Abstract
Background
Despite being chemosensitive, the majority of triple negative breast cancer (TNBC) patients recur. The primary study objectives were to compare disease free survival (DFS), and overall survival (OS) for TNBC after adjuvant chemotherapy, who underwent maintenance metronomic chemotherapy versus no maintenance therapy.
Methods
TNBC patients were eligible for enrolment if they had TNM stages II-III and fit with our inclusion criteria. Patients were assigned to either: group 1, 3 cycles FEC-100 then 3 cycles docetaxel, carboplatin, followed by maintenance metronomic chemotherapy for 1 year; and group 2, 3 cycles FEC-100 then 3 cycles docetaxel.
Results
Between November 2008 and December 2014, 158 patients (78 group 1, and 80 group 2) were enrolled. The mean age was 46 years. The median DFS for groups 1,2 were 28 and 24 months, respectively; P value 0.05. The median OS for groups 1,2 were 37 and 29 months, respectively; P values 0.04. Additionally, during the follow-up period, the overall distant metastasis recurrence rates for groups 1,2 were 26% and 37% respectively. Finally, treatment protocol was tolerated well in both groups with mild toxicity profiles.
Conclusions
Extended adjuvant metronomic chemotherapy achieved significant improvement in the survival and was well tolerated.
Keywords: Triple-negative breast cancer (TNBC), chemotherapy, adjuvant, metronomic, carboplatin, overall survival (OS), disease free survival (DFS), toxicity
Introduction
Triple-negative breast cancer (TNBC) is a heterogeneous subtype of breast cancer. It has a significant variability in morphological and pathological features (1).
TNBC accounts for 12% to 17% of all breast cancers. Clinicopathologic features of TNBC included young age, large tumor size, high grade and higher incidence of node positivity at presentation (2-6).
TNBC has showed an increased rate of breast cancer-related deaths within the first 5 years. Additionally, once metastatic, patients with TNBC experience shorter overall survival (OS) in comparison to patients with other subtypes. On the other hand, studies showed that the OS beyond 5 years is roughly equivalent to other subtypes of breast cancer (2,7).
Furthermore, patients with TNBC have a higher likelihood of recurrence within the first 3 years of diagnosis. TNBC patients are more often to develop visceral versus osseous metastases when compared to other subtypes. A large multi-centre study showed that women with TNBC were more likely to develop lung, and brain metastases as their first site of recurrence (8-11).
In the absence of a guideline and due to the increased risk of recurrence, the use of a third-generation chemotherapeutic regimen similar to that offered to other high-risk patients should be considered for the treatment of TNBC (9,12).
Although numerous large randomized trials have established the benefit of adjuvant anthracyclines and/or taxanes in TNBC, there are some recent randomized trials and pooled analysis which confirm the benefit of anthracyclines and/or taxanes in the adjuvant treatment of TNBC. Moreover, there are ongoing promising findings in favor of other new agents including capecitabine, platinum-based agents (especially for those with deficiencies in BRCA-associated DNA repair mechanisms) and ixabepilone (13-20).
TNBC has a specific biological profile with many potential molecular targets; including overexpression of vascular endothelial growth factors (VEGF), EGFR and a high rate of BRCA mutation or deficiency in BRCA function (a concept termed BRCAness). As a result, there is a growing body of data on the use of VEGF, EGFR, poly ADP-ribose polymerase (PARP), and mammalian target of rapamycin (mTOR) inhibitors for the treatment of TNBC (21).
Despite all of these efforts and despite the chemosensitivity and the promising initial response, unfortunately the majority of patients with TNBC have residual disease after treatment of early breast cancer, and for these patients, there is a high risk of relapse and a sharp decline in survival in the first 3-5 years. A high proportion of patients therefore eventually present with metastatic TNBC, and the majority of these patients relapse shortly following prior treatment. Subsequently, all of these findings raise the need to augment the initial response and to consolidate it with a maintenance therapy (2,22,23).
In the non-TNBC patients, the first 5 years after adjuvant chemotherapy are usually covered by the antitumoral activity of the hormonal treatment with or without the anti-Her2 therapy, which is not an option in TNBC, trying to offer an anti tumoral coverage during this period for those patients bringing back to mind the very suitable treatment option of giving an effective, tolerable and cheap chemotherapeutic agent at relatively low, non-toxic doses, with no prolonged, drug-free breaks in a Dose-dense/Metronomic schedule (24).
Metronomic chemotherapy is thought to exert anticancer activity by inhibiting tumor angiogenesis, a process which is not fully understood but may be due to reducing the circulating VEGF concentration, or by inducing significant endothelial-cell apoptotic death in tumor-associated microvasculature (25,26).
Another mechanism responsible for the anti-tumour effect of metronomic chemotherapy is through stimulation of the immune response, because they induce a profound and selective reduction in circulating regulatory T cells. This effect is associated with suppression of the inhibitory functions on conventional T and natural killer cells, leading to restoration of peripheral T-cell proliferation and innate killing activities (26).
