Abstract
Background To determine the prevalence of a high-riding jugular bulb (HRJB) in the endolymphatic hydrops population.
Methods This was a retrospective chart and radiology review of patients seen at a tertiary care medical center. Patients were identified using the International Classification of Diseases, 9th edition, code 386.xx (Meniere disease–unspecified), and were required to have undergone an imaging study that included views of the jugular bulb that were available for review. A radiologist then evaluated all of the imaging studies for evidence of HRJB or inner ear dehiscence with a jugular bulb abnormality.
Results The prevalence of a HRJB in all endolymphatic hydrops patients was 9.0% (7 of 78), and it was 4.5% (7 of 156) in all ears. The prevalence of HRJB ipsilateral to an ear with endolymphatic hydrops was 4.6% (4 of 88 ears); it was 4.4% (3 of 68 ears) in ears without endolymphatic hydrops. The incidence of inner ear dehiscence with a HRJB was 1.3% (1 of 78). Electrocochleography results were not correlated with jugular bulb volume.
Discussion The results of this study indicate that a small subset of patients treated for endolymphatic hydrops patients have a HRJB. Overall, these results suggest that HRJB does not play a major role in endolymphatic hydrops, although it may play a role in a few isolated patients.
Keywords: Meniere disease, dizziness, lateral skull base, hearing loss
Introduction
The jugular bulb is a dilatation of the internal jugular vein at its origin, which is located within the posterior compartment (pars vascularis) of the jugular foramen at the skull base.1 Most of the venous inflow to the jugular bulb comes from the transverse and sigmoid dural venous sinuses, whereas venous outflow is transmitted via the internal jugular vein. The jugular bulb does not exist in fetal life or infancy but develops in early childhood,1 2 and it may occur with the attainment of an upright posture after the age of 2 years.1 2 Further expansion of the jugular bulb occurs after initial formation of the jugular bulb and stabilizes in adulthood.1
In some individuals the jugular bulb can become pathologic when it is enlarged and causes local audiological or vestibular symptoms. This can occur with a high-riding jugular bulb (HRJB) that has been variably defined as a jugular bulb that rises to the level of the basal turn of the cochlea,3 within 2 mm of the internal auditory canal (IAC),4 or to the level of the superior tympanic annulus.5 A HRJB has been implicated as the cause of symptoms including dizziness, conductive hearing loss, sensorineural hearing loss, vertigo, and pulsatile tinnitus.3 5 6 7 Sensorineural hearing loss, vertigo, and dizziness may result from dehiscence of the jugular bulb with inner ear structures such as the semicircular canals or vestibular aqueduct. The prevalence of the HRJB has been cited from 8% to 32.5%,5 8 9 but dehiscence of the jugular bulb into inner ear structures is uncommon, with studies citing a prevalence between 1.5% and 11.5%.5 9 10
Meniere disease or endolymphatic hydrops has been associated with a HRJB in several articles.7 11 12 13 The hypothesized mechanism of pathophysiology is dehiscence of the HRJB with inner ear structures.11 13 14 Two authors demonstrated higher frequencies of jugular bulb abnormalities in people with Meniere disease than in the general population,13 15 suggesting a pathophysiologic link between abnormalities of the jugular bulb and Meniere disease. The purpose of the current study was to identify the prevalence of HRJB in patients with suspected Meniere disease, as well as to correlate jugular bulb anomalies with results of electrocochleography (ECOG) testing, a diagnostic tool in Meniere disease.
Materials and Methods
This study was approved by our institutional review board. The study was a retrospective review, and patients were selected from 2000 to 2012 using the International Classification of Diseases, 9th edition (ICD-9) code 386.xx (Meniere's disease–unspecified). The charts were then reviewed, and patients treated medically for definite Meniere disease were included in the study. All patients were required to have undergone an imaging study that included views of the bilateral jugular bulbs available for review. Basic demographics, audiological data, and ECOG results were recorded. ECOG values were retrospectively collected and recorded as the summation potential-to-action potential (SP:AP) ratio. Patients were excluded if they did not have SP:AP values available. A single radiologist then evaluated all of the imaging studies for dimensions of the jugular bulb and evidence of HRJB or jugular bulb abnormality dehiscence in the inner ear.
