Abstract
BACKGROUND
Cannabis users frequently report concurrent tobacco use, and tobacco use is associated with poorer outcomes during treatment for cannabis use disorders (CUD). Interventions that simultaneously target both tobacco and cannabis use disorders may enhance cessation outcomes for either or both substances.
METHODS
This study evaluated an intervention integrating highly effective treatments for cannabis and tobacco use disorders. Thirty-two participants meeting diagnostic criteria for CUD and reporting daily tobacco use were enrolled in a 12-week computer-assisted behavioral treatment for CUD. Participants were encouraged to participate in a tobacco intervention that included a computer-assisted behavioral treatment tailored for tobacco and cannabis co-users, and nicotine-replacement therapy (NRT). Cannabis and tobacco outcomes were evaluated using descriptive statistics and were compared to a historical control group that received treatment for CUD but not tobacco.
RESULTS
Participants achieved 3.6 ± 4.3 consecutive weeks of cannabis abstinence, which was comparable to the historical control group (3.1 ± 4.4). A majority of the sample (78%) completed at least one tobacco module and 44% initiated NRT. Over half (56%) initiated tobacco quit attempts, and 28% were tobacco abstinent for at least two consecutive weeks. Participants showed greater reduction in tobacco use (cigarettes per day) than the historical control group, but differences in tobacco abstinence rates during the final month of treatment were not statistically significant (12.5% vs 4%).
CONCLUSION
Findings suggest that providing a tobacco intervention during treatment for CUD is feasible and may positively impact tobacco use without negatively affecting cannabis use outcomes.
Keywords: Cannabis, Tobacco, Co-use, Computer-assisted, Treatment, Incentives
1. INTRODUCTION
Concurrent use of tobacco is common among cannabis users; over 60% of adult cannabis users report current tobacco use, and over a third of those with cannabis use disorders (CUD) meet criteria for nicotine dependence (Agrawal et al., 2012; Moore and Budney, 2001; NSDUH, 2014). The high prevalence of co-use is especially problematic when considering the adverse health-related effects of tobacco smoking (Center for Disease Control and Prevention, 2008) and the negative consequences associated with cannabis use (Volkow et al., 2014).
Co-use also appears to be associated with poorer cannabis and tobacco cessation outcomes. Tobacco use is a predictor of poorer outcomes during treatment for CUD (Peters et al., 2012), and cannabis use among tobacco smokers predicts poorer tobacco-cessation outcomes (Amos et al., 2004; Ford et al., 2002). Genetic, neurobiological, and behavioral mechanisms, and a shared route of administration (smoking), likely contribute to establishing learned patterns of co-use and to increased difficulty quitting use of either or both substances (Agrawal et al., 2012; Castañé et al., 2005; Cohen et al., 2005; Cooper and Haney, 2009; Penetar et al., 2005; Rabin and George, 2015; Ream et al., 2008).
These links between cannabis and tobacco use suggest that interventions targeting both substances may enhance cessation outcomes over interventions that target cannabis or tobacco alone. Almost all interventions targeting tobacco during treatment for alcohol and other illicit drugs have shown decreases in tobacco use with no adverse effects on outcomes for the primary substances (for review see: Baca and Yahne, 2009; Sullivan and Covey, 2002), however, this finding is not unequivocal (Joseph et al., 2004). Moreover, targeting tobacco may increase long-term abstinence from alcohol and other substances (Prochaska et al., 2004). Both simultaneous and sequential treatment approaches to co-use of tobacco and alcohol/illicit drugs have demonstrated positive outcomes (Kalman et al., 2010; Nieva et al., 2010; Prochaska et al., 2004). However, because simultaneous treatments decrease the resources required for treatment delivery (Kalman et al., 2010), and cannabis users frequently use tobacco and cannabis in close proximity (either together in blunts/spliffs, or chasing cannabis with tobacco), a simultaneous rather than sequential approach might provide the most effective treatment option for cannabis and tobacco co-use.
To date, only two uncontrolled studies have examined integrated treatments for cannabis and tobacco co-use. The first evaluated a 10-week program that included cognitive behavioral therapy (CBT) targeting both substances and transdermal nicotine replacement therapy (NRT; Hill et al., 2013). A reduction in cigarettes per day was reported, but no significant improvements in cannabis use were observed for the seven of 12 participants that completed the study. A second study tested an intervention in 77 participants consisting of 5 to 6 sessions of behavioral-based therapies (i.e., motivational interviewing, CBT, and self-control training) in a group setting (Becker et al., 2015). At end of treatment, 25% reported abstinence from cannabis, 33% reported abstinence from tobacco and 13% reported abstinence from both. These initial reports indicate good feasibility of interventions targeting both substances, but also suggest a need to investigate more potent treatment strategies using more rigorous methodology.
Arguably, the most effective outpatient treatments for CUD combine Motivational Enhancement Therapy (MET), CBT, and Contingency Management (CM; Budney et al., 2006; Carroll et al., 2006, 2012; Kadden et al., 2007; Litt et al., 2013). Although limitations have been raised associated with real-world implementation of such interventions due to availability and cost (Carroll, 2014), recent studies suggest that computer-assisted versions of these treatments may enhance access and reduce cost without sacrificing efficacy (Budney et al., 2011, 2015; Kay-Lambkin et al., 2009, 2011).
