Abstract
Study Design Randomized controlled trial.
Objective The aim of this study was to assess the efficacy of the bone grafting substitute silicate-substituted calcium phosphate (SiCaP) compared with recombinant human bone morphogenetic protein 2 (rhBMP-2) and to evaluate the clinical outcomes over a period of 2 years.
Methods Patients undergoing PLF surgery for DDD at a single center were recruited and randomized to one of two groups: SiCaP (n = 9) or rhBMP-2 (n = 10). One patient withdrew prior to randomization and another from the rhBMP-2 group after randomization. The radiologic and clinical outcomes were examined and compared. Fusion was assessed at 12 months with computed tomography and plain radiographs. Clinical outcomes were evaluated by recording measures of pain, quality of life, disability, and neurologic status from 6 weeks to 2 years postoperatively.
Results In the SiCaP and rhBMP-2 groups, fusion was observed in 9/9 and 8/9 patients, respectively. Pain and disability scores were reduced and quality of life increased in both groups. Leg pain, disability, and satisfaction scores were similar between the groups at each postoperative point; however, back pain was less at 6 weeks and quality of life was higher at 6 months in the SiCaP group than the rhBMP-2 group.
Conclusions SiCaP and rhBMP-2 were comparable in terms of achieving successful bone growth and fusion. Both groups achieved similar alleviation of pain and improved quality of life and neurologic, satisfaction, and return to work outcomes following PLF surgery.
Keywords: lumbar fusion, bone graft substitutes, Actifuse
Introduction
Recombinant human bone morphogenetic protein 2 (rhBMP-2) is used as an alternative to iliac crest bone in posterolateral lumbar fusion (PLF) surgery, but there are potential concerns about the cost and the associated complications including radiculitis, ectopic bone formation, and osteolysis.1 2 3 4 Silicate-substituted calcium phosphate (SiCaP) has been proposed as a viable option in spinal fusion procedures, in conjunction with appropriate stabilizing hardware.5 However, it is unclear whether it is as effective in achieving a solid arthrodesis, particularly in the setting of PLF.
Objective
To compare fusion success at 12 months and the clinical outcomes over a period of 2 years in patients with degenerative disk disease (DDD) who underwent instrumented PLF surgery with either SiCaP or rhBMP-2 as bone grafting materials.
Methods
Study Design
This phase IV randomized controlled trial studied patients undergoing instrumented PLF. Randomization was performed centrally on a 1:1 basis and conveyed by sealed envelope. Patients and radiologists were blinded to the surgical treatment. This study was reviewed and approved by St Vincent's & Holy Spirit Health Human Research Ethics Committee 2009.
Inclusion Criteria
Patients presenting to a single center between July 2009 and July 2010 were screened for eligibility. Patients aged 18 to 80 years with DDD of the lumbar spine that had failed to respond to nonoperative treatment for at least 6 months and who were considered suitable for PLF surgery were included.
Exclusion Criteria
Patients were excluded if they had undergone previous fusion attempts; required surgery at more than two levels; had recent or ongoing infection, osteoporosis (excluding osteopenia), or other significant medical condition; or were taking medication that may have interfered with bone metabolism.
Patient Population
Of the 33 patients assessed for eligibility, 14 were excluded and 19 underwent randomization. Nine patients were in the SiCaP group and 10 were in the rhBMP-2 comparator group, from which 1 patient withdrew. In total, 9 patients were analyzed for each group (Fig. 1).
Fig. 1.