Computed Tomography Imaging Protocol
We performed computed tomography (CT) examinations on the patient cohort in accordance with our routine brain, temporal bone, sinuses, or maxillofacial protocol. All CT examinations were obtained on a 64-detector row CT scanner (Lightspeed VCT; GE Healthcare, Milwaukee, Wisconsin, United States). CT brain examinations were axially acquired with a slice thickness of 1.25 mm with 5-mm-thick coronal reformats. The CT temporal bone examinations were helically acquired with a slice thickness of 0.0625 mm and a 0.3-mm reconstruction interval, with 1-mm-thick coronal reconstructions. CT sinus and maxillofacial examinations were helically acquired with a 1.25-mm slice thickness and reconstruction interval with 1.25-mm coronal and sagittal 1.5-mm-thick reformats.
Magnetic Resonance Imaging Protocol
Magnetic resonance imaging (MRI) examinations were performed with either of two 1.5-T MRI units (Intera or Achieva; Philips Medical Systems of North America, Cleveland, Ohio, United States) with and without intravenous contrast (0.1 mM/kg, Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey, United States, or MultiHance; Bracco Diagnostics Inc., Monroe Township, New Jersey, United States). Measurements were performed on axial postcontrast enhanced spin-echo T1-weighted images (MRI neck: 4 mm thick; MRI brain: 5 mm thick; MRI IAC: 2.5 mm thick).
Image-Based Measurements
Of the 79 patients included in this cohort, 38 patients had MRI examinations only, 9 patients had CT examinations only, and 32 patients had both CT and MRI examinations. Of the MRI examinations, 67 patients had an MRI using an IAC protocol, 2 patients had MRI utilizing a neck protocol, and one patient had a routine brain MRI. For CT, 25 patients had routine noncontrast CT brain examinations, 11 patients had temporal bone CT scans, 3 patients had CT sinus examinations, and 2 patients had a neck CT. Jugular bulb measurements were performed in both the axial and coronal planes on both the CT and MRI examinations for all patients with both imaging modalities, but we preferentially used the CT examination for measuring the jugular bulb diameters for data analysis due to superior bony resolution (Figs. 1 and 2). The jugular bulb was defined as the dilated portion of the upper jugular vein at the junction between the sigmoid sinus and the jugular vein.16 The anteroposterior and mediolateral diameters of the jugular bulb were measured on axial and coronal images. The presence of a HRJB, defined as a jugular bulb that extended into the middle ear, above the level of the floor of the IAC17 or tympanic annulus,18 was also recorded. Any associated dehiscence of inner ear structures was also recorded. After determining the mediolateral and anteroposterior dimensions of the jugular bulb, the estimated cross-sectional area of the jugular bulb was calculated using the formula for an ellipse. We used the standard formula of πab, where a is half the anteroposterior diameter and b is half of the mediolateral diameter of the ellipse.
Fig. 1.