Based on its encouraging efficacy [overall clinical benefit of 31.7% (95% CI, 20.6-44.6%)] and very tolerable toxicity [grade 1 and 2 neutropenia (20.6%), anemia (9.5%) and elevated liver enzymes (0.9%)] in the management of metastatic breast cancer, we selected oral methotrexate plus cyclophosphamide given in a metronomic schedule for 1 year after finishing the adjuvant treatment for patients with TNBC in an attempt to prolong their disease free interval (27).
The primary study objectives were to compare the disease free survival (DFS) and OS for TNBC patients after adjuvant chemotherapy, who underwent maintenance metronomic chemotherapy vs. no maintenance therapy. The secondary end point was toxicity.
Methods
Patients were eligible for inclusion in the current phase III randomized trial if they fit with its inclusion criteria.
Inclusion criteria
Female patients with previously untreated breast cancer had estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor (HER)-2 receptors negative status, with TNM stages II-III. Tumor size should be >1.0 cm, with positive or negative axillary lymph nodes. Patients should be between 17 and 65 years, and had Eastern Cooperative Oncology Group (ECOG) performance state <2. They should have adequate liver, renal, bone marrow reserves with baseline laboratory criteria included hemoglobin >9.0 g/dL, neutrophils ≥1.5×103/mL, platelet count ≥100×103/mL, creatinine ≤176 mmol/L (2 mg/mL); total bilirubin level less ≤1.5 the upper limit of normal; ALT, AST ≤3 the upper limit of normal. Patients should have normal cardiac function, as evidenced by a left ventricular ejection fraction (LVEF) ≥50 as per out institution guidance.
Written informed consent from patients needed before proceeding in the trial. Premenopausal patients should have negative pregnancy test (serum β-HCG) prior to inclusion, and must employ an adequate contraceptive method (e.g., non-hormonal intrauterine device, male condom, or surgical sterilization) during treatment and at least three months after treatment.
For group 1 patients (experimental group), patients should be free from metastatic, or recurrence (local, regional, or distant) disease prior to initiation of metronomic chemotherapy as evident by the CT imaging.
Exclusion criteria
Patients who unfit with the above criteria were excluded. Further to that, patients with radiological evidence of metastatic disease, patients with concurrent malignancy, except those with non-melanoma skin cancer, and those with previously treated malignancy at least 5 years with no evidence of recurrence, patients with inflammatory breast cancer, those with serious comorbidities were further excluded.
Settings
The study was run in 4 educational oncology hospitals in Egypt: Ain Shams University Hospital, Ain Shams Specialized Hospital, Ismailia Oncology Teaching Hospital, and El-Gomhoria Health Insurance Hospital.
Study design
Patients were randomly assigned to one of the following groups:
Group 1 (Experimental group): Patients underwent adjuvant chemotherapy in the form of FEC-100 [FEC-100 was given in the form of 5-flurouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2 (day 1)] for 3 cycles then docetaxel 80 mg/m2, carboplatin AUC 5 for 3 cycles, followed by postoperative radiotherapy (PORT) (if indicated), followed by maintenance metronomic chemotherapy.
Group 2: Patients underwent adjuvant chemotherapy in the form of FEC-100 protocol for 3 cycles then docetaxel 100 mg/m2, for 3 cycles followed by PORT (if indicated), followed by no more treatment. Chemotherapy cycles were given on day 1 and repeated every 21 days (Figure 1) (28,29).
Figure 1.
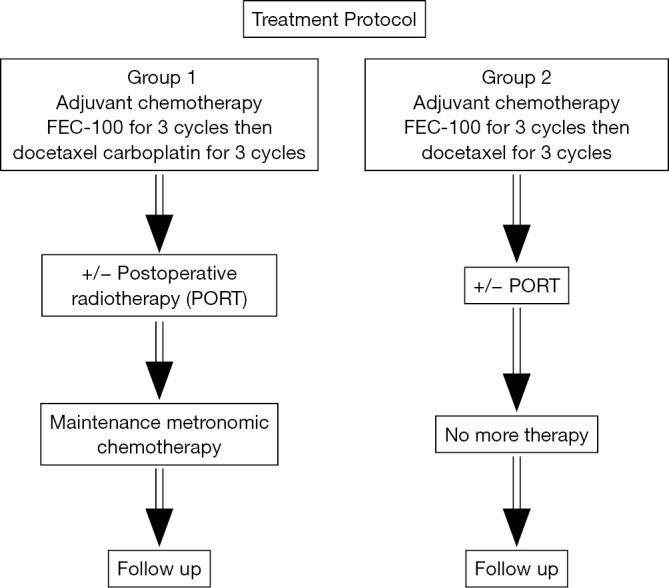
Treatment protocol of the current study.
