Abstract
Background
In the United States, African Americans (AA) have the highest incidence and mortality from colorectal cancer (CRC) of any racial group. Few studies have evaluated if AA who undergo colonoscopy are more likely to have aggressive neoplasia (polyp) tumor biology compared to Caucasians (C). The primary aim of the study was to compare polyp characteristics between AA and C undergoing outpatient colonoscopy.
Methods
A single center retrospective cohort study was performed at a single Veteran Administration (VA) health care system. The charts of 4,038 veterans undergoing colonoscopy from 2005 to 2008 were reviewed. After applying exclusion criteria, data was analyzed for 1,388 persons. Categorical variables were compared using the chi-square test and data were expressed as percentages. Continuous variables were compared using student’s t-test and the data were expressed as mean with standard deviation.
Results
A total of 37% of AA had proximal polyps compared to 21% of C (P<0.0001). Twenty-four percent of AA had polyps with villous histology compared to 16% of C (P=0.01). Twelve percent of AA had hyperplastic polyps compared to 20% of C (P=0.02). There was no difference in the overall prevalence of tubular adenomas, adenomatous polyps with high-grade dysplasia, number, size or polyp morphology between groups.
Conclusions
In an equal access healthcare system and under varying indications, AA have more proximal polyps and polyps with more aggressive histology compared to C. This could partially explain the higher incidence of CRC in AA, and the increased likelihood for AA to develop advanced proximal neoplasia.
Keywords: Neoplasia, polyps, race, colonoscopy, location
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related mortality among men and women in the United States (1). In 2014, an estimated 136,830 men and women will be newly diagnosed with CRC and 50,316 deaths will be attributable to the disease (1). Studies have shown that CRC incidence and mortality varies across racial/ethnic groups in the United States, which may eventually lead to racial/ethnic group based CRC screening strategies (2). African Americans (AA) and Caucasians (C) are 2 of the 3 largest US racial/ethnic groups and both have the highest CRC related mortality rates nationally with AA mortality consistently higher than C since 1980 (1,3-6). AA and C not only have the highest incidence and prevalence of CRC in U.S., but have the highest incidence and prevalence in the San Bernardino and Riverside counties, where our Veteran Administration (VA) hospital is located (7), and within our VA. For example, at our VA, from 1999-2009, 594 cancers were diagnosed and 500 of them were either African-American or Caucasian in race. Several etiologies have been proposed to explain this variation between the two groups ranging from socio-demographic factors to genetics and tumor biology (8).
When AA and C both participate in an equal access health care system, there is no difference in colonoscopy referral for CRC screening (9). These groups do not differ significantly in CRC screening status, when adjusted for confounding variables (10,11). Previous investigators have demonstrated that AA have an increased frequency of proximal colonic neoplasia (polyps) than C (12-15). Since CRC follows the adenoma-carcinoma sequence, a difference in tumor biology and genetics may explain the increased incidence and proximal location of CRC in AA. We hypothesized that AA patients undergoing outpatient colonoscopy may have a higher prevalence of proximal colonic neoplasia thereby explaining the increased frequency of proximal tumors. Therefore, we performed a retrospective study in an equal access VA health care system to compare multiple characteristics of colonic neoplasia between AA and C who underwent colonoscopy.
Materials and methods
Study population and study design
We conducted a single center retrospective study at Loma Linda VA Healthcare System in Loma Linda, California, USA. The study was approved by our institution’s review board. A total of 4,038 charts of veterans who had either colonoscopy or flexible sigmoidoscopy from 2005 to 2008 and had polyps removed were identified using the pathology database of our institution and then reviewed manually for study inclusion.
Consults originate from primary care physicians. They are located at the hospital or at five community-based outpatient clinics directly to the section of gastroenterology. The consultation is requested for either a symptom (bright red blood per rectum, abdominal pain, diarrhea, constipation or weight loss) or for screening. General screening is not encouraged due to limited capacity, but done for reasons like pre-transplant, family history of CRC or per patient request. A gastroenterologist reviewed the consult request and, if accepted, the patient was scheduled for a colonoscopy preparation class. Gender, race or any other demographic factor was not used to determine consult acceptance as a true equal access center. At the colonoscopy class, the patient learns about colonoscopy from a powerpoint presentation in a group session. The patient is evaluated individually upon completion of the presentation. This includes active medical history, past medical history and medications. Once the patient is considered to be appropriate for colonoscopy, the procedure is scheduled.
