Abstract
The global prevalence of renal transplants is increasing with time, and renal transplantation is the only definite treatment for end-stage renal disease. We have limited the acute and late acute rejection of kidney allografts, but the long-term survival of renal tissues still remains a difficult and unanswered question as most of the renal transplants undergo failure within a decade of their transplantation.
Among various histopathological changes that signify chronic allograft nephropathy (CAN), tubular atrophy, fibrous thickening of the arteries, fibrosis of the kidney interstitium, and glomerulosclerosis are the most important. Moreover, these structural changes are followed by a decline in the kidney function as well. The underlying mechanism that triggers the long-term rejection of renal transplants involves both humoral and cell-mediated immunity. T cells, with their related cytokines, cause tissue damage. In addition, CD 20+ B cells and their antibodies play an important role in the long-term graft rejection. Other risk factors that predispose a recipient to long-term graft rejection include HLA-mismatching, acute episodes of graft rejection, mismatch in donor-recipient age, and smoking.
The purpose of this review article is the analyze current literature and find different anti-proliferative agents that can suppress the immune system and can thus contribute to the long-term survival of renal transplants. The findings of this review paper can be helpful in understanding the long-term survival of renal transplants and various ways to improve it.
Keywords: renal transplant, rejection, drug targets
Introduction and background
Kidney transplantation is the only effective treatment option for managing end-stage renal disease. According to statistics, 75,000 kidney transplants were done globally in the year 2010, and these statistics are expected to rise to 350,000 (almost three to four times the baseline value) in the coming years [1]. Figure 1 depicts the kidney transplantation activities of 2012; Figure 2 depicts the region-wise rate of kidney transplantation.
Figure 1. Kidney transplantation activities, 2012.
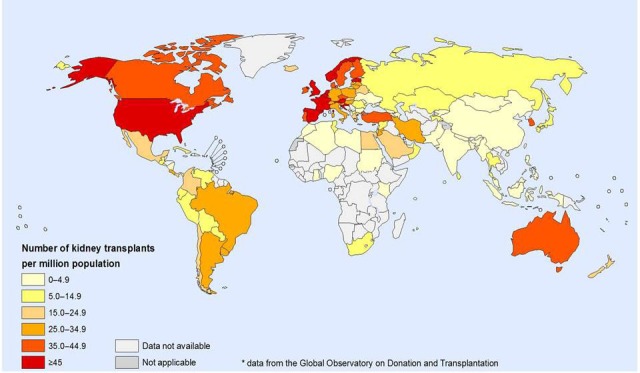
Data from Global Observatory on Donation and Transplantation (GODT) data, produced by the WHO-ONT collaboration
Figure 2. Kidney transplantation per region.
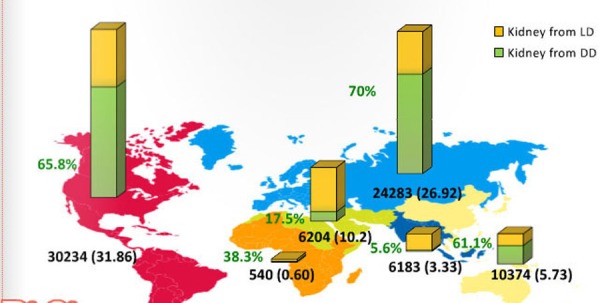
Data from Global Observatory on Donation and Transplantation (GODT) data, produced by the WHO-ONT collaboration
Transplant rejection is one of the biggest limitations in renal transplant procedures, where the kidney can undergo an acute, late acute, or chronic transplant rejection [2-3]. With the advancement in transplantation protocols, acute and long-term survival of renal transplants has improved [4], but long-term survival is still unsatisfactory. Acute transplant rejection has experienced a significant fall due to the use of immunosuppressant therapy, but most of the renal transplants develop chronic graft rejection within a decade [1, 5-6]. In addition to that, the long-term mortality rates among patients with renal transplants are observed to be significantly higher when compared to that seen in the general population [7]. Unfortunately, there is little we know when it comes to improving long-term survival of renal transplants. Therefore, time is of the utmost importance in understanding the underlying mechanism of long-term renal transplant rejection and explore different drug targets that can improve survival of both the graft and the patient.
Review
A successful kidney transplantation is a much better option to improve the quality and longevity of chronic and/or end-stage renal disease patients. This is especially true when dialysis is the only other option. Dialysis procedure is very time-consuming, expensive, and requires frequent hospital visits. Kidney transplantation is thus a viable alternative. However, transplantation also has a fair share of disadvantages, the major constraint being renal transplant rejection.
Histological and clinical features of chronic renal transplant rejection
Chronic renal transplant rejection is the result of a gradual decrease in the kidney function that starts to become evident three months after the transplantation surgery. Hypertension and proteinuria are the most important features of declining renal function [8-9]. Moreover, analysis of the serum creatinine concentration has shown that at least 80% of patients experience progressive loss of kidney function and start to exhibit signs of chronic allograft nephropathy (CAN) [10]. At least 50% of the patients with renal transplant develop features of CAN within 10 years of their transplant [11]. The major pathological features of CAN includes tubular atrophy, fibrous thickening of the arteries, fibrosis of the kidney interstitium, and glomerulosclerosis [12-13].
Transplant vasculopathy is the single most important feature of chronic renal transplant rejection [14]. Vasculopathy not only affects the large arteries but can also involve small peritubular capillaries [15]. The most important features of transplant vasculopathy include thickening of the fibrointima of the blood vessels, infiltration of the vessel walls with inflammatory cells, and breaks in the elastic layer of blood vessels. The subendothelial accumulation of smooth muscles in transplant vasculopathy was previously thought to be the result of migration of donor myofibroblasts from the media of the adjacent blood vessels. However, recent evidence has suggested that these smooth cells are from the recipient and are derived from the precursor cells present in the circulation [14-17]. The glomerular lesions seen in the biopsies obtained from the cases of CAN show wrinkling of the glomerular tuft of capillaries, focal glomerulosclerosis, hypertrophy of the glomeruli, and expansion of the mesangial matrix [18-20].