The most effective treatments for tobacco cessation typically include behavioral (e.g., behavioral counseling, internet-based interventions) and pharmacological (NRT, varenicline, bupropion) interventions (Eisenberg et al., 2008; Civljak et al., 2010; Herman and Sofuoglu, 2010; Strecher et al., 2005; Stapleton et al., 2008). Combining pharmacological and behavioral treatments is generally considered most effective (Fiore et al., 2008).
The aim of our current project is to combine and integrate highly effective treatments for cannabis and tobacco to produce a feasible treatment that simultaneously targets use of both substances. The intervention integrates computer-assisted MET/CBT/CM for CUD (Budney et al., 2011) with computer-assisted behavioral counseling and NRT via combination patch and gum or lozenge for tobacco use. A description of this intervention and outcomes from the first six participants were presented previously in a case series report (Lee et al., 2014). This manuscript extends that report by including a larger sample size, evaluating a priori outcome benchmarks, and comparing cannabis and tobacco outcomes to a historical control group.
Data from prior CUD studies and studies targeting tobacco use during treatment for alcohol dependence were used to formulate a-priori hypotheses to provide initial benchmarks of potential efficacy. We hypothesized that: 1) rates of cannabis abstinence would be comparable to the historical control group, which would suggest that targeting tobacco use does not negatively impact cannabis abstinence; 2) participants would complete at least one tobacco module and a substantial proportion (≥40%) would initiate NRT, indicating interest in the tobacco intervention, 3) the tobacco intervention would prompt quit/reduction attempts (i.e., >35% would make tobacco quit attempts with >24hrs abstinence) and 4) the tobacco intervention would result in sustained tobacco abstinence (>25% would achieve ≥2 weeks of abstinence, >15% would be tobacco abstinent during the final 30 days of treatment).
2. METHOD
2.1 Participants
The study was approved by the Dartmouth Committee for Protection of Human Subjects and registered on clinicaltrials.gov (NCT01834794). Participants were recruited from advertisements in newspapers, radio stations, and through Craigslist, notices to professionals and service agencies, and posters throughout the community. Advertisements described the intervention as a program for individuals seeking to quit cannabis who also smoked tobacco. Participants in the current study were enrolled between March of 2013 and September of 2014. Inclusion criteria were: 1) between 18 and 65 years of age, 2) diagnosed with DSM-IV cannabis abuse or dependence based on the Substance Use Disorders section of the Structured Clinical Interview for DSM-IV (First et al., 1995), 3) used cannabis on at least 45 of the previous 90 days, 4) daily use of tobacco cigarettes or daily simultaneous use of cannabis and tobacco (i.e., blunts or spliffs), and 5) reported some interest in quitting tobacco in the next 6 months (rating of 2 or more on a 5-point scale, with 1 = no interest and 5 = very interested).
Participants were excluded if they 1) met dependence criteria for alcohol or any drug other than nicotine and cannabis, with the exception of opiate dependence maintained by agonist replacement therapy, 2) currently used non-tobacco nicotine (i.e., NRT), 3) were currently enrolled in a psychosocial treatment program or behavioral counseling for substance abuse, 4) had a medical condition that prevented use of NRT, 5) had current, severe psychological distress (i.e., active suicidal ideation, uncontrolled psychosis, and/or debilitating panic disorder), 5) had a legal status that would interfere with participation, 6) lived more than 45 miles from the clinic, 7) were living with someone currently enrolled in the project, and 8) were not fluent in English.
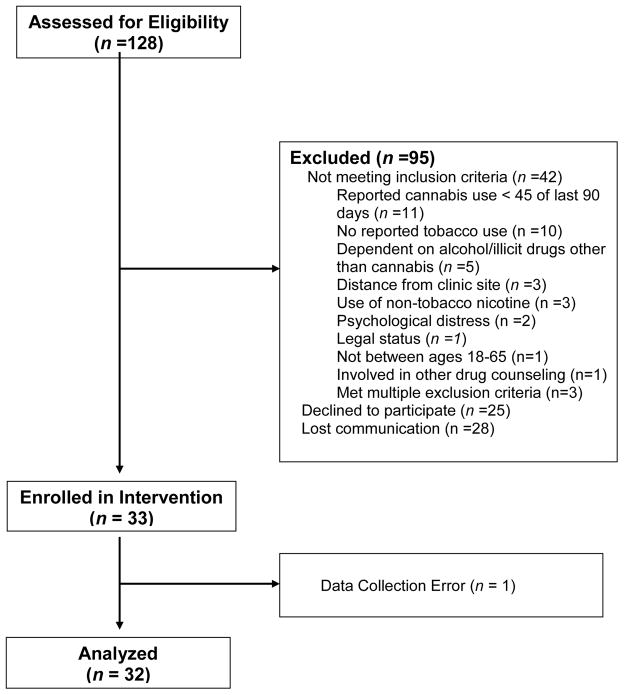
Of 128 individuals assessed for eligibility, 42 did not meet eligibility criteria, 28 did not respond to communication following initial telephone screening, and 25 declined to participate for reasons unrelated to eligibility (Figure 1). Thirty-three adults enrolled, including 3 with opiate dependence that had been stable on agonist replacement therapy for more than 6 months. No participants were excluded due to lack of interest in quitting tobacco. All participants reported daily use of smoked tobacco products (i.e., cigarettes/cigarillos); no participant reported exclusive use of blunts or spliffs. Abstinence data from one participant could not be confirmed due to a data collection error, resulting in a final sample size of 32.
