |
| Inflammation | 0 (100) | 0 (100) | 0 (100) | 0 (100) | |
| Small intestine | Loss of tinctoral quality | 3g (90) | 0h (80) | 2 (42) | 2 (36) |
| Edema or hemorrhage | 0 (60) | 0 (90) | 1i (75) | 1i (54) | |
| Inflammation | 0 (80) | 0 (80) | 1 (67) | 1 (72) | |
| Large intestine | Loss of tinctoral quality | 3 (80) | 0j (90) | 2 (33) | 2 (45) |
| Edema or hemorrhage | 0 (60) | 0 (90) | 1k (75) | 1k (54) | |
| Inflammation | 0 (70) | 0 (70) | 1 (58) | 1 (64) |
Because scores were not normally distributed, score frequencies were compared. Data are given as mode scores for each treatment group (percentage of mice represented by that score). Treatments included a pentobarbital–phenytoin combination (Pe/Ph), 0.9% saline, 70% ethanol diluted in saline, and 100% ethanol. Mode scores for uterus, seminal vesicle, and urinary bladder were all 0; these scores have been omitted from the table. In most cases, scores were higher for mice in the Pe/Ph group. Scores for edema in the small and large intestines were higher for mice in both the 70% and 100% ethanol groups when compared with saline but were not different from those for mice treated with Pe/Ph.
Significant (P < 0.0001) difference between score for Pe/Ph and those for all other treatment groups
Significant (P < 0.0001) difference between score for Pe/Ph and those for all other treatment groups
Significant (P < 0.0001) difference between score for Pe/Ph and those for all other treatment groups
Significant (P < 0.0001) difference between scores for 70% and 100% ethanol and that for the saline group
Significant (P < 0.0002) difference between score for Pe/Ph and all other treatment groups
Significant (P < 0.0001) difference between scores for 70% and 100% ethanol and that for the saline group
Significant (P = 0.02) difference between score for Pe/Ph and that for the 70% or 100% ethanol group
Significant (P < 0.004) difference between score for saline and those for all other treatment groups
Significant (P < 0.01) between scores for 70% and 100% ethanol and that for the saline group
Significant (P < 0.004) between score for the saline group and those for all other treatment groups
Significant (P < 0.02) between scores for the 70% and 100% ethanol groups and that for the saline group
Figure 1.
Photomicrographs showing representative images of each score level for loss of architecture or tinctoral quality for the pancreas of mice used in this study. (A) Score of 0, minimal to no change; (B) score of 1, mild increase in intralobular space and cell shrinkage; (C) score of 2, moderate increase in intralobular space, cellular fragmentation and loss of staining differential at the periphery; and (D) score of 3, severe loss of staining differential extending to deeper cell layers, loss of zymogen granule staining, indistinct cell boundaries and indistinct nuclear boundaries affecting at least 75% of tissue on the slide. Mice that received pentobarbital–phenytoin had significantly (P < 0.0001) higher scores for the loss of architecture or tinctoral quality. Mice treated with ethanol at either 70% or 100% had significantly (P < 0.0002) higher scores for loss of architecture or tinctoral quality than did mice treated with saline. Hematoxylin and eosin stain; scale bar, 100 µm.
Mice in both the 70% and 100% ethanol groups had higher scores for edema in the small intestine (P < 0.01) or large intestine (P < 0.02) than did mice treated with saline, but these scores were not significantly different from those of mice treated with Pe/Ph. In addition, the muscle and peritoneum of the left and right body walls were commonly affected by a focal or multifocal to focally extensive loss of tinctoral quality, loss of cellular architecture, or myocyte degeneration. Once again, the mice in the Pe/Ph treatment group had significantly higher scores than did the other groups (P < 0.03 for all comparisons). There were no differences between saline and either 70% or 100% ethanol. The uterus or seminal vesicle on the side of injection (left) occasionally was affected by a diffuse to focally extensive loss of tinctoral quality and loss of cellular detail.
