Abstract
Background
Lynch syndrome (LS) diagnosis is underestimated, and most of the patients remain undetected after colorectal resections. The study aims to assess the frequency of LS in patients undergoing surgical treatment for colorectal cancer (CRC).
Methods
A total of 458 CRC patients were operated from January 2005 to December 2008. Positive CRC family history (FH) was present in 118 (25.8%) patients. Histologic sections were reviewed for microsatellite instability (MSI) criteria (Bethesda guidelines), immunohistochemical (IHC) analysis for MLH1, MSH2, MSH6, PMS2 proteins, through the avidin-biotin-peroxidase complex, MSI (BAT-25, BAT-26, NR-21, NR-24 and MONO-27) and BRAF somatic mutation.
Results
Of the 118 patients with FH, 61 (51.69%) met at least one of the revised Bethesda criteria. IHC was abnormal in 8 (13.1%) and MSI in 12 patients (20%). BRAF was negative in all cases. MSI histopathological included: intratumoral lymphocytes (47.5%), expansive tumors (29.5%) mucinous component (27.8%) and Crohn’s like reaction in (14.7%). There was an association between the revised Bethesda criteria with: sex, mucinous histology and Crohn’s like reaction; MSI and IHC with PMS2 and MLH1. Revised Bethesda criteria 4 had 10.6 increased chances to display positive MSI. We have proposed a score to contribute as a practical tool in the diagnosis of LS.
Conclusions
The frequence of LS in resected CRC patients was 2.6%. The criterion 4 Revised Bethesda was associated more strongly with the presence of MSI.
Keywords: Hereditary nonpolyposis colorectal cancer (HNPCC), immunohistochemical (IHC), lynch syndrome (LS), microsatellite instability (MSI), B-raf
Introduction
Lynch syndrome (LS), also called hereditary nonpolyposis colorectal cancer (HNPCC) is an autosomal dominant disorder with penetrance between 80% and 90%, vertical transmission and no gender preference (1), affecting approximately 2-3% of all cases of colorectal cancer (CRC) patients (2,3). It is determined by germline mutations in DNA repair genes, including the most commonly affected genes: human mutS homolog 2 (hMSH2; chromosome 2p16) and human mutL homolog 1 (hMLH1; chromosome 3p21), in about 90% of cases. The remaining 10% are caused almost entirely by changes in MSH6 and PMS2 genes (4-6).
The loss of function of DNA repair genes causes accumulation of genetic mutations in the DNA of this individual, a process called genomic instability, involving areas of microsatellites (4-6).
The main clinical features of LS are: CRC at an early age (below 45 years), predominantly in the right colon (70%), higher incidence of synchronous tumors (3×) and metachronous (5-7×) the rapid development of cancer, originated from adenomas, increased risk of malignancies of the endometrium, ovary, small intestine, kidney upper tract, stomach, and biliary tract. It can be associated with skin tumors—sebaceous adenomas, sebaceous carcinoma and keratoacanthomas (also known as Muir-Torre syndrome) (4-9).
Amsterdam Criteria have been proposed to facilitate clinical identification of those patients (7). Subsequently, the Bethesda criteria were proposed to indicate the molecular “tracking” of HNPCC [Test of microsatellite instability (MSI) tumor, immunohistochemistry or gene sequencing]. However, a major challenge in the diagnosis of LS is the low diagnostic accuracy of clinical criteria (10-12).
The diagnosis of LS is underestimated, and most of the patients remain undetected after colorectal resections. Preventive measures, intraoperative management changes, adjuvant treatment, patient postoperative surveillance, patient and familial genetic counseling, and prognosis may be altered in this group of patients and families if they fulfill the criteria for LS.
Thus, the objectives of this study were: (I) to assess the frequency of LS in patients undergoing surgical treatment for CRC; (II) to evaluate which clinical, histopathological, immunohistochemical (IHC) criteria, and MSI would be more informative in the diagnosis of LS in Brazilian population; (III) to propose a score to facilitate the diagnosis of LS.
Patients and methods
This study was approved by the Institutional Ethics Committee with the number 0709/09, and all patients signed an informed consent.
From January 2005 until December 2008, 458 cases of CRC were operated at Coloproctology Service of the Hospital das Clinicas-University of São Paulo School of Medicine (HC-FMUSP). Of those, 118 had a family history (FH) of CRC or LS associated cancer. These patients were selected for interview and a standardized questionnaire was applied, which include the Amsterdam criteria I, II and revised Bethesda. After the questionnaire, 61 patients presented at least one of the revised Bethesda criteria or guidelines.
Patients with ulcerative colitis, Crohn’s disease, chemotherapy and preoperative radiotherapy, and poliposis adenomatosis familial were excluded.
