Abstract
Objectives Endovascular embolization of the middle meningeal artery (MMA) is currently considered one of the basic methods to treat acute bleeding and a posttraumatic aneurysm. The present research correlates the morphological characteristics of the MMA with individual skull shape.
Design A prospective cohort study.
Setting Hospital of University of Jordan (Amman, Jordan) from 2012 to 2013.
Participants A total of 50 patients without known vascular pathology in the carotid system underwent routine magnetic resonance angiography examination of the head and neck.
Main Outcome Measures The length and outer diameter of extracranial, intraosseous, and intracranial segments of the MMA were measured in patients with dolichocephalic, mesocephalic, and brachycephalic types of skulls.
Results The brachycephalic patients have the most inauspicious anatomical precondition for endovascular intervention of the MMA due to the narrowest lumen of the vessel, high probability of a tortuous extracranial part, and pronounced inflexion at the transmission of the intraosseous segment to the intracranial one.
Conclusions The morphological characteristics of the MMA have a close correlation with individual skull shape.
Keywords: middle meningeal artery, endovascular embolization, posttraumatic aneurism
Introduction
The middle meningeal artery (MMA) supplies a sizable part of the dura matter in the frontal, parietal, and temporal regions.1 It arises in the infratemporal fossa, branching off from the maxillary artery that is a terminal branch of the external carotid artery. Then it passes through the spinous foramen in the skull base and bends forward off its terminal dural branches.2
Due to its anatomical peculiarity, the MMA is comparatively exposed in the temporal region, which makes it quite vulnerable to outer injuries of the skull that result in acute epidural bleeding or the formation of a posttraumatic pseudoaneurysm.3 4 Studies by Vitorino et al revealed that the inefficient aneurismal wall has a strong tendency to rupture, carrying a great risk of delayed hemorrhage with a mortality rate up to 20%.5 According to Wang et al, early diagnosis and treatment beforehand of the posttraumatic pseudoaneurysm of the MMA are the most significant factors affecting mortality.6
During the last decade, conventional surgical options such as ligation of the MMA, coagulation of the vessel, or its resection were improved thanks to interventional minimally invasive methods of angioplasty and catheter-delivered stenting.7 The endovascular access through the common carotid artery allows any extra and intracranial vessels to be accessed without a preceding craniotomy and proper preoperative assessment. Studies by Suzuki et al revealed that endovascular embolization of the MMA is now considered the most efficient way to stop acute bleeding.8 Furthermore, earlier reports revealed that the best solution for prompt treatment of the endovascular obstruction of traumatic pseudoaneurysm is to deploy an occlusion device across it.9 10 Due to the last innovation proposed by Wallace et al,11 the occlusive treatment of arterial aneurisms by stent implantation is possible even for vessels with an internal diameter of ∼ 1 to 5 mm. To choose an appropriate tool and method of endovascular intervention, understanding the anatomical features and variability of the MMA is crucial.
Previous research concentrated mainly on the position and distribution of the branches of the intracranial segment of the MMA, providing mean values and ranges of their distribution for different age classes, sexes, and hemispheres.12 13 But the variability of the extradural parts of the MMA subject to skull shape was not described adequately.
The goal of our research was to determine and characterize the range of anatomical variability of the main trunk of the MMA along its extracranial (EC), interosseous (IO), and intracranial (IC) segments in correlation with individual skull features.
Materials and Methods
Fifty patients without known vascular pathology in the carotid system were included in our study (24 men and 26 women; mean age: 48 years). The patients underwent routine magnetic resonance angiography (MRA) examination of the head and neck in 2012–2013 under the direct supervision of one of the authors and in accordance with ethical standards.
Magnetic resonance (MR) images of the head had been acquired on a 3-T Magnetom Trio (Siemens AG, Germany). Twenty consecutive sagittal T1-weighted images of the head were taken (TR/TE, 260/2.6) for each patient. Images were obtained using a 6-mm slice thickness and 23-cm field of view (FOV) with a 0.10 distant factor. The axial slices of the head were scanned parallel to the axis of the lateral ventricle. The longitudinal diameter (LD) and transverse diameter (TD) of the skull were measured on the sagittal and transverse MR images of the head as the lengths between the most distant points of the skull bones (Figs. 1 and 2). The dimensions were equal to the distances from the glabella to the opisthocranion and from the euryon to euryon, respectively. The cranial index (CI) was counted as the ratio of TD to LD percentagewise according to standard anatomical descriptions.14 15 Then the studied group was divided into three subgroups: (1) dolichocephalic with a narrow skull (CI < 74.9%), (2) mesocephalic with a proportional skull (CI range: 75–79.9%), and (3) and brachycephalic with a wide skull (CI > 80%). The anatomical measurements of the artery and following statistical values were computed in each group separately with detection of the marginal forms for each concerned dimension.
Fig. 1.