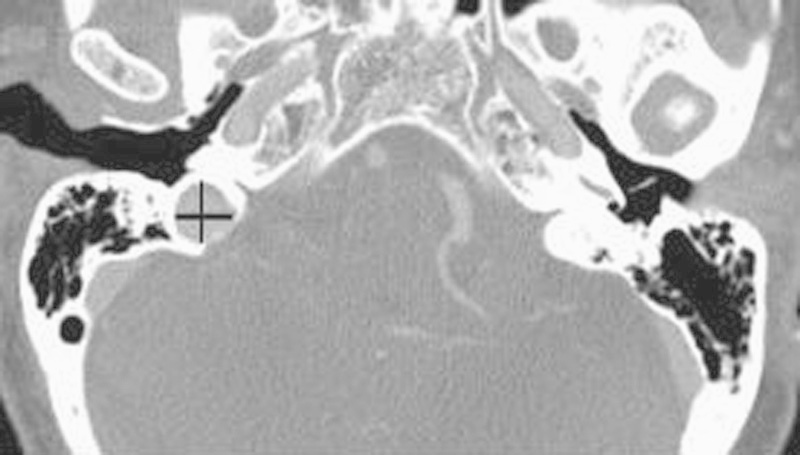
Axial contrast-enhanced computed tomography through the level of the jugular bulb with the transverse line depicting the mediolateral diameter of the right jugular bulb and the vertical line demonstrating the anteroposterior diameter of the right jugular bulb.
Fig. 2.
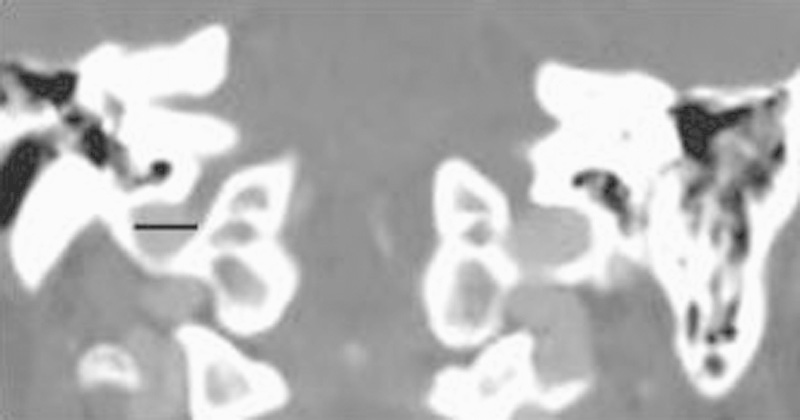
Coronal contrast-enhanced computed tomography through the level of the jugular bulb with the line illustrating the mediolateral diameter of the right jugular bulb.
A chi-square analysis was performed comparing the prevalence of HRJB in ears with endolymphatic hydrops symptoms and the prevalence of HRJB in ears without endolymphatic hydrops symptoms. A t test was used to compare ECOG results between ears with both endolymphatic hydrops and HRJB, and ears with endolymphatic hydrops and without HRJB.
Results
A total of 92 patients had the ICD-9 code 386.xx and had imaging through the temporal bone. Fourteen patients did not have ECOG results available for review and were excluded. A total of 78 patients (156 ears) were included in the final study. The average age was 48.31 years (range: 18–82 years); the male-to-female ratio was 1:2.25 (24 men and 54 women). Of the 78 patients, 7 met the criteria for probable or definite Meniere disease, 48 met the criteria for possible Meniere disease, and 23 patients were suspected of having Meniere disease and were treated medically with diet alteration or diuretics, but not enough retrospective clinical information was available to categorize them based on the American Academy of Otolaryngology criteria.19 The prevalence of HRJB in all suspected endolymphatic hydrops patients was 9.0% (7 of 78) and 4.5% (7 of 156) in all ears. The prevalence of HRJB ipsilateral to an ear with suspected endolymphatic hydrops was 4.6%, or 4 of 88 ears, which was not significantly different than in ears without suspected endolymphatic hydrops (4.4%, or 3 of 68 ears; p = 0.97). The prevalence of HRJB dehiscence with the inner ear was 1.3% (1 of 78) and was present in only one patient but in an ear with endolymphatic hydrops. All HRJBs in this study were right sided, and right-sided jugular bulb dominance was found in 74% of patients.