Histopathological evaluation
All the histopathological sections were reviewed by experienced pathologists and were analyzed according to the criteria for MSI as defined by the revised Bethesda Guidelines—(Intra-tumoral lymphocytes, Crohn’s disease reaction, mucinous differentiation or signet ring cell tumor, undifferentiated or medullary growth, and expansive characteristics of the tumor) (13).
Immunohistochemistry
We used primary monoclonal antibodies anti-human MLH1 purified G168-15 clone, Becton Dickinson, cod. 550838 (RR-002-12155), Lot 83115; anti-hum domestic MSH2, clone G2019-1129, Becton Dickinson, cod.556349 (RR-002-05885), Lot 23665; anti-MSH6 (GTBP) 150ug, Becton Dickinson, clone 44/MSH6, cod. 610 919 (RR-002-17297), Lot 64559; and anti-PMS2 hum/domestic cam, A16-4 clone, Becton Dickinson, cod. 556415 (RR-002-13805), Lot 76080. The internal controls of the reactions were stained intra-tumoral lymphocytes and/or stromal cells.
Microsatellite instability (MSI)
Samples of normal and tumor tissue fixed in buffered formaldehyde and embedded in paraffin were used for DNA extraction and the analysis of MSI, which included probes (primers) fluorescently labeled amplification for seven markers including five mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24 and MONO-27) and two repeated penta-nucleotides markers (Penta C and Penta D). The mononucleotide markers were used to determine the MSI and penta-nucleotides markers were used to control the reaction. The PCR products were separated by capillary electrophoresis.
BRAF analysis
DNA extraction
Formalin-fixed and paraffin-embedded tissue sections were digested overnight at 37 °C with Proteinase K after removing paraffin with xylene and ethanol. DNA was isolated with QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following manufacturer’s instructions.
DNA sequencing analysis
BRAF exon 15 was amplified by PCR using primers designed by Lee et al. [2010] to detect codon 600 V > E (T1799A) mutation. The PCR reaction contained 50 ng templates DNA, 0.2 mM of each primer, 0.2 mM dNTPs, 1.5 mM MgCl2, 1U Taq DNA polymerase and reaction buffer in a total volume of 25 µL. The initial denaturation at 95 °C for 5 min was followed by 40 cycles of 95 °C for 40 s, 55 °C for 40 s, 72 °C for 1 min, and a final cycle of 72 °C for 10 min. PCR products were then submitted to direct sequencing using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) and the ABI PRISM® 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA) (14).
Statistical analysis
Numeric variables were presented as mean ± standard deviation (SD). Fisher-exact test was utilized to determine the association between variables. Student t-test (quantitative)/Mann-Whitney were used to compare the mean/median age among groups. Univariate Binary Logistic Regression was performed to assess which factors are predictors of positivity of the variables of interest (MSH2, MLH1, MSH6, PMS2 and MSI), giving odds ratios and confidence intervals of 95%. Multivariate Binary Logistic Regression was used to verify the association with immunohistochemistry and MSI.
Score
A simple logistic regression was performed. Factors that had P values <0.20 were chosen to be evaluated in multiple logistic regression and that the most important variables were chosen by AIC criterion. The score was defined from the linear predictor coefficients have been rescaled so that the score was between 0 and 100. The cutoff point (43.63) was found from the Receiver operating characteristic curves (ROC), by maximizing Youden index. We calculated sensitivity, specificity, positive predictive value and negative predictive value. Finally, the optimism of internal validity was assessed through bootstrap samples.
Results
Sixty-one cases fulfilled the revised Bethesda criteria. Thirty-six were female (59%), and the aged varied from 22 to 87 years (mean, 53.18; SD =16.5). Nine patients fulfilled Amsterdam criteria I. The criterion 5 of the revised Bethesda was more common in this study, followed by criteria 1, 3, 4 and 2. At least one revised Bethesda criteria was present in 26 patients and two or more criteria, in 35 patients as seen in Table 1. Ten tumors were located in the right colon (16%) and 52 in the rest of the colon (84%). One patient had synchronous tumors, one in the right colon and the other in the left colon.
Table 1. Frequency of criteria for Lynch syndrome in 61 patients with familial history of colorectal cancer.
| Criteria | Number of cases (%) |
|---|---|
| Amsterdam I | 9 (14.7) |
| Bethesda 1 | 29 (47.5) |
| Bethesda 2 | 2 (4.9) |
| Bethesda 3 | 24 (39.3) |
| Bethesda 4 | 14 (22.9) |
| Bethesda 5 | 43 (70.5) |
| Revised Bethesda | |
| At least one revised Bethesda criterion | 26 (42.6) |
| Two or more revised Bethesda criterion | 35 (57.4) |
Immunohistochemistry was altered (no staining) in 13% of patients and no change (positive chromogen staining) in 53 cases (87%). Five of the cases presented altered for MSH2/MSH6 dimers, whereas staining was negative for MLH1/PMS2 dimer in three cases. Tables 1,2,3,4 show the main clinical, histopathological and molecular characteristics of this study.