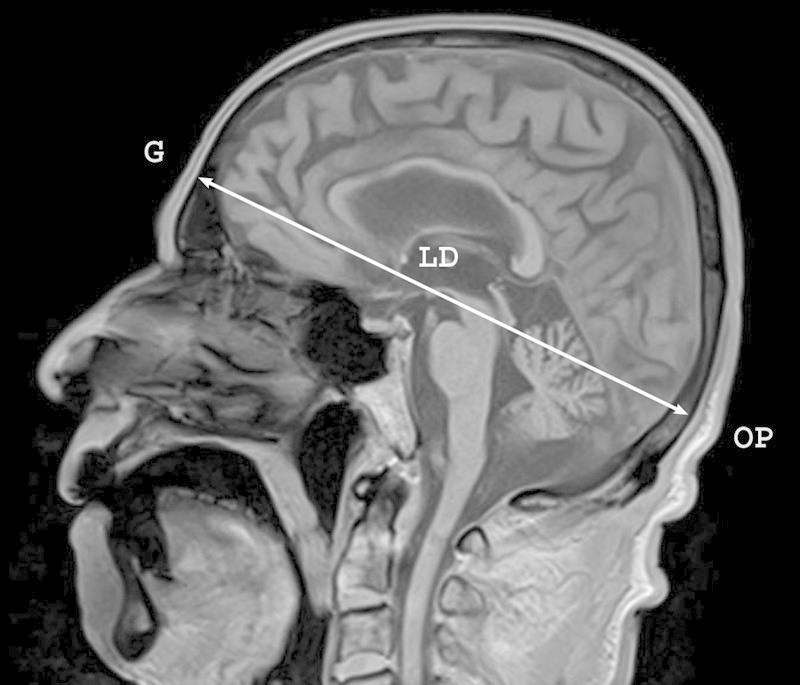
Craniological measurement of the longitudinal diameter (LD) of the skull performed on the sagittal magnetic resonance image of the head. G, glabella; OP, opisthocranion.
Fig. 2.
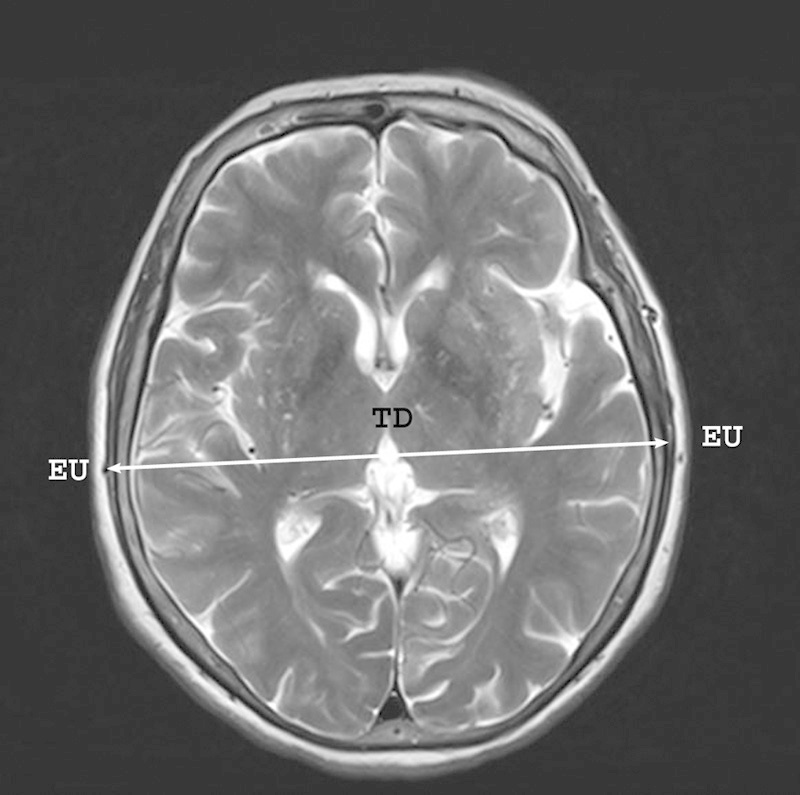
Craniological measurement of the transverse diameter of the skull performed on the transverse magnetic resonance image of the head. EU, euryon; TD, transverse diameter.
MRA was performed with the patients' heads in the neutral position. After injection of a low-osmolarity iodinated contrast material through a peripheral vein, the fast low-angle shot three-dimensional (3D) sequence scanning from the head to the aortic arch was triggered automatically 3 seconds later. The parameters for MRA acquisition were a 1.4-mm slice thickness, TR/TE, 3.2/1.2, and 34 cm FOV.
On the obtained MRA images, the course and morphology of the MMA were examined with members of the radiology department. On the anteroposterior view angiograms, the EC, IO, and IC segments of the MMA were considered according to their topography with measurements recorded of the length and outer diameter of each segment (Fig. 3). The length of the IC segment was measured in a direct line from the inner orifice of the spinous foramen to the point of the main trunk bifurcation. The morphological characteristics of each segment of the MMA were estimated separately in patients with narrow, wide, and normal shaped skulls.
Fig. 3.