Cycles of adjuvant chemotherapy were administered after checking CBC, renal function tests, liver function tests, bilirubin, before day 1 of each cycle, with subsequent dose modification based on the following Table 1 (28,29).
Table 1. Dose modification based on hematological, hepatic, and other toxicities.
| Conditions | Dose |
|---|---|
| Hematology: for both drugs (103/mL) | |
| Neutrophils (≥1.5) and platelets (≥90) | 100% |
| Neutrophils (1.0-1.49) or platelets (≥90) | 75% |
| Neutrophils (<1) or platelets (<100) | Delay for 1 week (or longer if needed), till recovery, then give 75% dose, consider giving filgrastim for subsequent cycles |
| Febrile neutropenia | 75% of dose for current and subsequent cycles, use G-CSF for 1st episode, and 50% of dose for 2nd episode |
| Creatinine clearance: for cyclophosphamide | |
| ≥10 | 100% |
| <10 | 75% |
| Hepatic function tests | |
| For epirubicin | |
| ALT (<2 ULN) and total bilirubin (≤1.17 mg/dL) | 100% |
| ALT (2-4× ULN) and total bilirubin (1.23-2.92 mg/dL) | 50% |
| ALT (>4× ULN) and total bilirubin (>2.92 mg/dL) | 25% |
| For docetaxel | |
| ALT/AST (<1.5× ULN) and alkaline phosphatase (<2.5× UNL) | 100 mg/m2 |
| ALT/AST (1.5-3.5 ULN) and alkaline phosphatase (2.5-6× UNL) | 75 mg/m2 |
| ALT/AST (>3.5× ULN), total bilirubin (>ULN) and alkaline phosphatase (>6× UNL) | Avoid use |
| Dose modification for other toxicities | |
| ≥grade 3 non-hematologic toxicities | Paclitaxel dose: hold treatment, re-evaluate treatment plan, consider discontinuing treatment with this protocol |
For group 1, following adjuvant treatment, patients received maintenance metronomic chemotherapy in the form of oral Cyclophosphamide (50 mg PO daily), and methotrexate (2.5 mg PO BID on days 1,2 of each week). Cycles were given every 28 days till toxicity, recurrence, or a total of 1 year. Patients may continue on the treatment for a longer duration as far no disease recurrence is detected. Treatment discontinued if disease progression after 3 cycles detected by radiological CT scanning. Cycles of metronomic chemotherapy were administered after checking CBC, renal function tests, liver function tests, bilirubin, before day1 of each cycle, with subsequent dose modification based on the following Table 2 (30).
Table 2. Dose modification for metronomic chemotherapy.
| Conditions | Dose |
|---|---|
| Hematology: for both drugs (103/mL) | |
| Neutrophils [≥1.5] and platelets [>100] | 100% |
| Neutrophils [1-1.49] or platelets [75-99] | Delay, then dose at 50% after recovery |
| Neutrophils [<1] or platelets [<75] | Delay, then dose at 50% after recovery |
| Creatinine clearance | |
| For methotrexate only | |
| >30 | 100% |
| 15-30 | 50% |
| <15 | Omit |
| For cyclophosphamide only | |
| ≥or | 100% |
| <10 | Omit |
| Hepatic function tests: methotrexate only | |
| ALT (2-3× ULN) and total bilirubin (2.98-4.97 mg/dL) | 2.5 mg daily on days 1 and 2 |
| ALT (>3× ULN) and total bilirubin (>4.97 mg/dL) | Avoid use |
Follow-up
After finish of the treatment protocol, patients of both groups were followed following the NCCN guideline; by regular clinic visits every 4-6 months for the first 5 years, then annually thereafter. In each visit, patients were evaluated by history, physical examination, annual X-ray mammography (31).
CT thorax, abdomen, and pelvis (TAP) was asked for patients of group 1 while they were on maintenance chemotherapy at baseline and after every 3-4 cycles, then following treatment as clinically indicated.
Toxicity
Toxic effects were graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0. Early toxicities were defined as toxicities that occurred during treatment till 8 weeks post treatment. Late toxicities referred to those occurred >8 weeks after finish of treatment protocol (32).
Statistical analysis
All calculations were carried out using prism 6 software for windows. All analyses were carried by intention to treat. All patients were included in their randomization group irrespective of whether they completed the planned treatment.
Mean, median, 95% CI values were used for the description of continuous data. For comparison between the 2 group characters, t-test, and P value were used. DFS and OS and for each arm were analyzed by the Kaplan-Meier method. Further, they were compared using the log rank and Wilcoxon tests.
DFS was measured from the time of randomization till relapse, recurrence, or the last follow-up visit. OS was measured from the time of randomization till death or the last follow-up visit. P value was significant at ≤0.05.
Results
Between January 2008 and December 2014, 158 patients were enrolled in the current study. A total of 78 patients were assigned to treatment group 1, and 80 patients were assigned to group 2. All the 158 patients fulfilled our eligibility criteria. The mean age was 46 years (95% CI, 32-62 years). The median performance status was 0 (range, 0-2) (Table 3).