Assessment of intraperitoneal injection technique.
Incorrect intraperitoneal injection technique was confirmed on postmortem exam in 7 of 85 mice by the presence of localized or segmental erythema, bruising, and distention of a structure; the absence of free fluid in the abdomen; and a prolonged time to loss of righting reflex and/or cardiac or respiratory arrest. Of these 7 mice, 5 mice received 70% ethanol and 2 mice received 100% ethanol. Both of the mice dosed with 100% ethanol and 3 mice dosed with 70% ethanol experienced an intraintestinal injection, one mouse from the 70% ethanol group experienced an intramuscular injection, and one mouse from the 70% ethanol group experienced a subcutaneous injection. No mice that received Pe/Ph or saline were confirmed to have had an incorrect intraperitoneal injection. Mice that received an incorrect intraperitoneal injection were analyzed as a separate group and compared with mice that received accurate intraperitoneal delivery of their injected substance. During injection, the proportions of mice that vocalized or kicked at the needle did not differ between mice that received an incorrect intraperitoneal injection (n = 7) and those that received a correct intraperitoneal injection (n = 78). During the postinjection observation period, there were no statistical differences in the proportions of mice that demonstrated behavioral signs of pain when correctly and incorrectly injected mice were compared. Mice that had an incorrect intraperitoneal injection (n = 7) still progressed to lose consciousness, but with a median time to loss of righting reflex of 105 s (Q1, 80; Q3, 285), which was significantly (P < 0.0001) longer than that of their correctly injected counterparts. In addition, incorrectly injected mice demonstrated significantly longer times to respiratory arrest (n = 7; median, 16 min; Q1, 4.3; Q3, 60; P = 0.006) and to cardiac arrest (n = 5); median, 17.5 min; Q1, 17.5; Q3, 45; P = 0.002) than did their correctly injected counterparts. All mice that received an incorrect intraperitoneal injection progressed to rigor mortis, and none of these mice recovered or regained consciousness after loss of the righting reflex.
Intraperitoneal injection of ethanol in rats.
Ethanol doses from 9.2 to 20.1 g/kg using both 70% ethanol and 100% ethanol concentrations were attempted in rats, with each dose repeated in duplicate. Of the 2 rats that received Pe/Ph, both lost the righting reflex at 75 s and experienced respiratory arrest at 4.5 min. Of the 2 rats that received 70% ethanol (9.2 g/kg; 3.9 mL; average body weight 233 g), only one lost consciousness during the 5-min observation period. Neither of the 70% ethanol rats experienced respiratory arrest due to the injection, and both were euthanized with CO2. Of the rats (n = 10) that received 100% ethanol at a dose of 13.3 g/kg (4.25 mL; average body weight, 249 g), 17.9 g/kg (4.5 mL; average body weight, 197 g), or 20.1 g/kg (7.13 mL; average body weight, 278 g), the mean time to loss of the righting reflex was 110 s for the 13.4-g/kg dose, 90 s for the 17.9-g/kg dose, and 82.5 s for the 20.1-g/kg dose. Only one of the rats in the lower dosage groups experienced respiratory arrest within the observation period (15 min); the other 3 subsequently were euthanized with CO2. The time to respiratory arrest for rats receiving the highest dose (20.1 g/kg; total volume, 7.13 mL) was 8 ± 5 min.
In addition, 8 of the 12 rats (66%) that received ethanol vocalized on injection, both rats that received Pe/Ph, and both that received saline vocalized on injection. Both of the rats (100%) given saline and 7 of the ethanol rats (58%) also kicked at the needle during injection. None of the rats exhibited overt signs of distress or abdominal pain during the observation period, but most rats in the ethanol group exhibited mild to moderate ataxia or incoordination and an increased respiratory rate. Given the technical challenge associated with delivering such a high volume by intraperitoneal injection to a conscious animal and the prolonged time from injection to respiratory arrest, we chose to discontinue further study on ethanol euthanasia in rats.