Primary exclusion criteria were: (I) veterans who underwent inpatient colonoscopy or flexible sigmoidoscopy for indications such as diarrhea, constipation, weight loss, abdominal pain, melena, or bright red blood per rectum; (II) familial adenomatous polyposis, hereditary non-polyposis CRC syndrome, or inflammatory bowel disease; (III) poor or inadequate bowel preparation; (IV) patients for whom race could not be determined (see Figure 1). No demographic data was recorded for patients meeting the primary exclusion criteria. After applying the primary exclusion criteria, a total of 2,337 charts were reviewed.
Figure 1.
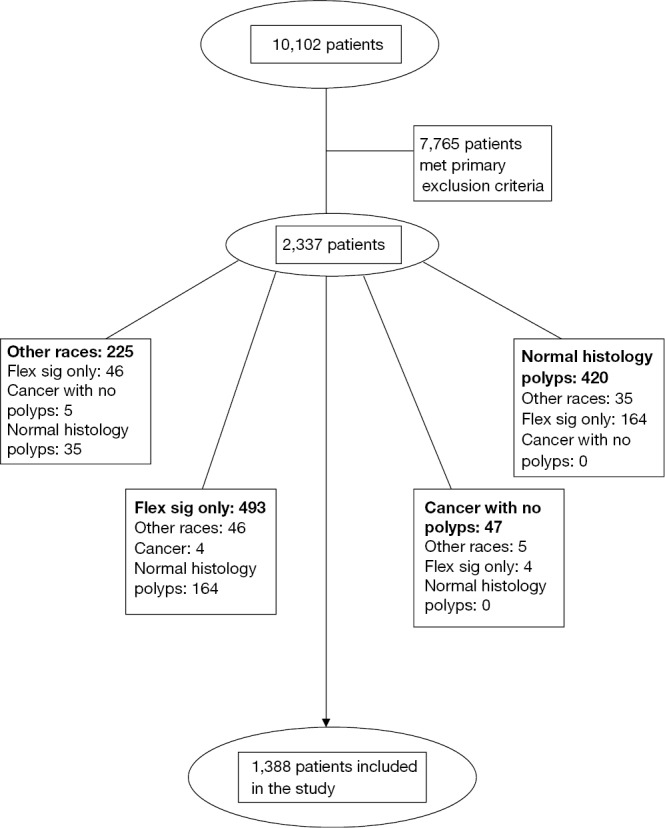
Flowsheet of exclusion criteria and subgroups.
Secondary exclusion criteria were: (I) race other than AA or C; (II) patients who only underwent flexible sigmoidoscopy; (III) patients with diagnosis of polyp on endoscopy but had normal histology; (IV) patients who had CRC and did not have any polyps (Figure 1). Demographic data on patients meeting secondary exclusion criteria was recorded.
After primary and secondary exclusion criteria were used, a total of 1,388 patients remained. These patients were then subdivided into five different subgroups based on the indication for the colonoscopy. The five subgroups were: (I) asymptomatic veterans, who were at average risk for CRC and underwent screening colonoscopy; (II) asymptomatic veterans, who had either a positive FOBT result or polyp detected on flexible sigmoidoscopy or barium enema; (III) asymptomatic veterans, who had a history of colon polyps or CRC and underwent surveillance colonoscopy; (IV) asymptomatic veterans who had a family history of CRC; and (V) symptomatic veterans who underwent outpatient colonoscopy for weight loss, constipation or diarrhea, overt gastrointestinal bleeding or anemia (Figure 1).
Procedure and polyp histology
Most of the patients were prepped with a standard lavage solution and all patients had undergone standard colonoscopy. Patients with poor or inadequate bowel preparation were excluded. Poor prep was defined as semi-solid stool that could not be suctioned or washed away but allowed for greater than 90% of the surface to be seen, and inadequate as solid stool obscuring mucosal detail and contour despite aggressive washing and suctioning; repeat preparation and colonoscopy needed. Data was collected based on age, sex, race, quality of preparation, BMI (<30 vs. ≥30), and various polyp characteristics such as location, histology, morphology, size and number. Polyps were identified by endoscopic landmarks. Polyps found in the cecum, ascending colon, hepatic flexure, or transverse colon were considered to be proximal. Distal polyps were defined as those found at the splenic flexure, descending colon, sigmoid colon, or rectum. The primary outcomes of interest were location, size, number, morphology, and histology of the polyps.