ECOG values were recorded as the SP:AP ratio. Of 78 patients with Meniere disease in this study, all patients had ECOG findings in at least one ear available for review, and of those, 64 had results in both ears. In all patients with only unilateral ECOG results, the test results were available in the ear with symptoms of Meniere disease. The average SP:AP ratio in active Meniere disease ears was 45.9; in ears without active disease (but in patients who still carried the diagnosis in the other ear), it was 32.1. The mean ECOG value for ears with active endolymphatic hydrops and HRJB was 41.2. A correlation coefficient was calculated between the jugular bulb cross-sectional area and ECOG SP:AP ratio, demonstrating no significant correlation (r = 0.06).
Jugular bulb volume was calculated on both sides for all patients. All HRJBs in this study were right sided, and right-sided jugular bulb dominance was found in 74% of patients. The average size of the jugular bulb in the right ear was 37.4 mm2; it was 27.0 mm2 on the left side (p ≤ 0.001).
Discussion
In this study we used CT and MRI to determine the prevalence of a HRJB in a cohort of patients with suspected Meniere disease. We found that the prevalence of HRJB (9.0%) and inner ear dehiscence (1.3%) to be similar to that reported in the literature for HRJB (8–32.5%5 8 9) and inner ear dehiscence (1.5–11.5%5 9 10) in the general population. Using asymptomatic ears on patients with Meniere disease as a control, we found no difference in the prevalence of HRJB between affected ears (4.6%) when compared with unaffected ears (4.4%); p = 0.97. Interestingly, the only incidence of inner ear dehiscence with a HRJB was ipsilateral to an ear with Meniere disease.
Other articles have examined the prevalence of HRJB in patients with Meniere disease.13 15 In one study, the authors found that 57.1% of 26 patients with definitive Meniere disease had evidence on a high-resolution CT scan of jugular bulb abnormalities,13 but, interestingly, HRJB and inner ear encroachment from the jugular bulb were not more common in affected Meniere ears. All patients in this series were being evaluated for surgical management of intractable Meniere disease, suggesting a severe clinical spectrum of symptoms that does not represent the overall course of disease for most patients in the current series.
In another article, the authors examined 200 ears of 167 patients with Meniere disease and 218 ears of 109 patients without symptoms of Meniere disease, finding a higher number of HRJBs, jugular bulb diverticula, and jugular bulbs in close proximity to the inner ear in the Meniere disease group.15 They did find an increase in HRJB ipsilateral to affected Meniere ears but no increase in inner dehiscence in the Meniere patients when compared with control patients. This contrasts with our findings and the article by Redfern et al13 that did not demonstrate a higher prevalence of HRJB ipsilateral to the affected Meniere disease ear.
Additionally, it should be noted that in a review of 30 patients with inner ear dehiscence, Friedmann et al20 reported that only 15 had any symptoms, and only 2 of these patients demonstrated symptoms of endolymphatic hydrops. Overall it would appear that a definitive relationship has not yet been identified between endolymphatic hydrops and Meniere disease. The current study does not demonstrate a higher prevalence of HRJB associated with Meniere disease, whereas in other studies there is an increase in jugular bulb abnormalities in patients with Meniere disease, but these abnormalities are variably associated with affected Meniere ears.13 15
To our knowledge, this is the first article to study ECOG findings, Meniere disease, and the relationship with the HRJB. It was not surprising to find that the ECOG SP:AP ratios in active Meniere ears were higher than in inactive ears because endolymphatic hydrops has been associated with elevated SP:AP ratios.21 22 Perhaps unsurprisingly as well, there was no difference in ECOG results between the HRJB ears with Meniere (average: 41.1) and Meniere ears without a HRJB (46.3) (p = 0.6). Correlation coefficients between jugular bulb area and ECOG were calculated demonstrating no significant correlation (r = 0.06) between jugular bulb volume and ECOG, suggesting there is not a strong relationship between the size of the jugular bulb and inner ear abnormalities resulting in elevation of the SP:AP ratio. The single ear with a dehiscent jugular bulb recorded a markedly elevated SP:AP ratio of 80, but without a larger series of inner ear dehiscence we cannot make assumptions about a relationship between dehiscence and the SP:AP ratio.