Discussion
This study validates and extends the initial investigation into the use of ethanol for euthanasia of mice.31 Our data show that intraperitoneal injection of ethanol results in a rapid loss of consciousness followed by respiratory and then cardiac arrest in mice. None of the mice in the groups that received ethanol or Pe/Ph regained consciousness after loss of the righting reflex, and all mice progressed to death. Furthermore, our experiments revealed no statistical difference in the time of progression from injection through loss of righting reflex and ultimately death between treatment with either of 2 concentrations of ethanol and treatment with Pe/Ph. Similarly, pain assessment measures did not differ significantly between ethanol groups and Pe/Ph, demonstrating that the intraperitoneal injection of ethanol is not more painful than is injection of a pentobarbital–phenytoin product, which is currently listed as an acceptable method of euthanasia.2
The working group of the European Commission currently lists the use of intraperitoneal injection of ethanol for euthanasia of laboratory mice as acceptable for use only in anesthetized or sedated animals.9,10 Their rationale for this conditional use was out of concern that alcohol would be irritating to the peritoneal cavity. The AVMA's Guidelines, however, impose no such restriction.2 In the current study we were unable to identify positive signs of pain or distress as a result of intraperitoneal ethanol administration. Using the MGS,28 we found no differences between treatment groups in the average preinjection MGS scores. For both the Pe/Ph and 100% ethanol groups, the postinjection score increased relative to the preinjection score, albeit by a small amount. However, only the 100% ethanol group had significantly higher MGS scores than did mice in the saline group. Interestingly, the saline-treated group had the highest incidence of behavioral signs of pain (licking or chewing at the abdomen, hunched posture, abdominal constriction or writhing, pressing the abdomen against the floor).11,12,19
The absence of behavioral signs of pain may be due to the sedative and hypnotic effects of ethanol and Pe/Ph treatment. Ethanol exerts effects on the body and nervous system through a number of mechanisms, many of which are similar to the mechanisms of various anesthetic agents.3,15,22,38,43,47,48 Similar to ketamine, ethanol antagonizes N-methyl-d-aspartate receptors in a concentration-dependent fashion.15,22 In addition, ethanol enhances the inhibitory actions of γ-aminobutyric acid (GABA) and may exert a direct stimulatory effect on the GABA receptor, as do diazepam, midazolam, and propofol; all of these effects can contribute to feelings of relaxation and euphoria in humans.3,15,22 Furthermore, ethanol has been shown to potentiate the neuroinhibitory function of the glycine receptor, as do volatile anesthetics like isoflurane.30,48 Through these various interactions, ethanol has been shown to produce both hypnotic and anesthetic effects at doses as low as 0.88 to 1.80 g/kg.22 Considering that the dose we used in this study was much higher (8.5 to 17.8 g/kg), it is highly likely that the sedative and anesthetic effects of ethanol occurred before irritation or peritonitis was perceived. Therefore the mice may be sedated or anesthetized before any noteworthy pain response can be mounted.
The most common histologic changes were loss of tinctoral quality and mild to moderate edema in the lamina propria of the intestine. Interestingly, a mild, diffuse, lymphoplasmacytic and histiocytic enteritis or enterocolitis occurred consistently in this study. It seems unlikely that this symptom was associated with the treatments used, because affected mice were present in all groups and because the infiltration of lymphocytes, plasma cells, and macrophages into a tissue usually requires 24 to 48 h and typically is associated with a subacute to chronic insult.14 Considering that cardiac arrest occurred in less than 10 min for 85% of our mice, these types of cells had insufficient time to respond and accumulate. Whether the histologic changes we observed might be painful or whether these represent artifactual or incidental changes imperceptible to the mouse before it lost consciousness is unclear. Given that the tissue changes we noted were consistent with prior reports on changes induced by pentobarbital euthanasia18,24 and that almost all changes were more pronounced in the Pe/Ph-treated mice than in either the 70% or 100% ethanol group, we conclude that the tissue changes induced by ethanol are unlikely to be more painful than are those caused by Pe/Ph treatment. Importantly, for studies that require intact-quality tissue for microscopic evaluation, our study verifies that a euthanasia choice other than Pe/Ph or ethanol should be used. We further postulate that ethanol or pentobarbital–phenytoin that enters the lymphatic, portal, or systemic circulation27,37,44 might induce changes in organs and tissues distant from the abdominal cavity. Characterizing these changes was beyond the scope of the current study, and additional studies are needed to elucidate ethanol's effect on tissues and tests commonly used in research.