Transplant glomerulopathy can be distinguished from other forms of glomerulopathy like membranoproliferative glomerulonephritis (MPGN), based on the results of electron and immune-fluorescence testing. MPGN is characterized by electron-dense deposits, whereas the deposits seen in transplant glomerulopathy are electron lucent. Moreover, the immune deposits seen in MPGN patients are predominantly C3, whereas the main type of deposits in patients with transplant glomerulopathy is of the IgM type [21].
Risk factors for chronic renal transplant rejection
The risk factors for chronic renal transplant rejection are described in Table 1.
Table 1. Comparison of the major risk factors governing survival of renal transplants.
| Factors for Increased Graft Rejection | Survival of the Graft | Factors for Better Graft Survival | Survival of the Graft |
| Acute transplant rejection episodes | 6.6 years | No acute transplant rejection episodes | 12.5 years |
| Non- HLA matched grafts | 8.6 years | HLA matched graft | 12.4 years |
| Recipient age <14 years | Less chances of <5 years survival | Recipient age >14 years but <70 years | More chances of >5 years survival |
| Donor-Recipient Mismatch (Young recipient-Old donor) | 8.7 years | Donor-Recipient Match (Young recipient-Young donor) | 11.64 years |
| Black Race | 7.2 years | White Race | 13.3 years |
| Antibodies to both class 1 and class 2 HLA antigens (2 years survival) | 71% | Antibodies to either HLA class 1 or class 2 antigens (2 years survival) | 77% and 79% respectively |
Acute transplant rejection episodes
The long-term survival of renal transplants is relatively shorter in those patients who experience episodes of acute transplant rejection as compared to patients who do not experience such episodes. The mean survival in patients with acute rejection patients vs. patients with no such episodes is 6.6 years and 12.5 years, respectively [22].
Human leukocyte antigen (HLA) mismatching
Major histocompatibility complex (MHC) molecules on the transplanted kidney are the primary targets of the kidney recipient's immune system. Therefore, HLA matching can save a lot of transplants from rejection. The mean survival for the HLA-mismatched vs. HLA-matched transplants is 8.6 years and 12.4 years, respectively [23].
Sensitization to HLA antigen
Pre-sensitization to Class 1 and Class 2 HLA is another important risk factor for chronic transplant rejection. Patients who are positive for the antibodies against Class 1 and Class 2 HLA usually have a poor long-term survival rate [24-26]. The two-year survival rate in individuals positive for anti-HLA Class 1 antibodies, anti-HLA Class 2 antibodies, and both Class 1 and Class 2 HLA antibodies is 77%, (vs. 84% anti-HLA Class 1 antibody-negative individuals), 79% (vs. 84% anti-HLA Class 2 antibody-negative individuals) and 71% (vs. no deleterious effects in individuals with a well-matched kidney), respectively [27].
Recipient age
Younger recipients are more likely to suffer chronic renal transplant failure as compared to older individuals. Younger patients have a more responsive immune system and show poor compliance to immunosuppressive therapy [28-29]. A recent study has demonstrated that the five-year survival of grafts in younger children (< 14 years) is much lower when compared to that of individuals of other age groups [30].
Match of donor-recipient age
Better matching of donor-recipient age is another important factor that can improve the outcomes of renal transplant. The mean survival of renal transplants in younger recipients receiving transplants from younger donors is 11.64 years as compared to the mean survival of 8.7 years in younger patients who receive grafts from older donors [31].
Race
The chances of graft rejection are greater among blacks as compared to whites [32-33]. This difference is perhaps due to the difference in immune responsiveness among two groups [34]. The mean survival of grafts in blacks as compared to whites is 7.2 years and 13.3 years, respectively [4].
Other risk factors
Other factors that may increase the risk of chronic renal transplant failure include loss of renal function [35], hypertension [36], proteinuria one-year post-transplantation [37], hyperlipidemia [38], and smoking [39].
Pathophysiology of chronic renal transplant rejection
The fundamental component of chronic graft rejection is the detection of antigens on the donor’s tissues as “foreign entities” by the recipient’s immune system. However, the degree of immune reaction, and thereby degree and speed of graft rejection, depends on the histocompatibility between donor and recipient, as HLA matched grafts survive longer as compared to HLA-mismatched grafts [40-41]. The activation of the immune system involves two distinct pathways: the direct and the indirect pathway (Figure 3). The direct way involves the activation of CD4+ T cells by the donor’s antigen presenting cells (APC). The indirect pathway involves the processing of the donor’s graft antigens by recipient’s APC that then activates the immune cells [42]. The indirect activation can stimulate the activity of activated B cells, which then leads to the production of antibodies against the graft tissues. These antibodies seem to play a very important role in chronic graft rejection [43-44].
Figure 3. A scheme showing direct and indirect pathways for allograft rejection.
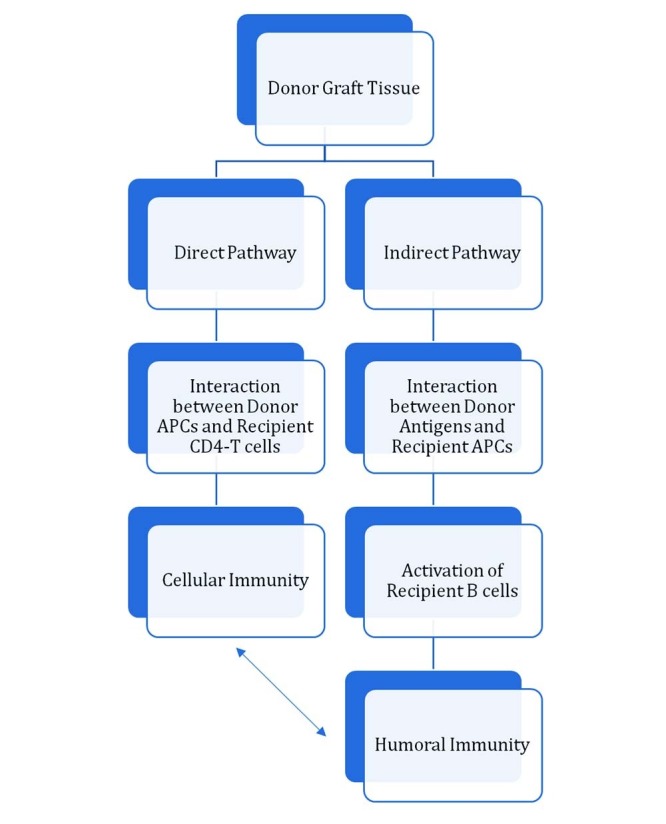
APCs: Antigen Presenting Cells
Cellular immunity has only a minor role in the chronic allograft rejection. Studies have shown that an indirect pathway is the main predictor for chronic allograft rejection [45-46]. The only role T cells play in chronic transplant rejection is via cytokines secreted by Type 2 helper lymphocytes (Th2). Several studies have shown that the cytokines secreted by Th2, like IL-4, IL-5, IL-6, IL-10, and IL-13, are responsible for reactions, such as tissue fibrosis and chronic rejection [47-48]. This fact has been further validated in studies where the injection of Th2 cells in immune-deficient recipients resulted in chronic graft rejection [49]. Blocking the effect of Th2-released cytokines can seemingly slow down the process of allograft rejection, which has been demonstrated through studies where the fibrosis in skin grafts was prevented via injection of anti-IL-4 antibodies [5]. The cytokines produced by Th2 cells causes allograft rejection through different ways. For instance, IL-4 stimulates the activity of fibroblast cells that increase the production of extracellular matrix and speed up the process of fibrosis. Similarly, a high titer of IL-10 inhibits the production of metalloproteinase by macrophages, the basic function of which is to digest excessive extracellular matrix [51-52]. Moreover, the cytokines produced by Th2 cells also promote the production of antibodies [53].