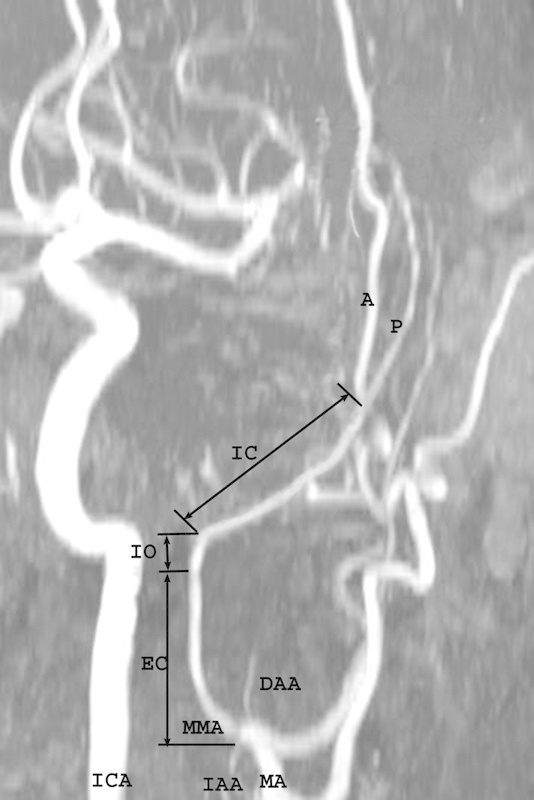
Magnetic resonance angiography of the head and neck demonstrating the segmentation of the medial meningeal artery (MMA) into the extracranial (EC), interosseous (IO), and intracranial (IC) segments according to topography of the vessel. A, anterior dural branch of the MMA; DAA, deep auricular artery; IAA, inferior alveolar artery; ICA, internal carotid artery; MA, maxillary artery; P, posterior dural branch of the medial meningeal artery.
Definitive analysis of the data obtained was performed using SPSS for Windows v.10.1. The linear regression and correlation analyses with the Student paired t tests were performed to investigate the correlation of each parameter with the CI. A p value ≤ 0.05 was considered statistically significant.
Results
The extracranial part (EC) of the MMA runs from the point of its origin up and medially reaching the external surface of the skull base. In most cases, the EC segment had a straight course, but the sinuous curves along its extension were recorded in 4% of the dolichocephalic patients; some abnormally tortuous vessels were registered in 8% of cases in the brachycephalic group (Figs. 4 5 6). According to our findings, the mean value of the EC length was 20.9 ± 3.66 mm without statistically significant side differences (Table 1). The dolichocephalic group of patients possessed the maximal length of the segment (21.46 ± 0.57 mm); the lowest value was found in the brachycephalic group (20.23 ± 0.68 mm). The mean outer diameter of the EC part of the MMA was equal to 2.7 ± 0.64 mm with a slight prevalence on the left. Subject to the CI, the parameter was larger in the mesocephalic study group (2.96 ± 0.36 mm) and was minimal in the brachycephalic one (2.38 ± 0.3 mm) (Table 2).
Fig. 4.
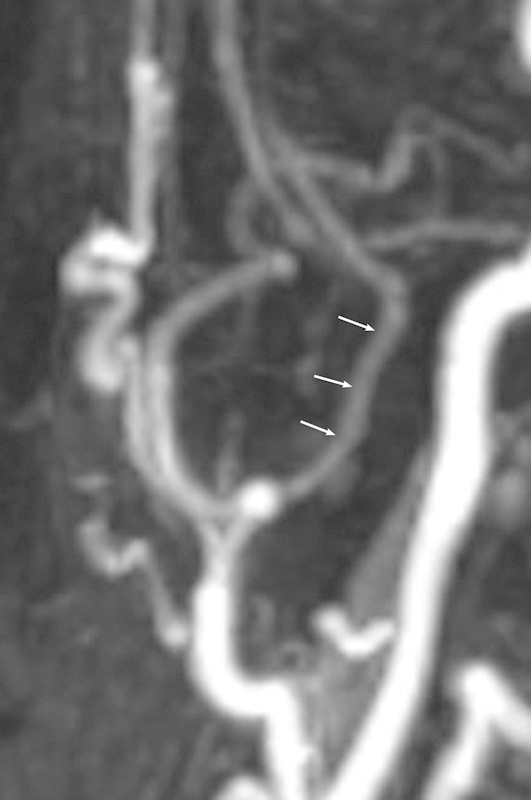
Magnetic resonance angiography of the head and neck demonstrates the extracranial segment of the medial meningeal artery in a dolichocephalic patient. The arrows point to sinuous curves along its length.
Fig. 5.
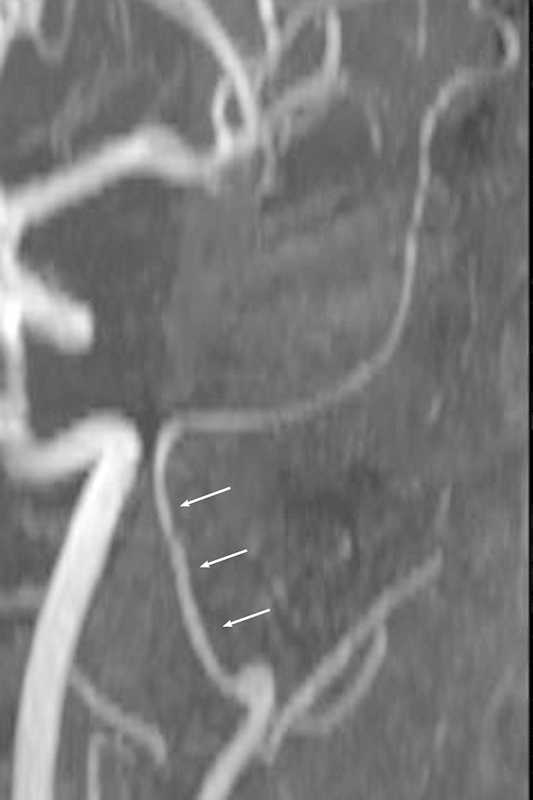
Typical view of the extracranial part of the medial meningeal artery in the mesocephalic group (arrows).