Polyps were classified histologically as follows: an adenoma contained at least low-grade dysplasia. The adenomas contained mild hyperchromasia with enlarged oval nuclei with basal polarization of the nuclei. If high-grade dysplasia was present, pseudostratification or stratification of neoplastic nuclei was present. The crypts had a back-to-back configuration with increased mitotic activity and marked loss of polarity. Villous adenomas were classified as adenomas that contained at least 75% villous epithelium. Tubular adenomas were classified as containing less than 25% villous epithelium. Tubulovillous adenomas contained between 25% and 75% villous epithelium.
Statistical analysis
Categorical variables were compared using the chi-square test and data were expressed as percentages. Continuous variables were compared using student’s t-test and the data were expressed as mean with standard deviation. A multivariate approach was not done, as the mean age, gender distribution, BMI and quality of bowel preparation were so similar. Statistical analysis was performed using JMP version 9.03 for Mac. A P value less than 0.05 was considered statistically significant.
Results
Demographics
AA and C composed 91% (AA 11%, C 80%) of the 2,337 patients who were evaluated after the primary exclusion criteria were used. This is similar to the national demographic data with AA constituting 13% and C 72% of the general population (16). Data collected on 1,388 patients, after applying the primary and secondary exclusion criteria, was included for the final analysis, 152 (11%) AA and 1,236 (89%) C. Patient characteristics are shown in Table 1. There was no statistical difference between AA and C when subject distribution of age, gender, bowel preparation or BMI was compared. The mean age of patients referred for colonoscopy was 65±10 years old.
Table 1. Patients’ demographics by race/ethnicity.
| Characteristics | C |
AA |
P value | |||
|---|---|---|---|---|---|---|
| N=1,236 | Percent (%) | N=152 | Percent (%) | |||
| Age | 0.47 | |||||
| <50 | 34 | 3 | 8 | 5 | ||
| 50 to 54 | 109 | 9 | 19 | 13 | ||
| 55 to 59 | 260 | 21 | 28 | 18 | ||
| 60 to 64 | 276 | 22 | 32 | 21 | ||
| 65 to 69 | 163 | 13 | 20 | 13 | ||
| 70 to 75 | 195 | 16 | 23 | 15 | ||
| Over 75 | 199 | 16 | 22 | 14 | ||
| Mean age (SD) | 64.9 | 9.4 | 63.8 | 9.9 | 0.17 | |
| Gender | 0.17 | |||||
| Male | 1,185 | 96 | 142 | 93 | ||
| Female | 51 | 4 | 10 | 7 | ||
| BMI | 0.41 | |||||
| <30 | 721 | 58 | 94 | 62 | ||
| ≥30 | 515 | 42 | 58 | 38 | ||
| Preparation* | 0.51 | |||||
| Good | 941 | 76 | 112 | 74 | ||
| Fair | 295 | 24 | 40 | 26 | ||
*, patients with poor bowel preparation were excluded. C, Caucasians; AA, African Americans.
The comparison of colonic neoplasia for all five subgroups is shown in Table 2. Thirty-seven percent of AA had proximal polyps, compared to 21% of C (P<0.0001). Twenty-four percent of AA had polyps of villous histology, as compared to 16% of C (P=0.01). Polyps with villous histology were more likely to be proximal in AA (31%) than in C (16%), P=0.02. Twelve percent of AA had hyperplastic polyps compared to 20% of C (P=0.02). There was no statistical difference in tubular adenomas (P=0.88) or polyps with high-grade dysplasia (P=0.93). Also, there was no statistical difference in morphology or number of polyps between the two racial groups.