Although only a single experienced researcher handled and delivered treatments to the mice in this study, 7 of the 85 mice (8.2%) experienced incorrect intraperitoneal delivery of the injected substance. Despite incomplete intraperitoneal injection, these mice still lost consciousness at a median time of 105 s and went on to experience respiratory and cardiac arrest, albeit with a marked delay. In the current study, the most common reason for failure of intraperitoneal injection was inadvertent intraintestinal injection. The rate of incorrect intraperitoneal injection in the current study (8.2%) is lower than most previously published rates (10% to 24%)4,8,21,39,50 but higher than the rate (1.2%) associated with the use of a 2-person injection technique.4 Indeed, many previous reports have questioned the suitability of intraperitoneal injection for use in research studies, considering that partial or complete failure of delivery of the injected substance into the peritoneal cavity can have profound effects on data generated as well as on the wellbeing of the animal receiving the injection.8,21,39,50,52 Although some authors argue that improved training and technique can effectively reduce the incidence of intraperitoneal injection failure,4,39 others have not found this to be the case.21,50 Despite the controversy surrounding intraperitoneal injection, this administration route continues to be popular for use in research. In addition, any technical challenges encountered with this technique are not confined to the use of ethanol for euthanasia. Moreover, the error rate we report in this study (8.2%) is lower than is the unsuccessful euthanasia rate recently reported for cervical dislocation in mice (21%).5 Considering that the technique of intraperitoneal injection is in common use and that our study did not show that incorrect intraperitoneal delivery of ethanol was more painful than was correct injection, we conclude that intraperitoneal injection of ethanol for euthanasia is an acceptable technique. However, it seems prudent to inform all personnel using any form of intraperitoneal injection for euthanasia of the possibility of inadequate injection and the likely consequences of such an event and to be prepared to use an alternative or adjunctive method of euthanasia.
The use of intraperitoneal ethanol injection has been suggested as a method of euthanasia for studies involving vaccine development, antibody production, and serologic assays.32,33 However, many other possible applications for this euthanasia agent exist. For example, in remote locations or field studies where using CO2 euthanasia is impractical or unfeasible, researchers could instead use intraperitoneal ethanol injection. Similarly, investigators traveling across borders might carry a bottle of ethanol to euthanize mice for study in regions where barbiturate drugs are not easily obtained, transported, or securely stored. Perhaps most importantly, ethanol's stability at room temperature, relatively low cost, and ease of transport make it an appealing alternative for use under emergency conditions.
In the wake of natural disasters like hurricanes Sandy, Katrina, Ike, and others, the Office of Laboratory Animal Welfare, the Guide, and the USDA Animal and Plant Health Inspection Service all have recently emphasized the need for research institutions to have disaster or contingency plans in place.,26,45,46,55 In many cases, this disaster plan requires institutions to be prepared to euthanize animals to prevent undue pain or distress under conditions in which they can no longer be cared for and when evacuation is impossible.16,17,20,23,29,41,51,53 During dire circumstances such as these, the supply chains for food, bedding, and compressed CO2 tanks often are disrupted as a result of the disaster. Because ethanol is a common reagent in biomedical research, it likely would already be present on a research campus and could readily be adapted for use as a euthanasia agent during a crisis. Alternatively, institutions might purchase sufficient quantities of ethanol in advance and store it with other emergency supplies.