As previously mentioned, the basic role in allograft rejection - whether acute of chronic - is played by B cell and anti-HLA antibodies. The presence of plasma cells and CD20+ B cells in the allografts have been found to be associated with irreversible allograft injury [54-55]. The diagnosis of chronic (or acute) antibody-mediated allograft rejection is based on the presence of three different features: (i) presence of anti-donor antibodies, as indicated by serology, (ii) C4d, a complement split product, positive staining in the peritubular capillaries, and (iii) morphological features of chronic (or acute) renal tissue injury [56-57]. As already mentioned, cellular immunity accentuates humoral immunity, and humoral immunity accentuates cellular immunity. Humoral immunity damages the graft by the production of anti-graft antibodies via activation of T cells through an indirect pathway [58]. Moreover, stimulation of B cells, in the presence of T cells, drives the naïve B cells to differentiate into memory B and plasma cells, which provides long-lasting immunity against grafts [59-60].
Drug targets for chronic renal transplant rejection
Different drugs that might be helpful in reducing chronic renal transplant rejection have been summarized in Table 2. Details of these drugs are as follows.
Table 2. Major drugs, their group, mechanism of actions and effects.
| Drug | Category | Mechanism | Effect |
| Mycophenolate mofetil | Immunosuppressive (Anti-proliferative) | Inhibitor of inosine monophosphate dehydrogenase (IMPDH) | Decreases proliferation of B and T cells. |
| Rapamycin (Sirolimus) | Immunosuppressive (Anti-proliferative) | Blocks Cells Cycle at the Junction of G1 and S phase by interacting with intracellular protein, FKBP12 and blocking cell specific kinase TOR (Target of rapamycin) | Decreases proliferation of B cells, T cells, smooth muscles and decreases antibody production |
| Everolimus | Immunosuppressive (Anti-proliferative) | Same as Rapamycin (Sirolimus) | Same as Rapamycin (Sirolimus) |
| Leflunomide | Immunosuppressive (Anti-proliferative) | Blocks the action of dihydroorotate dehydrogenase, which is a rate-limiting enzyme in the production of uridine monophosphate (UMP). | Decreases proliferation and differentiation of activated lymphocytes |
| Azithioprine | Immunosuppressive (Anti-proliferative) | Blocks de novo purine synthesis | Blocks T cell activation |
| Methylprednisolone | Immunosuppressive (Anti-proliferative and anti-inflammatory) | Causes redistribution of T cells and blocks inflammatory pathways | Decreases circulating T cells and inflammatory cytokines (for instance IL-6) |
| Tacrolimus (FK506) | Immunosuppressive (Anti-proliferative and antibiotic) | Causes decrease in gene expression | Decreases both cell-mediated and humoral immunity |
| Rituximab | Immunosuppressive (Anti-proliferative, anti-CD20 monoclonal antibody) | Antibody-dependent cellular cytotoxicity, direct signaling and antibody-mediated cytotoxicity | Decreases the population of CD20 B cells. |
Mycophenolate Mofetil
Mycophenolate mofetil (MMF) is a pro-drug for mycophenolic acid (MPA). It is an inhibitor of the inosine monophosphate dehydrogenase (IMPDH). It is an enzyme that controls the rate-limiting steps in the de novo production of guanosine nucleotides. T and B cells are more dependent on this pathway than any other cell [61-62]. Mycophenolate has been shown to have far more superior immunosuppressive properties as compared to azathioprine, and it can significantly reduce the chances of acute graft rejection [63]. However, its benefits in terms of long-term graft survival still need to be elucidated. Multiple studies have shown that the use of mycophenolate mofetil over an extended period can significantly reduce the chance of long-term graft rejection and can increase the mean survival of the transplant [64-65].
Rapamycin
Rapamycin (sirolimus), which was initially discovered as an antifungal agent, has now been shown to have significant anti-cancer and immunosuppressant activities. It blocks cell cycle at the junction of G1 and S phase by interacting with intracellular protein, FKBP12, and blocking cell specific kinase TOR (target of rapamycin) [66-67]. Several studies have shown that use of rapamycin in the maintenance regime after transplants can lead to immune suppression, decrease in smooth muscle proliferation, a decrease in the chances of acute and sub-acute transplant rejection, and improve the long-term survival and function of renal allografts [68-70].
Everolimus
Everolimus is a more polar version of rapamycin (sirolimus). Its mechanism of action and effects are essentially the same as rapamycin [71-73]. Data collected from pre-clinical studies have shown that everolimus not only improves the survival in response to acute graft rejection but also helps in the long-term survival of the grafts [72-74].