Table 3. Patient and disease characteristics of each treatment group.
| Characteristics | Group 1 |
Group 2 |
P value | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | |||
| Age | ||||||
| 17-<40 | 13 | 17 | 10 | 13 | 0.06 | |
| 40-49 | 41 | 52 | 45 | 55 | 0.07 | |
| 50-60 | 22 | 28 | 23 | 29 | 0.10 | |
| 61-65 | 2 | 3 | 2 | 3 | 0.09 | |
| Mean age | 46 | – | 47 | – | 0.10 | |
| Median age | 46 | – | 46 | – | 0.10 | |
| Performance status (ECOG) | ||||||
| 0 | 50 | 64 | 48 | 60 | 0.07 | |
| 1 | 21 | 27 | 25 | 31 | 0.06 | |
| 2 | 7 | 9 | 7 | 9 | 0.09 | |
| Performance status (median) | 0 | – | 0 | – | 0.10 | |
| Pathological classification | ||||||
| Ductal | 59 | 76 | 60 | 75 | 0.10 | |
| Lobular | 19 | 24 | 20 | 25 | 0.10 | |
| Other | 0 | – | 0 | – | – | |
| Pathological grade | ||||||
| 1 | 2 | 3 | 2 | 2.5 | 0.08 | |
| 2 | 66 | 84 | 66 | 82 | 0.07 | |
| 3 | 10 | 13 | 12 | 15 | 0.08 | |
| Grade median | 2 | – | 2 | – | 0.10 | |
| Tumor stage | ||||||
| pT0N1 | 0 | 0 | 0 | 0 | – | |
| pT2N0 | 4 | 5 | 6 | 7 | 0.09 | |
| pT1N1 | 9 | 12 | 5 | 6 | 0.05 | |
| pT2N1 | 23 | 29 | 23 | 28 | 0.09 | |
| pT3N1 | 4 | 5 | 7 | 8 | 0.07 | |
| pT4N0-1 | 3 | 4 | 2 | 2 | 0.06 | |
| pT1-3N2 | 28 | 36 | 29 | 36 | 0.10 | |
| pT1-3N3 | 7 | 9 | 8 | 10 | 0.09 | |
| Tumor stage median | pT2N2 | pT2N2 | 0.10 | |||
| Surgery | ||||||
| Modified radical mastectomy (MRM) | 60 | 77 | 60 | 75 | 0.09 | |
| Breast conservative surgery (BCS) | 18 | 23 | 20 | 25 | 0.08 | |
| Others | 0 | 0 | 0 | 0 | - | |
| Other risk features | ||||||
| Lymph node capsular invasion | ||||||
| Absent | 70 | 89 | 71 | 89 | 0.10 | |
| Present | 8 | 11 | 9 | 11 | 0.10 | |
| Lymphovascular invasion | ||||||
| Absent | 73 | 93 | 75 | 93 | 0.10 | |
| Present | 5 | 7 | 5 | 7 | 0.10 | |
| Perinural invasion | ||||||
| Absent | 74 | 95 | 76 | 95 | 0.09 | |
| Present | 4 | 5 | 4 | 5 | 0.10 | |
| Extensive intraducatal component | ||||||
| Absent | 68 | 87 | 69 | 86 | 0.09 | |
| Present | 10 | 13 | 11 | 14 | 0.10 | |
| Surgical margin: for BCS | ||||||
| Negative | 13 | 72 | 15 | 75 | 0.08 | |
| Positive | 5 | 28 | 5 | 25 | 0.10 | |
| Post-operative radiotherapy | ||||||
| Yes | 71 | 91 | 73 | 91 | 0.10 | |
| No | 7 | 9 | 7 | 9 | 0.10 | |
Treatment protocol
Out of the 158 patients, 152 underwent our treatment protocol (96%). The remaining 6 patients (3 group 1, and 3 group 2) lost follow up.
Adjuvant chemotherapy
For group 1, a total of 428 cycles were infused. The median cycles per patient was 6 (95% CI, 4-6). For group 2, a total of 444 cycles were given. The median cycles per patient were 6 (95% CI, 4-6).
Treatment discontinuation occurred in 12 patients from group 1 (2 at cycle 4, 6 at cycle 5, 4 at cycle 6), and in 11 patients from group 2 (1 at cycle 4, 5 at cycle 5, 5 at cycle 6). Treatment delay occurred in 4.7%, and 3.8% of cycles of group 1, and group 2 respectively (1 week 80%, >1 week 20%). Dose reduction occurred in 22%, and 17% of cycles of group 1, and group 2 respectively (Table 4).
