Abstract
Although a standardized approach to radiotherapy has been used to treat breast cancer, regardless of subtype (e.g., luminal, basal), recent clinical data suggest that radiation response may vary significantly among subtypes. We hypothesized that this clinical variability may be due, in part, to differences in cellular radiation response. In this study, we utilized RNA samples for microarray analysis from two sources: 1. Paired pre- and postirradiation breast tumor tissue from 32 early-stage breast cancer patients treated in our unique preoperative radiation Phase I trial; and 2. Sixteen biologically diverse breast tumor cell lines exposed to 0 and 5 Gy irradiation. The transcriptome response to radiation exposure was derived by comparing gene expression in samples before and after irradiation. Genes with the highest coefficient of variation were selected for further evaluation and validated at the RNA and protein level. Gene editing and agonistic antibody treatment were performed to assess the impact of gene modulation on radiation response. Gene expression in our cohort of luminal breast cancer patients was distinctly different before and after irradiation. Further, two distinct patterns of gene expression were observed in our biologically diverse group of breast cancer cell lines pre- versus postirradiation. Cell lines that showed significant change after irradiation were largely luminal subtype, while gene expression in the basal and HER2+ cell lines was minimally impacted. The 100 genes with the most significant response to radiation in patients were identified and analyzed for differential patterns of expression in the radiation-responsive versus nonresponsive cell lines. Fourteen genes were identified as significant, including FAS, a member of the tumor necrosis factor receptor family known to play a critical role in programed cell death. Modulation of FAS in breast cancer cell lines altered radiation response phenotype and enhanced radiation sensitivity in radioresistant basal cell lines. Our findings suggest that cell-type-specific, radiation-induced FAS contributes to subtype-specific breast cancer radiation response and that activation of FAS pathways may be exploited for biologically tailored radiotherapy.
INTRODUCTION
Radiotherapy is a routine component of multidisciplinary care for patients with breast cancer. However, despite increasingly detailed knowledge of breast tumor biology, the daily prescription of radiotherapy has remained relatively constant over many decades. It is now well known that breast tumors are composed of a heterogeneous group of distinct subtypes with characteristic clinical outcomes and patterns of gene expression (1). Moreover, it has been demonstrated repeatedly that breast cancer subtypes vary in their response to chemotherapy (2–4). Although less is known about the relationship between radiation response and breast cancer subtypes, previously reported clinical data have suggested that the more biologically aggressive phenotypes may display greater radiation resistance (5–7). In an analysis of 2,985 breast tumors, Voduc et al. noted clear variation in locoregional recurrence risk. These inherent subtype-specific differences in locoregional relapse were quite clear in patients treated with mastectomy, largely without radiation therapy. However, the subtype-specific patterns of locoregional recurrence persisted even in the breast conservation setting where radiation was uniformly delivered, suggesting that the more biologically aggressive subtypes are also more resistant to radiotherapy (7). Mechanisms underlying a differential response to radiotherapy in breast cancer subtypes are not well understood. Amundson et al. previously characterized a 60-cell-line panel from the National Cancer Institute for survival, apoptosis and gene expression in response to radiation exposure. This data suggested that the basal gene expression pattern was superior to radiation response signatures in discriminating radiosensitive from radioresistant cell lines. However, this panel incorporated only a few breast cancer cell lines and not all subtypes were included (8). Helland et al. (9) reported on changes in gene expression using pre- and postirradiation tumor biopsies from 19 patients with breast cancer. They identified four genes with consistent induction, two of which were involved in DNA repair and cell cycle control. However, all patients in this series had locally advanced or metastatic tumors, a setting in which inherent biologic differences may not be as clear.
Our overall hypothesis is that clinical differences in locoregional control among breast cancer subtypes are associated with unique radiation response gene expression profiles. Because breast cancer radiotherapy is typically delivered almost exclusively in the postoperative setting (10), opportunities to study this response are rare. However, we have generated a unique cohort of paired pre- and postirradiation samples from our clinical trial of patients with biologically favorable early-stage breast cancer treated with preoperative radiation therapy. To the best of our knowledge, our group was the first to propose delivery of radiation therapy prior to surgical resection, and we have since optimized this technique (11). In the current study, using our patient samples and a biologically diverse panel of breast tumor cell lines, we examined the mechanisms underlying observed biological diversity in breast cancer radiation response and looked for subtype-specific patterns of radiation response that could act as a starting point to link radiation response phenotype to potentially targetable biologic pathways (12).
METHODS
Breast Cancer Patients
Patients with biologically favorable [estrogen-receptor-(ER) or progesterone-receptor-(PR) positive, HER2-negative] early-stage breast cancer participating in an Institutional Review Board (IRB) approved Phase I protocol (no. Pro00015617) at Duke University Medical Center were included in the study (trial registration no. NCT00944528). Patients (n = 32) 55 years or older with cT1N0 invasive carcinomas (n = 25) or low/intermediate grade ductal carcinoma in situ <2 cm (n = 7) were enrolled after providing informed consent. Formalin fixed and paraffin embedded (FFPE) tumor samples were obtained at the time of diagnosis. Patients were enrolled consecutively in cohorts of eight to receive a 15, 18 and 21 Gy dose to determine the maximum tolerated dose of preoperative partial breast radiotherapy. Surgical resection was performed on patients within 10 days of treatment and postirradiation FFPE tumor samples were obtained at the time of surgical excision.
Microarray Analysis of Human Samples
Of 32 patients enrolled in the clinical trial, 26 had sufficient paired tumor tissue to be used for microarray analysis. In addition, we had a total of 9 biologic replicates to test for reproducibility of our data. RNA extraction and labeling was performed using the RNeasy FFPE kit (cat. no. 73504; QIAGEN®, Valencia, CA), and the Sensation-Plus™ FFPE Amplification and Labeling Kit (cat. no. 902312; Affymetrix Inc., Santa Clara, CA). All total RNA samples were assessed for quality using a NanoDrop™ 8000 spectrophotometer for absorbance ratios and the Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). Whole transcriptome expression analysis was evaluated with HTA 2.0 arrays (cat. no. 902162; Affymetrix). All samples were fully annotated and linked to clinical data.
The patients’ genomic data discussed in this article has been deposited in the NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) (13). The deposited data are accessible through the GEO Series accession no. GSE65505 (http://1.usa.gov/1JQFl9x).
Breast Cancer Cell Lines
A total of 16 breast cancer cell lines displaying gene expression patterns consistent with distinct clinical breast subtypes were selected (12, 14). Those cell lines were as follows: for luminal, MCF7, T47D, ZR751, CAMA-1, BT474 (Her2+), SKBR3 (Her2+), AU565 (Her2+); for basal, SUM149, SUM159, MDA-MB-231, MCF10A, MCF12A, BT549, HBL100, HCC1954 (Her2+) and DKAT. These cell lines reflect the heterogeneity of human breast cancer, thereby providing a valid biological model for study of phenotype-specific mechanisms. Cell lines were obtained from the American Type Culture Collection (ATCC®, Gaithersburg, MD) with the exception of DKAT, ZR751, MCF10A and the SUM lines, which were a gift from Drs. Victoria Seewaldt and Gayathri Devi (15, 16). For full details of culture conditions, please see the Supplementary Information (http://dx.doi.org/10.1667/RR14089.1.S1).
Microarray Analysis of Breast Cancer Cell Lines
Total RNA was collected from approximately 1 × 106 cells in each of the 16 cell lines. RNA was isolated from the irradiated cell lines (5 Gy) 24 h after treatment using the RNeasy Mini Isolation Kit (QIAGEN). The same protocol was used for control cell lines (0 Gy). Samples were run in triplicate. RNA was hybridized to Affymetrix U133A2 arrays and processed in the Microarray Core Facility (Duke University Medical Center, Durham, NC).
The genomic data for the breast cancer cell lines discussed in this article have been deposited in the NCBI GEO (http://www.ncbi.nlm.nih.gov/geo/) (13). The deposited date are accessible though the GEO Series accession no. GSE59732 (http://1.usa.gov/1JQJF4j).