Leflunomide
Leflunomide is a drug that is widely used for the treatment of auto-immune disorders, like rheumatoid arthritis (RA), due to its potent immune-suppressive effects. The metabolites from leflunomide block the action of dihydroorotate dehydrogenase, which is a rate-limiting enzyme in the production of uridine monophosphate (UMP). Activated lymphocytes need UMP for proliferation and differentiation [75-77]. Leflunomide has been shown to decrease acute graft rejection [78-80]. Moreover, its use in animal models has been shown to decrease the development and progression of CAN in the transplanted tissues [81-82]. Results of a study showed that leflunomide was superior to azathioprine or mycophenolate mofetil in improving renal functions in transplant patients with deteriorating kidney functions. Moreover, its use decreased the progression and rather initiated the reversal of CAN features [83].
Azathioprine
Azathioprine is a purine analog that functions at the level of DNA [84-85]. It is quickly metabolized into 6-mercaptopurine (6-MP), which gets incorporated into the DNA and thereby decreases the de novo purine synthesis [86]. Azathioprine blocks CD28 signaling and T cell activation [87]. Studies have shown that shifting patients from the conventional cyclosporine A therapy to azathioprine therapy can improve the survival of graft and can decrease the chances of nephrotoxicity seen with cyclosporine [88].
Methylprednisolone
Methylprednisolone decreases the chances of chronic graft rejection by suppressing several immunological and inflammatory mechanisms. The exact mechanism by which the methylprednisolone accomplishes this feat is still uncertain, but two mechanisms are worth mentioning here. First, the administration of steroids causes the redistribution of T cells from the circulation into other body compartments (for instance, to bone marrow), which renders these cells almost ineffective [89-90]. Second, the administration of methylprednisolone also seems to decrease the production of inflammatory cytokines [91]. There are some reports that favor short, but not long-term, use of methylprednisolone as it reduces the chances of acute graft rejection and thereby, rather indirectly, improves the long-term survival of transplant patients [92].
Tacrolimus
Tacrolimus (FK506) is a macrolide antibiotic with immunosuppressive activity as well. Although its structure is different from cyclosporine, its mechanism of action is essentially the same as that of cyclosporine. It causes impairment in the expression of targeted genes in the targeted cells. Tacrolimus binds to an immunophilin, FK506 binding protein (FKBP), which then inhibits the activity of calcineurin phosphatase. Inhibition of calcineurin phosphatase suppresses the activity of several genes, such as genes involved in cell degranulation, interleukin-2 transcription, and so on. These effects of tacrolimus then inhibit the proliferation of T cells and their related cytokines. In addition, it also decreases the proliferation of B cells and antibody formation through an indirect effect, i.e. decrease in the activity of T cells, and suppresses the activation of B cells as well [93-94]. Tacrolimus has shown its efficacy over different conventional immunosuppressive agents in different clinical studies. It is less nephrotoxic as compared to cyclosporine and ensures the long-term conservation of kidney structure and function [95]. Moreover, it has an enhanced efficacy when used in combination with other immunosuppressive agents, such as MMF [96].
Rituximab
Rituximab is an anti-CD20 monoclonal antibody that significantly thins down the B cell population. Antibody-dependent cellular cytotoxicity, direct signaling, and antibody-mediated cytotoxicity are all the important pieces in its mechanism of action [97-99].There is some evidence that support the use of rituximab for improving the long-term survival of the kidneys [100-101].
Conclusions
To conclude, the long-term survival of renal transplants is still poor. Different risk factors, like HLA-mismatching, acute episodes of rejection, mismatch of the donor-recipient age, the age of transplant, and race, contribute the most towards decreasing the long-term survival of kidneys. Moreover, both pillars of the immune system, i.e. cell-mediated immunity and humoral immunity, play a part in the rejection of kidneys in the long run. Therefore, improving the long-term survival of kidneys should include two important things. The first proactive step is to minimize the known risk factors before the actual renal transplantation. The second step is the usage of different anti-proliferative agents that can decrease the proliferation and action of immune cells to decrease the chances of graft rejection in the longer run. The choice of drugs for the same should be made only after vigilant consideration of multiple factors that are discussed in the review and should always be patient-specific.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.What are the key challenges we face in kidney transplantation today? Chapman JR. Transplantation Res. 2013;2:0. doi: 10.1186/2047-1440-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Late acute kidney transplant rejection: clinicopathological correlates and response to corticosteroid therapy. Nair R, Agrawal N, Lebaeau M, Tuteja S, Chandran PK, Suneja M. Transplant Proc. 2009;41:4150–4153. doi: 10.1016/j.transproceed.2009.09.074. [DOI] [PubMed] [Google Scholar]