Figure 1.
Consort Diagram.
The historical control group comprised 54 participants from two previous studies that evaluated therapist-delivered (N=28) or computer-delivered (N=26) MET/CBT/CM interventions for CUD that were highly similar to the treatment used in the current study, but did not include any intervention for tobacco use (NCT00594659; Budney et al., 2011, 2015). Participants in these studies were enrolled between May, 2008 and June, 2011. Recruitment methods were similar to those used in the current study. Inclusion criteria were also similar with the exception that participants did not need to be regular tobacco users or interested in quitting tobacco. The 54 participants from those studies who served as the historical control group in the present study were the subset of the study sample who reported daily tobacco use in the 30 days prior to the baseline assessment. Note that one opiate dependent individual stable on agonist replacement therapy was also included in this group.
2.2 Intervention
As previously described in Lee et al. (2014), the intervention consisted of a 12-week, 9 session computer-assisted version of MET/CBT/CM for CUD integrated with a tobacco intervention consisting of five computer modules tailored for tobacco and cannabis co-use, and NRT. The CUD components of the treatment are described in detail in Budney et al. (2011). Briefly, nine computer-delivered MET/CBT modules were provided at the clinic during weeks 1–12. Three supportive, 15–30 minute counseling sessions with a therapist occurred during weeks 1, 4 and 12. The CM program targeting cannabis abstinence provided monetary incentives contingent upon twice-weekly observed urine tests. Participants could earn up to $435 for continuous abstinence throughout the intervention. Incentives were delivered after each session via electronic deposits to a MasterCard provided to participants at the beginning of the study. Participants attended twice-weekly clinic visits for a total of 24 possible visits.
The tobacco intervention was delivered concurrently with the cannabis program. The intervention included access to NRT as well as five computer-delivered psychoeducation and behavioral counseling modules integrated with the cannabis modules on the same platform. These tobacco modules were developed specifically for this program, partially drawing from an internet-based tobacco intervention (Stop Tabac; Etter, 2005, 2009; http://www.stop-tobacco.ch/en/), and modified to address issues of co-use of cannabis and tobacco. Participants were encouraged by staff, but not required, to complete the first tobacco module during the second visit, and to complete additional modules at each visit during the 12-week program. The first module focused on the pros and cons of smoking tobacco. Participants completed a personalized assessment using Stop Tabac and were encouraged to set a tobacco quit date. Module 2 provided information about co-use of cannabis and tobacco, including potential additive health risks, difficulties with quitting one substance while continuing to smoke the other, planning to stop use of both substances, and roadblocks to quitting both. Module 3 provided NRT education and instruction. Module 4 focused on planning for change and setting a quit date. Module 5 provided reduction strategies for those interested in reducing rather than quitting.
Participants were offered free NRT, provided on a bi-weekly schedule, which could be initiated as early as the first week of treatment. NRT options included a combination of patch and gum or lozenges, following standard guidelines for dosing (Stead et al., 2012). Research staff monitored adverse effects at each visit. NRT rather than other pharmacotherapies was chosen for use in this study for three reasons: 1) easy access - it is widely available without a prescription, 2) strong safety profile with minimal side effects (Stead et al., 2012), and 3) few contraindications. In contrast, varenicline and bupropion require prescriptions and physician monitoring, have more potential side-effects, and have potential interactions with cannabis-related withdrawal symptoms (Haney et al., 2001, Stapleton et al., 2008).
2.3 Measures
Substance use: Self-reported substance use was obtained at intake for the preceding 90 days and at twice-weekly study visits during treatment using a timeline follow-back (TLFB) procedure (Sobell and Sobell, 1992). During treatment, participants were asked to report on daily substance use that occurred since the preceding study visit. TLFB assessments included cannabis, cigarettes, other tobacco and nicotine products (including smokeless tobacco and ecigs), alcohol, other drug use, and NRT (if initiated). Nicotine dependence was assessed at baseline using the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991). Interest in quitting cannabis was assessed by the action subscale of the Readiness to Change Questionnaire – Treatment Version (Heather and Hönekopp, 2008).
Cannabis abstinence was verified twice-weekly via observed collection of urine specimens which were tested immediately after collection. Cannabis and adulterant testing were performed using dipstick tests (http://www.americanscreeningcorp.com). Failure to submit a specimen without an excused absence was treated as a cannabis-positive result.