Table 2. Distribution of 61 patients according to immunohistochemistry findings and clinicopathologic variables.
| Variables | Immunohistochemistry |
P value* | |
|---|---|---|---|
| Not altered (%) | Altered (%) | ||
| Histopathologic characteristics: present | 37 (69.81) | 7 (87.5) | 0.423 |
| Mucinous component | 12 (22.64) | 5 (62.5) | 0.032 |
| Signet ring component | 6 (11.32) | 2 (25.0) | 0.280 |
| Medular component | 1 (1.89) | 0 (0) | 1.000 |
| Intra-tumoral lymphocytes | 22 (41.51) | 7 (87.5) | 0.022 |
| Crohn’s-like reaction | 6 (11.32) | 3 (37.5) | 0.087 |
| Expansive | 15 (28.30) | 3 (37.5) | 0.683 |
| Localization: right colon | 4 (8.16) | 5 (41.7) | 0.013 |
| Bethesda 1 | 24 (45.28) | 5 (62.5) | 0.460 |
| Bethesda 2 | 2 (3.77) | 1 (12.5) | 0.349 |
| Bethesda 3 | 18 (33.96) | 6 (75.0) | 0.048 |
| Bethesda 4 | 9 (16.98) | 5 (62.5) | 0.012 |
| Bethesda 5 | 38 (71.70) | 5 (62.5) | 0.683 |
| Bethesda >1 | 27 (50.94) | 8 (100.0) | 0.016 |
| Amsterdam I | 4 (7.55) | 5 (62.5) | 0.001 |
*, Fisher’s exact test.
Table 3. Distribution of 61 patients according to the findings of MSI and the clinico-pathological variables.
| Variables | MSI |
P value* | |
|---|---|---|---|
| Not altered (%) | Altered (%) | ||
| Histopathologic characteristics: present | 34 (69.39) | 10 (83.33) | 0.481 |
| Mucinous component | 12 (24.49) | 5 (41.67) | 0.287 |
| Signet ring component | 6 (12.24) | 2 (16.67) | 0.65 |
| Medular component | 0 (0) | 1 (8.33) | 0.197 |
| Intra-tumoral lymphocytes | 19 (38.78) | 10 (83.33) | 0.009 |
| Crohn’s-like reaction | 6 (12.24) | 3 (25.00) | 0.361 |
| Expansive | 13 (26.53) | 5 (41.67) | 0.313 |
| Localization: right colon | 5 (9.43) | 4 (50.00) | 0.011 |
| Bethesda 1 | 24 (48.98) | 5 (41.67) | 0.753 |
| Bethesda 2 | 2 (4.08) | 1 (8.33) | 0.488 |
| Bethesda 3 | 18 (36.73) | 6 (50.00) | 0.513 |
| Bethesda 4 | 7 (14.29) | 7 (58.33) | 0.003 |
| Bethesda 5 | 34 (69.39) | 9 (75.00) | 1.000 |
| Bethesda >1 | 25 (51.02) | 10 (83.33) | 0.001 |
| Amsterdam I | 3 (6.12) | 6 (50.00) | 0.055 |
*, Fisher’s exact test. MSI, microsatellite instability.
Table 4. Distribution of IHC, MSI and BRAF mutation in 12 patients with lynch syndrome.
| Cases | BAT25 | BAT26 | NR21 | NR24 | Mono27 | Penta C | Penta D | IHC | BRAF |
|---|---|---|---|---|---|---|---|---|---|
| 14 | + | + | + | + | + | + | – | Altered | Negative |
| 15 | + | + | + | + | + | + | + | Altered | Negative |
| 22 | + | + | + | + | + | – | + | Altered | Negative |
| 31 | + | – | + | + | + | + | + | Altered | Negative |
| 33 | + | – | + | + | + | + | – | Altered | Negative |
| 42 | + | + | + | + | + | – | + | Altered | Negative |
| 50 | + | + | + | + | + | + | – | Altered | Negative |
| 58 | + | + | + | + | + | – | – | Altered | Negative |
| 26 | + | + | + | + | + | – | – | Normal | Negative |
| 32 | – | – | + | – | – | + | – | Normal | Negative |
| 40 | – | + | + | – | – | + | + | Normal | Negative |
| 60 | – | + | + | + | – | – | + | Normal | Negative |
+, MSI positive; –, MSI negative. IHC, immunohistochemistry; MSI, microsatellite instability.