Table 4. Treatment modification for both groups.
| Modifications | Group 1 (cycle numbers) |
Group 2 (cycle numbers) |
P value | |||
|---|---|---|---|---|---|---|
| FEC-100 | Taxotere 80 carboplatin | FEC-100 | Taxotere 100 | |||
| Treatment discontinuation | 0 | 22 | 0 | 18 | 0.08 | |
| Treatment delay | 5 | 15 | 6 | 11 | 0.09 | |
| Dose reduction | 21 | 73 | 20 | 55 | 0.04 | |
Group 1
CT TAP was performed at median of 2 weeks after the last adjuvant chemotherapy cycle. A total of 70 patients were eligible for enrollment. For the remaining 5 patients (1 selected not to continue on more chemotherapy, further missed follow up, 2 had grade 3 toxicities and were unfit for more chemotherapy, and 2 had disease relapse). Metronomic chemotherapy was initiated at median time of 4 weeks after the last chemotherapy cycle (95% CI, 3-4 weeks). A total of 680 cycles were given. The median cycles per patient were 10 (95% CI, 8-12). Treatment discontinuation occurred in 10 patients (2 patients at cycle 5, 3 patients at cycle 6, and 5 patients at cycle 7). Dose reduction occurred in 17% cycles. Treatment delay occurred in 11% of cycles (80% for 1 week, and 20% for >1 week).
Survival data
The median follow up period was 52 months (95% CI, 46-60 months). The median DFS for groups 1,2 were 28 and 24 months respectively (95% CI were 9-36 and 8-34 months, respectively) (P value 0.05). The mean DFS for groups 1,2 were 28 and 23.5 months, respectively. The median OS for groups 1,2 were 37 and 29 months respectively (95% CI were 12-40+ and 10-38 months, respectively) (P value 0.04). The mean OS for groups 1,2 were 37.3 and 29.3 months respectively. The 4-year DFS were 63% and 42%, while the 4-year OS were 74% and 55% for groups 1,2 respectively. P values were 0.05 and 0.04 respectively (Figures 2,3).
Figure 2.
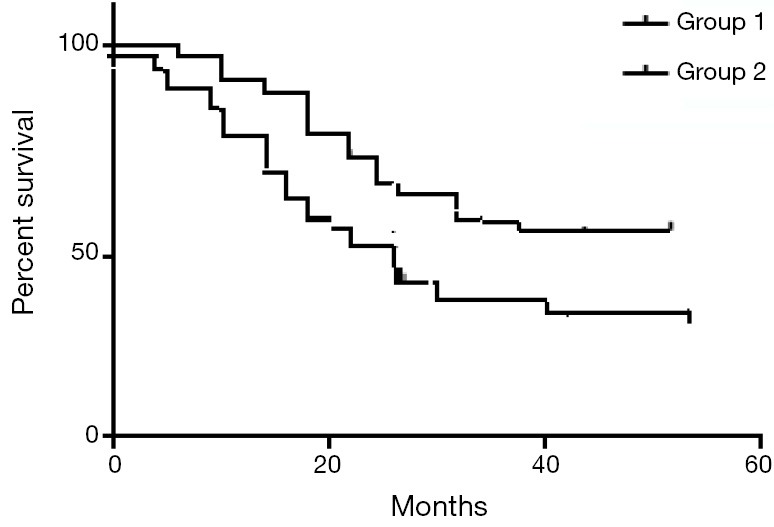
The DFS for the study group—P value: 0.05. DFS, disease free survival.
Figure 3.
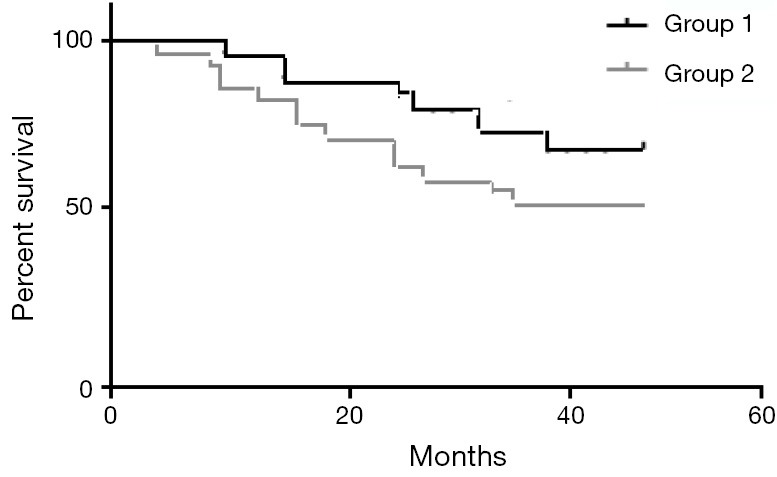
The OS for the study group—P value 0.03. OS, overall survival.
Recurrence data
During the follow-up period, 22 patients from group 1 developed breast cancer recurrence (1 locoregional, 18 metastatic, and 3 both), and 30 patients from group 2 had recurrence (1 locoregional, 23 metastatic, and 6 both). The overall distant metastasis rates for groups 1,2 were 26% and 37%, respectively.