- 3.Chronic renal allograft rejection: pathophysiologic considerations. Joosten SA, Sijpkens YW, van Kooten C, Paul LC. Kidney Int. 2005;68:1–13. doi: 10.1111/j.1523-1755.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- 4.Improved graft survival after renal transplantation in the United States, 1988 to 1996. Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. N Engl J Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 5.Chronic allograft nephropathy: An update. Paul LC. Kidney Int. 1999;56:783–793. doi: 10.1046/j.1523-1755.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- 6.Clayton P, Campbell S, Hurst K, McDonald S, Chadban S. ANZDATA Registry Report 2010, Australia and New Zealand Dialysis and Transplant Registry Adelaide, South Australia. ANZDATA Registry Report ; [Nov;2015 ]. 2011. Transplantation; pp. 0–31. [Google Scholar]
- 7.Patient survival after renal transplantation; more than 25 years follow-up. Arend SM, Mallat MJ, Westendorp RJ, van der Woude FJ, van Es LA. Nephrol Dial Transplant. 1997;12:1672–1679. doi: 10.1093/ndt/12.8.1672. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic criteria for chronic rejection/accelerated graft atherosclerosis in heart and kidney transplants: joint proposal from the Fourth Alexis Carrel Conference on Chronic Rejection and Accelerated Arteriosclerosis in Transplanted Organs. Paul LC, Hayry P, Foegh M, Dennis MJ, Mihatsch MJ, Larsson E, Fellström B. Transplant Proc. 1993;25:2022–2023. [PubMed] [Google Scholar]
- 9.A thirty percent chronic decline in inverse serum creatinine is an excellent predictor of late renal allograft failure. Kasiske BL, Andany MA, Danielson B. Am J Kidney Dis. 2002;39:762–768. doi: 10.1053/ajkd.2002.31996. [DOI] [PubMed] [Google Scholar]
- 10.Chronic renal allograft rejection: Immunologic and nonimmunologic risk factors. Massy ZA, Guijarro C, Wiederkehr MR, Ma JZ, Kasiske BL. Kidney Int. 1996;49:518–524. doi: 10.1038/ki.1996.74. [DOI] [PubMed] [Google Scholar]
- 11.The natural history of chronic allograft nephropathy. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 12.Mauiyyedi S, Colvin RB. Kidney Transplantation: Principles and Practice, 5th edition. Oxford: WB Saunders Company; 2001. Pathology of kidney transplantation; pp. 343–376. [Google Scholar]
- 13.Chronic allograft nephropathy–biopsy findings and outcome. Freese P, Svalander CT, Mölne J, Nordén G, Nyberg G. Nephrol Dial Transplant. 2001;16:2401–2406. doi: 10.1093/ndt/16.12.2401. [DOI] [PubMed] [Google Scholar]
- 14.Donor and recipient contribution to transplant vasculopathy in chronic renal transplant dysfunction. Boersema M, Rienstra H, van den Heuvel M, van Goor H, van Luyn MJ, Navis GJ, Popa ER, Hillebrands JL. Transplantation. 2009;88:1386–1392. doi: 10.1097/TP.0b013e3181bca1e4. [DOI] [PubMed] [Google Scholar]
- 15.Transplant capillaropathy and transplant glomerulopathy: Ultrastructural markers of chronic renal allograft rejection. Ivanyi B. Nephrol Dial Transplant. 2003;18:655–660. doi: 10.1093/ndt/gfg139. [DOI] [PubMed] [Google Scholar]
- 16.Neointimal and tubulointerstitial infiltration by recipient mesenchymal cells in chronic renal-allograft rejection. Grimm PC, Nickerson P, Jeffery J, Savani RC, Gough J, McKenna RM, Stern E, Rush DN. N Engl J Med. 2001;345:93–97. doi: 10.1056/NEJM200107123450203. [DOI] [PubMed] [Google Scholar]
- 17.Chronic transplant vasculopathy microenvironment present in the renal allograft reprograms macrophage phenotype. Lepage S, Cailhier JF. Transplant Proc. 2009 ;41:3311–3313. doi: 10.1016/j.transproceed.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Transplant glomerulopathy: Evolution of morphologically distinct changes. Maryniak RK, First MR, Weiss MA. Kidney Int. 1985;27:799–806. doi: 10.1038/ki.1985.83. [DOI] [PubMed] [Google Scholar]
- 19.Focal segmental glomerulosclerosis in renal allografts with chronic nephropathy: implications for graft survival. Cosio FG, Frankel WL, Pelletier RP, Pesavento TE, Henry ML, Ferguson RM. Am J Kidney Dis. 1999;34:731–738. doi: 10.1016/S0272-6386(99)70400-2. [DOI] [PubMed] [Google Scholar]
- 20.The pathobiology of chronic allograft nephropathy: immune-mediated damage and accelerated aging. Joosten SA, van Kooten C, Sijpkens YW, de Fijter JW, Paul LC. Kidney Int. 2004;65:1556–1559. doi: 10.1111/j.1523-1755.2004.05410.x. [DOI] [PubMed] [Google Scholar]
- 21.Immunohistological and ultrastructural differences between recurrent type I membranoproliferative glomerulonephritis and chronic transplant glomerulopathy. Andresdottir MB, Assmann KJ, Koene RA, Wetzels JF. Am J Kidney Dis. 1998;32:582–588. doi: 10.1016/s0272-6386(98)70020-4. [DOI] [PubMed] [Google Scholar]
- 22.The impact of acute rejection episodes on long-term graft function and outcome in 1347 primary renal transplants treated by 3 cyclosporine regimens. Lindholm A, Ohlman S, Albrechtsen D, Tufveson G, Persson H, Persson NH. Transplantation. 1993;56:307–315. doi: 10.1097/00007890-199308000-00010. [DOI] [PubMed] [Google Scholar]
- 23.New immunosuppressants and HLA matching. Opelz G. Transplant Proc. 2001;33:467–468. doi: 10.1016/s0041-1345(00)02095-9. [DOI] [PubMed] [Google Scholar]
- 24.Anti-HLA antibodies after solid organ transplantation. McKenna RM, Takemoto SK, Terasaki PI. Transplantation. 2000;69:319–326. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 25.All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, Tsai A, Lei HY. Transplantation. 2002;74:1192–1194. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 26.Impact of donor-specific antibodies on chronic rejection occurrence and graft loss in renal transplantation: posttransplant analysis using flow cytometric techniques. Piazza A, Poggi E, Borrelli L, Servetti S, Monaco PI, Buonomo O, Valeri M, Torlone N, Adorno D, Casciani CU. Transplantation. 2001;71:1106–1112. doi: 10.1097/00007890-200104270-00017. [DOI] [PubMed] [Google Scholar]
- 27.Kidney graft failure and presensitization against HLA class I and class II antigens. Süsal C, Opelz G. Transplantation. 2002;73:1269–1273. doi: 10.1097/00007890-200204270-00014. [DOI] [PubMed] [Google Scholar]