Fig. 6.
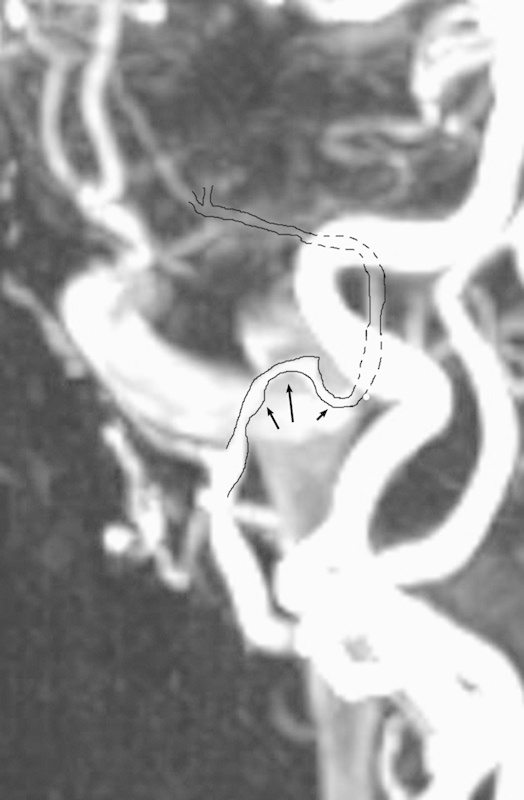
Magnetic resonance angiography of the head and neck demonstrating the abnormally tortuous extracranial segment of the medial meningeal artery shown in a brachycephalic patient (arrows).
Table 1. Quantitative characteristics of the extracranial, interosseous, and intracranial parts of the middle meningeal arterya .
| EC | IO | IC | ||||
|---|---|---|---|---|---|---|
| Length | Diameter | Length | Diameter | Length | Diameter | |
| Left | 20.9 ± 3.66* | 2.91 ± 0.72** | 4.83 ± 0.06* | 2.47 ± 0.53** | 30.74 ± 10.93NS | 2.38 ± 0.5* |
| Right | 21.3 ± 2.75* | 2.61 ± 0.62** | 4.79 ± 0.04** | 2.3 ± 0.48* | 30.86 ± 20.46NS | 2.17 ± 0.43** |
Abbreviations: EC, extracranial, IC, intracranial; IO, interosseous.
Depending on body side, mean values, and ranges of their distribution (standard deviation), mm.
Significance levels: ***p < 0.001; ** p < 0.01; * p < 0.5; NS, not significant.
Table 2. Diameters and lengths of the extracranial, interosseous, and intracranial segments of the middle meningeal artery subject to shape of skull and sidea .
| EC | IO | IC | ||||
|---|---|---|---|---|---|---|
| Diameter | Length | Diameter | Length | Diameter | Length | |
| Dolichocephalic CIb ≤ 74.9% | 2.77 ± 0.54** | 21.46 ± 0.57* | 2.46 ± 0.31** | 5.62 ± 0.15* | 2.33 ± 1.13 ** | 30.43 ± 20.48NS |
| Mesocephalic CIb (75–79.9%) | 2.96 ± 0.36* | 21.24 ± 1.04* | 2.61 ± 0.48** | 4.73 ± 0.18* | 2.45 ± 0.52*** | 20.76 ± 20.83NS |
| Brachycephalic CIb ≥ 80% | 2.38 ± 0.3** | 20.23 ± 0.68** | 2.12 ± 0.44*** | 4.13 ± 0.21** | 2.04 ± 0.43** | 30.82 ± 10.86NS |
Abbreviations: CI, cranial index; EC, extracranial, IC, intracranial; IO, interosseous.
Mean (μ) ± SD (σn-1), mm.
Confidence interval for CI (0.9140–0.9464) at p ≤ 0.05 significance level.
Significance levels: ***p < 0.001; ** p < 0.01; * p < 0.5; NS, no statistical significance.
The intraosseous part (IO) of the MMA extends between the external and internal orifices of the spinous foramen of the greater wing of the sphenoid bone passing through it. This segment possesses two laterally directed inflection points at the EC-IO and IO-IC transmissions; those help distinguish it on angiograms (Fig. 7). The IC inflection exhibits varying curvature magnitude from a gentle bend in the dolichocephalic group to an abrupt curve in the brachycephalic one. The mean outer diameter of the IO varied from 2.3 to 2.47 mm with the greater value on the left (Table 1). The former parameter gained its superior limit in the mesocephalic group (2.61 ± 0.48 mm) and showed the inferior limit in the brachycephalic group (2.12 ± 0.44 mm). The mean length of the IO segment of the MMA extended from 4.1 up to 5.6 mm (4.82 ± 0.75 mm) with the maximum value in the dolichocephalic study group and the minimal value in the brachycephalic group (Table 2).
Fig. 7.