Toxicities
The mainly encountered side effects were neutropenia. For group 1,2, grades 3,4 neutropenia occurred in 90 and 75 cycles respectively. The second common side effect was febrile neutropenia (FN). Grades 3,4 FN were observed in 51 and 40 cycles respectively. Of them, almost 75% of these events happened in the first 2 cycles for both groups.
Early toxicities
Tables 5,6 summarizes early grades 3,4 early toxicities for groups 1,2 encountered while they were on the adjuvant chemotherapy, as well as metronomic chemotherapy for group 1.
Table 5. Summarizes grades 3,4 early toxicities for groups 1,2.
| Side effect | Group 1 |
Group 2 |
||
|---|---|---|---|---|
| Grade 3 (%) | Grade 4 (%) | Grade 3 (%) | Grade 4 (%) | |
| Leuconeutropenia | 19 | 1.9 | 17 | 0 |
| Anemia | 1 | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 12 | 0 | 9 | 0 |
| Nausea, vomiting, GIT upset | 12 | 0 | 4 | 0 |
| Diarrhea | 6 | 0 | 4 | 0 |
| Mucositis | 4 | 0 | 3 | 0 |
| Fatigue | 4 | 0 | 2 | 0 |
| Lower limb edema | 1 | 0 | 0 | 0 |
| Elevated hepatic transaminases | 2 | 0 | 1 | 0 |
Table 6. Summarize grade 3,4 early side effects, and their percentage in group 1 for metronomic chemotherapy.
| Side effect | Group 1 |
|
|---|---|---|
| Grade 3 (%) | Grade 4 (%) | |
| Leuconeutropenia | 2.8 | 0 |
| Anemia | 1.5 | 0 |
| Thrombocytopenia | 0 | 0 |
| Febrile neutropenia | 0 | 0 |
| Nausea, vomiting, GIT upset | 0 | 0 |
| Mucositis | 0 | 0 |
| Increased hepatic transaminases (ALT/AST) | 11 | 0 |
Late toxicities
Generally treatment protocol tolerated well. All the early side effects recovered within 4-6 weeks after finish of the adjuvant chemotherapy.
During the 52 months follow-up period, there were no grades 3,4 late morbidities.
Discussion
Management of TNBC is a challenge. Studies showed that patients with TNBC were more likely to die than patients with other breast cancer subtypes (42.2% vs. 28%, respectively; P<0.0001). Also, TNBC has an earlier tendency to relapse with distant metastases than other forms of breast cancer (2,33).
Furthermore, treatment options are limited by the fact that TNBC is resistant to new targeted therapies by absence of ER, PR, and lack of overexpression of HER2. On the other hand, TNBC is responsive to chemotherapy. So that, it is evident that improvement of chemotherapy in TNBC may improve the outcome (33).
Studies showed promising results for adding platinum based agents to neoadjuvant chemotherapy in TNBC, based on the evidence that TNBC is strongly associated with germline mutations in the BRCA1 gene, and cells with BRCA1 mutations are deficient in DNA repair mechanisms, which make them sensitive to platinum agents. A study by Silver et al., 6 (21%) out of the 28 patients with TNBC achieved pCR with single-agent neoadjuvant cisplatin (4 cycles of cisplatin at 75 mg/m2 every 21 days) (34,35).
Moreover, metronomic chemotherapy has gained some popularity recently.
Few recent trials address them in the adjuvant/neoadjuvant settings. They act through stimulation of the immune system and have antiangiogenic mode of action (33,36,37).
They achieved promising results in metastatic breast cancer. The study of Kontani et al. (37) showed that metronomic low dose cyclophosphamide and methotrexate achieved significant improvement of OS, and progression free survival (PFS), with mild toxicity profile. And there results. In the adjuvant/neoadjuvant settings, although few publications are available, there results are encouraging. They showed improved survival and mild toxicity profiles (36).
To our knowledge, till the date of publication, our study is probably the only one which included in its protocol both carboplatin, and metronomic chemotherapy altogether in the adjuvant setting. Our aim was to find an improvement in the results of each drug separately, looking for a new hope in this aggressive disease.
In our prospective phase III study, we selected our patients by randomization using simple randomization. There was no significant difference between the two groups. The power of the study was 0.85.
Group 1 received 3 FEC-100 then 3 docetaxel, carboplatin followed by metronomic chemotherapy, while group 2 received 3 FEC-100, then 3 docetaxel (standard arm). We selected DFS, and OS as our primary end point for this early; non-metastatic disease. Our study included a larger number of patients and over a longer follow up period as compared to other trials. Therefore, the power of our study reached 90% (using SPSS version 22). Further, there was no geographical or ethnic difference among our patients.
Our result showed that group 1 achieved better survival results than group 2. The difference was clinically, and statistically significant as evident by the P value result. Group 1 patients achieved a 4 year DFS of 74%, and OS of 79%.