Self-reported tobacco abstinence was verified using expired carbon monoxide (CO; coVita Micro + Smokerlyzer: http://covita.net/micro+.html). Participants with a CO level ≤ 5 were considered tobacco abstinent. In the event of self-reported tobacco abstinence, a breath CO > 5, and self-reported cannabis use, which could increase CO level, tobacco abstinence was verified further using urine cotinine (ONESCREEN Cotinine Test; http://www.americanscreeningcorp.com). In the event of a positive CO, self-reported cannabis use, and NRT use (which would increase cotinine levels), tobacco abstinence was verified by urine anatabine and anabasine levels ≤ 2 ng/ml (Jacob et al., 2002).
2.4 Data Analysis
The primary outcomes assessed included 1) longest duration of cannabis abstinence measured by the mean number of consecutive cannabis-negative urine specimens, 2) number of tobacco modules completed, 3) initiation of NRT, 4) self-reported tobacco quit attempts, and 5) tobacco abstinence verified by expired CO and, if necessary, urinalysis. All a priori hypotheses were evaluated using descriptive statistics.
Cannabis and tobacco outcomes for the current study sample were then compared to those achieved by the historical control group. Demographic and baseline drug use characteristics were compared using chi-square and t-tests. Multivariate linear and logistic regression were used to test for group differences on (a) number of consecutive cannabis-negative urine screens, and (b) number of tobacco-abstinent participants during the final 30 days of treatment while controlling for age, sex, race, and baseline cannabis and tobacco use. A generalized estimating equations (GEE) approach was used to compare the current study and historical control groups on change in cigarettes per day between baseline (i.e., 30 days prior to intake) and final 30 days of treatment while controlling for age, sex, race, and baseline cannabis use. Significant interactions were examined using Tukey-Kramer adjusted differences of least-squared means.
Cannabis abstinence was analyzed using an intent-to-treat approach with missing specimens and dropouts counted as positive. For tobacco abstinence, calculation of percent abstinent at end of treatment used all participants in the denominator and dropouts were considered to still be smoking. All participants were included in both analyses. For change in tobacco cigarettes per day, mean cigarettes per day was calculated using daily TLFB data collected at intake (for baseline) and during the final 30 days of treatment (or at ETX assessment for those that did not complete treatment). Cigarettes per day during the final 30 days of treatment were counted as missing for participants that did not attend either the final 30 days of treatment or ETX assessment (current study N=11, historical control N=15).
3. RESULTS
3.1 Participants
Table 1 presents demographic information and baseline cannabis and tobacco use characteristics. Participants in the present sample (n=32) were 78% male and 78% Caucasian, with a mean age of 29. All participants reported using cannabis in the 30 days preceding treatment enrollment (M = 26.7 ± 5.0 days), and on average used cannabis on 3.6 ± 1.8 occasions per day. All participants were regular cigarette smokers (with the exception of one individual with daily use of cigarillos), with mean age of initiation of 14.5 ± 3.0 years, mean cigarettes per day of 12 ± 7.3 cigarettes per day, and a mean FTND score of 3.3 ± 2.2. Interest in quitting tobacco was high (i.e., mean of 4.7 on a 5 point scale), and 72% of the sample had made at least one previous tobacco quit attempt (mean 5.9 ± 10.5 attempts). Compared to participants in the current study, participants in the historical control group (N=54) were older, less likely to be male, more likely to be a person from a racial or ethnic minority, and had fewer days of cannabis use during the 30 days prior to intake. No data on interest in quitting tobacco use was available for the historical control group.
Table 1.
Participant characteristics and substance use at intake
| Characteristic | Current Study | Historical Control | p-valuea |
|---|---|---|---|
|
| |||
| M (SD) or % (N) | M (SD) or % (N) | ||
| N | 32 | 54 | |
| Age | 29.0 (11.8) | 34.0 (10.8) | 0.05 |
| Sex (Male) | 78% | 52% | 0.02 |
| Race | 0.01 | ||
| Caucasian | 78% | 50% | |
| Other | 22% | 50% | |
| Age of Initiation (Cannabis) | 15.4 (3.5) | 14.6 (2.9) | 0.21 |
| Past 30 Days | |||
| Cannabis (days of use) | 26.7 (5.0) | 22.2 (8.7) | 0.01 |
| Tobacco (cigarettes per day) | 12.0 (7.3) | 13.0 (8.8) | 0.58 |
Note
t-tests and chi square tests were used to assess group differences
3.2 Retention and Attendance
Independent samples t-tests revealed that compared to the historical control group, participants in the current study completed more of the possible 9 MET/CBT cannabis treatment sessions (6.7 ± 3.0 vs. 5.1 ± 3.4, p<.05), but the number of urine specimens collected during treatment did not significantly differ between the two groups (15.5 ± 9.0 vs.12.0 ± 9.3, p=.09). End of treatment attendance was similar across groups, with 66% attending the end of treatment assessment in the current study compared to 63% of the historical control group (p=.80).