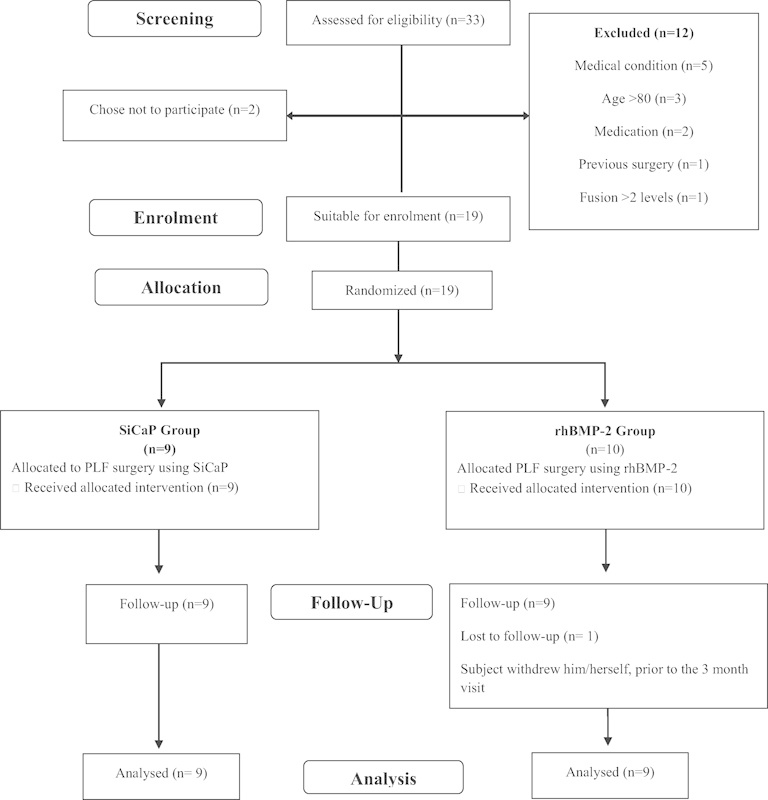
CONSORT diagram of patient selection. Abbreviations: PLF, posterolateral lumbar fusion; rhBMP-2, recombinant human bone morphogenetic protein 2; SiCaP, silicate-substituted calcium phosphate.
Intervention
Through a midline approach, pedicle screws were inserted. The transverse processes and facet joints were exposed and decorticated. SiCaP in the form of Actifuse (Baxter, Elstree, Hertfordshire, United Kingdom) or rhBMP-2 in the form of Infuse (Medtronic, Minneapolis, Minnesota, United States) and Mastergraft (Medtronic) was packed in the facet joints and posterolateral gutters. With Actifuse, 10 to 15 mL was used per level per side. One large Infuse sponge (12 mg rhBMP-2, 1.5 mg/mL) wrapped around 15 cc Mastergraft calcium phosphate ceramic granules was used per level, with half in each posterolateral gutter.
Outcomes
Fusion success was assessed with computed tomography (CT) scans 12 months after surgery and with plain radiographs taken at 3, 6, 12, and 24 months after surgery. The images were assessed by two radiographic reviewers blinded to the intervention. Solid fusion on CT was based on bridging trabecular bone between transverse processes in the posterolateral gutters and graded according to previous methodology.2 Grade 4 (solid unilateral fusion) or grade 5 (solid bilateral fusion) was deemed solid fusion. Solid fusion on plain X-ray was based on the absence of motion, which was defined as no more than 3 mm translation or 5 degrees of angulation on flexion extension views. To be considered fused, both imaging modalities needed to demonstrate fusion.
Data was recorded before and at 6 weeks and 3, 6, 12, and 24 months after surgery for visual analogue scale (VAS) of back and leg (left and right) pain status,6 the Oswestry Disability Index (ODI),7 8 quality-of-life outcomes using the 36-Item Short Form Health Survey,9 neurologic status, patients' general satisfaction, and changes in work status. The neurologic status was based on physical examination of four measurements: motor function, sensory function, reflexes, and straight leg raise. Satisfaction and change in work status were assessed based on patient's response to specific questions. Safety was assessed in terms of adverse events.