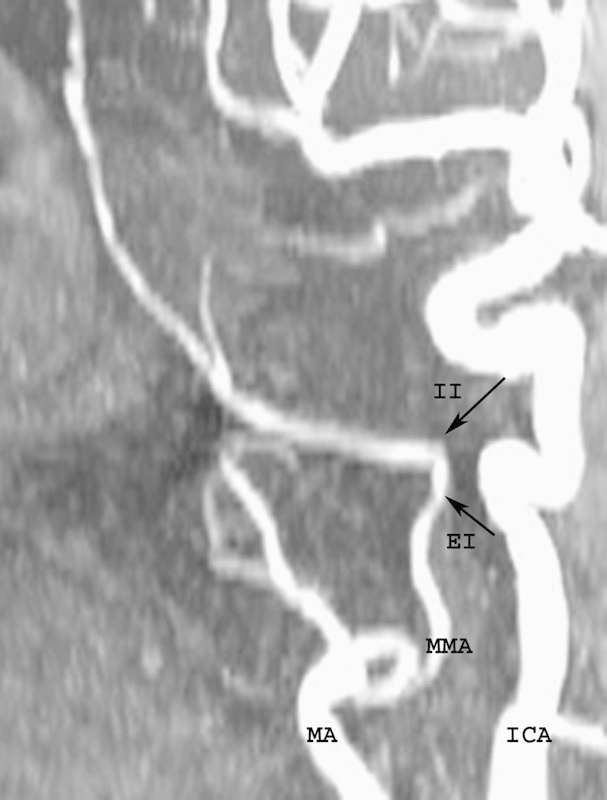
Magnetic resonance angiography of the head and neck demonstrating the Interosseous segment (IO) of the medial meningeal artery (MMA) bounded by extracranial and intracranial lateral inflections (EI and II, respectively). ICA, internal carotid artery; MA, maxillary artery.
The intracranial segment (IC) of the MMA upon leaving the foramen spinosum passes at first outward, either directly or inclined somewhat backward or forward. The segment had a gentle outward curve in the patients with a narrow shaped skull. In the mesocephalic study group, the bending much more decidedly approximated a right angle, whereas in the brachycephalic group it was even greater than a right angle (Figs. 8 9 10). The measures of the IC segment revealed a decrease of the vessel's outer diameter up to 2.27 ± 0.16 mm in comparison with the width of the IO and EC segments (Table 1). The greater value was found on the left and in the mesocephalic tested group (2.45 ± 0.52 mm); the minimal value of the sign was detected in the brachycephalic group (2.04 ± 0.43 mm) (Table 2). The range of the length of the IC segment was distributed in a wide range from 1.8 to 5.6 cm without a statistically significant correlation either with the right-left side or the shape of the skull.
Fig. 8.
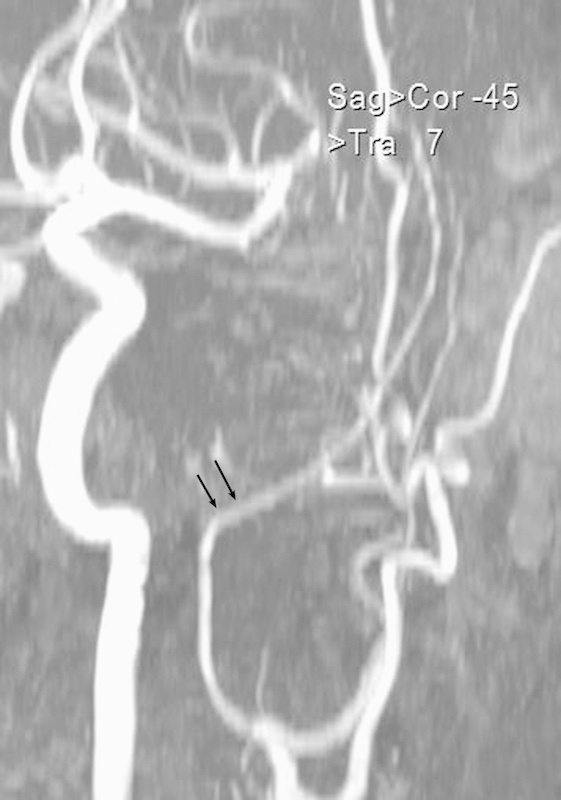
Typical view of the intracranial inflection of the medial meningeal artery observed in the dolichocephalic studied group (arrows).
Fig. 9.
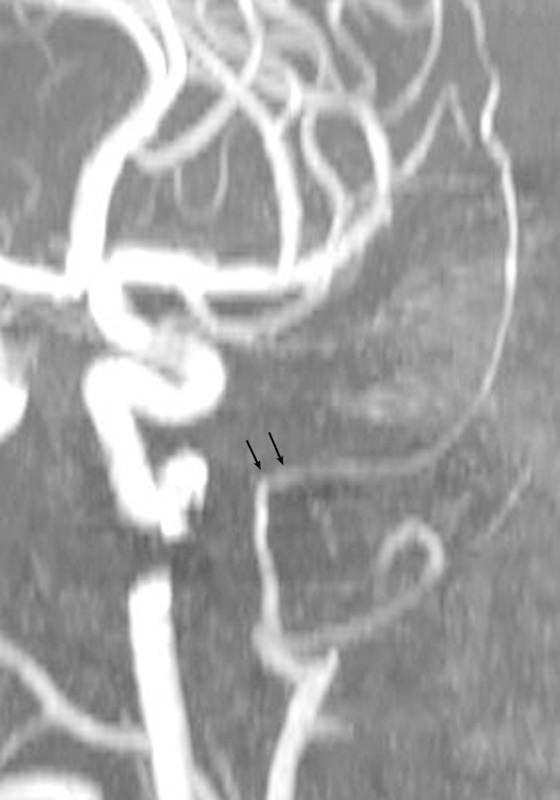
Typical view of the intracranial inflection of the medial meningeal artery observed in the mesocephalic studied group (arrows).