Although successful in mice, intraperitoneal injection of ethanol was an unsuitable method of euthanasia in rats at the dose range and age of rats tested. We used linear, rather than allometric, scaling to predict a dose for rats from that for mice, given that linear scaling tends to overdose larger animals and that an effective overdose was the goal for our purposes.25 From the scaled starting dose for rats, we incrementally increased the dosage to identify an effective dose for euthanasia. In our study, a relatively large volume of fluid was required (approximately 7 mL), which made the injection itself technically challenging to administer. The increased volume load meant that the rat had to be restrained longer to dispense the full dose, which caused visible distention of the abdomen. Younger rats are known to be resistant to ethanol intoxication, compared with rats older than 10 mo.57 However, our current study did not investigate a variety of age ranges (for example, neonatal to geriatric) in either rats or mice. Future studies will be needed to investigate whether an acceptable and efficacious dose and route can be identified; until then, alternative euthanasia methods to intraperitoneal ethanol should be used for rats.
No method of euthanasia is without drawbacks, and all methods of euthanasia must be carefully evaluated for each combination of circumstances, species, personnel, and available equipment. The mice in this study likely experienced brief or momentary pain or distress associated with restraint and injection, but predictably no more severe than that associated with other types of injections.36 Although inhaled agents might avoid the stress of prolonged handling and the risk of incorrect intraperitoneal injection, they carry the risk of being aversive or distressful to mice.13,34,40,49,54,56 Overall, our study demonstrated that intraperitoneal ethanol injection for mice resulted in rapid and irreversible loss of consciousness, followed by death, and appears to meet 10 of the 14 criteria for the evaluation of euthanasia methods presented in the AVMA Guidelines;2 our study did not address the emotional effect on observers or operators, safety for predators or scavengers, legal requirements, or environmental impacts.2 Considering the low risk of incorrect technique, we suggest that intraperitoneal injection of 70% ethanol, as a method of euthanasia, continue to be listed as ‘acceptable with conditions’ and that these conditions include specific training in the technique of intraperitoneal injection, demonstrated proficiency, and preparation to use an adjunctive method of euthanasia in the event of prolonged time to respiratory or cardiac arrest.
Acknowledgments
Studies involving mice were supported by a grant from the Johns Hopkins Center for Alternatives to Animal Testing (CAAT, http://caat.jhsph.edu/). This work was supported in part by the Office of the Vice Provost for Research at the University of Pennsylvania. We thank Dr Gui-Shuang Ying for statistical consultation and analysis. We appreciate the efforts and skill of Dr Blythe Philips, Dr Rebecca Erickson, and Dr Jessica Johnston in scoring the Mouse Grimace Scale images. We also thank the ULAR training staff for their help in identifying laboratories willing to donate animals to this project and the investigators at the University of Pennsylvania who donated animals for use in this study: Dr Michael May, Dr Kelly McCorkell, Dr Abigail Smith, and Dr Marcus Handy.