Statistical Consideration
All statistical analyses were conducted using the R statistical environment and extension packages from CRAN and the Bio-conductor project (17, 18). Gene level expression estimates were obtained with Affymetrix Expression Console software (v.1.3) using RMA-Sketch workflow. To check for sample outliers and batch effects, principal component analysis of the global gene expression was conducted. Batch effects were corrected by scale-shift normalization prior to analysis (19). For the human tumor samples, differential expression for paired samples was evaluated using the Bioconductor limma package with correction for multiple comparisons (20). Genes with false discovery rate (FDR) adjusted P values (Q values) less than 0.05 were selected as differentially expressed in response to radiation exposure (21). In the breast cancer cell lines, predefined genes of interest were selected to represent major cellular signaling pathways of interest in cancer progression and radiation response. A two-sample t test, followed by multiple hypotheses testing, was performed to identify differentially expressed probe sets caused by radiation exposure. To link the human data to our tissue culture findings, we utilized a two-way multiplicative linear mixed-effects model (22) (using the cell line as a random effect) to identify interactions between the 100 human genes whose expression was most significantly changed by exposure to radiation and differentially expressed genes in the radiation-responsive versus nonresponsive breast cancer cell lines. Implementation was provided by the linear mixed effects (lme) function in the nonlinear mixed effects (nlme) extension package and the inference was conducted using the restricted maximum likelihood (REML) approach (23). The null sampling distribution for testing the marginal hypothesis of each fixed effect was assumed to follow a t distribution with appropriate degrees of freedom. The analyses were conducted under the implicit assumption of homogeneity with respect to the variance of the random effects and the variance of the measurement errors. Multiplicity was addressed within the FDR framework as above. The interaction effect between hypothesized radiation response status and radiation treatment is considered significant for those probe sets with FDR adjusted P values (Q values) less than 0.05.
The Cancer Genome Atlas (TCGA)
We used TCGA for external confirmation of our results. TCGA mRNA data were retrieved from the Cancer Genomic Data Server (CGDS) through the Computational Biology Center Portal (cBio; http://www.cbioportal.org/). The cdgsr extension package (https://cran.r-project.org/web/packages/cgdsr/cgdsr.pdf) was used to execute the retrieval. We used a validated radiation response molecular signature (AR, cJun, STAT1, PKC, RelA, cABL, SUMO1, CDK1, HDAC1 and IRF1) (24) to calculate the radiosensitivity index (RSI) for 81 basal-like TCGA breast tumor samples. The model predicts a continuous RSI that is based on the survival fraction at 2 Gy (SF2). We did a linear regression of FAS expression values over RSI for all basal samples.
Immunohistochemistry (IHC)
FFPE tissue samples were processed via standard histologic procedures and 5 μm sections were stained with the intelliPATH® automated immunostainer (Biocare Medical Inc., Concord, CA). After an antigen retrieval step (35 min in sub-boiling citrate buffer, pH 6.0), the anti-FAS primary antibody [Ab-FAS (C20); no. sc715; Santa Cruz Biotechnology Inc., Dallas, TX] was applied for 2 h (1:800 dilution). The MDA-MB-468 cell line and placental tissue were used as positive controls. A negative control stain without primary antibody was also performed for all experiments. Each section was scored for average staining intensity (+ = weak, 2+ = moderate; there was no strong staining) and percentage of positive cells in both the cell membrane and the cytoplasm by a breast pathologist (JG). Those two factors were then multiplied together to create a modified histoscore. The membrane and cytoplasmic scores were added to generate a combined score. If the difference in combined score was >100, the expression level was considered significantly altered.
Quantitative Real-Time RT-PCR Analysis
Total RNA was harvested from approximately 1 × 106 irradiated cell lines (0 and 5 Gy) 24 h after irradiaton using the RNeasy Mini Isolation Kit (QIAGEN). RNA was reverse transcribed with MuLV reverse transcriptase and Oligo d(T) primers (Applied Biosystems, Foster City, CA). The SYBR® Green PCR Kit (Applied Biosystems) was used for quantitative real-time RT-PCR analysis. The primers were synthesized by Integrated DNA Technologies (Coralville, Iowa). Human primer sequences are listed as below. Relative differences in gene expression among groups were determined from cycle time (Ct) values. These values were first normalized to 18S or GAPDH in the same sample (ΔCt) and expressed as fold over control (2−δδCt). Real-time fluorescence detection was performed using an ABI PRISM® 7900 Sequence Detector (Applied Biosystems). The following primers were used:
Forward: FAS - 5′-TCA GTA CGG AGT TGG GGA AG-3′ and FAS L - 5′-GCA CTT TGG GAT TCT TTC CA-3′; reverse: FAS - 5′-CAG GCC TTC CAA GTT CTG AG-3′ and FAS L - 5′-CCT CCA TTT GTC TGG CTC AT-3′.
Western Blot Analyses
Protein expression levels were analyzed using Western blot assays. Cell lysates were prepared from cells at 50–70% confluence. Cells were harvested by trypsinization, washed once in PBS and resuspended in mammalian cell lysis buffer (MCLB) [50 mM Tris-HCl (pH 8.0), 100 mM NaCl, 5 mM EDTA, 2 mM DTT, 1% NP40, 10 mM β-glycerophosphate, 1 mM Na3VO4] supplemented with 1× protease inhibitor cocktail (cat. no. P8340; Sigma-Aldrich LLC, St. Louis, MO). After clarifying the extract by centrifugation, protein concentration was determined using the Bio-Rad DC protein assay (Bio-Rad Laboratories, Inc., Hercules, CA). Samples containing equal amounts of protein were mixed with equal volumes of 2× Laemmli sample buffer [125 mM Tris-HCl (pH 6.8), 4% SDS, 20% glycerol] containing 5% β-mercaptoethanol, boiled and separated by SDS-PAGE. Proteins were transferred to nitrocellulose and probed with antibodies against: FAS (cat. no. 8023; Cell Signaling Technology, Danvers, MA), FAP1 (cat. no. sc-15356; Santa Cruz Biotechnology), TP53 (cat. no. MS-187-PO; Lab Vision/NeoMarkers, Fremont, CA), cleaved (active) caspase 3 (Asp175, cat. no. 9661S; Cell Signaling Technology), cleaved PARP [c-PARP (Asp214, cat. no. 9541, Cell Signaling Technology], cleaved caspase 8 (cat. no. NBP1-71399; Novus Biologicals LLC, Littleton, CO) and cleaved caspase 9 antibody (cat. no. AB3629; EMD Millipore, Billerica, MA).
Modulation of FAS Expression
shRNA-mediated FAS knockdown
To evaluate the effect of FAS knockdown on radiosensitivity we used the radiation-responsive MCF7 cell line, which showed high levels of FAS induction in response to radiation exposure. We utilized a lentivirus-based approach to knockdown our gene of interest (cat. no. 9606102–9606106; Thermo Scientific Open Bio Systems, Huntsville, AL). The shRNA lentiviral vectors contained a U6 RNA polymerase III-constitutive promoter that drives short hairpin RNA expression. The MCF7 cells were infected with a single human FAS shRNA (cat. no. 9606104; Thermo Scientific Open Bio Systems) or negative control shRNA lentiviral vectors and 72 h post infection were placed under puromycin (1 μg/ml) selection. A pool of five human FAS shRNAs (cat. no. 9606102–9606106) was also utilized in a separate experiment. Cells were maintained for 3–5 days in selection media. Roughly 70% of the cells survived. The surviving cells were considered to be stably expressing shRNA due to the high efficiency integration of lentiviral vector into the mammalian genome and puromycin resistance. Changes in FAS protein expression were confirmed by Western blot.
FAS overexpression
Overexpression of FAS was achieved using a lentivirus-based approach (cat. no. EX-G0198-Lv105; GeneCopoeia™, Rockville, MD) in two basal cell lines with low levels of FAS expression, MDA-MB-231 and AU565. The lentiviral vector contains a CMV promoter that derives human FAS expression. The cells were infected with FAS-vector or negative control lentiviral vectors and 72 h post infection the cells were placed under puromycin selection (1 mg/ml). Cells were selected further for another 3–5 days, and roughly 70% of cells survived. The surviving cells were considered to be stably expressing FAS due to high efficiency of lentiviral vector integration into the mammalian genome. Change in FAS protein expression was confirmed by Western blot. FAS overexpressing cells were then treated with radiation and a FAS agonistic antibody (CH11, cat. no. 05-201; EMD Millipore) to evaluate downstream FAS signaling.