- 28.Rejection and recipient age. Bradley BA. Transpl Immunol. 2002;10:125–132. doi: 10.1016/s0966-3274(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 29.Medication compliance following renal transplantation. Raiz LR, Kilty KM, Henry ML, Ferguson RM. Transplantation. 1999;68:51–55. doi: 10.1097/00007890-199907150-00010. [DOI] [PubMed] [Google Scholar]
- 30.Inferior allograft outcomes in adolescent recipients of renal transplants from ideal deceased donors. Levine MH, Reese PP, Wood A, Baluarte JH, Huverserian A, Naji A, Abt PL. Ann Surg. 2012;255:556–564. doi: 10.1097/SLA.0b013e3182471665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donor-recipient age matching improves years of graft function in deceased-donor kidney transplantation. Lim WH, Chang S, Chadban S, Campbell S, Dent H, Russ GR, McDonald SP. Nephrol Dial Transplant. 2010 ;25:3082–3089. doi: 10.1093/ndt/gfq127. [DOI] [PubMed] [Google Scholar]
- 32.Delayed function reduces renal allograft survival independent of acute rejection. Feldman HI, Gayner R, Berlin JA, Roth DA, Silibovsky R, Kushner S, Brayman KL, Burns JE, Kobrin SM, Friedman AL, Grossman RA. Nephrol Dial Transplant. 1996;11:1306–1313. [PubMed] [Google Scholar]
- 33. Determinants of chronic renal allograft rejection in cyclosporine-treated recipients. Flechner SM, Modlin CS, Serrano DP, Goldfarb DA, Papajcik D, Mastroianni B, Goormastic M, Novick AC. Transplantation. 1996;62:1235–1241. doi: 10.1097/00007890-199611150-00009. [DOI] [PubMed] [Google Scholar]
- 34.Renal transplantation in black Americans. Young CJ, Gaston RS. N Engl J Med. 2000;343:1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 35.Glomerular hypertension—An under-appreciated aspect of chronic rejection. Paul LC. Nephrol Dial Transplant. 2001;16:213–215. doi: 10.1093/ndt/16.2.213. [DOI] [PubMed] [Google Scholar]
- 36.Arterial hypertension and renal allograft survival. Mange KC, Cizman B, Joffe M, Feldman HI. JAMA. 2000;283:633–638. doi: 10.1001/jama.283.5.633. [DOI] [PubMed] [Google Scholar]
- 37.Surrogate markers of chronic allograft nephropathy. van Es LA, Sijpkens YW, Paul LC. Ann Transplant. 2000;5:7–11. [PubMed] [Google Scholar]
- 38.Clinical correlation between renal allograft failure and hyperlipidemia. Guijarro C, Massy ZA, Kasiske BL. Kidney Int Suppl. 1995;52:0–59. [PubMed] [Google Scholar]
- 39.Excess risk of renal allograft loss associated with cigarette smoking. Sung RS, Althoen M, Howell TA, Ojo AO, Merion RM. Transplantation. 2001;71:1752–1757. doi: 10.1097/00007890-200106270-00009. [DOI] [PubMed] [Google Scholar]
- 40.Permanent detrimental effect of nonimmunological factors on long-term renal graft survival: a parsimonious model of time-dependency. Smits JM, van Houwelingen HC, De Meester J, le Cessie S, Persijn GG, Claas FH, Frei U. Transplantation. 2000;70:317–323. doi: 10.1097/00007890-200007270-00015. [DOI] [PubMed] [Google Scholar]
- 41.Twelve years' experience with national sharing of HLA-matched cadaveric kidneys for transplantation. Takemoto SK, Terasaki PI, Gjertson DW, Cecka JM. N Engl J Med. 2000;343:1078–1084. doi: 10.1056/NEJM200010123431504. [DOI] [PubMed] [Google Scholar]
- 42.Mechanism of cellular rejection in transplantation. Ingulli E. Pediatr Nephrol. 2010;25:61–74. doi: 10.1007/s00467-008-1020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Why do we reject a graft? Role of indirect allorecognition in graft rejection. Sayegh MH. Kidney Int. 1999;56:1967–1979. doi: 10.1046/j.1523-1755.1999.00751.x. [DOI] [PubMed] [Google Scholar]
- 44.Indirect allorecognition of donor class I and II major histocompatibility complex peptides promotes the development of transplant vasculopathy. Womer KL, Stone JR, Murphy B, Chandraker A, Sayegh MH. J Am Soc Nephrol. 2001;12:2500–2506. doi: 10.1681/ASN.V12112500. [DOI] [PubMed] [Google Scholar]
- 45.Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. Ciubotariu R, Liu Z, Colovai AI, Ho E, Itescu S, Ravalli S, Hardy MA, Cortesini R, Rose EA, Suciu-Foca N. J Clin Invest. 1998;101:398–405. doi: 10.1172/JCI1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction. Vella JP, Spadafora-Ferreira M, Murphy B, Alexander SI, Harmon W, Carpenter CB, Sayegh MH. Transplantation. 1997;64:795–800. doi: 10.1097/00007890-199709270-00001. [DOI] [PubMed] [Google Scholar]
- 47. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Chan SY, DeBruyne LA, Goodman RE, Eichwald EJ, Bishop DK. Transplantation. 1995;59:1155–1161. [PubMed] [Google Scholar]
- 48.Predominant expression of T helper 2 cytokines and altered expression of T helper 1 cytokines in long-term allograft survival induced by intrathymic immune modulation with donor class I major histocompatibility complex peptides. Shirwan H, Barwari L, Khan NS. Transplantation. 1998;66:1802–1809. doi: 10.1097/00007890-199812270-00039. [DOI] [PubMed] [Google Scholar]
- 49.Transfusion of polarized TH2-like cell populations into SCID mouse cardiac allograft recipients results in acute allograft rejection. VanBuskirk AM, Wakely ME, Orosz CG. Transplantation. 1996;62:229–238. doi: 10.1097/00007890-199607270-00014. [DOI] [PubMed] [Google Scholar]
- 50.Critical roles for IL-4, IL-5, and eosinophils in chronic skin allograft rejection. Le Moine A, Flamand V, Demoor FX, Noël JC, Surquin M, Kiss R, Nahori MA, Pretolani M, Goldman M, Abramowicz D. J Clin Invest. 1999;103:1659–1667. doi: 10.1172/JCI5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. Postlethwaite AE, Holness MA, Katai H, Raghow R. J Clin Invest. 1992;90:1479–1485. doi: 10.1172/JCI116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. J Clin Invest. 1995;96:2304–2310. doi: 10.1172/JCI118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 54.Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Hippen BE, DeMattos A, Cook WJ, Kew CE 2nd, Gaston RS. Am J Transplant. 2005;5:2248–2252. doi: 10.1111/j.1600-6143.2005.01009.x. [DOI] [PubMed] [Google Scholar]