Analysis
The Fisher exact test and t test (using SAS software version 9.1.3, SAS Institute, Cary, North Carolina, United States) were used to obtain p values for categorical and continuous data, respectively. Improvement in back and leg VAS, ODI, and quality-of-life outcomes using 36-Item Short Form Health Survey were analyzed based on achievement of minimum clinically important difference (MCID) at all postoperative times.10
Results
Patient Characteristics
Nineteen patients were enrolled; one patient withdrew from the rhMBP-2 group, leaving nine patients in each group (Fig. 1). A summary of demographic information is provided in Table 1. All patients had DDD. In all cases, the primary diagnosis was spinal stenosis with or without degenerative spondylolisthesis.
Table 1. Patient characteristics.
| Sample characteristic | SiCaP (n = 9) | BMP-2 (n = 10) | Total (n = 19) |
|---|---|---|---|
| Age (y)a | 65.8 ± 7.17 | 66.9 ± 6.54 | 66.4 ± 6.68 |
| Height (cm)a | 173.8 ± 11.68 | 167.0 ± 11.31 | 170.4 ± 11.64 |
| Weight (kg)a | 88.1 ± 23.36 | 81.5 ± 15.08 | 84.8 ± 19.30 |
| Body mass index (kg/m2)a | 28.9 ± 5.82 | 30.1 ± 2.93 | 29.4 ± 4.49 |
| Obese (n) | 4 | 3 | 7 |
| Sex, n (%) | |||
| Male | 6 (67%) | 3 (30%) | 9 (47%) |
| Female | 3 (33%) | 7 (70%) | 10 (53%) |
| Smoker, n (%) | |||
| No | 8 (89%) | 10 (100%) | 18 (95%) |
| Yes | 1 (11%) | 0 (0%) | 1 (5%) |
Abbreviations: rhBMP-2, recombinant human bone morphogenetic protein 2; SiCaP, silicate-substituted calcium phosphate.
Note: Obesity was defined as having a body mass index > 30 kg/m2.
Values are expressed as mean ± standard deviation.
Primary Outcome Results
Fusion occurred in 9/9 patients in the SiCaP group (Fig. 2) and 8/9 patients in the rhBMP-2 group (p = not significant; Fig. 3).
Fig. 2.
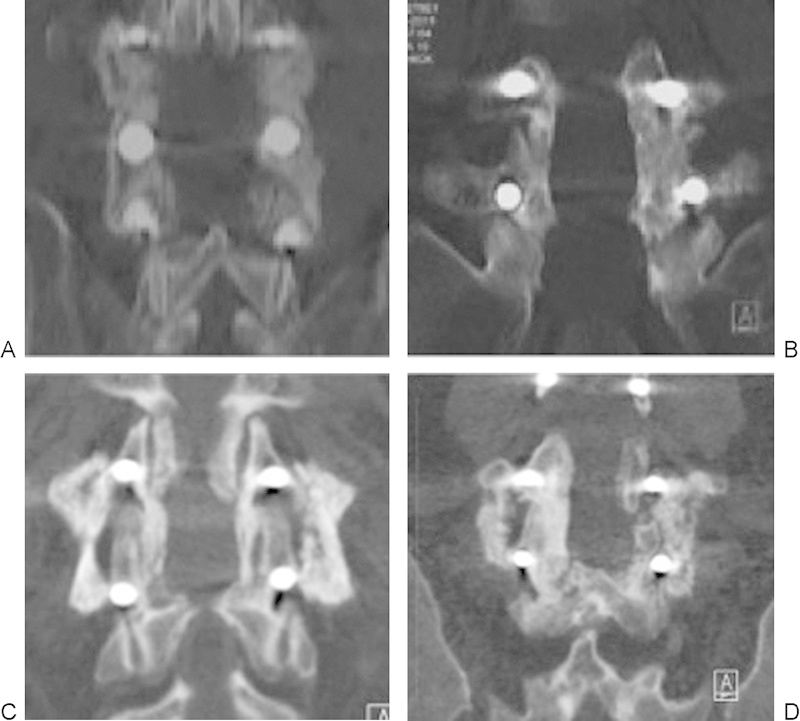
(A–D) Illustrative computed tomography images acquired 12 months after posterolateral fusion surgery with silicate-substituted calcium phosphate in four patients who achieved a solid fusion.