FAS Stimulating Antibody
To activate FAS, we used a FAS agonistic (stimulating) antibody, CH11 (monoclonal mouse antibody, cat. no. 05-201; EMD Millipore), at a concentration of 0.1 μg/ml. The goal was to determine if FAS activity would change radiation sensitivity in a radiation-nonresponsive cell line. SUM159 was pretreated with CH11 for 6 h prior to irradiation. The antibody-containing media was then removed and replaced with fresh media without antibody. Clonogenic cell survival assays were conducted as described below. Cell survival in the treated lines was compared to the untreated cell lines.
Clonogenic Survival Assays
Cell lines of interest were plated and allowed to adhere overnight at 37°C. Plates were exposed to variable doses of radiation using a Cesium-137 Mark-I irradiator (JL Shepherd, San Fernando, CA). Media was removed and replaced 24 h after irradiation. The cells were allowed to grow into colonies over a period of 10–14 days or until the control plates grew visible colonies. The plates were then washed with 1× PBS, fixed with 10% methanol–10% acetic acid for 10 min and stained with a 0.4% solution of crystal violet for 10 min. A ColCount™ colony counter (Oxford Optronix Ltd., Abingdon, UK) was used to image and count the number of colonies per plate using fixed sensitivity settings so that only colonies of >50 cells were counted. Plating efficiencies (PE) were calculated using the formula: PE = number of colonies/number of cells seeded and normalized to the control/sham-irradiated plates. Surviving fraction (SF) was calculated using the formula: SF = number of colonies/number of cells seeded × PE. End points included the surviving fraction at each dose level.
shRNA-Mediated TP53 Knockdown
We utilized a retrovirus-based approach to knockdown TP53 in MCF7 cell lines (25) (gift from Dr. Xiaohu Tang). Briefly, the shRNA retroviral vector pRetroSuperpuro contained a H1 RNA polymerase III-constitutive promoter that drives short hairpin RNA expression. The MCF7 cells were infected with human TP53 shRNA or negative control shRNA retroviral vectors and 72 h after infection were placed under puromycin (1 μg/ml) selection. Cells were selected further for another 3–5 days, and roughly 70% of the cells survived. The surviving cells were considered to be stably expressing shRNA due to high-efficiency integration of the retroviral vector into the mammalian genome and puromycin resistance. Changes in TP53 protein expression were confirmed by Western blot.
ImageStream Flow Cytometry
In addition to the effect of radiation on FAS expression at the mRNA and protein levels, exposure to radiation may also affect FAS localization in the cells. We used the ImageStreamX® Mark II imaging flow cytometer (Amnis/EMD Millipore, Seattle WA) to evaluate radiation-induced changes in the localization of: 1. FAS; 2. FAS-associated phosphatase 1 (FAP1); and 3. FAS ligand. Human breast cancer cell lines (radiation-responsive cell line MCF7 and radiation-nonresponsive cell line SUM159) were 0 or 5 Gy irradiated and harvested 24 h after irradiation. Cells were stained with mAbs for human FAS (FAS PE; eBioscience Inc., San Diego, CA) or FAS ligand (FASL, PE; BioLegend® Inc., San Diego, CA). After Ab staining the cells were fixed with 1% formaldehyde. For the cytoplasmic immunostaining, the cells were then permeabilized and blocked in permeabilization buffer (3% fetal calf serum, 0.1% Triton™ X-100) at room temperature for 1 h and incubated with anti-FAS Ab, anti-FAS ligand Ab or anti-FAP1 Ab (H-300, Santa Cruz Biotechnology) followed by incubation with goat-anti-rabbit IgG FITC Ab (Santa Cruz Biotechnology) in permeabilization buffer. Anti-DRAQ5 DNA dye (Cell Signaling Technology) was used for nuclear staining. A total of 5,000–8,000 cells were then collected and analyzed by ImageStreamX Mark II imaging flow cytometer. Membrane and cytoplasmic masks were used to analyze FAS and FAS ligand localization. FAS and FAP1 co-localization was quantified by calculating the bright detail similarity of the FAS and FAP1 intensity.
Details on the cell line growth conditions can be found in the Supplementary Information (http://dx.doi.org/10.1667/RR14089.1.S1).
RESULTS
Gene Expression Response to Radiation in Human Breast Tumors
Thirty-two women with early-stage ER-positive breast cancer were enrolled in our novel clinical trial evaluating preoperative radiation therapy (Fig. 1A). After pathologic confirmation of a ≤2 cm in situ or invasive breast cancer with clinically negative lymph nodes, patients received a single fraction dose of 15, 18 and 21 Gy. Within 10 days, patients were taken to the operating room for surgical excision. Twenty-six patients had tumor tissue from the pretreatment diagnostic core biopsy and post-treatment from the surgical specimen collected for further analysis of gene expression.
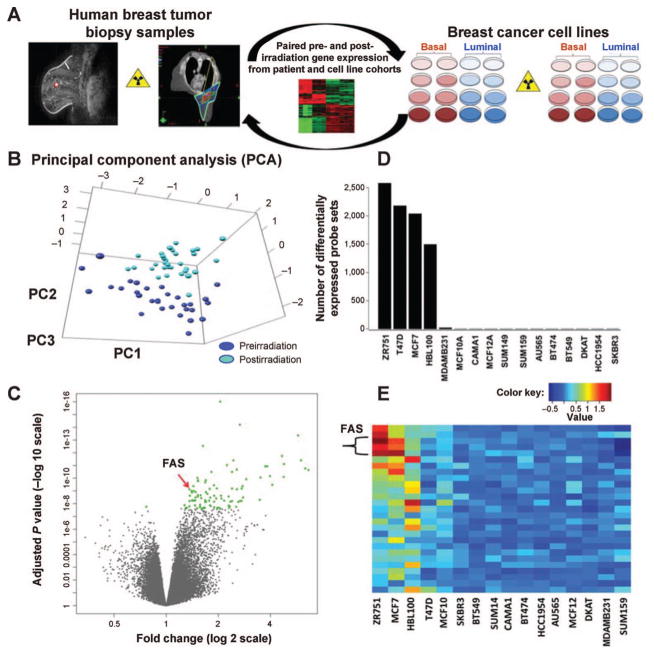
FIG. 1.
Response to radiation in paired pre- and postirradiation breast tumor samples and a panel of diverse breast cancer cell lines. Panel A: Overview schematic of research plan. Panel B: Principal component analysis on human breast cancers suggests that gene expression profiles after irradiation are significantly and consistently distinct from that noted prior to radiation. Panel C: Induction of gene expression dominates repression in these samples. The 100 genes with the most significant change in response to radiation are illustrated in green. Panel D: 16 cell lines representing diverse biologic phenotypes: luminal HER2− (MCF7, T47D, ZR751, CAMA-1), luminal-HER2+ (BT474, SKBR3, AU565), HER2+ (HCC1954) and basal (SUM149, SUM159, MDA-MB-231, MCF10A, MCF12A, BT549, HBL100 and DKAT) were evaluated for response to a single dose of 5 Gy. Four of the cell lines (3 HER2− luminal lines: MCF7, ZR751, T47D, and 1 basal: HBL100) had a large number of genes with significant induction or repression after irradiation. In contrast, the remaining twelve basal and HER2+ luminal cell lines revealed a striking absence of response to radiation. Panel E: Fourteen genes associated with 27 probe sets were identified as having significant Q values for differential expression in both the human data and in the cell line data (Q < 0.002). FAS is significantly induced with an effect conserved across multiple probe sets.
Radiotherapy to the intact breast tumor resulted in marked changes in gene expression (Fig. 1B). Principal component analysis showed distinct separation between pre- and postirradiation breast tumor samples with minimal overlap. Furthermore, gene induction, rather than repression, appears to be the dominant effect, with a large number of genes induced by radiation exposure (Fig. 1C). From this data, 100 genes with the most significant change in radiation response (Q < 2.57E-08) were selected for further evaluation (Fig. 1C, green dots). Genes in this list included those involved in various biological processes such as apoptosis, cell cycle and MAPK signaling pathways.