Table 2. Comparison of polyps among AA and C.
| Variables | Caucasians, N=1,236 (%) | AA, N=152 (%) | Total, N=1,388 (%) | P value | Sub-group analyses |
|---|---|---|---|---|---|
| Location | <0.0001 | ||||
| Proximal | 21 | 37 | 23 | <0.0001 | |
| Distal | 50 | 40 | 49 | 0.03 | |
| Proximal & distal | 29 | 23 | 29 | 0.11 | |
| Histology | 0.02 | ||||
| Hyperplastic | 20 | 12 | 19 | 0.02 | |
| Tubular adenoma | 61 | 61 | 61 | 0.88 | |
| Villous | 16 | 24 | 16 | 0.01 | |
| High grade | 3.8 | 4 | 3.8 | 0.93 | |
| Morphology | 0.92 | ||||
| Sessile | 72 | 72 | 72.1 | ||
| Pedunculated | 19 | 18 | 18.7 | ||
| Flat | 9 | 10 | 9.2 | ||
| Size | 0.98 | ||||
| Under 5 mm | 59 | 59 | 59 | ||
| 5 to 10 mm | 28 | 28 | 28 | ||
| Over 10 mm | 14 | 13 | 14 | ||
The comparison of colonic neoplasia between AA and C was done in each of the five subgroups (as mentioned in the methods section). This is shown in Table 3. Proximal polyps were more common in AA who had colonoscopy performed for CRC screening (P<0.016), after a positive FOBT, polyp detected on the flexible sigmoidoscopy or after barium enema (P=0.009) when compared to C. Polyps with villous histology were more prevalent in AA in each of the subgroups but the difference was not statistically significant (Table 3). In addition, Table 3 shows histology, morphology, size or the number of polyps in individual subgroups. However, the sample sizes in the subgroups were too small for further analysis.
Table 3. Location distribution of hyperplastic and villous polyps in AA and C.
| Polyp | Proximal, N=316 (%) | Distal, N=675 (%) | Proximal and distal, N=397 (%) | Total, N=1,388 (%) | P value |
|---|---|---|---|---|---|
| Hyperplastic, N=259 | 11 | 28 | 9 | 19 | <0.0001 |
| Villous, N=228 | 16 | 14 | 20 | 16 | 0.03 |
Subgroup analysis for hyperplastic polyps: P<0.0001 for prox. vs. distal; 0.32 prox. vs. prox. and distal; <0.0001 distal vs. prox. and distal. Subgroup analysis for villous polyps: P=0.43 for prox. vs. distal; 0.14 prox. vs. prox. and distal; <0.009 distal vs. prox. and distal. AA, African Americans; C, Caucasians.
Discussion
Evidence suggests that a racial gap still exists in CRC incidence and mortality despite availability of colonoscopy (1). Studies evaluating tumor biology have shown racial differences in polyps and CRC (13). Polyps have been shown to be more proximally located and are larger in size in AA (13,15,17). However, these studies have evaluated only certain polyp characteristics and very few were done in an equal access health care system. Our study is unique as it evaluates multiple anatomical and histological characteristics of colon polyps between AA and C in the setting of an equal access health care system. These results could have important implications in the effort to maximize colonoscopy use.
The results of our study are consistent with previous investigations showing that AA have more proximally located polyps compared to C (13,15). It is unclear why AA have more proximally located neoplasms, as compared to other races. On subgroup analysis, the predominance of proximal polyps seen in AA persisted. In addition, AA had fewer hyperplastic polyps and 1.5 times more villous adenomas than C histologically. This was seen in all subgroups except the group with occult positive/polyps on flexible sigmoidoscopy. Not only were AA more likely to have villous adenomas, they were more likely to have villous adenomas in the proximal colon when compared to C. This is an important finding because villous histology is a sensitive indicator of polyp transformation to malignancy (18). Another independent indicator for transformation of polyp to malignancy is the polyp size. Lieberman et al. showed higher prevalence of large adenomas among AA (17). However, our study did not reveal any difference in polyp size between the two racial groups. There was also no difference in polyp number or morphology.
There are some strengths of our study. In this study we compared multiple polyp characteristics between the AA and C. Factors such as histology, shape, size and number of polyps are important in determining the risk of development of CRC and hence optimal surveillance interval. One of the reasons given for the higher CRC incidence and mortality in AA is poor access to health care system secondary to greater lack of medical insurance, and lower socioeconomic status. Our study was performed solely in a VA hospital, which is equally accessible to veterans of all racial/ethnic groups. Even in an equal access system, differences between the two races still exist.