This study was done retrospectively at a single academic institution, so it may not be possible to extrapolate this information to all populations. Imaging evaluation was not standardized, with the patients undergoing different CT scanning protocols, different image slice thicknesses, and differences in contrast administration. Additionally, 38 of 78 patients underwent MRI only, which has less bony detail compared with CT. Even with these limitations, HRJB was identified in three patients with MRI only and in four patients with CT scan. No inner ear dehiscence was identified on MRI, and it may be that this modality is not as reliable in identifying inner ear dehiscence with a jugular bulb. Overall, the incidence of inner ear dehiscence may be slightly underrepresented in this study.
The diagnostic criteria for Meniere disease were not standardized. Patients were selected for inclusion based on medical treatment for Meniere disease with diuretics or diet alterations. Most patients (53 of 78) fit into established American Academy of Otolaryngology criteria,19 but a sizable percentage could not be characterized due to limited retrospective information. This is a large spectrum of clinical disease, and it may be that in patients with definite Meniere disease, the prevalence of HRJB, or jugular bulb abnormalities is significantly higher.
Conclusion
In this study Meniere disease does not appear to be correlated with a higher prevalence of HRJB or jugular bulb abnormalities. The SP:AP ratio in ECOG does not appear related to the size of the jugular bulb. Jugular bulb abnormalities do not appear in most patients with suspected Meniere disease, and the relationship between the two entities remains poorly elucidated.
References
- 1.Okudera T, Huang Y P, Ohta T. et al. Development of posterior fossa dural sinuses, emissary veins, and jugular bulb: morphological and radiologic study. AJNR Am J Neuroradiol. 1994;15(10):1871–1883. [PMC free article] [PubMed] [Google Scholar]
- 2.Friedmann D R, Eubig J, McGill M, Babb J S, Pramanik B K, Lalwani A K. Development of the jugular bulb: a radiologic study. Otol Neurotol. 2011;32(8):1389–1395. doi: 10.1097/MAO.0b013e31822e5b8d. [DOI] [PubMed] [Google Scholar]
- 3.Wadin K, Thomander L, Wilbrand H. Effects of a high jugular fossa and jugular bulb diverticulum on the inner ear. A clinical and radiologic investigation. Acta Radiol Diagn (Stockh) 1986;27(6):629–636. doi: 10.1177/028418518602700603. [DOI] [PubMed] [Google Scholar]
- 4.Rauch S D, Xu W Z, Nadol J B Jr. High jugular bulb: implications for posterior fossa neurotologic and cranial base surgery. Ann Otol Rhinol Laryngol. 1993;102(2):100–107. doi: 10.1177/000348949310200204. [DOI] [PubMed] [Google Scholar]
- 5.Hourani R, Carey J, Yousem D M. Dehiscence of the jugular bulb and vestibular aqueduct: findings on 200 consecutive temporal bone computed tomography scans. J Comput Assist Tomogr. 2005;29(5):657–662. doi: 10.1097/01.rct.0000175499.34213.5d. [DOI] [PubMed] [Google Scholar]
- 6.Friedmann D R, Le B T, Pramanik B K, Lalwani A K. Clinical spectrum of patients with erosion of the inner ear by jugular bulb abnormalities. Laryngoscope. 2010;120(2):365–372. doi: 10.1002/lary.20699. [DOI] [PubMed] [Google Scholar]
- 7.Gopen Q, Zhou G, Poe D, Kenna M, Jones D. Posterior semicircular canal dehiscence: first reported case series. Otol Neurotol. 2010;31(2):339–344. doi: 10.1097/MAO.0b013e3181be65a4. [DOI] [PubMed] [Google Scholar]