Fig. 3.
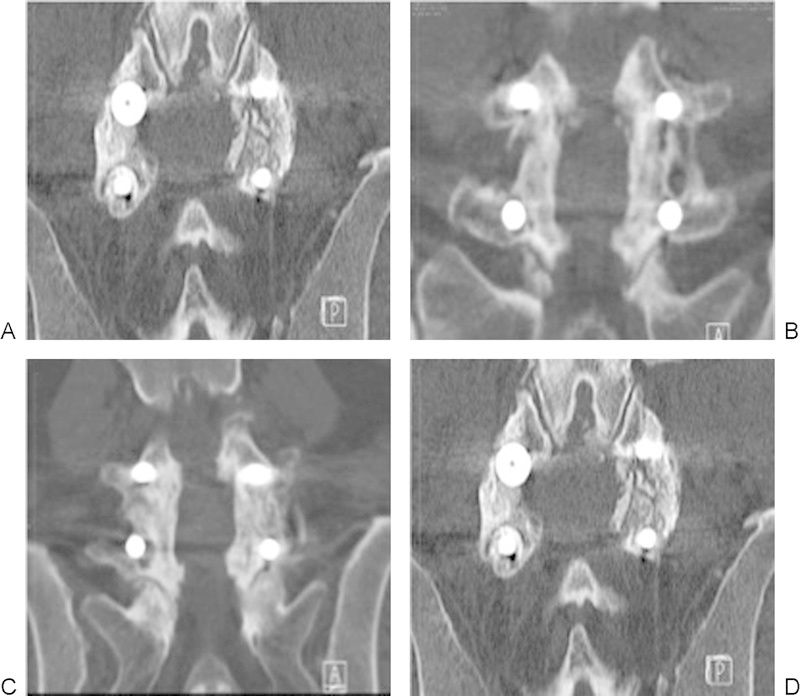
(A–D) Illustrative computed tomography images acquired 12 months after posterolateral fusion surgery with recombinant human bone morphogenetic protein 2 in four patients who achieved a solid fusion.
Secondary Outcome Results
MCID for ODI was achieved by a greater proportion of patients in the SiCaP group at all postoperative time points. However, this difference was not significant (Table 2).
Table 2. Comparison of ODI and quality-of-life SF-36 Physical Component Summary scores.
| Time | SiCaP | rhBMP-2 | p Value |
|---|---|---|---|
| ODIa | |||
| 6 wk | 75.0% (6/8) | 25.0% (2/8) | 0.13 |
| 3 mo | 85.7% (6/7) | 50.0% (4/8) | 0.28 |
| 6 mo | 87.5% (7/8) | 44.4% (4/9) | 0.13 |
| 12 mo | 100% (8/8) | 55.6% (5/9) | 0.08 |
| 24 mo | 87.5% (7/8) | 66.7% (6/9) | 0.58 |
| Quality-of-life SF-36 Physical Component Summaryb | |||
| 6 wk | 87.5% (7/8) | 75% (6/8) | > 0.99 |
| 3 mo | 100% (7/7) | 87.5% (7/8) | > 0.99 |
| 6 mo | 100% (8/8) | 100% (9/9) | > 0.99 |
| 12 mo | 100% (8/8) | 100% (8/8) | > 0.99 |
| 24 mo | 100% (8/8) | 100% (9/9) | > 0.99 |
Abbreviations: MCID, minimum clinically important difference; ODI, Oswestry Disability Index; rhBMP-2: bone morphogenetic protein-2; SF-36, 36-Item Short Form Health Survey; SiCaP: silicated calcium phosphate bone graft.