Fig. 10.
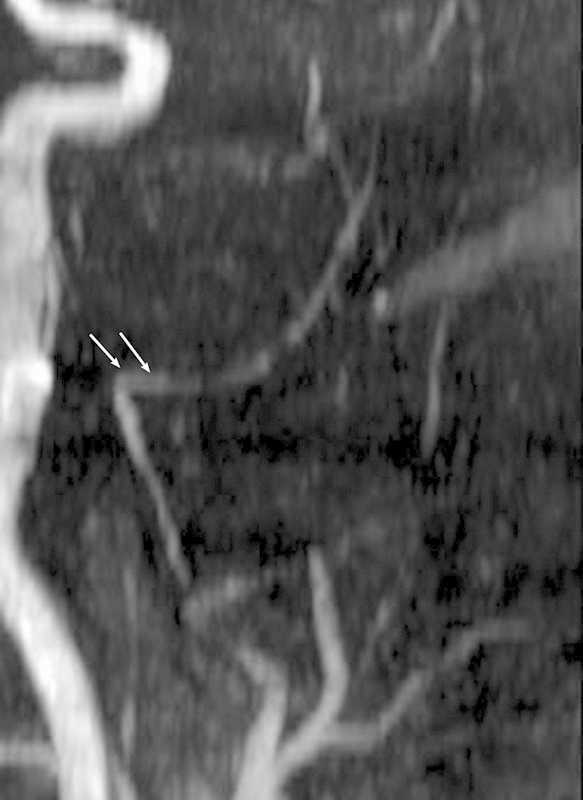
Typical view of the intracranial inflection of the medial meningeal artery observed in the brachycephalic studied group (arrows).
Influence of the age factor vis-à-vis the diameter of the vessel was analyzed in each study group (Table 3). In the dolichocephalic group the mean diameter of the EC varied from 2.69 ± 0.21 mm in patients 35 to 44 years of age to 2.78 ± 0.3 mm in patients ≥ 84 years; the minimal width of the IC segment of the MMA was disclosed in the age group from 65 to 74 years. Among the mesocephalic patients the narrowest IO segment of the artery was also found in this age group, whereas the diameter of the IC segment was distributed from 2.48 ± 0.22 mm in the youngest patients (18–34 years) to 2.46 ± 0.16 mm in the ≥ 84 years age group. The distribution of the diameter of the EC segment among the different age groups of patients had a sinusoidal pattern. The brachycephalic patients had an EC width gradually decreasing from 2.37 ± 0.32 mm to 2.26 ± 0.35 mm along the age line with an unexpected increment up to 2.38 ± 0.17 mm in the patients > 84 years of age. Analysis of the IO and IC diameters in different age categories did not show any unidirectional tendency in this craniological group (Table 3).
Table 3. Age distribution of diameters of the extracranial, interosseous, and intracranial parts of the middle meningeal arterya .
| Age, y | 18–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–84 | 84–95 |
|---|---|---|---|---|---|---|---|
| Dolichocephalic; CI 1 ≤ 74.9% | |||||||
| n | 0 | 2 | 3 | 4 | 6 | 2 | 2 |
| EC | – | 2.69 ± 0.21 | 2.73 ± 0.15 | 2.67 ± 0.27 | 2.77 ± 0.09 | 2.93 ± 0.25 | 2.78 ± 0.3 |
| IO | – | 2.28 ± 0.27 | 2.31 ± 0.15 | 2.46 ± 0.18 | 2.52 ± 0.32 | 2.82 ± 0.22 | 2.35 ± 0.11 |
| IC | – | 2.32 ± 0.24 | 2.38 ± 0.18 | 2.43 ± 0.35 | 2.18 ± 0.22 | 2.46 ± 0.14 | 2.22 ± 0.25 |
| Mesocephalic; CI 1 (75–79.9%) | |||||||
| n | 1 | 2 | 1 | 4 | 4 | 2 | 0 |
| EC | 2.84 ± 0.32b | 3.15 ± 0.21 | 2.97 ± 0.13 | 2.94 ± 0.28 | 2.73 ± 0.31 | 3.14 ± 0.43 | – |
| IO | 2.63 ± 0.16 | 2.75 ± 0.32 | 2.63 ± 0.24 | 2.81 ± 0.31 | 2.27 ± 0.22 | 2.62 ± 0.24 | – |
| IC | 2.48 ± 0.22 | 2.46 ± 0.16 | 2.44 ± 0.21 | 2.43 ± 0.33 | 2.53 ± 0.27 | 2.46 ± 0.16 | – |
| Brachycephalic; CI 1 ≥ 80% | |||||||
| n | 0 | 1 | 4 | 4 | 6 | 2 | 0 |
| EC | – | 2.37 ± 0.32 | 2.44 ± 0.33 | 2.33 ± 0.41 | 2.26 ± 0.35 | 2.38 ± 0.17 | – |
| IO | – | 2.14 ± 0.25 | 1.98 ± 0.17 | 2.17 ± 0.47 | 1.96 ± 0.65 | 1.97 ± 0.38 | – |
| IC | – | 2.01 ± 0.26 | 2.04 ± 0.16 | 2.13 ± 0.23 | 1.84 ± 0.14 | 2.02 ± 0.27 | – |
Abbreviations: CI, cranial index; EC, extracranial, IC, intracranial; IO, interosseous.