References
- 1.American Veterinary Medical Association 2007. AVMA guidelines for the euthanasia of animals, 7th ed. Schaumburg (IL): American Veterinary Medical Association. [Google Scholar]
- 2.American Veterinary Medical Association 2013. AVMA guidelines for the euthanasia of animals, 8th ed. Schaumburg (IL): American Veterinary Medical Association. [Google Scholar]
- 3.Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. 2002. GABAA receptors as molecular sites of ethanol action: direct or indirect actions? Curr Top Med Chem 2:869–885. [DOI] [PubMed] [Google Scholar]
- 4.Arioli V, Rossi E. 1970. Errors related to different techniques of intraperitoneal injection in mice. Appl Microbiol 19:704–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone L, Carbone ET, Yi EM, Bauer DB, Lindstrom KA, Parker JM, Austin JA, Seo Y, Gandhi AD, Wilkerson JD. 2012. Assessing cervical dislocation as a humane euthanasia method in mice. J Am Assoc Lab Anim Sci 51:352–356. [PMC free article] [PubMed] [Google Scholar]
- 6.Caro AC, Hankenson FC, Marx JO. 2013. Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. J Am Assoc Lab Anim Sci 52:577–583. [PMC free article] [PubMed] [Google Scholar]
- 7.Cartner SC, Barlow SC, Ness TJ. 2007. Loss of cortical function in mice after decapitation, cervical dislocation, potassium chloride injection, and CO2 inhalation. Comp Med 57:570–573. [PubMed] [Google Scholar]
- 8.Claassen V. 1994. Intraperitoneal injection, p 46–58. In Huston JP. Neglected factors in pharmacology and neuroscience research techniques in the behavioral and neurosciences, vol 12 New York (NY): Elsevier. [Google Scholar]
- 9.Close B, Banister K, Baumans V, Bernoth E-M, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H, Morton D, Warwick C. 1996. Recommendations for euthanasia of experimental animals: part 1. Lab Anim 30:293–316. [DOI] [PubMed] [Google Scholar]
- 10.Close B, Banister K, Baumans V, Bernoth E-M, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H, Morton D, Warwick C. 1997. Recommendations for euthanasia of experimental animals: part 2. Lab Anim 31:1–32. [DOI] [PubMed] [Google Scholar]
- 11.Collier HOJ, Dinneen LC, Johnson CA, Schneider C. 1968. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother 32:295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collier HOJ, Hammond AR, Horwood-Barrett S, Schneider C. 1964. Rapid induction by acetylcholine, bradykinin, and potassium of a nociceptive response in mice and its selective antagonism by aspirin. Nature 204:1316–1318. [DOI] [PubMed] [Google Scholar]
- 13.Conlee KM, Stephens ML, Rowan AN, King LA. 2005. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim 39:137–161. [DOI] [PubMed] [Google Scholar]
- 14.Cotran RS, Kumar V, Robbins SL. 1989. Robbins’ pathologic basis of disease, 4th ed. Philadelphia (PA): W B Saunders. [Google Scholar]
- 15.Criswell HE, Ming Z, Griffith BL, Breese GR. 2003. Comparison of effect of ethanol on N-methyl-d-aspartate- and GABA-gated currents from acutely dissociated neurons: absence of regional differences in sensitivity to ethanol. J Pharmacol Exp Ther 304:192–199. [DOI] [PubMed] [Google Scholar]
- 16.Dupepe LM. 2013. Rebuild, restore, renew. Lab Anim (NY) 42:395. [DOI] [PubMed] [Google Scholar]
- 17.Durkee SJ. 2013. Planning for the continued humane treatment of animals during disaster response. Lab Anim (NY) 42:F8–F12. [DOI] [PubMed] [Google Scholar]
- 18.Feldman DB, Gupta BN. 1976. Histopathologic changes in laboratory animals resulting from various methods of euthanasia. Lab Anim Sci 26:218–221. [PubMed] [Google Scholar]
- 19.Fichna J, Lapointe T, Chapman K, Janecka A, Vergnolle N, Altier C, Storr MA. 2012. New neostigmine-based behavioral mouse model of abdominal pain. Pharmacol Rep 64:1146–1154. [DOI] [PubMed] [Google Scholar]