3.3 Cannabis Outcomes
Mean continuous weeks of cannabis abstinence among current study participants was 3.6 ± 4.3 out of a possible 12 weeks, with 50%, 38% and 16% achieving at least 2, 4, and 10 continuous weeks of verified abstinence (i.e., consecutive cannabis-negative urine screens), respectively. Approximately 44% were cannabis abstinent at the end of treatment assessment, compared to 35% of the historical control group. Table 2 presents results from a multiple linear regression analysis comparing weeks of continuous cannabis abstinence in the current study and historical control group (controlling for age, sex, race and baseline days of cannabis and tobacco use). Mean consecutive cannabis-negative urine screens in the current study (M = 3.6 ± 4.3) did not significantly differ from the historical control group (M = 3.1 ± 4.4).
Table 2.
Multiple Regression Results for Cannabis Abstinence
| Variable | b | SE | β | t | p-value |
|---|---|---|---|---|---|
| Constant | 2.72 | 5.74 | 0.47 | 0.64 | |
| Group | 1.97 | 2.09 | 0.11 | 0.94 | 0.35 |
| Age | 0.14 | 0.08 | 0.19 | 1.71 | 0.09 |
| Sex | 0.90 | 1.93 | 0.05 | 0.47 | 0.64 |
| Race | −3.94 | 1.90 | −0.23 | −2.08 | 0.04 |
| Cannabis Use | −0.18 | 0.12 | −0.17 | −1.55 | 0.13 |
| Tobacco Use | 0.09 | 0.11 | 0.10 | 0.87 | 0.39 |
Note: Overall model: R = 0.39, F(6,79) = 2.35, p<.05. Group is current study (N = 32) vs. historical control (N = 54), Race is white vs. other, cannabis and tobacco use refer to the mean number of days of cannabis and tobacco use during the 30 days prior to intake).
3.4 Participation in the Tobacco Intervention
Over three-quarters of participants (78%) completed the first tobacco module, and fourteen participants (44%) initiated NRT. Among those that completed at least one module, the mean number completed was 2.8 ± 1.1 out of a possible 5 modules. The mean duration of NRT use was 46.9 ± 31.5 days. All participants that initiated NRT were provided with a combination of the patch and gum or lozenges. With the exception of one participant who reported gum use alone, all participants reported using combined NRT. No participant discontinued NRT due to adverse effects.
3.5 Tobacco Outcomes
Twenty-one participants (66%) set a tobacco quit date while completing the first tobacco computer module and nineteen (56%) made at least one self-reported tobacco quit attempt of 24 hours or longer. Quit attempts were verified by expired CO or cotinine for 11 out of the 19 participants; eight of the 19 participants had returned to smoking prior to their next clinic visit so biological verification of quit attempts could not be determined. Nine participants (28%) achieved biologically confirmed tobacco abstinence for at least two consecutive weeks at some point during the study, and four participants (12.5%) were tobacco abstinent during the final 30 days of treatment as verified by CO or cotinine. With the exception of tobacco abstinence over the final 30 days of treatment, we exceeded a priori hypotheses for tobacco quit attempts (>35% predicted and 56% observed) and tobacco abstinence for two or more consecutive weeks (>25% predicted and 28% observed). Of note, although no a priori hypothesis was established a for reduction in tobacco use, eight participants (25%) that did not achieve abstinence reduced cigarettes per day by at least 50%, as indicated by comparing use during the final 30 days of treatment with use during the 30 days prior to enrollment.
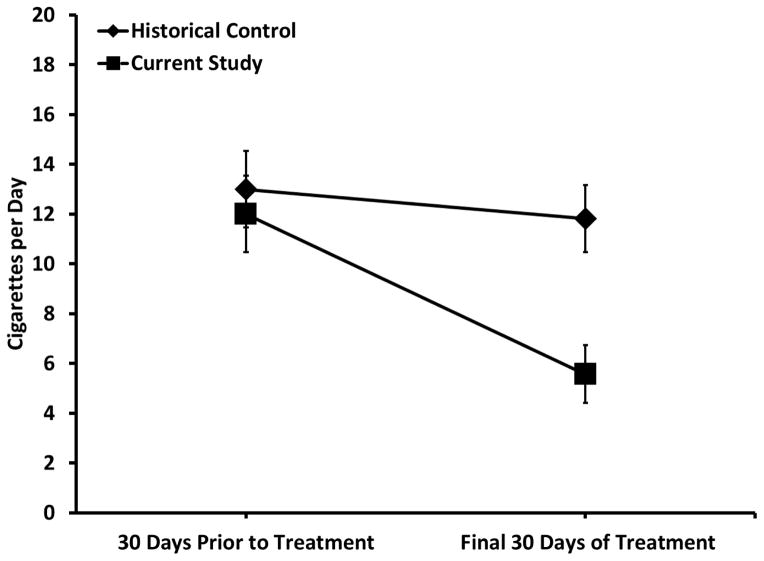
Table 3 presents the results of the GEE analysis of change in cigarettes per day from baseline to the final 30 days of treatment. A significant group x time interaction emerged, with pairwise comparisons indicating that participants in the current study smoked fewer cigarettes per day during the final 30 days of treatment compared historical controls (p<.05; Figure 2). A logistic regression (controlling for age, sex, race and baseline days of cannabis and tobacco use) indicated that group was not a significant predictor of tobacco abstinence during the final month of treatment (p = 0.13: 12.5 vs. 4.0% abstinent for current study and historical control, respectively).