Mean values and dispersions, mm.
Difference of the diameters on the right and left.
During the estimation of the tortuosity of the different segments of the MMA, particular attention was paid to the EC part that is unfixed by the dura matter. In the dolichocephalic patients, gentle curves along this part emerged sporadically in the 45 to 54 age groups and also from 65 to 75 years. In the mesocephalic studied group, the trunk of MMA including its EC part was relatively straight even in the oldest patients. Ascertained evidence of pathologically tortuous courses of the EC segment primarily appeared in brachiocephalic patients from 55 to 64 years of age (25%); however, the cases were concentrated in the patients > 65 years (75%).
Discussion
The MMA is the branch of the retromandibular part of the maxillary artery, one of the two terminal branches of the external carotid artery. The extracranial origin and direct course of the MMA to the cranial cavity make it an irreplaceable endovascular access to any of its intracranial dural branches, avoiding the risks of potential complication of open cranial surgery.5
Analysis of the recent clinical reports shows that the rate of acute epidural hematomas and posttraumatic pseudoaneurysms of the MMA treated by nonconventional endovascular therapy is steadily increasing.8 9 A wide range of efficient radiologic techniques and devices, such as 3D coils, balloons, and stent-assisted coiling, are available for the treatment of intracranial vascular pathology today. According to the recommendations of Wallace et al, the intracranial delivery catheter for endovascular treatment should be inserted via the aorta into the common carotid, external carotid, maxillary, and into the MMA passing through its extracranial and intraosseous parts and actually reaching the intracranial trunk and its dural branches.11 This effectual and facile procedure requires that specialists have a detailed understanding of the anatomy and possible variations of the touched vessels.
We assume that close interrelation of the cranial mesenchymal derivatives during antenatal development will lead to ascertainment of certain correspondences between the variability of skull and adjacent vascular structures in adults.16 Therefore, certain types of the skull constitution correlate with a definite variety of the head's vascular bed making vascular variability foreseeable for specialists. The variability of the MMA along the EC, IO, and IC lengths and its correlation with the individual shape of the skull is not described in the literature.
According to our findings, the dolichocephalic shape of the skull is associated with elongation of the extradural part of the MMA that sometimes has sinuous curves on its EC extension. Surprisingly, the vessel did not have any constrictions at the point of crossing the skull base, and its outer diameter gradually decreased from the EC segment to the IC segment with the medial value at its IO part. Independently from its length, the IC segment possesses a mild outward curve reflecting an inner relief of the skull base. The EC-IO and IO-IC transitions were presented as broad curves that are absolutely favorable for bringing an endovascular delivery system into the cranial cavity.
Regarding the greatest outer diameters and the moderate values of the lengths of all segments of the artery, the patients with a mesocephalic shaped skull have the most providential anatomical conditions for endovascular treatment of the pathology on the MMA. Generally, the vessel had a straight course along the EC extension with a slight external deviation at the crossing of the skull. However, its IC segment bent at a right angle laterally entering the cranial cavity.
Thanks to recent technical developments, the 90-degree flexion at the IO-IC point should not impede delivery of the endovascular devices into the cranial cavity, but the specialist should be careful to reduce the risk of iatrogenic damages at the crossing of the IO segment of the artery during endovascular procedures.
The brachycephalic group of patients is characterized by the most negative anatomical precondition, such as the narrowest lumen of the vessel along all its length, the number of pathologically tortuous courses of its EC segment, and the sharp intracranial inflection at the IOIC transmission. These facts allow defining this type of constitution as undesirable for the interventional manipulations on the MMA, and preference should be given to conventional surgical methods. In addition, studies by Bullitt et al revealed that the brachycephalic type of constitution is associated with a high risk of thrombosis and tearing of the artery.17
It was proved in previous research that aging is associated with various changes in the vascular system such as increased vascular calcification, generalized stiffening of the arterial tree, and narrowing of the vascular lumen.18 19 Virmani et al showed that the thickness of the intimal layer of the vascular wall increases threefold between 20 and 90 years of age, which is an important cardiovascular risk factor.20 Because most of the investigated patients in our study were > 45 years, the age factor did not exert a significant influence on the diameter distribution in each craniological group. However, all pathologically tortuous EC segments were found in patients > 55 years.
The extremely wide range of distribution of the length of the IC segment of the MMA found in all our studied groups confirms the data repeated in the previous researches.2 12 17 The high anatomical variability of the intradural part of the MMA should be taken into account by experimenters such as Ustün et al proposing the revascularization technique such as MMA-to-petrous internal carotid artery bypass.21
A final point is that we recommend that dolichocephalic and mesocephalic patients with acute or chronic pathology along the MMA or its dural branches be treated with interventional minimally invasive methods of angioplasty and catheter-delivered stenting, as opposed to the patients with a brachycephalic shaped skull. All patients with a wide skull should undergo thorough investigation on the brain vascular bed and be treated conventionally.