Gene Expression Response to Radiation in Breast Cancer Cell Lines
Our clinical cohort consisted entirely of patients with luminal subtype breast cancer, since individuals with more aggressive subtypes were not felt to be optimal candidates for the clinical trial. To better understand the biologic relevance of our findings across tumor subtypes, we next evaluated gene expression patterns after irradiation in a large panel of biologically diverse breast cancer cell lines (Fig. 1A). Microarray analysis indicated markedly different radiation response profiles among the breast cancer cell lines after irradiation, with two dominant patterns emerging (Fig. 1D). In four of the 16 cell lines, there were a large number of genes exhibiting significant change in expression as a result of radiation exposure. Three of the four HER2− luminal cell lines (MCF7, T47D, ZR751) and one basal line (HBL100) were included in this radiation-responsive cohort. In contrast, using the same filtering criteria, we noted a striking absence of change in gene expression in the remaining 12 basal and HER2+ luminal cell lines.
Candidate genes were evaluated to determine which specific pathways might be driving the unique radiation response in these two cohorts. The most significant differential pattern of expression was seen in the induction of FAS after irradiation. FAS (CD95, APO-1) a critical modulator of programed cell death, was seen to increase significantly in three of the four radiation-responsive cell lines (MCF7, ZR751 and HBL100). In contrast, despite highly variable levels of FAS expression at baseline, none of the radiation-nonresponsive cell lines demonstrated significant FAS induction. This effect was conserved across multiple FAS probe sets. Additional genes important in programed cell death, TP5313 (p53-inducible protein 3) and caspase 9, were also noted to be differentially affected by radiation in the radiation-responsive versus nonresponsive cell lines.
Concordant Gene Expression Response in Breast Tumors and Cell Lines
To link the human tumor samples and biologically diverse breast cancer cell lines, we assessed for differential expression of the 100 most radiation-responsive human genes in the radiation-responsive versus nonresponsive cell lines. Fourteen genes associated with 27 probe sets were identified as having significant Q values for differential expression in both the human data and in the cell line data (Q < 0.002). FAS was again identified as highly significant (Q = 7.42E-10) (Fig. 1E). These findings suggested a role for the differential activation of the apoptosis pathway as a biologically plausible explanation for clinical differences in radiation response (10) that deserved further exploration.
Validation of FAS Induction in Human Tissue
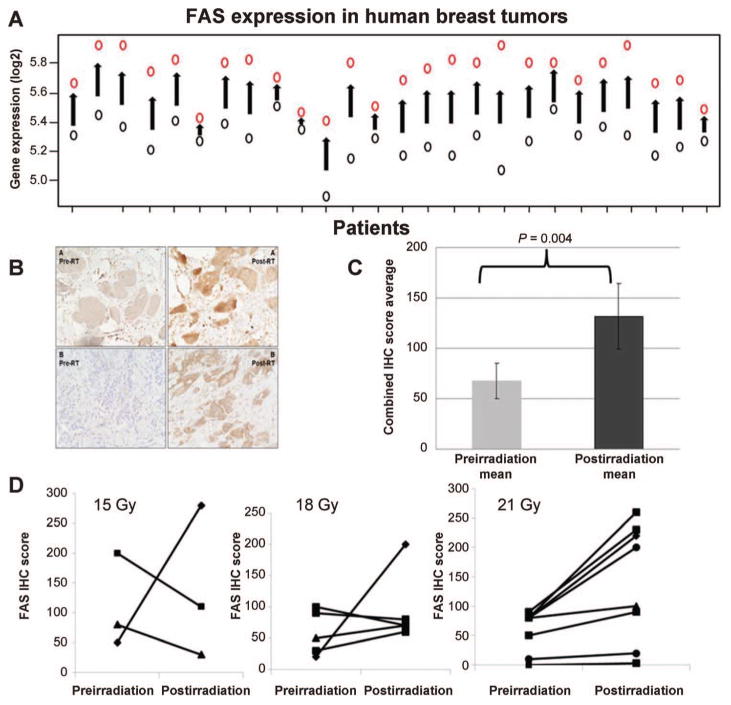
To validate our human microarray results (Fig. 2A) showing that in each patient FAS was induced in response to radiation exposure, we utilized immunohistochemistry (IHC) to confirm FAS induction at the protein level. We again saw significant FAS induction with radiation exposure (Fig. 2B). For evaluable preirradiation patient samples (n = 27), the mean score was 68, while evaluable postirradiation samples (n = 20) had a mean FAS score that was nearly double at 132 (P = 0.004; Fig. 2C). Sixteen patients had paired pre- and postirradiation IHC results. Six of those (38%) demonstrated upregulation of FAS that was identified as significant (change in histoscore >100; P = 0.01). Although induction was not as uniform at the protein level, tumors were assessed at a variable time window (1–10 days) between radiation treatment and surgical resection in this initial proof-of-concept dose-escalation trial, and IHC is known to be a more subjective measure. Of the six cases with significant induction, four were in the highest radiation dose cohort of 21 Gy suggesting that significant FAS induction may be dose dependent (Fig. 2D).
FIG. 2.
Patterns of FAS induction in response to radiation treatment in human breast tumor samples. Panel A: FAS response to radiation in the paired pre- and postirradiation gene expression samples from our clinical trial (Q = 7.42 E-10). Black circles represent preirradiation expression levels and red circles represent postirradiation expression. Panel B: FAS immunohistochemistry (IHC) in two selected patients (panels A and B) pre- (left side) and postirradiation (right side). Photos were taken at 200×. Panel C: Mean FAS IHC scores nearly doubled (P = 0.004) when including all interpretable patient samples preirradiation (n = 27) and postirradiation (n = 20). Panel D: Sixteen of 32 patients had paired pre- and postirradiation FAS IHC. Six of 16 patients showed significant FAS induction (change in histoscore >100) and 4 were in the highest dose cohort (21 Gy) suggesting a dose-response relationship.
FAS Validation in Cell Lines
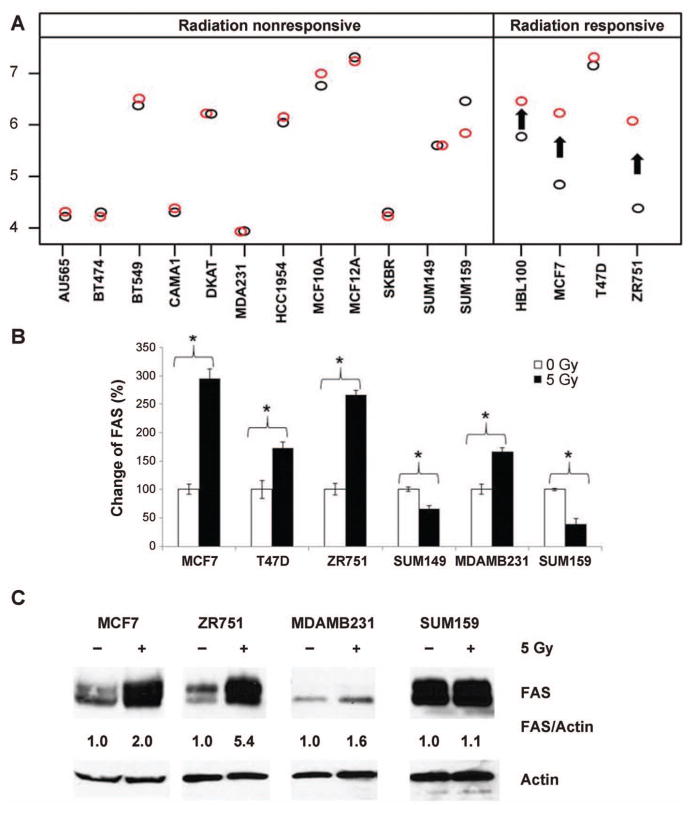
To confirm our cell line microarray findings (Fig. 3A) showing induction of FAS in predominantly luminal cell lines, we selected representative subsets of cell lines from the radiation-responsive and nonresponsive groups for further study. Although FAS induction was seen in one of the nonresponsive cell lines (MDAMB231), qPCR analysis also confirmed significant induction of FAS in three of the radiation-responsive lines (MCF7, ZR751 and T47D). In contrast, the two SUM lines did not demonstrate FAS induction. Similarly, at the protein level, Western blot analysis demonstrated that the radiation-responsive cell lines (MCF7 and ZR751) had increased FAS signal in response to radiation while there were no significant changes in MDAMB231 and SUM159 (radiation-nonresponsive subset) despite significant variability in baseline FAS levels (Fig. 3C). Our findings of FAS induction in the radiation-responsive cell line MCF7 and no change or repression in the nonresponsive line SUM159 were confirmed over multiple experiments [Fig. 3 and Supplementary Fig. S1 (http://dx.doi.org/10.1667/RR14089.1.S1)].