Table 3.
GEE Model for Change in Cigarettes per Day (Baseline vs Final 30 Days of Treatment)
| Variable | Estimate | SE | p-value |
|---|---|---|---|
| Intercept | 0.06 | 3.56 | 0.99 |
| Group | 5.21 | 1.59 | <0.01 |
| Time | 5.80 | 1.59 | <0.001 |
| Group*Time | −4.02 | 1.81 | 0.03 |
| Age | 0.19 | 0.08 | 0.02 |
| Sex | 2.09 | 1.63 | 0.20 |
| Race | −2.37 | 1.50 | 0.11 |
| Cannabis Use | −0.02 | 0.09 | 0.83 |
Note: GEE: Generalized Estimating Equations. Group is current study (N = 32) vs. historical control (N = 54), Race is white vs. other, cannabis use refers to the mean number of days of cannabis and tobacco use during the 30 days prior to intake.
Figure 2.
Mean number of cigarettes per day for the 30 days prior to treatment and the final 30 days of treatment. Participants in the current study (N = 32) smoked fewer cigarettes per day during the final 30 days of treatment compared to the historical control group (N = 54).
4. DISCUSSION
Consistent with the a priori hypotheses, the majority of participants seeking treatment for CUD showed interest in receiving an intervention for tobacco, nearly half initiated NRT, and a substantial subset initiated tobacco quit and reduction attempts. Compared to a historical control group that was not offered a tobacco intervention, participants in the current study achieved comparable rates of cannabis abstinence, showed a greater reduction in tobacco use over the course of treatment, but did not show a significantly greater incidence of tobacco abstinence at the end of treatment.
Previous uncontrolled studies evaluating simultaneous interventions for cannabis and tobacco co-use suggested that combined interventions were feasible but because these studies did not include comparison groups their cessation outcomes were difficult to interpret (Becker et al., 2015; Hill et al., 2013). The finding that cigarettes per day decreased from pre-treatment to the end of treatment is similar to outcomes reported in Hill et al. (2013), though more individuals achieved abstinence from cannabis and tobacco in the current study. This study extends those findings, and findings from our previously published case series report, by demonstrating that combining two highly effective interventions for tobacco and cannabis (i.e., MET/CBT/CM and combination NRT) decreased tobacco use without negatively impacting the effects of treatment for cannabis abstinence. Cannabis abstinence outcomes were generally consistent with outcomes reported in previous studies using similar computer-assisted and therapist-delivered treatments, two of which included CM and two that did not (i.e., Budney et al., 2011, 2015; Kay-Lambkin et al., 2009, 2011).
The majority of the sample participated in the tobacco intervention and reported quit and reduction attempts, suggesting that the tobacco intervention was effective in motivating smokers to try to quit tobacco. However, fewer participants than expected achieved documented tobacco abstinence over the final four weeks of treatment, suggesting that more intensive strategies for increasing sustained tobacco abstinence merit consideration.
One alternative is to integrate an incentive program for tobacco abstinence. This strategy has been utilized successfully in other tobacco-smoking, high-risk populations (Donatelle et al., 2000; Dunn et al., 2010; Halpern et al., 2015; Shoptaw et al., 2002), and while the initial costs would be greater than the current tobacco intervention, the increase in efficacy may prove cost effective. Similarly, incentives for completing tobacco modules or adhering to NRT might increase tobacco quit attempts and abstinence. Another possibility would be to offer the tobacco intervention following completion of the CUD treatment for those reluctant to change cannabis and tobacco use at the onset of treatment, (i.e., a sequential approach). Last, alternative pharmacotherapies to NRT for tobacco dependence (i.e., varenicline) could be offered, either as a choice at treatment initiation, or if NRT is unsuccessful.
A number of limitations of the current study warrant highlighting. First, the use of a historical control group rather than comparing outcomes in a randomized trial limits confidence in conclusions related to the efficacy of the approach. While differences in age, sex, race, and baseline cannabis use between the historical and current sample were controlled in the statistical model, limitations associated with using multivariate regression to control confounders, such as statistical assumptions about the covariates, unequal group sizes, and measurement error in the variables may have contributed to observed group differences. In addition, other differences that were not measured may have contributed to the observed differences in tobacco outcomes between groups and cannot be ruled out. For example, the current sample, but not the historical controls, knew that treatment would be offered for tobacco use. Second, inclusion of participants in the current study was limited to those with some interest in quitting tobacco in the next six months; data on interest in quitting tobacco was not collected in the historical control group. Third, comparative data on other psychiatric disorders was not available. Fourth, data from the historical control group was collected from 2008 to 2011, compared to 2013 to 2014 in the present study. Historical trends in tobacco and cannabis use likely changed between studies, which might have impacted group differences observed between studies. The relatively small sample size also limited generalizability and the ability to detect smaller changes in cannabis and tobacco use during treatment. Last, this study did not include a follow-up assessment. Our group is currently conducting a randomized controlled trial comparing this intervention to a sequential intervention for cannabis and tobacco with multiple follow-up assessments to provide a more methodologically rigorous test of the combined intervention.