Histopathological characteristics related to LS were present in 72% of cases. Intra-tumoral lymphocytes (47.54%), mucinous component (27.87%) and expansive characteristics of the tumor (29.51%) were the most important findings in the histopathological evaluation of these tumors (Tables 2,3). There was no association between immunohistochemistry and the presence of intra-tumoral lymphocytes and mucinous component (Table 2). Only one patient presented negative histopathological characteristic for LS (12.5%) when immunohistochemistry was altered. In 10/44 cases with histopathological criteria for LS tumors occurred in the right colon. All the cases that did not have these criteria were located on the left colon (P=0.051). Among patients with only one revised Bethesda criterion, none had abnormal immunohistochemistry, while patients with more than one revised Bethesda criterion, 22.86% had changes in immunohistochemistry (P=0.016).
Three of the eight (37.5%) patients with altered immunohistochemistry had tumor location in the right colon, and 6/53 (12.8%) patients had not altered tumor location in the right colon (P=0.013). MSI positive was present in 12 (20%) cases, 4 cases with IHC not altered. Of these, 3 were found in the left colon and 1 in the right colon. Of the 12 MSI positive cases, 11 were classified as MSI-H (2 or more unstable markers), and 1 MSI-L (an unstable marker). Three patients presented four histopathological criteria for the LS (25%), two with three histopathological criteria for LS (16.7%), three with two histopathological criteria for LS (25%), two with one criterion for the LS (16.7%) and two with negative histopathology (16.7%) (Tables 3,4).
Histopathological alterations were present in ten patients (83%) with MSI positive, and absent in two cases (17%). Negative immunohistochemistry was found in four patients with age ≥50 years, who were positive for MSI. Of these four cases, all patients were older than 60 years [63, 64, 69 and 70], three with tumors located elsewhere in the colon, and one in the right colon.
BRAF somatic mutation analysis was carried out in the 12 MSI altered cases to exclude hyper-methylation in the promoter region of the MLH1 gene that is found in approximately 15% of sporadic cancers. In all cases of this research the results were negative, confirming that they were probable LS.
There was an association of the Amsterdam criteria with MSI, and MLH1 with PMS2 in immunohistochemistry. The revised Bethesda criteria were associated with age, mucinous component, intra-tumor lymphocytes and Crohn’s like reaction in histopathology. There was also an association of these criteria with MLH1 and PMS2 in immunohistochemistry and MSI. Using Univariate Binary Regression, we found that patients who presented the revised Bethesda criteria four (B4), had 8.4 higher chance of having positive MSI (Table 5). Multiple Binary Regression also confirmed that among all the revised Bethesda criteria, the most informative about the MSI is B4, with a greater chance of 10.59 times the individual who presents this criterion have this MSI (Table 6).
Table 5. Simple binary regression of 62 patients with familial history of colorectal cancer.
| Variable | Group | OR | 95% CI | P value |
|---|---|---|---|---|
| Gender | Male | 2.41 | 0.67-8.72 | 0.180 |
| Bethesda 1 | Present | 0.74 | 0.21-2.67 | 0.650 |
| Bethesda 2 | Present | 2.14 | 0.18-25.74 | 0.550 |
| Bethesda 3 | Present | 1.72 | 0.48-6.15 | 0.402 |
| Bethesda 4 | Present | 8.40 | 2.07-34.04 | 0.003 |
| Bethesda 5 | Present | 1.32 | 0.31-5.59 | 0.703 |
| Mucinous component | Present | 2.20 | 0.59-8.24 | 0.241 |
| Signet ring cell component | Present | 1.43 | 0.25-8.19 | 0.685 |
| Medular component | Present | – | – | 0.197 |
| Intra-tumoral lymphocytes | Present | 7.89 | 1.56-40 | 0.013 |
| Crohn’s like reaction | Present | 2.39 | 0.50-11.37 | 0.274 |
| Expansive | Present | 1.98 | 0.53-7.34 | 0.308 |
| Location | Right colon | 8.33 | 1.72-33.33 | 0.008 |
| Amsterdam I | Present | 15.33 | 3.01-78.01 | 0.001 |
| Revised Bethesda | >1 | 4.80 | 0.95-24.24 | 0.057 |
| Histopathologic component | Positive | 2.21 | 0.43-11.31 | 0.343 |
| Histopathologic component | 1 | 0.75 | 0.09-5.95 | 0.785 |
| Histopathologic component | >1 | 4.28 | 0.77-23.76 | 0.096 |
Table 6. Multivariate binary regression of 62 patients with familial history of colorectal cancer.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Bethesda 1 present | 0.68 | 0.05-8.65 | 0.764 |
| Bethesda 2 present | 1.03 | 0.06-18.38 | 0.984 |
| Bethesda 3 present | 4.31 | 0.42-44.36 | 0.221 |
| Bethesda 4 present | 10.59 | 2.04-54.95 | 0.005 |
| Bethesda 5 present | 1.67 | 0.26-10.72 | 0.588 |
Figures 1,2 show the ROC for IHC and MSI using all the clinicopathological data. Tables 7,8 demonstrate the sensitivity, specificity, predictive positive and negative values of the proposed score (Figures 1,2; Tables 7,8).