FIG. 3.
FAS response to radiation in breast cancer cell lines. Panel A: FAS induction is noted in 3 of the 4 radiation-responsive cell lines (black circles represent preirradiation gene expression; red circles represent postirradiation gene expression; arrows represent statistically significant findings). This is not seen in the nonresponsive cell lines, despite highly variable baseline levels of FAS. Panel B: qPCR confirms differential patterns of FAS response to radiation in phenotypically distinct cell lines (*P < 0.05). Panel C: At the protein level, FAS protein is again increased in the radiation-responsive (MCF7, ZR751) group and does not significantly change in the nonresponsive (MDAMB231, SUM159) cell lines. All results shown are for 24 h after a single dose of 5 Gy.
FAS Localization
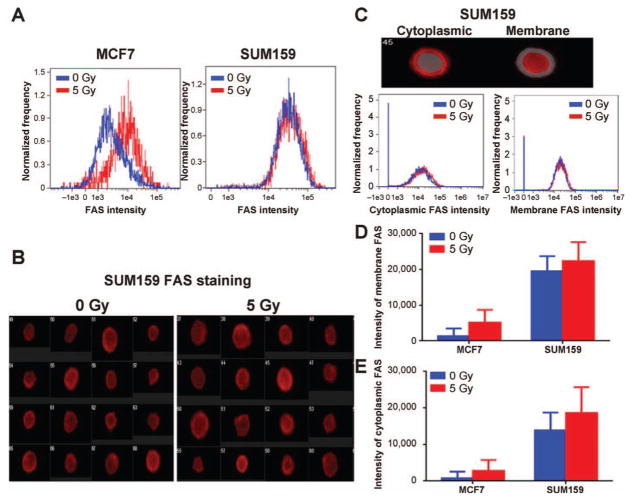
It has been shown that in some human tumors, export of FAS to the cell surface is impaired due to FAS association with FAS-associated phosphatase 1 (FAP1), thereby inactivating FAS ligand-mediated apoptosis (29, 30). To investigate if radiation exposure affected FAP1 expression and FAS retention differently in radiation-responsive versus nonresponsive cell lines, we used ImageStream flow cytometry to analyze FAS trafficking and FAS/FAP1 co-localization. We found that radiation exposure increased total FAS intensity in the radiation-responsive cell line MCF7, but had little effect on the radiation-nonresponsive cell line, SUM159 (Fig. 4A). In SUM159 cells, FAS was intensely expressed on both the cell surface and in the cytoplasm before as well as after exposure to radiation (Fig. 4B–E).
FIG. 4.
Effects of radiation on FAS trafficking. MCF7 and SUM159 cells were treated with a 0 and 5 Gy dose of radiation and stained with anti-FAS Ab 24 h after irradiation. FAS expression was analyzed by ImageStream flow cytometry. Panel A: Radiation exposure increased the total FAS intensity in the radiation-responsive cell line MCF7, but had little effect on the radiation-nonresponsive cell line, SUM159. Panel B: Images of FAS staining in SUM159 cells demonstrated that FAS was expressed both on the cell surface and in the cytoplasm. Panel C: To evaluate the localization of FAS, we generated masks for cytoplasmic and membrane FAS to quantify their intensity. Panels D and E: In response to radiation, both cytoplasmic and membrane FAS increased in the MCF7 cell line. In contrast, SUM159 had high levels of membrane and cytoplasmic FAS before and after irradiation.
FAP1 was also highly expressed in the cytoplasm of the radiation nonresponsive cell line SUM159. Radiation exposure increased FAP1 expression in MCF7, but not in SUM159 (Supplementary Fig. S2A–C; http://dx.doi.org/10.1667/RR14089.1.S1). In both cell lines, the similarity scores of FAP1 and total FAS or FAP1 and cytoplasmic FAS were low (less than 1) despite radiation exposure, indicating there was low co-localization of FAP1 and FAS (Supplementary Fig. S2D–F; http://dx.doi.org/10.1667/RR14089.1.S1) and as a result, that FAS was not retained in the cytoplasm through association with FAP-1.
Finally, some increase in total FAS ligand expression was noted after irradiation in both cell lines (Supplementary Fig. S3A–D; http://dx.doi.org/10.1667/RR14089.1.S1). However, FAS ligand was contained primarily in the cytoplasm with minimal surface expression (Supplementary Fig. S3E and F; http://dx.doi.org/10.1667/RR14089.1.S1) both before and after irradiation. SUM159 did exhibit a small increase in surface expression after irradiation but no clear relationship could be discerned between FAS ligand localization and patterns of FAS expression. Similarly, no easily discernible pattern could be identified in FAS ligand mRNA expression and induction or repression of FAS (data not shown) across all 16 cell lines.
TP53 and FAS Induction
Next, we investigated whether the induction of FAS after irradiation was driven by TP53, which is a known key mediator of cell cycle control, programed cell death and radiation response, as well as an upstream regulator of FAS. Although TP53 is commonly mutated in breast cancers overall, only 12% of luminal A tumors contain TP53 mutations compared to 80% of basal-like tumors.
The MCF7 cell line is known to express wild-type TP53 and induce both TP53 and FAS in response to radiation exposure (Supplementary Fig. S4; http://dx.doi.org/10.1667/RR14089.1.S1). Therefore, we used shRNA to knockout TP53 in the MCF7 cell line. However, despite the loss of TP53, FAS induction was persistent in the TP53 null cell line. The overall level of FAS expression was reduced, but the magnitude of radiation response was similar to that seen in the parent MCF7 cell line [FAS expression (normalized to actin): 1.0–1.8 after irradiation in TP53wt; 0.1–0.4 in TP53 null]. It is important to note that in addition to TP53 there are other known transcriptional regulators that can affect FAS expression, including nuclear factor of kappa light polypeptide gene enhancer in B cells 1 (Nfkb1 or NF-kappaB), signal transducer and activator of transcription 3 (STAT3) and the jun proto-oncogene (JUN, also known as c-Jun) (31–33). Therefore, although FAS radiation response occurs in the absence of TP53 in our study, further study will be required to determine the role of additional regulators of FAS transcription.
Impact of FAS Modulation on Radiation Sensitivity
Although FAS induction appeared to be differentially induced in the more favorable, luminal-type breast cancers, it was not clear that this could then be utilized as a tool to modify radiation response. Therefore, we selected one cell line from the responsive cohort (luminal: MCF7) and one from the nonresponsive cohort (basal: SUM159) to determine if sensitivity to radiation would change as a result of FAS modulation.
We selected the radiation-responsive MCF7 cell line to determine if radiation sensitivity could be reversed in the absence of FAS expression. We used Western blotting to first verify that shRNA effectively knocked down the FAS receptor (Fig. 5A). Radiation was also applied and no FAS induction was seen in the knockdown. Clonogenic cell survival assays were then performed and compared to the control shRNA MCF7 cell line with intact FAS (Fig. 5B). Both cell lines were equally radiosensitive at 4 Gy, but increased radioresistance was observed with FAS knockdown for the lower doses of 1 and 2 Gy.