In summary, findings from this study suggest that integrating established treatments for tobacco and cannabis to simultaneously target both substances is feasible and may positively impact tobacco use without negatively affecting cannabis use outcomes. Two of the strengths of this multicomponent intervention, computer-assisted treatment combined with over the counter NRT, likely made treatment more accessible and increased the potential for high treatment fidelity. Future studies that use more rigorous methodological designs, test alternative strategies for targeting tobacco, and that include follow-up assessments are needed to confirm and extend these findings.
Highlights.
An intervention simultaneously targeting tobacco and cannabis is feasible
Including an intervention for tobacco did not negatively impact cannabis abstinence
A majority of the sample participated in the tobacco intervention
Participants smoked fewer cigarettes at end of treatment compared to a historical control group
Acknowledgments
Role of Funding Source: Funding for this study was provided by NIH-NIDA grants R01-DA032243, R01-DA023526, and T32-DA037202; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We thank Gray Norton, Bonita Basnyat, Stanley See and Hao Yang who assisted with all aspects of the conduct of the study.
Footnotes
Contributors: AJB and CS designed the study, wrote the protocols, and directed the study. DCL assisted with management of the study, conducted the analyses of the data, and wrote the initial draft of the manuscript. MB contributed to study design, and managed NRT dosing schedules and side-effects reports. JRH and JFE assisted in the design of the tobacco intervention and development of the computer modules. All authors contributed to the writing and have approved the final manuscript.
Conflict of Interest: Dr. Hughes has received consulting and speaking fees from several companies that develop or market pharmacological and behavioral treatments for smoking cessation or harm reduction and from several non-profit organizations that promote tobacco control. Some of these market nicotine replacement products or their competitors. All other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction. 2012 doi: 10.1111/j.1360-0443.2012.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A. ‘You can’t go without a fag…you need it for your hash’--a qualitative exploration of smoking, cannabis and young people. Addiction. 2004;99:77–81. doi: 10.1111/j.1360-0443.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36:205–219. doi: 10.1016/j.jsat.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Becker J, Haug S, Kraemer T, Schaub MP. Feasibility of a group cessation program for co-smokers of cannabis and tobacco. Drug Alcohol Rev. 2015 doi: 10.1111/dar.12244. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Fearer S, Walker DD, Stanger C, Thostensen J, Grabinski MJ, Bickel WK. An initial trial of a computerized behavioral intervention for cannabis use disorder. Drug Alcohol Depend. 2011;115:74–79. doi: 10.1016/j.drugalcdep.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.74.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Stanger C, Tilford JM, Scherer EB, Brown PC, Li Z, Li Z, Walker DD. Computer-assisted behavioral therapy and contingency management for cannabis use disorder. Psychol Addict Behav. 2015 doi: 10.1037/adb0000078. http://dx.doi.org/10.1037/adb0000078. [DOI] [PMC free article] [PubMed]
- Carroll KM. Lost in translation? Moving contingency management and cognitive behavioral therapy into clinical practice. Ann NY Acad Sci. 2014;1327:94–111. doi: 10.1111/nyas.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J Consult Clin Psychol. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Lapaglia DM, Peters EN, Easton CJ, Petry NM. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction. 2012;107:1650–1659. doi: 10.1111/j.1360-0443.2012.03877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañé A, Berrendero F, Maldonado R. The role of the cannabinoid system in nicotine addiction. Pharmacol Biochem Behav. 2005;81:381–386. doi: 10.1016/j.pbb.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Tobacco-Related Diseases. 2004 (Accessed at http://www.cdc.gov/tobacco/data_statistics/sgr/2004/index.htm)
- Civljak M, Sheikh A, Stead LF, Car J. Internet-based interventions for smoking cessation. Cochrane Database System Rev. 2010;9:CD007078. doi: 10.1002/14651858.CD007078.pub3. [DOI] [PubMed] [Google Scholar]
- Cohen C, Kodas E, Griebel G. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol Biochem Behav. 2005;81:387–395. doi: 10.1016/j.pbb.2005.01.024. http://dx.doi.org/10.1016/j.pbb.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. 2009;103:107–113. doi: 10.1016/j.drugalcdep.2009.01.023. S0376-8716(09)00104–5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomized controlled trial using social support and financial incentives for high risk pregnant smokers: significant other supporter (SOS) program. Tob Control. 2000;9(Suppl 3):iii67–iii69. doi: 10.1136/tc.9.suppl_3.iii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST. A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Exp Clin Psychopharmacol. 2010;18:37–50. doi: 10.1037/a0018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Bélisle P, Mottillo S, Joseph L, Gervais A, O’Loughlin J, Paradis G, Rinfret S, Pilote L. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135–144. doi: 10.1503/cmaj.070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF. Comparing the efficacy of two Internet-based, computer-tailored smoking cessation programs: a randomized trial. J Med Internet Res. 2005;7:e2. doi: 10.2196/jmir.7.1.