Note: p values for between-group comparisons. MCID calculation was based on 12.8 points for ODI and 4.9 points for SF-36 PCS (Physical Component Summary).10
Percentage achieving MCID, compared with preoperative score.
Percentage achieving MCID, compared with preoperative score.
There was no significant difference between the SiCaP and rhBMP-2 groups in the percentage of patients achieving MCID for Physical Component Summary scores at any of the postoperative times (Table 2).
When compared with their preoperative scores, the majority of patients in both the SiCaP and rhBMP-2 groups reported a reduction in back and leg VAS at the 6-month postoperative review. However, not all of these patients achieved an MCID. There does not appear to be a difference between groups, but there does appear to be a trend toward a higher preoperative VAS relating to a greater reduction in VAS at the 6-month postoperative review (Figs. 4, 5, and 6).
Fig. 4.
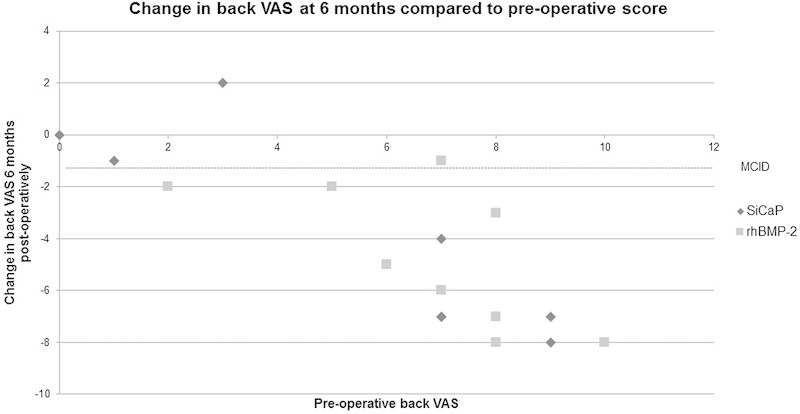
VAS for back pain. The VAS score was assessed from 0 (no pain) to 10 (greatest pain). MCID demonstrated at −1.2.10 Abbreviations: MCID, minimum clinically important difference; rhBMP-2, recombinant human bone morphogenetic protein 2; SiCaP, silicate-substituted calcium phosphate; VAS, visual analog scale.
Fig. 5.
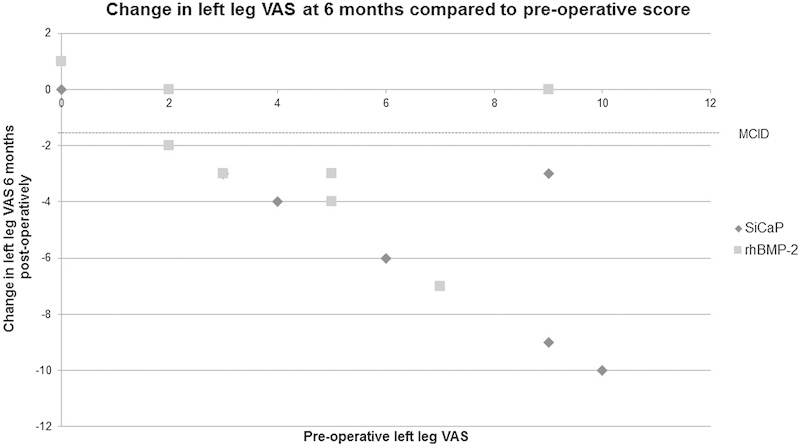
VAS for left leg pain. The VAS score was assessed from 0 (no pain) to 10 (greatest pain). MCID demonstrated at −1.6.10 Abbreviations: MCID, minimum clinically important difference; rhBMP-2, recombinant human bone morphogenetic protein 2; SiCaP, silicate-substituted calcium phosphate; VAS, visual analog scale.
Fig. 6.