Figure 1.
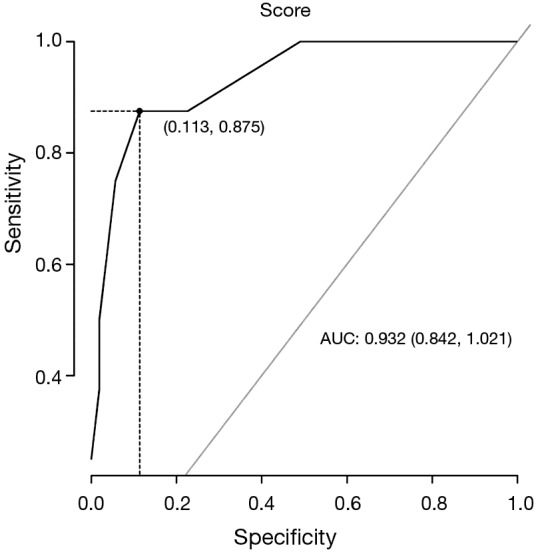
ROC curve for immunohistochemistry using clinicopathological data of 61 patients with familial colorectal cancer history. If the score is >49.4%, sensibility (Se) =88% (47-100%); specificity (Sp) =89% (77-96%); predictive positive value (PPV) =54% (33-98%); predictive negative value (NPV) =98% (86-99%) for detection of LS. ROC, receiver operating characteristic curves; LS, lynch syndrome.
Figure 2.
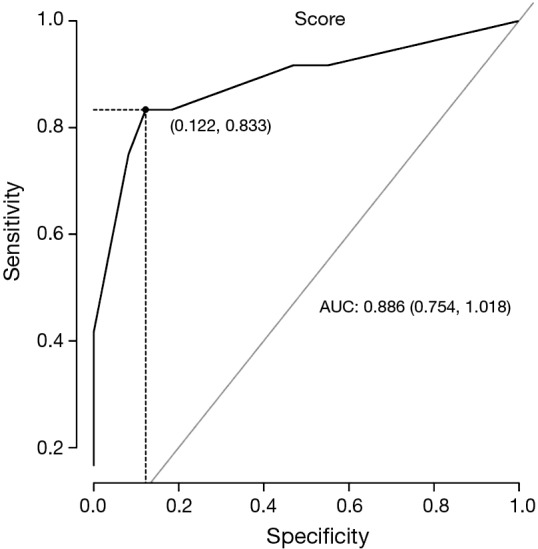
ROC curve for microsatellite instability using clinicopathological data of 61 patients with familial colorectal cancer history. If the score is >43.6%, sensibility (Se) =83% (52-98%); specificity (Sp) =88% (75-95%); predictive positive value (PPV) =62% (41-94%); predictive negative value (NPV) =96% (82-98%) for detection of LS. ROC, receiver operating characteristic curves; LS, lynch syndrome.
Table 7. Proposed score for IHC for detection of LS (>49.4 points).
| Variable | Score (points) |
|---|---|
| Bethesda 4 present | 14.75 |
| Mucinous component present | 22.41 |
| Intratumoral lymphocytes present | 15.25 |
| Localization right colon | 17.69 |
| Amsterdam I present | 29.88 |
IHC, immunohistochemistry; LS, lynch syndrome.
Table 8. Proposed score for MSI for detection of LS (>43.6 points).
| Variable | Score (points) |
|---|---|
| Bethesda 4 present | 20.95 |
| Intratumoral lymphocytes present | 22.68 |
| Localization right colon | 26.72 |
| Amsterdam I present | 29.64 |
MSI, microsatellite instability; LS, lynch syndrome.
Discussion
LS is the most common category of familial CRC, however, the clinical diagnostic criteria are still flawed, as well as the patient selection for performing genetic testing. In these surgical series, we reported a 20% of FH patient detection rate of histologic, immunohistochemistry and molecular alterations compatible with LS, and 2.6% of total group of operated patients. The lack of initial diagnosis of LS in patients undergoing surgical treatment can lead patients and their families to be neglected regarding correct intraoperative management, adjuvant treatment, patient postoperative surveillance, patient and familial genetic counseling, and prognosis.