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF. Comparing computer-tailored, internet-based smoking cessation counseling reports with generic, untailored reports: a randomized trial. J Health Commun. 2009;14:646–657. doi: 10.1080/10810730903204254. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview For DSM-IV Axis 1 Disorders--Patient Edition SCID I/P. Biometricts Research Department: New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Fiore M, Jaén CR, Baker TB, Bailey WC, Bennett G, Benowitz NL, et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DE, Vu HT, Anthony JC. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug Alcohol Depend. 2002;67:243–248. doi: 10.1016/s0376-8716(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, Loewenstein G, Brennan TA, Asch DA, Volpp KG. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372:2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology. 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Heather N, Hönekopp J. A revised edition of the Readiness to Change Questionnaire [Treatment Version] Addict Res Theory. 2008;16:421–433. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Bri J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Herman AI, Sofuoglu M. Comparison of available treatments for tobacco addiction. Curr Psychiatry Rep. 2010;12:433–440. doi: 10.1007/s11920-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KP, Toto LH, Lukas SE, Weiss RD, Trksak GH, Rodolico JM, Greenfield SF. Cognitive behavioral therapy and the nicotine transdermal patch for dual nicotine and cannabis dependence: a pilot study. Am J Addict. 2013;22:233–238. doi: 10.1111/j.1521-0391.2013.12007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, 3rd, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol Biomark Prev. 2002;11:1668–1673. [PubMed] [Google Scholar]
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. J Stud Alcohol. 2004;65:681–691. doi: 10.15288/jsa.2004.65.681. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav. 2007;32(6):1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Kim S, DiGirolamo G, Smelson D, Ziedonis D. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programs. Clin Psychol Rev. 2010;30:12–24. doi: 10.1016/j.cpr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay-Lambkin FJ, Baker AL, Kelly B, Lewin TJ. Clinician-assisted computerised versus therapist-delivered treatment for depressive and addictive disorders: a randomised controlled trial. Med J Aust. 2011;195:S44–50. doi: 10.5694/j.1326-5377.2011.tb03265.x. [DOI] [PubMed] [Google Scholar]
- Kay-Lambkin FJ, Baker AL, Lewin TJ, Carr VJ. Computer-based psychological treatment for comorbid depression and problematic alcohol and/or cannabis use: a randomized controlled trial of clinical efficacy. Addiction. 2009;104:378–388. doi: 10.1111/j.1360-0443.2008.02444.x. [DOI] [PubMed] [Google Scholar]
- Lee DC, Budney AJ, Brunette MF, Hughes JR, Etter JF, Stanger C. Treatment models for targeting tobacco use during treatment for cannabis use disorder: case series. Addict Behav. 2014;39:1224–1230. doi: 10.1016/j.addbeh.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: randomized trial of contingency management and self-efficacy enhancement. Addict Behav. 2013;38:1764–1775. doi: 10.1016/j.addbeh.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Budney AJ. Tobacco smoking in marijuana dependent outpatients. J Subst Abuse. 2001;13:583–596. doi: 10.1016/S0899-3289(01)00093-1. [DOI] [PubMed] [Google Scholar]
- Nieva G, Ortega LL, Mondon S, Ballbe M, Gual A. Simultaneous versus delayed treatment of tobacco dependence in alcohol-dependent outpatients. Eur Addict Res. 2010;17:1–9. doi: 10.1159/000321256. [DOI] [PubMed] [Google Scholar]
- Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, Lukas SE. Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend. 2005;79:211–223. doi: 10.1016/j.drugalcdep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Peters Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404–1417. doi: 10.1111/j.1360-0443.2012.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Rabin RA, George TP. A review of co-morbid tobacco and cannabis use disorders: possible mechanisms to explain high rates of co-use. Am J Addict. 2015;24:105–116. doi: 10.1111/ajad.12186. [DOI] [PubMed] [Google Scholar]
- Ream GL, Benoit E, Johnson BD, Dunlap E. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 2008;95:199–208. doi: 10.1016/j.drugalcdep.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Rawson RA, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, Sutherland G. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103:146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database System Rev. 2012 doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- Strecher VJ, Marcus A, Bishop K, Fleisher L, Stengle W, Levinson A, Fairclough DL, Wolfe P, Morra M, Davis S, Warnecke R, Heimendinger J, Nowak M. A randomized controlled trial of multiple tailored messages for smoking cessation among callers to the cancer information service. J Health Commun. 2005;10(Suppl 1):105–118. doi: 10.1080/10810730500263810. U14XV6LR81G5W37J [pii] [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Covey LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4:388–396. doi: 10.1007/s11920-002-0087-5. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services; Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health, ICPSR35509-v1. Inter-university Consortium for Political and Social Research [distributor]; Ann Arbor, MI: 2013. 2014-11-18 http://doi.org/10.3886/ICPSR35509.v1. [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]