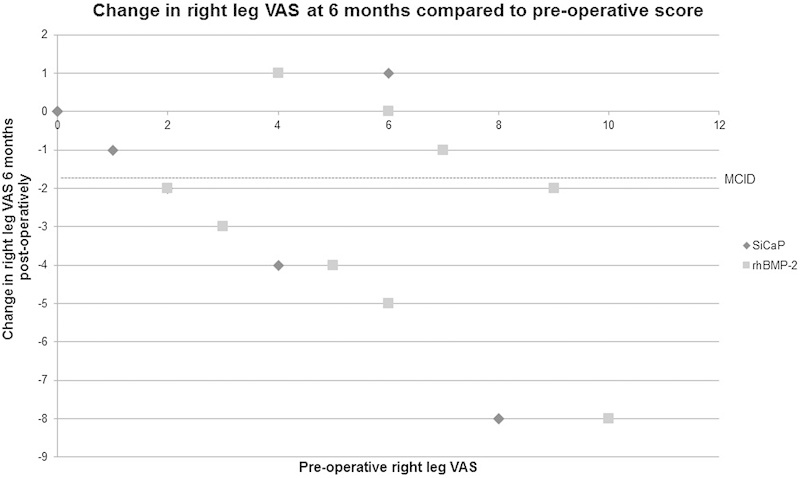
VAS for right leg pain. The VAS score was assessed from 0 (no pain) to 10 (greatest pain). MCID demonstrated at −1.6.10 Abbreviations: MCID, minimum clinically important difference; rhBMP-2, recombinant human bone morphogenetic protein 2; SiCaP, silicate-substituted calcium phosphate; VAS, visual analog scale.
Patients reported improved neurologic status, general satisfaction, and no change in employment after surgery. There were no significant differences between SiCaP and rhBMP-2 groups at any of the postoperative times.
There were no adverse events attributable to the bone graft material in either group.
Discussion
rhBMP-2 is widely used in bone graft applications in the lumbar spine and demonstrates higher-grade fusions than iliac crest bone graft.2 3 However, questions regarding the safety profile of rhBMP-2 in spinal surgery have been raised.4 The osteoconductive bone graft material SiCaP has recently gained considerable attention and has been used in a case series of 42 patients undergoing PLF surgery, with reported fusion rates of 76%.11
This study is the first randomized trial to evaluate the effects of using SiCaP relative to rhBMP-2 in this particular PLF surgical setting. We observed postoperative fusion rates of 100% at 12 months with SiCaP, confirming the usefulness of SiCaP in the context of PLF for patients with DDD. These results suggest similar rates of fusion and clinical outcomes in patients receiving PLF procedures with SiCaP or rhBMP-2.
The small number of patients in this study was largely due to patients being excluded after being screened and deemed suitable for interbody fusion rather than posterolateral fusion. Posterolateral fusion was chosen as the surgical technique in this study as it provides a more demanding environment for fusion.
Further investigations using a greater numbers of subjects will be beneficial in providing information about the efficacy and safety of SiCaP as a bone-grafting substitute.
Conclusion
SiCaP and rhBMP-2 substitute bone graft biomaterials were comparable in terms of achieving successful bone growth and bone fusion and similarly alleviated pain and improved quality of life, neurologic outcomes, general satisfaction, and work status change following PLF surgery.
Acknowledgments
Baxter Healthcare sponsored this investigation. The study was managed by Roz Mazey. Protocol and analysis support was provided by Therasa Curtis and Steve Czop. Queensland University of Technology research assistants Laura Ewing and Michelle Johnston assisted with data collection and analysis.
Disclosures Paul Licina, none Marc Coughlan, none Emma Johnston, none Mark Pearcy, Meeting expenses: Nuvasive
Notes
Funding for this study was provided by Apatech Ltd (Actifuse).