Few studies have evaluated the effectiveness of the revised Bethesda criteria (10,11,15) in the diagnosis of LS. Despite this, the revised Bethesda criteria are most often used in the diagnosis of CRC before age 50 (16,17).
In this study, it was observed that the criterion four of the revised Bethesda guidelines was the most informative on the presence of MSI, thus, the individual who presents this criterion has a greater chance of 10.6 of bearer LS (P>0.005; 95% CI, 2.04-54.95).
A recent study (18) comparing the investigation of patients with CRC using molecular testing for LS and the revised Bethesda criteria, demonstrated that routine molecular studies in patients with CRC using immunohistochemistry or MSI has better sensitivity to detect mutation carriers than the clinical revised Bethesda criteria. Of 486 (23.2%) patients who had at least one revised criteria Bethesda, only 180 patients (8.6%) showed a loss of expression of the protein in mismatch repair genes and/or MSI. Gene sequencing revealed that 14 patients (0.7%) had mutations in the mismatch repair genes, of which 12 met at least one of the revised Bethesda criteria and 2 (14.3%) did not fulfill.
Serrano et al. (19) evaluated and compared the Bethesda criteria with the revised Bethesda criteria for the detection of MSI-H and subsequent identification of mutations in mismatch repair genes. Of the 174 cases of CRC with an indication for the analysis of MSI in accordance with the criteria of Bethesda and revised Bethesda, 114 (65.5%) met the criteria for Bethesda and all 174 revised Bethesda criteria. MSI-H were detected in 37/114 (32.5%) and mutational analysis in 14/37 (37.8%) of patients with Bethesda criteria and in cases of revised Bethesda 49/174 (28.2%) and the presence of mutations in mismatch repair genes in 16/49 (32.7%) patients. The authors concluded that the Bethesda guideline showed similar total percentage for detecting mutations and MSI-H when compared to the revised Bethesda criteria. The revised Bethesda criteria analysis resulted in more patients; however, they contributed to increased detection of two cases of LS. This difference has a significant impact on the establishment of preventive measures and in all mutation carriers belonging to these families.
In this series, Bethesda 4 criteria were more informative as the presence of MSI. Similarly, Serrano et al. (19) found that the first criterion of Bethesda (Individuals with cancer in families that fulfill the Amsterdam criteria) and the revised Bethesda 4 criteria (or tumor associated with LS, diagnosed in one or more first degree relatives with one of the cancers diagnosed below 50 years) as the most frequently associated with the identification of MSI-H tumors.
Different studies show that CRC in LS differs in location, histology, and natural history of those of the sporadic type. Usually, show clinicopathological features that are suggestive of MSI, including severe lymphocytic infiltration, large areas with poorly differentiated tumors and mucinous histology component (20-22). In this study the histology was considered positive when one or more of the histological features of MSI-H were present and that occurred in 72% of cases.
It has been suggested that histology could be useful in selecting patients with CRC for molecular testing in patients with LS (23,24). Histology limit molecular tests in patients with pathologic features of MSI-H, thus reduce the overhead of molecular testing in 60%, when compared to test all patients who meet the revised Bethesda criteria. The most important is that histology could help identify the LS among patients with late onset and no FH of cancer (21).
A typical aspect of LS associated CRC is the predominance of proximal cancers in 56-62% (25-27). In this series, 16% of tumors were located in the right colon and 84% in the left colon. Of the eight cases without loss of immunoreactivity of repair proteins, i.e., immunohistochemistry not altered and MSI stable, four were located in the right colon and four in the left colon. This result may be explained by the fact that there are sporadic tumors with familial aggregation would present clinicopathologic features similar to LS, but with mutations in genes not identified up to now.
Immunohistochemistry can identify only a loss of proteins due to mutations in genes known to repair, however, the analysis of MSI may indicate other repair genes potentially pathogenic (28). In this study, four cases with IHC not altered were older than 50 years, and were positive for MSI.
The sensitivity for IHC repair proteins ranged from 77% to 83% (29-33). An analysis of 1,381 tumors in families with LS revealed CRC as the most frequent malignancy and 78% of patients with mutations in MLH1 and MSH2 in 65%. There were large numbers of CRCs in the right colon (60%), and the number of CRC was 21% in patients with mutations in MLH1 and 20% in MSH2 (32).
The reported sensitivity of MSI is 89% for MLH1/MSH2 and MSH6 to 77% (34). The specificity of the MSI and IHC test decreases from 90% to 87% to 83% for patients diagnosed with CRC at ages of 50, 60 and 70 years, respectively (35). This is the result of an increase in the prevalence of somatic MLH1 hypermethylation of which is associated with BRAF mutations in the tumor DNA, and the vast majority of patients over 70 years need not predictive tests.