- 55.Nodular B-cell aggregates associated with treatment refractory renal transplant rejection resolved by rituximab. Lehnhardt A, Mengel M, Pape L, Ehrich JH, Offner G, Strehlau J. Am J Transplant. 2006;6:847–851. doi: 10.1111/j.1600-6143.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 56.Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 57.Banff 07 classification of renal allograft pathology: updates and future directions. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 58.Cellular mechanisms underlying acute graft rejection: time for reassessment. Alegre ML, Florquin S, Goldman M. Curr Opin Immunol. 2007;19:563–568. doi: 10.1016/j.coi.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 59.Understanding and making use of human memory B cells. Lanzavecchia A, Bernasconi N, Traggiai E, Ruprecht CR, Corti D, Sallusto F. Immunol Rev. 2006;211:303–309. doi: 10.1111/j.0105-2896.2006.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maintenance of serological memory by polyclonal activation of human memory B cells. Bernasconi NL, Traggiai E, Lanzavecchia A. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 61.Mycophenolate mofetil and its mechanisms of action. Allison AC, Eugui EM. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 62.Mycophenolate mofetil: An update. Villarroel MC, Hidalgo M, Jimeno A. Drugs Today. 2009 ;45:521–532. doi: 10.1358/dot.2009.45.7.1384878. [DOI] [PubMed] [Google Scholar]
- 63.Reduced kidney transplant rejection rate and pharmacoeconomic advantage of mycophenolate mofetil. Wüthrich RP1, Weinreich T, Ambühl PM, Schwarzkopf AK, Candinas D, Binswanger U. Nephrol Dial Transplant. 1999;14:394–399. doi: 10.1093/ndt/14.2.394. [DOI] [PubMed] [Google Scholar]
- 64.Mycophenolate mofetil versus azathioprine therapy is associated with a significant protection against long-term renal allograft function deterioration. Meier-Kriesche HU, Steffen BJ, Hochberg AM, Gordon RD, Liebman MN, Morris JA, Kaplan B. Transplantation. 2003;75:1341–1346. doi: 10.1097/01.TP.0000062833.14843.4B. [DOI] [PubMed] [Google Scholar]
- 65.Long-term use of mycophenolate mofetil is associated with a reduction in the incidence and risk of late rejection. Meier-Kriesche HU, Steffen BJ, Hochberg AM, Gordon RD, Liebman MN, Morris JA, Kaplan B. Am J Transplant. 2003;3:68–73. doi: 10.1034/j.1600-6143.2003.30112.x. [DOI] [PubMed] [Google Scholar]
- 66.Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae. Crespo JL, Hall MN. Microbiol Mol Biol Rev. 2002;66:579–591. doi: 10.1128/MMBR.66.4.579-591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sirolimus its discovery, biological properties, and mechanism of action. Sehgal SN. Transplant Proc. 2003;35:7–14. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 68.Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in caucasian recipients of mismatched primary renal allografts: a phase II trial. Rapamune Study Group. Kahan BD, Julian BA, Pescovitz MD, Vanrenterghem Y, Neylan J. Transplantation. 1999;68:1526–1532. doi: 10.1097/00007890-199911270-00016. [DOI] [PubMed] [Google Scholar]
- 69.Long-term renal allograft function on a tacrolimus-based, pred-free maintenance immunosuppression comparing sirolimus vs. MMF. Gallon L, Perico N, Dimitrov BD, Winoto J, Remuzzi G, Leventhal J, Gaspari F, Kaufman D. Am J Transplant. 2006;6:1617–1623. doi: 10.1111/j.1600-6143.2006.01340.x. [DOI] [PubMed] [Google Scholar]
- 70.Long-term improvement in renal function with sirolimus after early cyclosporine withdrawal in renal transplant recipients: 2-year results of the Rapamune Maintenance Regimen Study. Oberbauer R, Kreis H, Johnson RW, Mota A, Claesson K, Ruiz JC, Wilczek H, Jamieson N, Henriques AC, Paczek L, Chapman J, Burke JT, Rapamune Maintenance Regimen Study Group. Transplantation. 2003;76:364–370. doi: 10.1097/01.TP.0000074360.62032.39. [DOI] [PubMed] [Google Scholar]
- 71.Clinical pharmacokinetics of everolimus. Kirchner GI, Meier-Wiedenbach I, Manns MP. Clin Pharmacokinet. 2004;43:83–95. doi: 10.2165/00003088-200443020-00002. [DOI] [PubMed] [Google Scholar]
- 72.Everolimus: an update on the mechanism of action, pharmacokinetics and recent clinical trials. Sánchez-Fructuoso AI. Expert Opin Drug Metab Toxicol. 2008;4:807–819. doi: 10.1517/17425255.4.6.807. [DOI] [PubMed] [Google Scholar]
- 73.Antirestenotic mechanisms of everolimus on human coronary artery smooth muscle cells: inhibition of human coronary artery smooth muscle cell proliferation, but not migration. Lavigne MC, Grimsby JL, Eppihimer MJ. J Cardiovasc Pharmacol. 2012;59:165–174. doi: 10.1097/FJC.0b013e31823a39c7. [DOI] [PubMed] [Google Scholar]
- 74.Review of the proliferation inhibitor everolimus. Nashan B. Expert Opin Investig Drugs. 2002;11:1845–1857. doi: 10.1517/13543784.11.12.1845. [DOI] [PubMed] [Google Scholar]
- 75.Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Herrmann ML, Schleyerbach R, Kirschbaum BJ. Immunopharmacology. 2000;47:273–289. doi: 10.1016/s0162-3109(00)00191-0. [DOI] [PubMed] [Google Scholar]
- 76.Mechanism of action for leflunomide in rheumatoid arthritis. Fox RI, Herrmann ML, Frangou CG, Wahl GM, Morris RE, Strand V, Kirschbaum BJ. Clin Immunol. 1999;93:198–208. doi: 10.1006/clim.1999.4777. [DOI] [PubMed] [Google Scholar]