There are limitations to this study that should be considered when interpreting our findings. First, it is a retrospective study. Second, since the majority of patients were men, our findings may not be generalizable to women. Furthermore, our study population did not have an equivalent number of AA and C patients. However, the percentage of AA was similar to that of the national population. This could have impacted our results, which show no difference in size, number and shape of the polyps amongst the two ethnic groups. There is strong epidemiologic evidence suggesting that lifestyle factors play an important role in CRC incidence (19). We only included BMI in our study but did not include other characteristics such as diabetes, smoking, alcohol, diet, exercise, aspirin, or non-steroidal anti-inflammatory use, supplements or sun exposure, which could have impacted observed results.
Conclusions
To conclude, our study demonstrates that in an equal access healthcare system and under varying indications, AA have proximal colon polyps more commonly than C and in addition, villous polyps with increased frequency. This could explain the higher incidence of CRC in AA, and the increased likelihood for AA to develop advanced proximal neoplasia. Our findings emphasize that further investigation to completely understand these differences is essential to reducing CRC in AA.
Acknowledgements
This research was approved by the institutional review board at the Loma Linda VA Medical Center.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Howlader N, Noone AM, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute, 2009. [Google Scholar]
- 2.Rex DK, Khan AM, Shah P, et al. Screening colonoscopy in asymptomatic average-risk African Americans. Gastrointest Endosc 2000;51:524-7. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008;9:730-56. [DOI] [PubMed] [Google Scholar]
- 4.Alteri R, Barnes C, Brooks D, et al, editors. Cancer Facts & Figures for African Americans. Atlanta, GA: American Cancer Society, 2012. [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49. [DOI] [PubMed] [Google Scholar]
- 6.Rim SH, Seeff L, Ahmed F, et al. Colorectal cancer incidence in the United States, 1999-2004: an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115:1967-76. [DOI] [PubMed] [Google Scholar]
- 7.Morgan JW, Koch SM, Imai C, et al. Cancer incidence and mortality in Inyo, Mono, Riverside, and San Bernardino Counties, 1988-2007. Region 5 of the California Cancer Registry, Loma Linda University Medical Center (June 2009). Retrieved from http://www.DSCCSP.org or http://CCR.CA.gov
- 8.Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol 2005;100:515-23; discussion 514. [DOI] [PubMed] [Google Scholar]
- 9.Dolan NC, Ferreira MR, Fitzgibbon ML, et al. Colorectal cancer screening among African-American and white male veterans. Am J Prev Med 2005;28:479-82. [DOI] [PubMed] [Google Scholar]
- 10.Fisher DA, Dougherty K, Martin C, et al. Race and colorectal cancer screening: a population-based study in North Carolina. N C Med J 2004;65:12-5. [PubMed] [Google Scholar]
- 11.Fisher DA, Jeffreys A, Coffman CJ, et al. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev 2006;15:1232-5. [DOI] [PubMed] [Google Scholar]
- 12.Thomas CR, Jr, Jarosz R, Evans N. Racial differences in the anatomical distribution of colon cancer. Arch Surg 1992;127:1241-5. [DOI] [PubMed] [Google Scholar]
- 13.Thornton JG, Morris AM, Thornton JD, et al. Racial variation in colorectal polyp and tumor location. J Natl Med Assoc 2007;99:723-8. [PMC free article] [PubMed] [Google Scholar]
- 14.Francois F, Park J, Bini EJ. Colon pathology detected after a positive screening flexible sigmoidoscopy: a prospective study in an ethnically diverse cohort. Am J Gastroenterol 2006;101:823-30. [DOI] [PubMed] [Google Scholar]
- 15.Ozick LA, Jacob L, Donelson SS, et al. Distribution of adenomatous polyps in African-Americans. Am J Gastroenterol 1995;90:758-60. [PubMed] [Google Scholar]
- 16.Humes KR, Jones NA, Ramirez RR, editors. Overview of Race and Hispanic Origin: 2010. Washington, DC: U.S. Census Bureau, 2011. [Google Scholar]
- 17.Lieberman DA, Holub JL, Moravec MD, et al. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA 2008;300:1417-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itzkowitz SH, Kim YS. Polyps and benign neoplasm’s of the colon. In: Sleisenger MH, Fordtran JS, editors. Gastrointestinal disease. 5th ed. Philadelphia: WB Saunders, 1993:1402-29. [Google Scholar]
- 19.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138:2029-2043.e10. [DOI] [PMC free article] [PubMed]