Ferreira et al. (29) studying CRC detected MSI-H in 33/41 families (80%), the remaining families were MSI-S. In families with suspected X syndrome it was recorded fewer cases of CRC and lower frequency of extra-colonic tumors from LS spectrum. MSI tumors predominated in the distal/rectum had lower mucus production and decreased peri-tumoral lymphocytic infiltration. In 70% of families with MSI-H, mutations in DNA repair genes were identified compared to none in the MSI-S. The authors concluded that families with stable Amsterdam criteria had particular characteristics, reinforcing the existence of an entity other than the SL.
The study of somatic mutations for BRAF was valuable to differentiate cases of promoter hyper-methylation in the MLH1 gene, which occurs in 15% of cases of sporadic CRC type (28). In this series, all cases investigated were negative for this mutation, emphasizing the possible presence of LS.
This study also shows that CRC patients with MSI phenotype need to be better characterized as the presence of other target genes not yet discovered and who might be involved in the molecular pathway that determines this syndrome. Or the existence of other routes or subgroups of sporadic tumors similar to LS with clinical, histopathological and phenotypic characteristics, but do not share the same pathway in carcinogenesis.
Currently, several approaches are available to identify individuals and families at risk for LS. The selection of individuals is based on the fulfillment of the clinical criteria and molecular analysis of tumors for evidence of MSI. The Universal approach tests all cases of CRC patients with less than or equal to 70 years old, while the targeted approach (based on age or FH criteria), followed by genetic testing for those considered at increased risk (12,13,33-36).
Although the universal strategy is cost-effective, it may fail to identify mutations in mismatch repair genes that disrupt its function, but results in MSI, as seen in the case of mutations in MSH6, or when the results of IHC are normal despite of a nonfunctional protein (13).
Predictive models were developed to quantify the risk of detecting a mutation based on personal and FH, helping to decide who could be referred for further assessment and/or genetic testing (36). Risk quantification based on predictive models provides additional means to identify individuals at high risk of carrying mutations if affected or not affected by cancer.
In this research it is proposed a new score for risk stratification based on the main clinicopathologic variables, IHC and MSI presence. This score may be incorporated in the clinical setting; however, it must be validated in other Services and/or through the expansion of our sample. This score also proved to be a good predictor for identifying negative cases of the LS.
Moreover, cheaper, high accurate, and easy to perform genetic tests will be incorporated in daily practice in short period of time. The identification of new genes or other epigenetic changes will promote better understanding of the clinical course of LS patients and adequate therapeutic guidance.
Conclusions
The frequency of LS in patients undergoing resection for cancer was 2.6%. The fourth criterion of revised Bethesda (CRC in one or more first-degree relatives or tumor associated with HNPCC, one of the tumors diagnosed before age 50) was associated more strongly with the presence of MSI in the studied population. The score developed in this study may contribute as a practical tool in the diagnosis of LS.
Acknowledgements
Funding: This work was supported by a grant from the Foundation of Support to the Research of the State of São Paulo (FAPESP, No. 2010/06045-8).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lynch HT, Smyrk T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer 1996;78:1149-67. [DOI] [PubMed] [Google Scholar]
- 2.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919-32. [DOI] [PubMed] [Google Scholar]
- 3.de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer 2004;4:769-80. [DOI] [PubMed] [Google Scholar]
- 4.Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453-6. [DOI] [PubMed] [Google Scholar]
- 5.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst 1997;89:1758-62. [DOI] [PubMed] [Google Scholar]
- 7.Vasen HF, Mecklin JP, Khan PM, et al. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 1991;34:424-5. [DOI] [PubMed] [Google Scholar]
- 8.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005;352:1851-60. [DOI] [PubMed] [Google Scholar]
- 9.Lynch HT, Shaw MW, Magnuson CW, et al. Hereditary factors in cancer. Study of two large midwestern kindreds. Arch Intern Med 1966;117:206-12. [PubMed] [Google Scholar]
- 10.Piñol V, Castells A, Andreu M, et al. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA 2005;293:1986-94. [DOI] [PubMed] [Google Scholar]