As far, there is no other published trial which included the same protocol, as ours, so we decided to compare our results with other trials that included somewhat similar protocols in the adjuvant/neoadjuvant settings.
When compared our results with that of Alagizy et al. (33), who used capecitabine as metronomic chemotherapy after FEC-100 for 6 cycles. They showed their 4-year DFS and OS of 72%, 78% respectively.
Our results are relatively comparable with them. Probably, their small number, and their usage of metronomic capecitabine instead of our metronomic regimes may explain this difference. Taking into consideration their shorter follow-up period and their results were preliminary rather than definite.
Our results were compared with that of Ma et al. (38), who underwent a phase II trial on 31 patients with TNBC. They gave them 6 cycles of carboplatin and paclitaxel. They observed that after 3 years of follow up, the 3-year DFS and OS were 62% and 74.7%, respectively. Our results were by far better than theirs. Probably, the explanation is related to their protocol as they didn’t include anthracycline which is an essential component in the adjuvant setting of this aggressive cancer.
A final comparison was made with that of Torrisi et al. (39). They underwent a phase II trial through treating 30 women with T2-T3 N 0-3 TNBC by 3 cycles of ECF (epirubicin, cisplatin, and fluorouracil as continuous infusion) followed by 3 cycles of weekly paclitaxel, then adjuvant metronomic chemotherapy by cyclophosphamide and methotrexate for 6 months. The 2-year DFS and OS were 87.5% and 90.3% respectively. Although, there relatively shorter follow-up duration, our results are relatively comparable with them.
A striking difference between ours and them is the toxicity profile. Their grade 3,4 non-hematological toxicities were 20%.
In accordance with our data, extended adjuvant metronomic chemotherapy was tolerated well, with no severe or life threatening adverse effects. The main side effect that encountered those in the experimental arm was elevated transaminases. This side effect necessitates careful selection of patients, and further it needs more confirmation from other trials.
An important question may arise here about the chemotherapy resistance that expected to occur by giving metronomic long duration of chemotherapy. The answer comes from the study of Emmenegger et al. (40), from their in vitro study, they observed that tumors that have low dose metronomic cyclophosphamide resistance remained sensitive to further chemotherapy.
Conclusions
Extended adjuvant metronomic chemotherapy achieved significant improvement in the OS, DFS, and further they were well tolerated. Further trials are needed to confirm our promising results looking for a new hope for patients with such aggressive disease.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Part of the study was presented in American Society of Clinical Oncology (ASCO) Annual Meeting, May 2015, USA, and subsequently published in the J Clin Oncol 33, 2015 (suppl; abstr e12087).
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938-48. [DOI] [PubMed] [Google Scholar]
- 2.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-34. [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathological features and sites of recurrence according to breast cancer subtype in the National Comprehensive Cancer Network (NCCN). J Clin Oncol 2009;27:abstr 543.
- 4.Dawood S, Broglio K, Esteva FJ, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol 2009;20:621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irvin WJ, Jr, Carey LA. What is triple-negative breast cancer? Eur J Cancer 2008;44:2799-805. [DOI] [PubMed] [Google Scholar]
- 6.Lund M, Butler E, Hair B, et al. A first report of incidence rates (not prevalence) by breast cancer subtypes. Cancer Res 2009;69:3065. [Google Scholar]
- 7.Gronwald J, Byrski T, Huzarski T, et al. Neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. J Clin Oncol (Meeting Abstracts) 2009;27:502. [DOI] [PubMed] [Google Scholar]
- 8.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 2005;11:5678-85. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Ver. 2. 2011. Fort Washington, PA: NCCN; 2011. Available online: www.nccn.org/professionals/physician_gls/pdf/breast.pdf; cited January 31, 2011.
- 10.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13:2329-34. [DOI] [PubMed] [Google Scholar]
- 11.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275-81. [DOI] [PubMed] [Google Scholar]
- 12.Cardoso F, Senkus-Konefka E, Fallowfield L, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v15-9. [DOI] [PubMed] [Google Scholar]
- 13.Cheang M, Chia SK, Tu D, et al. Anthracyclines in basal breast cancer: The NCIC-CTG trial MA5 comparing adjuvant CMF to CEF. J Clin Oncol 2009;27:abstr 519.