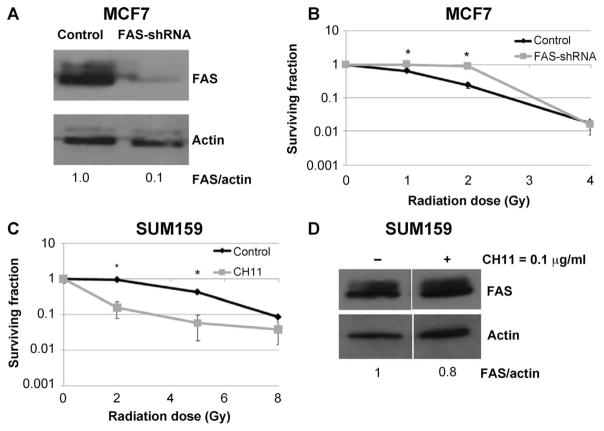
FIG. 5.
Effect of FAS modulation on radiation response. Panel A: FAS was silenced using shRNA in the radiation-responsive MCF7 cells. Panel B: Clonogenic assays revealed an increase in radiation resistance at 1 and 2 Gy dose levels in the absence of FAS (*P < 0.05). At 4 Gy, survival was equivalent suggesting activation of pathways other than FAS-mediated apoptosis, possibly dose-dependent, in this radiation-sensitive cell line. Panel C: In contrast, stimulation of FAS increased sensitivity to radiation in the radiation-nonresponsive cell line, SUM159. The dose to achieve a surviving fraction of 0.1 was approximately 8 Gy in the control cells and 3.25 Gy in the CH11-treated SUM159 cells, yielding a dose-modifying factor of 2.5. Panel D: Enhanced radiation sensitivity occurred despite a lack of significant change in FAS expression levels after pre-treatment with CH11, a FAS activating antibody (0.1 μg/ml).
In contrast, when FAS was activated by FAS-stimulating antibody CH11, the radiation-nonresponsive SUM159 demonstrated significantly greater radiosensitivity (Fig. 5C). The dose to achieve a surviving fraction of 0.1 was approximately 8 Gy in the control cells and 3.25 Gy in the CH11-treated SUM159 cells, yielding a dose-modifying factor of 2.5. This occurred despite a lack of visible FAS induction (Fig. 5D).
FAS as a Therapeutic Target in Basal Cancers
Based on our findings that SUM159 could be sensitized to radiation using a FAS-activating antibody (despite a lack of FAS induction), we hypothesized that basal cell lines with high levels of FAS may retain an intact apoptosis signaling pathway while those with low-level expression do not. Western blot analysis revealed that in the cell lines with high baseline levels of FAS (SUM159, HCC1954) downstream activation of cleaved caspases 3, 9 and PARP did not occur in response to radiation, but could be induced with FAS stimulation via CH11. In contrast, those cell lines with low levels of FAS (MDA231, AU565) at baseline did not exhibit evidence of apoptosis after irradiation or stimulation with CH11 (Fig. 6).
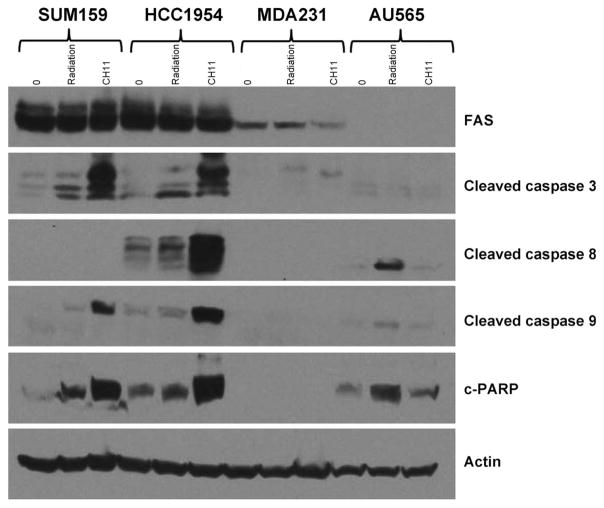
FIG. 6.
FAS signaling in basal cell lines with variable levels of baseline FAS. After pre-treatment with CH11, a FAS activating antibody (0.1 μg/ml), we observed induction of apoptosis proteases caspases 3, 8 and 9, as well as c-PARP in two cell lines with high baseline levels of FAS, SUM159 and HCC1954, suggesting an intact apoptosis pathway. In contrast, this was not observed in the two cell lines with low levels of baseline FAS, MDAMB231 and AU565.
FAS was then overexpressed in the two cell lines with low baseline FAS expression, MDA231 and AU565, to determine if this could restore FAS function and as a result, radiation response. Western blot analysis revealed that significant induction of apoptosis proteases caspases 3, 8 and 9 and c-PARP could be seen in response to treatment with CH11 after FAS overexpression (Fig. 7A). Furthermore, reintroduction of FAS signaling was able to enhance radiation response (Fig. 7B) in both basal cell lines.
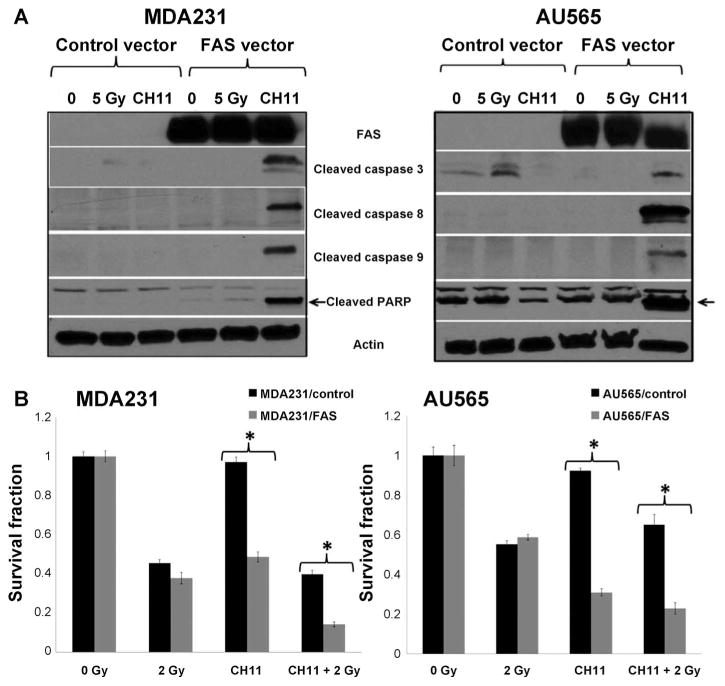
FIG. 7.
Impact of FAS overexpression on radiation response phenotype in basal cell lines with low levels of baseline FAS. Panel A: FAS overexpressing MDMBA231 and AU565 cell lines treated with CH11 demonstrate induction of the apoptosis proteases, caspases 3, 8 and 9 and c-PARP. Panel B: Reintroduction of FAS signaling enhanced radiation response in both basal cell lines.
Finally, to further test our theory that baseline FAS expression may be linked to radiation sensitivity in the basal subtype, we utilized the validated radiation response molecular signature from Eschrich et al. (24) to calculate the radiosensitivity index (RSI) for 81 TCGA basal breast tumor samples. A linear regression model of FAS expression values over RSI demonstrated significance for all of the samples (P = 0.002844) with a negative regression coefficient (−0.83) meaning that FAS expression decreased as RSI increased; an increasing RSI score indicates radiation resistance (Supplementary Fig. S5; http://dx.doi.org/10.1667/RR14089.1.S1).
DISCUSSION
Delivery of radiation therapy in the treatment of breast cancer is currently based on historic data that predated modern molecular techniques and an understanding of breast subtype-specific biology. For almost 300,000 women in the U.S. diagnosed with breast cancer each year, the “one size fits all” radiotherapy approach is a routine part of multidisciplinary breast cancer care. However, clinical data are accumulating to support the idea that radiation response can be linked to breast cancer phenotype (6, 7). A recent Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) publication, with pooled data on over 10,000 breast cancer patients from 17 historical randomized clinical trials, found that while radiation therapy was effective in both groups, the degree of risk reduction for any first recurrence was substantially greater in the estrogen receptor (ER+) patients receiving radiation than those with ER− tumors (10). This data suggest that there is some biologic feature common to ER+ tumors that enhances their sensitivity to ionizing radiation (10). If this characteristic can be identified, it can lead to a greater understanding of breast cancer radiation response and potentially influence radiotherapeutic approaches for all breast tumor subtypes.