- 8.Friedmann D R, Eubig J, Winata L S, Pramanik B K, Merchant S N, Lalwani A K. Prevalence of jugular bulb abnormalities and resultant inner ear dehiscence: a histopathologic and radiologic study. Otolaryngol Head Neck Surg. 2012;147(4):750–756. doi: 10.1177/0194599812448615. [DOI] [PubMed] [Google Scholar]
- 9.Wadin K, Wilbrand H. The jugular bulb diverticulum. A radioanatomic investigation. Acta Radiol Diagn (Stockh) 1986;27(4):395–401. doi: 10.1177/028418518602700405. [DOI] [PubMed] [Google Scholar]
- 10.Atilla S, Akpek S, Uslu S, Ilgit E T, Işik S. Computed tomographic evaluation of surgically significant vascular variations related with the temporal bone. Eur J Radiol. 1995;20(1):52–56. doi: 10.1016/0720-048x(95)00619-2. [DOI] [PubMed] [Google Scholar]
- 11.Bilgen C, Kirazli T, Ogut F, Totan S. Jugular bulb diverticula: clinical and radiologic aspects. Otolaryngol Head Neck Surg. 2003;128(3):382–386. doi: 10.1067/mhn.2003.32. [DOI] [PubMed] [Google Scholar]
- 12.Couloigner V, Grayeli A B, Bouccara D, Julien N, Sterkers O. Surgical treatment of the high jugular bulb in patients with Ménière's disease and pulsatile tinnitus. Eur Arch Otorhinolaryngol. 1999;256(5):224–229. doi: 10.1007/s004050050146. [DOI] [PubMed] [Google Scholar]
- 13.Redfern R E, Brown M, Benson A G. High jugular bulb in a cohort of patients with definite Ménière's disease. J Laryngol Otol. 2014;128(9):759–764. doi: 10.1017/S0022215114001820. [DOI] [PubMed] [Google Scholar]
- 14.Schmerber S, Lefournier V, Lavieille J P, Boubagra K. Endolymphatic duct obstruction related to a jugular bulb diverticulum: high resolution CT and MR imaging findings. Clin Radiol. 2002;57(5):424–428. doi: 10.1053/crad.2001.0919. [DOI] [PubMed] [Google Scholar]
- 15.Park J J Shen A Keil S Kuhl C Westhofen M Jugular bulb abnormalities in patients with Meniere's disease using high-resolution computed tomography Eur Arch Otorhinolaryngol 2014; March 20 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 16.Hollinshead W H. Hagerstown, MD: Harper & Row; 1982. Anatomy for Surgeons: The Head and Neck. [Google Scholar]
- 17.Schuknecht H R. Philadelphia, PA: Lea and Febiger; 1993. Pathology of the Ear. 2nd ed. [Google Scholar]
- 18.Levine S B, Snow J B Jr. Pulsatile tinnitus. Laryngoscope. 1987;97(4):401–406. doi: 10.1288/00005537-198704000-00001. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Otolaryngology-Head and NeckFoundation . Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere's disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113(3):181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 20.Friedmann D R, Eubig J, Winata L S, Pramanik B K, Merchant S N, Lalwani A K. A clinical and histopathologic study of jugular bulb abnormalities. Arch Otolaryngol Head Neck Surg. 2012;138(1):66–71. doi: 10.1001/archoto.2011.231. [DOI] [PubMed] [Google Scholar]
- 21.Margolis R H, Rieks D, Fournier E M, Levine S E. Tympanic electrocochleography for diagnosis of Menière's disease. Arch Otolaryngol Head Neck Surg. 1995;121(1):44–55. doi: 10.1001/archotol.1995.01890010032007. [DOI] [PubMed] [Google Scholar]
- 22.Adams M E, Heidenreich K D, Kileny P R. Audiovestibular testing in patients with Meniere's disease. Otolaryngol Clin North Am. 2010;43(5):995–1009. doi: 10.1016/j.otc.2010.05.008. [DOI] [PubMed] [Google Scholar]