- 14.Gennari A, Sormani MP, Pronzato P, et al. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trials. J Natl Cancer Inst 2008;100:14-20. [DOI] [PubMed] [Google Scholar]
- 15.Steger GG, Barrios C, O’Shaughnessy J, et al. Review of Capecitabine for the Treatment of Triple-Negative Early Breast Cancer [abstract PD01-03]. San Antonio, TX: San Antonio Breast Cancer Symposium; 2010. [Google Scholar]
- 16.O’Shaughnessy J, Paul D, Stokoe C, et al. First efficacy results of a randomized, open-label, phase iii study of adjuvant doxorubicin plus cyclophosphamide, followed by docetaxel with or without capecitabine, in high-risk early breast cancer [abstract S4-2]. San Antonio, TX: San Antonio Breast Cancer Symposium; 2010. [Google Scholar]
- 17.Lluch A, Gomez H, Ruiz-Borrego M, et al. First Safety Data from a Randomised Phase III (CIBOMA 2004- 01/GEICAM 2003-11) Trial Assessing Adjuvant Capecitabine Maintenance Therapy after Standard Chemotherapy for Triple-Negative Early Breast Cancer. Cancer Res 2010;70:P5-10-15.
- 18.Sirohi B, Arnedos M, Popat S, et al. Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol 2008;19:1847-52. [DOI] [PubMed] [Google Scholar]
- 19.Perez EA, Moreno-Aspitia A, Aubrey Thompson E, et al. Adjuvant therapy of triple negative breast cancer. Breast Cancer Res Treat 2010;120:285-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. FinXX Final 5-Year Analysis: Results of the Randomised, Open-Label, Phase III Trial in Medium-to-High Risk Early Breast Cancer. Cancer Res 2005;70:S4-1. [Google Scholar]
- 21.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer 2004;4:814-19. [DOI] [PubMed] [Google Scholar]
- 22.Cleere DW. Triple-negative breast cancer: a clinical update. Community Oncol 2010;7:203-11. [Google Scholar]
- 23.Dent R, Hanna WM, Trudeau M, et al. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 2009;115:423-8. [DOI] [PubMed] [Google Scholar]
- 24.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol 2010;7:455-65. [DOI] [PubMed] [Google Scholar]
- 25.Colleoni M, Rocca A, Sandri MT, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol 2002;13:73-80. [DOI] [PubMed] [Google Scholar]
- 26.Scharovsky OG, Mainetti LE, Rozados VR. Metronomic chemotherapy: changing the paradigm that more is better. Curr Oncol 2009;16:7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mross K, Steinbild S. Metronomic anti-cancer therapy – an ongoing treatment option for advanced cancer patients. J Cancer Ther Res 2012;1:32. [Google Scholar]
- 28.BCCA Protocol Summary for Adjuvant Therapy for Breast Cancer using Dose Dense Therapy: DOXOrubicin and Cyclophosphamide followed by PACLitaxel. Available online: http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Breast/BRAJACTG_Protocol_1Aug2014.pdf
- 29.BCCA Protocol Summary for Adjuvant Therapy for Breast Cancer Using Fluorouracil, Epirubicin and Cyclophosphamide Followed by DOCEtaxel and Trastuzumab (HERCEPTIN). Available online: http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Breast/BRAJFECDT_Protocol_1Dec2014.pdf
- 30.BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer Using Metronomic Low-Dose Oral Cyclophosphamide and Methotrexate. Available online: http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Breast/BRAVCMPO_Protocol_1Jul2012.pdf
- 31.NCCN clinical practice guidelines in oncology, breast cancer, version 3, 2014. Available online: http://www.nccn.org/ professionals/physician_gls/pdf/breast.pdf
- 32.Common Toxicity Criteria (CTC) Version 2.0. Available online: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf
- 33.Alagizy HA, Shehata MA, Hashem TA, et al. Metronomic capecitabine as extended adjuvant chemotherapy in women with triple negative breast cancer. Hematol Oncol Stem Cell Ther 2015;8:22-7. [DOI] [PubMed] [Google Scholar]
- 34.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010;28:1145-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chacón RD, Costanzo MV. Triple-negative breast cancer. Breast Cancer Res 2010;12 Suppl 2:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penel N, Adenis A, Bocci G. Cyclophosphamide-based metronomic chemotherapy: after 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol 2012;82:40-50. [DOI] [PubMed] [Google Scholar]
- 37.Kontani K, Hashimoto SI, Murazawa C, et al. Metronomic chemotherapy for metastatic breast cancer to prolong time to treatment failure to 12 months or more. Mol Clin Oncol 2013;1:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma WY, Zhang P, Zhang BL, et al. Phase II clinical trial of neoadjuvant therapy with carboplatin plus paclitaxel for locally advanced triple-negative breast cancer. Zhonghua Zhong Liu Za Zhi 2012;34:770-4. [DOI] [PubMed] [Google Scholar]
- 39.Torrisi R, Balduzzi A, Ghisini R, et al. Tailored preoperative treatment of locally advanced triple negative (hormone receptor negative and HER2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel. Cancer Chemother Pharmacol 2008;62:667-72. [DOI] [PubMed] [Google Scholar]
- 40.Emmenegger U, Francia G, Chow A, et al. Tumors that acquire resistance to low-dose metronomic cyclophosphamide retain sensitivity to maximum tolerated dose cyclophosphamide. Neoplasia 2011;13:40-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