- 20.Fishell G. 2013. Hurricane Sandy: after the deluge. Nature 496:421–422. [DOI] [PubMed] [Google Scholar]
- 21.Gaines Das R, North D. 2007. Implications of experimental technique for analysis and interpretation of data from animal experiments: outliers and increased variability resulting from failure of intraperitoneal injection procedures. Lab Anim 41:312–320. [DOI] [PubMed] [Google Scholar]
- 22.Garfield JM, Bukusoglu C. 1996. Propofol and ethanol produce additive hypnotic and anesthetic effects in the mouse. Anesth Analg 83:156–161. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin BS, Donaho JC. 2010. Tropical storm and hurricane recovery and preparedness strategies. ILAR J 51:104–119. [DOI] [PubMed] [Google Scholar]
- 24.Grieves JL, Dick EJ, Schlabritz-Loutsevich NE, Butler SD, Leland MM, Price SE, Schmidt CR, Nathanielsz PW, Hubbard GB. 2008. Barbiturate euthanasia solution-induced tissue artifact in nonhuman primates. J Med Primatol 37:154–161. [DOI] [PubMed] [Google Scholar]
- 25.Hunter RP, Isaza R. 2008. Concepts and issues with interspecies scaling in zoological pharmacology. J Zoo Wildl Med 39:517–526. [DOI] [PubMed] [Google Scholar]
- 26.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 27.Kraft AR, Tompkins RK, Jesseph JE. 1968. Peritoneal electrolyte absorption: analysis of portal, systemic venous, and lymphatic transport. Surgery 64:148–153. [PubMed] [Google Scholar]
- 28.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AMJM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. [DOI] [PubMed] [Google Scholar]
- 29.Levy LS. 2006. Lessons learned from Katrina. Lessons Learned from Katrina, Rita, and Their ‘Sisters’: evacuating, euthanizing, rescuing, and rebuilding. PRIM&R/ARENA 2006 Annual IACUC Conference. Boston (MA): Office of Laboratory Animal Welfare. [Google Scholar]
- 30.Lobo IA, Harris RA. 2005. Sites of alcohol and volatile anesthetic action on glycine receptors. Int Rev Neurobiol 65:53–87. [DOI] [PubMed] [Google Scholar]
- 31.Lord R. 1989. Use of ethanol for euthanasia of mice. Aust Vet J 66:268. [DOI] [PubMed] [Google Scholar]
- 32.Lord R. 1991. Humane killing. Nature 350:456. [DOI] [PubMed] [Google Scholar]
- 33.Lord R, Jones GL, Spencer L. 1991. Ethanol euthanasia and its effect on the binding of antibody generated against an immunogenic peptide construct. Res Vet Sci 51:164–168. [DOI] [PubMed] [Google Scholar]
- 34.Makowska J, Golledge HDR, Marquardt N, Weary DM. 2012. Sedation or inhalant anesthesia before euthanasia with co2 does not reduce behavioral or physiologic signs of pain and stress in mice. J Am Assoc Lab Anim Sci 51:396–400. [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. 2012. Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci 51:42–49. [PMC free article] [PubMed] [Google Scholar]
- 36.Meijer MK, Spruijt BM, van Zutphen LFM, Baumans V. 2006. Effect of restraint and injection methods on heart rate and body temperature in mice. Lab Anim 40:382–391. [DOI] [PubMed] [Google Scholar]
- 37.Mikhaylova K, Vasilev V. 1988. A study of the 2-way transport of horseradish peroxidase across the visceral pleura. Histochemistry 88:583–586. [DOI] [PubMed] [Google Scholar]
- 38.Miller KW, Firestone LL, Forman SA. 1987. General anesthetic and specific effects of ethanol on acetylcholine receptors. Ann N Y Acad Sci 492:71–87. [DOI] [PubMed] [Google Scholar]
- 39.Miner NA, Koehler J, Greenaway L. 1969. Intraperitoneal injection of mice. Appl Microbiol 17:250–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moody CM, Chua B, Weary D. 2014. The effect of carbon dioxide flow rate on the euthanasia of laboratory mice. Lab Anim 48:298–304. [DOI] [PubMed] [Google Scholar]
- 41.Mortell N, Nicholls S. 2013. Practical considerations for disaster preparedness and continuity management in research facilities. Lab Anim (NY) 42:F18–F24. [DOI] [PubMed] [Google Scholar]