- 77.Leflunomide mode of action in the treatment of rheumatoid arthritis. Breedveld F, Dayer J. Ann Rheum Dis. 2000;59:841–849. doi: 10.1136/ard.59.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leflunomide in experimental transplantation. Control of rejection and alloantibody production, reversal of acute rejection, and interaction with cyclosporine. Williams JW, Xiao F, Foster P, Clardy C, McChesney L, Sankary H, Chong AS. Transplantation. 1994;57:1223–1231. [PubMed] [Google Scholar]
- 79.Effect of leflunomide in control of acute rejection in hamster-to-rat cardiac xenografts. Xiao F, Chong A, Foster P, Sankary H, McChesney L, Williams JM, Frieders D, Williams JW. Transplant Proc. 1994;26:1263–1265. [PubMed] [Google Scholar]
- 80.Leflunomide immunosuppression in rat small intestinal transplantation. Foster PF, Xiao F, Kociss K, Chong AS, Sankary HN, McChesney L, Cohn S, Williams JW. Transplant Proc. 1994;26:1599–1600. [PubMed] [Google Scholar]
- 81.Pharmacologically induced regression of chronic transplant rejection. Xiao F, Chong A, Shen J, Yang J, Short J, Foster P, Sankary H, Jensik S, Mital D, McChesney L. Transplantation. 1995;60:1065–1072. doi: 10.1097/00007890-199511270-00001. [DOI] [PubMed] [Google Scholar]
- 82.Studies in experimental models of chronic rejection: use of rapamycin (sirolimus) and isoxazole derivatives (leflunomide and its analogue) for the suppression of graft vascular disease and obliterative bronchiolitis. Morris RE, Huang X, Gregory CR, Billingham ME, Rowan R, Shorthouse R, Berry GJ. Transplant Proc. 1995;27:2068–2069. [PubMed] [Google Scholar]
- 83.Prospective, pilot, open-label, short-term study of conversion to leflunomide reverses chronic renal allograft dysfunction. Hardinger KL, Wang CD, Schnitzler MA, Miller BW, Jendrisak MD, Shenoy S, Lowell JA, Brennan DC. Am J Transplantation. 2002;2:867–871. doi: 10.1034/j.1600-6143.2002.20909.x. [DOI] [PubMed] [Google Scholar]
- 84.The George Hitchings and Gertrude Elion Lecture. The pharmacology of azathioprine. Elion GB. Ann N Y Acad Sci. 1993;685:400–407. doi: 10.1111/j.1749-6632.1993.tb35897.x. [DOI] [PubMed] [Google Scholar]
- 85.Thiopurine biology and pharmacology. Aarbakke J, Janka-Schaub G, Elion GB. Trends Pharmacol Sci. 1997;18:3–7. doi: 10.1016/s0165-6147(96)01007-3. [DOI] [PubMed] [Google Scholar]
- 86.Azathioprine: old drug, new actions. Jonathan MS, Koretzky GA. J Clin Invest. 2003;111:1122–1124. doi: 10.1172/JCI18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, Mudter J, Hildner K, Bartsch B, Holtmann M, Blumberg R, Walczak H, Iven H, Galle PR, Ahmadian MR, Neurath MF. J Clin Invest. 2003;111:1133–1145. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Long-term graft survival after conversion from cyclosporin to azathioprine 1 year after renal transplantation. A prospective, randomized study from 1 to 6 years after transplantation. Pedersen EB, Hansen HE, Kornerup HJ, Madsen S, Sørensen AW. Nephrol Dial Transplant. 1993;8:250–254. [PubMed] [Google Scholar]
- 89.Fauci AS, Dale DC. J Clin Invest. Vol. 55. Jan: 1975. Alternate-day prednisone therapy and human lymphocyte subpopulations; pp. 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Methylprednisolone dosage effects on peripheral lymphocyte subpopulations and eicosanoid synthesis. Rota S, Rambaldi A, Gaspari F, Noris M, Daina E, Benigni A, Perna A, Donadelli R, Remuzzi G, Garattini S. Kidney Int. 1992;42:981–990. doi: 10.1038/ki.1992.377. [DOI] [PubMed] [Google Scholar]
- 91.Preoperative methylprednisolone administration maintains coagulation homeostasis in patients undergoing liver resection: importance of inflammatory cytokine modulation. Pulitanò C, Aldrighetti L, Arru M, Finazzi R, Catena M, Guzzetti E, Soldini L, Comotti L, Ferla G. Shock. 2007;28:401–405. doi: 10.1097/shk.0b013e318063ed11. [DOI] [PubMed] [Google Scholar]
- 92.Early treatment of acute rejections gives favorable long-term function after renal transplantation in small children. Qvist E, Their M, Krogerus L, Holmberg C, Jalanko H. Pediatr Transplant. 2007 ;11:895–900. doi: 10.1111/j.1399-3046.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 93.Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Thomson AW, Bonham CA, Zeevi A. Ther Drug Monit. 1995;17:584–591. doi: 10.1097/00007691-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 94.Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and leflunomide. Brazelton TR, Morris RE. Curr Opin Immunol. 1996;8:710–720. doi: 10.1016/s0952-7915(96)80090-2. [DOI] [PubMed] [Google Scholar]
- 95.Tacrolimus versus ciclosporin immunosuppression: long‐term outcome in renal transplantation. Jurewicz WA. Nephrol Dial Transplant. 2003;18:0. doi: 10.1093/ndt/gfg1028. [DOI] [PubMed] [Google Scholar]
- 96.Long-term renal allograft function on a tacrolimus-based, pred-free maintenance immunosuppression comparing sirolimus vs MMF. Gallon L, Perico N, Dimitrov BD, Winoto J, Remuzzi G, Leventhal J, Gaspari F, Kaufman D. Am J Transplant. 2006;6:1617–1623. doi: 10.1111/j.1600-6143.2006.01340.x. [DOI] [PubMed] [Google Scholar]
- 97.Rituximab: mechanism of action. Weiner GJ. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rituximab mechanism of action and resistance. Maloney DG, Smith B, Rose A. Semin Oncol. 2002;29:2–9. doi: 10.1053/sonc.2002.30156. [DOI] [PubMed] [Google Scholar]
- 99.Mechanism of action of rituximab. Cerny T, Borisch B, Introna M, Johnson P, Rose AL. Anticancer Drugs. 2002;13:0–10. doi: 10.1097/00001813-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 100.Drug Insight: rituximab in renal disease and transplantation. Salama AD, Pusey CD. Nat Clin Pract Nephrol. 2006;2:221–230. doi: 10.1038/ncpneph0133. [DOI] [PubMed] [Google Scholar]
- 101.Long-term results of ABO-incompatible kidney transplantation with antigen-specific immunoadsorption and rituximab. Genberg H, Kumlien G, Wennberg L, Tydén G. Transplantation. 2007;84:0. doi: 10.1097/01.tp.0000296031.41424.f8. [DOI] [PubMed] [Google Scholar]