- 11.Wolf B, Gruber S, Henglmueller S, et al. Efficiency of the revised Bethesda guidelines (2003) for the detection of mutations in mismatch repair genes in Austrian HNPCC patients. Int J Cancer 2006;118:1465-70. [DOI] [PubMed] [Google Scholar]
- 12.Gologan A, Krasinskas A, Hunt J, et al. Performance of the revised Bethesda guidelines for identification of colorectal carcinomas with a high level of microsatellite instability. Arch Pathol Lab Med 2005;129:1390-7. [DOI] [PubMed] [Google Scholar]
- 13.Grover S, Syngal S. Risk assessment, genetic testing, and management of Lynch syndrome. J Natl Compr Canc Netw 2010;8:98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HJ, Choi J, Hwang TS, et al. Detection of BRAF mutations in thyroid nodules by allele-specific PCR using a dual priming oligonucleotide system. Am J Clin Pathol 2010;133:802-8. [DOI] [PubMed] [Google Scholar]
- 15.Gologan A, Krasinskas A, Hunt J, et al. Performance of the revised Bethesda guidelines for identification of colorectal carcinomas with a high level of microsatellite instability. Arch Pathol Lab Med 2005;129:1390-7. [DOI] [PubMed] [Google Scholar]
- 16.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol 2008;26:5783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Carbonell L, Ruiz-Ponte C, Guarinos C, et al. Comparison between universal molecular screening for Lynch syndrome and revised Bethesda guidelines in a large population-based cohort of patients with colorectal cancer. Gut 2012;61:865-72. [DOI] [PubMed] [Google Scholar]
- 19.Serrano M, Lage P, Belga S, et al. Bethesda criteria for microsatellite instability testing: impact on the detection of new cases of Lynch syndrome. Fam Cancer 2012;11:571-8. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins MA, Hayashi S, O'Shea AM, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology 2007;133:48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truta B, Chen YY, Blanco AM, et al. Tumor histology helps to identify Lynch syndrome among colorectal cancer patients. Fam Cancer 2008;7:267-74. [DOI] [PubMed] [Google Scholar]
- 22.Koehler-Santos P, Izetti P, Abud J, et al. Identification of patients at-risk for Lynch syndrome in a hospital-based colorectal surgery clinic. World J Gastroenterol 2011;17:766-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright CL, Stewart ID. Histopathology and mismatch repair status of 458 consecutive colorectal carcinomas. Am J Surg Pathol 2003;27:1393-406. [DOI] [PubMed] [Google Scholar]
- 24.Kakar S, Aksoy S, Burgart LJ, et al. Mucinous carcinoma of the colon: correlation of loss of mismatch repair enzymes with clinicopathologic features and survival. Mod Pathol 2004;17:696-700. [DOI] [PubMed] [Google Scholar]
- 25.Urso E, Pucciarelli S, Agostini M, et al. Proximal colon cancer in patients aged 51-60 years of age should be tested for microsatellites instability. A comment on the Revised Bethesda Guidelines. Int J Colorectal Dis 2008;23:801-6. [DOI] [PubMed] [Google Scholar]
- 26.Hendriks YM, de Jong AE, Morreau H, et al. Diagnostic approach and management of Lynch syndrome (hereditary nonpolyposis colorectal carcinoma): a guide for clinicians. CA Cancer J Clin 2006;56:213-25. [DOI] [PubMed] [Google Scholar]
- 27.Lynch HT, Lynch JF, Lynch PM. Toward a consensus in molecular diagnosis of hereditary nonpolyposis colorectal cancer (Lynch syndrome). J Natl Cancer Inst 2007;99:261-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J Mol Diagn 2008;10:301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira S, Lage P, Sousa R, et al. Familial colorectal cancer type X: clinical, pathological and molecular characterization. Acta Med Port 2009;22:207-14. [PubMed] [Google Scholar]
- 30.Jass JR, Smyrk TC, Stewart SM, et al. Pathology of hereditary non-polyposis colorectal cancer. Anticancer Res 1994;14:1631-4. [PubMed] [Google Scholar]
- 31.Vasen HF, Mecklin JP, Watson P, et al. Surveillance in hereditary nonpolyposis colorectal cancer: an international cooperative study of 165 families. The International Collaborative Group on HNPCC. Dis Colon Rectum 1993;36:1-4. [DOI] [PubMed] [Google Scholar]
- 32.Goecke T, Schulmann K, Engel C, et al. Genotype-phenotype comparison of German MLH1 and MSH2 mutation carriers clinically affected with Lynch syndrome: a report by the German HNPCC Consortium. J Clin Oncol 2006;24:4285-92. [DOI] [PubMed] [Google Scholar]
- 33.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005;352:1851-60. [DOI] [PubMed] [Google Scholar]
- 34.Palomaki GE, McClain MR, Melillo S, et al. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med 2009;11:42-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kastrinos F, Syngal S. Screening patients with colorectal cancer for Lynch syndrome: what are we waiting for? J Clin Oncol 2012;30:1024-7. [DOI] [PubMed] [Google Scholar]
- 36.Kastrinos F, Steyerberg EW, Mercado R, et al. The PREMM(1,2,6) model predicts risk of MLH1, MSH2, and MSH6 germline mutations based on cancer history. Gastroenterology 2011;140:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]