Individualization of therapy is particularly relevant in breast cancer. Systemic therapy has moved from a more uniform therapeutic approach to biologically based treatment over the last 5–10 years. Chemotherapy delivered prior to surgery has revolutionized our understanding of subtype-specific chemotherapy response and dramatically impacted clinical practice (34). While initial trials evaluating preoperative chemotherapy grouped all patients with breast cancer together, it has quickly become apparent that this is not an optimal approach. Modern trials now routinely separate patients from different chemotherapy response groups into distinct clinical trials with unique end points.
Therefore, it seems feasible that a better understanding of breast cancer subtype-specific radiation biology could similarly impact clinical practice. Radiation dose could potentially be decreased in patients with more radiosensitive tumors, thus decreasing radiation-related normal tissue damage. Conversely, supplemental therapies could be utilized in patients with more radioresistant tumors to reduce the unacceptably high rate of tumor recurrence with associated morbidity and mortality. However, molecular insight into this differential radiation response is needed to lay the groundwork for clinical trials evaluating individualized radiotherapy.
In this work, we set out to determine whether there were variations in radiation response, using RNA microarray data from a rare cohort of paired pre- and postirradiation breast tumor tissue and a library of 16 diverse breast tumor cell lines. We specifically noted the differential induction of FAS (also known as APO-1 or CD95), a member of the tumor necrosis factor receptor (TNF-R) family, in the luminal human samples and the four radiation-responsive cell lines, three of which were luminal and HER2−. FAS is a cell surface receptor that contains an intracellular “death domain” and plays a critical role in the initiation of apoptotic cell death. Differential expression of FAS has been implicated in tumor aggression, metastasis and resistance to both chemotherapy and radiation therapy (35–39). In breast cancer patients, Botti et al. noted significantly shorter disease-free and overall survival periods in 167 FAS-negative and FASL-positive stage I/II breast cancer patients (40). In contrast, higher expression levels of FAS are associated with smaller tumor size, negative lymph nodes and prolonged disease-free survival (41).
In addition to its prognostic role, FAS has also been shown to predict response to therapy. In human myeloma and T-cell leukemia cells, resistance to mitoxantrone and doxorubicin has been associated with reduced FAS expression. In tumors with epigenetically repressed FAS that are rescued, tumor growth can be suppressed and chemosensitivity restored (42). Furthermore, radiation sensitivity has been linked to FAS expression levels in patients with malignant lymphoma. In patients with rapid clinical tumor regression, FAS was identified at relatively low radiation doses. This was in contrast to the low or absent expression seen in patients with squamous cell tumors from the same anatomic region (43). While FAS induction is viewed as contributing to differences in radiation sensitivity among tumors of diverse origin, it may be that differential FAS induction also helps to explain the clinical differences in response to radiation that exist within a heterogeneous group of breast tumors.
In addition to showing that FAS is induced in response to radiation in a subset of biologically favorable breast cancer cell lines and patient tumors, we have demonstrated, more importantly, that we can alter radiation response by modulating FAS. In the initially radiation-responsive MCF7 cell line, we were able to show that reduction of FAS increased radiation resistance. Furthermore, we were able to demonstrate that the induction seen in response to radiation was reduced, but not dependent on TP53. This finding is supported by the work of Oh et al., who also identified FAS induction despite using multiple mouse models that were TP53 deficient (45). In parallel, we were also able to demonstrate that the radiation response of resistant cell lines (e.g., SUM159) with high baseline levels of FAS expression can be enhanced by a FAS-stimulating antibody. Importantly, this was seen even when FAS induction was not apparent, suggesting that baseline FAS levels may serve as a biomarker of radiation response in the basal tumor subtype. Further supporting this hypothesis, we were able to restore FAS signaling and enhance radiation response by overexpressing FAS in basal cell lines with low baseline expression.
However, our study does have limitations. For example, the classification of cell lines as radiation responsive versus radiation nonresponsive is a working model based on the number of genes differentially expressed in response to radiation exposure. Our analysis of radiation effect in these two cohorts may be biased since we are using the same dataset to classify cells into responsive versus nonresponsive. Furthermore, in our study, the relationship between breast cancer subtype and radiation response was not perfectly aligned. One basal cell line had significant response to radiation and one radiation-responsive luminal cell line did not have significant FAS induction. It is possible that some additional clinical or tumor feature is needed to determine radiation response. Indeed, in human breast cancers, although we observed FAS mRNA induction with radiation exposure in all samples, at the protein level we observed FAS induction in only a subset of samples. Furthermore, the patterns of FAS-dependent cell death in vivo may also differ significantly from what we have observed in vitro due to significantly higher levels of FAS-ligand secretion from tumor-associated macrophages (26, 27, 28). Additional in vivo work will be required to further delineate the impact of the tumor microenvironment on subtype-specific programed cell death. Most importantly, clinical proof that FAS can be used as biomarker to individualize breast radiotherapy will require robust clinical trials with long-term locoregional response end points.
Given the disparate rates of local control in breast tumor subtypes, we would anticipate that subtype-specific radiation knowledge would be utilized differently depending on the subtype. In radiation-responsive breast cancers with significant induction of FAS, clinical trials evaluating dose-reduction strategies to limit the toxicity of therapy could be designed. In contrast, more nonresponsive tumors with high levels of FAS could be selected for concurrent targeted therapy. The use of clinical antibodies targeting the FAS receptor has thus far been limited by normal tissue toxicity (46), but alternative strategies are under investigation. One such strategy involves the use of short structured RNA molecules, known as aptamers, which have several advantages over antibody therapy including the potential to develop aptamer antidotes that reverse toxicity (47).
Our ongoing projects and future studies include using in vitro and in vivo assays to validate and further evaluate additional radiation response candidate genes, as well as to elucidate FAS interaction partners and their involved pathways in the radiation-responsive versus nonresponsive cell lines.
CONCLUSION
We have linked FAS induction to luminal breast tumors, confirmed that this appears to be a subtype-specific phenomenon in our diverse cohort of breast cancer cell lines and demonstrated that FAS can be used to favorably affect radiation sensitivity in resistant subtypes. Based on this data we believe that FAS has the potential to act as a predictive and therapeutic biomarker for radiation response in breast cancer patients.
Supplementary Material
Fig. S1. Radiation-induced FAS expression patterns in SUM159 cell line.
Fig. S2. FAP1 and FAS co-localization.
Fig. S3. Effects of radiation on FAS ligand expression and cellular location in response to 0 and 5 Gy irradiation.
Fig. S4. Relationship between TP53 and FAS induction.
Fig. S5. Relationship between FAS and RSI in TCGA database.
Acknowledgments
We would like to thank the Duke Microarray Core facility (a Duke NCI Cancer Institute and a Duke Institute for Genome Sciences and Policy shared resource facility) for their technical support, microarray data management and feedback on the generation of the microarray data reported in this manuscript. In addition, Dr. Kouros Owzar provided critical supervision and guidance in the analysis of genomic data. The National Cancer Institute and Integrative Cancer Biology Program supported the distribution, via the American Tissue Type Collection, of breast cancer cell lines from the NCI-ICBP-45 kit. Varian Medical Systems provided research funding to support the development of the clinical trial objectives. Salary support for Dr. Horton and research support was provided for the preclinical portions of this project by K12HD043446 - Building Interdisciplinary Research Careers in Women’s Health and Susan G. Komen for the Cure, CCR12225923. We also wish to acknowledge the generous financial support from the NIH (grant nos. CA125618 and CA106520 to JTC) and Department of Defense (grant no. W81XWH-12-1-0148 to JTC). This work was performed under trial registration no. NCT00944528.