Thus the morphological characteristics of the MMA have a close correlation with the individual shape of the skull. Estimating the shape of the skull offers involved specialists valuable information, helping them predict probable types of variability of the MMA and foresee the possibility of the manipulation and risk of iatrogenic trauma at the early diagnostic stage. This is especially important for emergent patients with acute epidural bleeding requiring prompt lifesaving solutions.
References
- 1.Mantini S, Bruner E, Colaiacomo B. et al. The anatomical variability and the functional role of the middle meningeal artery. Ital J Anat Embryol. 2010;115:101. [Google Scholar]
- 2.Talib J A. Anatomical variation in the course, branches, and metrical measurement of the grooves, of the middle meningeal artery inside the skull. Tikrit Medical Journal. 2009;15(2):38–41. [Google Scholar]
- 3.Bruneau M Gustin T Zekhnini K Gilliard C Traumatic false aneurysm of the middle meningeal artery causing an intracerebral hemorrhage: case report and literature review Surg Neurol 2002573174–178.; discussion 178 [DOI] [PubMed] [Google Scholar]
- 4.Babu M L, Bhasin S K, Kumar A. Extradural hematoma—an experience of 300 cases. JK Science. 2005;7(4):205–207. [Google Scholar]
- 5.Araujo J L, Aguiar UdoP, Todeschini A B, Saade N, Veiga J C. Epidemiological analysis of 210 cases of surgically treated traumatic extradural hematoma. Rev Col Bras Cir. 2012;39(4):268–271. doi: 10.1590/s0100-69912012000400005. [DOI] [PubMed] [Google Scholar]
- 6.Wang C H Lee H C Cho D Y Traumatic pseudoaneurysm of the middle meningeal artery: possible indicators for early diagnosis in the computed tomography era Surg Neurol 2007686676–681.; discussion 681–682 [DOI] [PubMed] [Google Scholar]
- 7.Kiyosue H, Hori Y, Okahara M. et al. Treatment of intracranial dural arteriovenous fistulas: current strategies based on location and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics. 2004;24(6):1637–1653. doi: 10.1148/rg.246045026. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki S, Endo M, Kurata A. et al. Efficacy of endovascular surgery for the treatment of acute epidural hematomas. AJNR Am J Neuroradiol. 2004;25(7):1177–1180. [PMC free article] [PubMed] [Google Scholar]
- 9.Shah Q A Hurst R W Endovascular treatment of a traumatic pseudo aneurysm of the middle meningeal artery Available at: http://radiology.casereports.net/index.php/rcr/article/view/22/171 [DOI] [PMC free article] [PubMed]
- 10.Bortoluzzi M, Pavia M. Endovascular treatment of incoercible epistaxis and epidural cerebral hematoma. A case report. Interv Neuroradiol. 2006;12(3):233–236. doi: 10.1177/159101990601200305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace G Clemente S Lenker J et al. Intracranial stent and method of use. US patent 0243224 A1 October 2, 2008
- 12.Mantini S, Bruner E, Colaiacomo B. et al. An angio-tomographic approach to the study of the variation of the middle meningeal artery in humans. Ital J Anat Embryol. 2011;116(1):110. [Google Scholar]
- 13.Klisovic D, Sikic E, Krmpotic-Nemanic J. Variations of the middle meningeal artery: Significance for surgery and practice. Clin Anat. 1993;6(5):289–294. [Google Scholar]
- 14.Banister M. London, UK: Elbs with Churchill Livingston; 1995. Skeletal system; pp. 607–612. [Google Scholar]
- 15.Kolar J C, Salter E M. Springfield, IL: Charles C Thomas; 1997. Craniofacial Anthropometry: Practical Measurements of the Head and Face for Clinical, Surgical and Research Use. [Google Scholar]
- 16.Kornieieva M A. Kharkov, Ukraine: Kharkiv National Medical University; 2007. Morphological Interrelations of the Dural Sinuses of the Skull Base with the Venous Plexuses of Face in the Early Period of Human Ontogenesis [PhD dissertation] [Google Scholar]
- 17.Bullitt E, Gerig G, Pizer S M, Lin W, Aylward S R. Measuring tortuosity of the intracerebral vasculature from MRA images. IEEE Trans Med Imaging. 2003;22(9):1163–1171. doi: 10.1109/TMI.2003.816964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jani B, Rajkumar C. Ageing and vascular ageing. Postgrad Med J. 2006;82(968):357–362. doi: 10.1136/pgmj.2005.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacic J C, Moreno P, Nabel E G, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123(17):1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- 20.Virmani R, Avolio A P, Mergner W J. et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139(5):1119–1129. [PMC free article] [PubMed] [Google Scholar]
- 21.Ustün M E, Büyükmumcu M, Seker M, Karabulut A K, Uysal I I, Ziylan T. Possibility of middle meningeal artery-to-petrous internal carotid artery bypass: an anatomic study. Skull Base. 2004;14(3):153–156. doi: 10.1055/s-2004-832258. [DOI] [PMC free article] [PubMed] [Google Scholar]