Editorial Perspective
Evidence-Based Spine-Care Journal (EBSJ) reviewers unanimously commended the authors for performing a prospective randomized controlled trial with thoughtfully designated patient cohorts and a relevant surgical technique-related study question about osteobiologics and their osseous healing in a posterolateral instrumented lumbar fusion setting. Although some reviewers were concerned about the small sample size, EBSJ decided to proceed with publication to show an example of a well-executed pilot study with a combination of meaningful patient-reported outcomes in addition to surgeon-reported variables such as radiographic data and even CT scans at 12 months to objectively rate fusion mass. It was apparent to our reviewers that the study authors sought to create a “clean” study without the usual variables influencing outcomes—for instance, by selecting patients who did not require an anterior interbody fusion or by avoiding a left-to-right comparison of a posterolateral bone graft bed with different bone graft materials. Perhaps most importantly, patient selection appears to have been done in a very controlled fashion to keep extraneous influences on bone healing of a posterolateral fusion to a minimum. Reporting was also done in a very transparent fashion, allowing a reviewer to evaluate each patient's outcomes scores individually. There are three findings EBSJ would like to underscore:
Fusion studies are hard to do. Making sure that there is indeed a solid bony fusion takes a long time for follow-up and usually more than plain radiographs—as was done in this study where the investigators were able to get a CT scan at the 1-year postoperative follow-up.
The authors have shown equivalence of a silicate calcium phosphate material to a recombinant bone morphogenic protein in a small group setting, which basically represents a pilot efficacy trial.
In the bigger picture, simple questions like the one posed here (Can one achieve similar fusion results with a much less expensive anorganic bone graft substitute compared with a genetically engineered recombinant implant?) are often the most difficult to answer as the number of variables to consider can be daunting. The cleaner the study design, the more difficult it will be to recruit patients to achieve adequate sample sizes. More-definitive answers can only be generated from larger multicenter efforts and eventually are probably better answered using a registry format to answer efficiency-related questions at the cost of precision relative to the focus of the question. For the basic questions at hand, smaller pilot studies such as this one are ideal to establish initial patient safety and efficacy.
EBSJ and Global Spine Journal hope that this study will inspire many further prospective investigations by AOSpine going forward.
References
- 1.Boden S D, Kang J, Sandhu H, Heller J G. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 2002;27(23):2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Glassman S D, Dimar J R, Carreon L Y, Campbell M J, Puno R M, Johnson J R. Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine (Phila Pa 1976) 2005;30(15):1694–1698. doi: 10.1097/01.brs.0000172157.39513.80. [DOI] [PubMed] [Google Scholar]
- 3.Glassman S D, Carreon L Y, Djurasovic M. et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: a randomized, controlled trial in patients over sixty years of age. Spine (Phila Pa 1976) 2008;33(26):2843–2849. doi: 10.1097/BRS.0b013e318190705d. [DOI] [PubMed] [Google Scholar]
- 4.Carragee E J, Hurwitz E L, Weiner B K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Nagineni V V, James A R, Alimi M. et al. Silicate-substituted calcium phosphate ceramic bone graft replacement for spinal fusion procedures. Spine (Phila Pa 1976) 2012;37(20):E1264–E1272. doi: 10.1097/BRS.0b013e318265e22e. [DOI] [PubMed] [Google Scholar]
- 6.Price D D, McGrath P A, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 7.Fairbank J C, Couper J, Davies J B, O'Brien J P. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–273. [PubMed] [Google Scholar]
- 8.Fairbank J C Pynsent P B The Oswestry Disability Index Spine (Phila Pa 1976) 200025222940–2952., discussion 2952 [DOI] [PubMed] [Google Scholar]
- 9.Ware J E Jr, Sherbourne C D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 10.Copay A G, Glassman S D, Subach B R, Berven S, Schuler T C, Carreon L Y. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8(6):968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Jenis L G, Banco R J. Efficacy of silicate-substituted calcium phosphate ceramic in posterolateral instrumented lumbar fusion. Spine (Phila Pa 1976) 2010;35(20):E1058–E1063. doi: 10.1097/BRS.0b013e3181df196f. [DOI] [PubMed] [Google Scholar]