References
- 1.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 3.Rody A, Karn T, Solbach C, Gaetje R, Munnes M, Kissler S, et al. The erbB2+ cluster of the intrinsic gene set predicts tumor response of breast cancer patients receiving neoadjuvant chemotherapy with docetaxel, doxorubicin and cyclophosphamide within the GEPARTRIO trial. Breast. 2007;16:235–40. doi: 10.1016/j.breast.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–85. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 5.Kyndi M, Sorensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–26. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–8. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 7.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 8.Amundson SA, Do KT, Vinikoor LC, Lee RA, Koch-Paiz CA, Ahn J, et al. Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Res. 2008;68:415–24. doi: 10.1158/0008-5472.CAN-07-2120. [DOI] [PubMed] [Google Scholar]
- 9.Helland A, Johnsen H, Froyland C, Landmark HB, Saetersdal AB, Holmen MM, et al. Radiation-induced effects on gene expression: an in vivo study on breast cancer. Radiother Oncol. 2006;80:230–5. doi: 10.1016/j.radonc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists’ Collaborative G. Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–16. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palta M, Yoo S, Adamson JD, Prosnitz LR, Horton JK. Preoperative single fraction partial breast radiotherapy for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;82:37–42. doi: 10.1016/j.ijrobp.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets—update. Nucl Acids Res. 2013;41:D991–5. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Amato NC, Ostrander JH, Bowie ML, Sistrunk C, Borowsky A, Cardiff RD, et al. Evidence for phenotypic plasticity in aggressive triple-negative breast cancer: human biology is recapitulated by a novel model system. PLoS One. 2012;7:e45684. doi: 10.1371/journal.pone.0045684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans MK, Tovmasyan A, Batinic-Haberle I, Devi GR. Mn porphyrin in combination with ascorbate acts as a pro-oxidant and mediates caspase-independent cancer cell death. Free Radic Biol Med. 2014;68:302–14. doi: 10.1016/j.freeradbiomed.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentlemen R, Carey V, Huber W, Irizarry R, Duboit S. Bioinformatics and computational biology solutions using R and bioconductor. New York: Springer; 2005. [Google Scholar]
- 18.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owzar K, Barry WT, Jung SH, Sohn I, George SL. Statistical challenges in preprocessing in microarray experiments in cancer. Clin Cancer Res. 2008;14:5959–66. doi: 10.1158/1078-0432.CCR-07-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owzar K, Barry WT, Jung SH. Statistical considerations for analysis of microarray experiments. Cts-Clin Transl Sci. 2011;4:466–77. doi: 10.1111/j.1752-8062.2011.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinheiro JC, Bates DM. Mixed effects models in S and S-PLUS. Berlin Heidelberg New York: Springer; 2000. [Google Scholar]
- 23.Pinheiro J, Bates D, DebRoy S, Sarkar D R Development Core Team. nlme: linear and nonlinear mixed effects models. R package version 3.1-102. 2011 2013. http://CRAN.R-project.org/package=nlme.
- 24.Eschrich SA, Fulp WJ, Pawitan Y, Foekens JA, Smid M, Martens JW, et al. Validation of a radiosensitivity molecular signature in breast cancer. Clin Cancer Res. 2012;18:5134–43. doi: 10.1158/1078-0432.CCR-12-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 26.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 27.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiener PA, Davis PM, Starling GC, Mehlin C, Klebanoff SJ, Ledbetter JA, et al. Differential induction of apoptosis by fas–fas ligand interactions in human monocytes and macrophages. J Exp Med. 1997;185:1511–6. doi: 10.1084/jem.185.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290–3. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- 30.Ivanov VN, Lopez Bergami P, Maulit G, Sato TA, Sassoon D, Ronai Z. FAP-1 association with Fas (Apo-1) inhibits Fas expression on the cell surface. Mol Cell Biol. 2003;23:3623–35. doi: 10.1128/MCB.23.10.3623-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieckowski E, Atarashi Y, Stanson J, Sato TA, Whiteside TL. FAP-1-mediated activation of NF-kappa B induces resistance of head and neck cancer to Fas-induced apoptosis. J Cell Biochem. 2007;100:16–28. doi: 10.1002/jcb.20922. [DOI] [PubMed] [Google Scholar]
- 32.Ivanov VN, Bhoumik A, Krasilnikov M, Raz R, Owen-Schaub LB, Levy D, et al. Cooperation between STAT3 and c-Jun Suppresses Fas Transcription. Mol Cell. 2001;7:517–28. doi: 10.1016/s1097-2765(01)00199-x. [DOI] [PubMed] [Google Scholar]
- 33.Ivanov VN, Krasilnikov M, Ronai Z. Regulation of Fas expression by STAT3 and c-Jun is mediated by phosphatidylinositol 3-kinase-AKT signaling. J Biol Chem. 2002;277:4932–44. doi: 10.1074/jbc.M108233200. [DOI] [PubMed] [Google Scholar]
- 34.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 35.Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DC, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–9. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landowski TH, GleasonGuzman MC, Dalton WS. Selection for drug resistance results in resistance to Fas-mediated apoptosis. Blood. 1997;89:1854–61. [PubMed] [Google Scholar]
- 37.Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med. 1996;2:574–7. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 38.Kykalos S, Mathaiou S, Karayiannakis AJ, Patsouras D, Lambropoulou M, Simopoulos C. Tissue expression of the proteins fas and fas ligand in colorectal cancer and liver metastases. J Gastrointest Cancer. 2012;43:224–8. doi: 10.1007/s12029-011-9252-6. [DOI] [PubMed] [Google Scholar]
- 39.Toillon RA, Descamps S, Adriaenssens E, Ricort JM, Bernard D, Boilly B, et al. Normal breast epithelial cells induce apoptosis of breast cancer cells via Fas signaling. Exp Cell Res. 2002;275:31–43. doi: 10.1006/excr.2002.5490. [DOI] [PubMed] [Google Scholar]
- 40.Botti C, Buglioni S, Benevolo M, Giannarelli D, Papaldo P, Cognetti F, et al. Altered expression of FAS system is related to adverse clinical outcome in stage I–II breast cancer patients treated with adjuvant anthracycline-based chemotherapy. Clin Cancer Res. 2004;10:1360–5. doi: 10.1158/1078-0432.ccr-1092-03. [DOI] [PubMed] [Google Scholar]
- 41.Mottolese M, Buglioni S, Bracalenti C, Cardarelli MA, Ciabocco L, Giannarelli D, et al. Prognostic relevance of altered Fas (CD95)-system in human breast cancer. Int J Cancer. 2000;89:127–32. doi: 10.1002/(sici)1097-0215(20000320)89:2<127::aid-ijc5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Maecker HL, Yun Z, Maecker HT, Giaccia AJ. Epigenetic changes in tumor Fas levels determine immune escape and response to therapy. Cancer Cell. 2002;2:139–48. doi: 10.1016/s1535-6108(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa Y, Nishioka A, Hamada N, Terashima M, Inomata T, Yoshida S, et al. Expression of fas (CD95/APO-1) antigen induced by radiation therapy for diffuse B-cell lymphoma: immunohistochemical study. Clin Cancer Res. 1997;3:2211–6. [PubMed] [Google Scholar]
- 44.Li Y, Kanki H, Hachiya T, Ohyama T, Irie S, Tang G, et al. Negative regulation of Fas-mediated apoptosis by FAP-1 in human cancer cells. Int J Cancer. 2000;87:473–9. doi: 10.1002/1097-0215(20000815)87:4<473::aid-ijc3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 45.Oh DS, Cheang MC, Fan C, Perou CM. Radiation-induced gene signature predicts pathologic complete response to neoadjuvant chemotherapy in breast cancer patients. Radiat Res. 2014;181:193–207. doi: 10.1667/RR13485.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gasparian ME, Chernyak BV, Dolgikh DA, Yagolovich AV, Popova EN, Sycheva AM, et al. Generation of new TRAIL mutants DR5-A and DR5-B with improved selectivity to death receptor 5. Apoptosis. 2009;14:778–87. doi: 10.1007/s10495-009-0349-3. [DOI] [PubMed] [Google Scholar]
- 47.Rusconi CP, Roberts JD, Pitoc GA, Nimjee SM, White RR, Quick G, Jr, et al. Antidote-mediated control of an anticoagulant aptamer in vivo. Nat Biotechnol. 2004;22:1423–8. doi: 10.1038/nbt1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Radiation-induced FAS expression patterns in SUM159 cell line.
Fig. S2. FAP1 and FAS co-localization.
Fig. S3. Effects of radiation on FAS ligand expression and cellular location in response to 0 and 5 Gy irradiation.
Fig. S4. Relationship between TP53 and FAS induction.
Fig. S5. Relationship between FAS and RSI in TCGA database.