- 42.Morton DB, Jennings M, Buckwell A, Ewbank R, Godfrey C, Holgate B, Inglis I, James R, Page C, Sharman I, Verschoyle R, Westall L, Wilson AB. 2001. Refining procedures for the administration of substances. Lab Anim 35:1–41. [DOI] [PubMed] [Google Scholar]
- 43.Mukherjee S, Das SK, Vaidyanathan K, Vasudevan DM. 2008. Consequences of alcohol consumption on neurotransmitters—an overview. Curr Neurovasc Res 5:266–272. [DOI] [PubMed] [Google Scholar]
- 44.Nagy JA. 1992. Lymphatic and nonlymphatic pathways of peritoneal absorption in mice: physiology versus pathology. Blood Purif 10:148–162. [DOI] [PubMed] [Google Scholar]
- 45.Office of Laboratory Animal Welfare 2002. Public Health Service policy on humane care and use of laboratory animals, IV A 1. Bethesda (MD): Office of Laboratory Animal Welfare. [Google Scholar]
- 46.Office of Laboratory Animal Welfare. [Internet] 2015. Office of Laboratory Animal Welfare, Division of Policy and Education. Frequently Asked Questions PHS Policy on Humane Care and Use of Laboratory Animals “G. Institutional Responsibilities, 3. Do awardee institutions need animal facility disaster plans?” National Institutes of Health Office of Extramural Research. [Cited 21 October 2015]. Available at: http://grants.nih.gov/grants/olaw/faqs.htm.
- 47.Pagala M, Ravindran K, Amaladevi B, Namba T, Grob D. 1995. Effect of ethanol on function of the rat heart and skeletal muscles. Alcohol Clin Exp Res 19:676–684. [DOI] [PubMed] [Google Scholar]
- 48.Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. 2010. Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol Ther 127:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raj ABM, Leach MC, Morton DB. 2004. Carbon dioxide for euthanasia of laboratory animals. Comp Med 54:470–471. [PubMed] [Google Scholar]
- 50.Steward JP, Ornellas EP, Beernink KD, Northway WH. 1968. Errors in the technique of intraperitoneal injection of mice. Appl Microbiol 16:1418–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stokes WS. 2006. Disaster planning for laboratory animal facilities: lessons learned from hurricane Katrina. Lessons learned from Katrina, Rita, and their ‘sisters’: evacuating, euthanizing, rescuing, and rebuilding. PRIM&R/ARENA 2006 Annual IACUC Conference. Boston (MA): Office of Laboratory Animal Welfare. [Google Scholar]
- 52.Svendsen O. 2005. Ethics and animal welfare related to in vivo pharmacology and toxicology in laboratory animals. Basic Clin Pharmacol Toxicol 97:197–199. [DOI] [PubMed] [Google Scholar]
- 53.Swearengen JR, Vargas KJ, Tate MK, Linde NS. 2010. Disaster preparedness in biocontainment animal research facilities: developing and implementing an incident response plan (IRP). ILAR J 51:120–126. [DOI] [PubMed] [Google Scholar]
- 54.Thomas AA, Flecknell PA, Golledge HDR. 2012. Combining nitrous oxide with carbon dioxide decreases the time to loss of consciousness during euthanasia in mice—refinement of animal welfare? PLoS One 7:e32290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.United States Department of Agriculture, Animal and Plant Health Inspection Service 2012. Final rule: handling of animals; contingency plans. Fed Regist 77:76815–76816. [Google Scholar]
- 56.Valentine H, Williams WO, Maurer KJ. 2012. Sedation or inhalant anesthesia before euthanasia with CO2 does not reduce behavioral or physiologic signs of pain and stress in mice. J Am Assoc Lab Anim Sci 51:50–57. [PMC free article] [PubMed] [Google Scholar]
- 57.Wiberg GS, Trenholm HL, Coldwell BB. 1970. Increased ethanol toxicity in old rats: changes in ld50, in vivo and in vitro metabolism, and liver alcohol dehydrogenase activity. Toxicol Appl Pharmacol 16:718–727. [DOI] [PubMed] [Google Scholar]