Abstract
Background
Cytokinins are plant-specific hormones that affect plant growth and development. The endogenous level of cytokinins in plant cells is regulated in part by irreversible degradation via cytokinin oxidase/dehydrogenase (CKX). Among the 11 rice CKXs, CKX2 has been implicated in regulation of rice grain yield.
Results
To specifically down-regulate OsCKX2 expression, we have chosen two conserved glycosylation regions of OsCKX2 for designing artificial short hairpin RNA interference genes (shRNA-CX3 and -CX5, representing the 5′ and 3′ glycosylation region sequences, respectively) for transformation by the Agrobacterium-mediated method. For each construct, 5 independent transgenic lines were obtained for detailed analysis. Southern blot analysis confirmed the integration of the shRNA genes into the rice genome, and quantitative real time RT-PCR and northern blot analyses showed reduced OsCKX2 expression in the young stem of transgenic rice at varying degrees. However, the expression of other rice CKX genes, such as CKX1 and CKX3, in these transgenic lines was not altered. Transgenic rice plants grown in the greenhouse were greener and more vigorous with delayed senescence, compared to the wild type. In field experiments, both CX3 and CX5 transgenic rice plants produced more tillers (27–81 %) and grains (24–67 %) per plant and had a heavier 1000 grain weight (5–15 %) than the wild type. The increases in grain yield were highly correlated with increased tiller numbers. Consistently, insertional activation of OsCKX2 led to increased expression of CKX2 and reduced tiller number and growth in a gene-dosage dependant manner.
Conclusions
Taken together, these results demonstrate that specific suppression of OsCKX2 expression through shRNA-mediated gene silencing leads to enhanced growth and productivity in rice by increasing tiller number and grain weight.
Electronic supplementary material
The online version of this article (doi:10.1186/s12284-015-0070-5) contains supplementary material, which is available to authorized users.
Keywords: Rice, Cytokinin oxidase/dehydrogenase, shRNA, Growth, Productivity
Background
Cytokinins are a class of plant hormones that play an essential role in various developmental and physiological processes, including cell differentiation, apical dominance, leaf senescence, nutrient signaling, and shoot and chloroplast differentiation (Mok 1994; Letham 1994). One of the mechanisms that controls the endogenous level of cytokinins is mediated by the enzyme cytokinin oxidase/dehydrogenase (CKX) (Galuszka et al. 2001), which irreversibly degrades cytokinins in plants (Jones and Schreiber 1997). Genes coding for CKXs have been cloned and characterized from model plants, such as Arabidopsis (Werner et al. 2003; Werner et al. 2001) and rice (Ashikari et al. 2005). The enzyme is encoded by a multigene family, including 7 in Arabidopsis (AtCKX) and 11 in rice (OsCKX). The CKX proteins encoded by the Arabidopsis gene family vary in their biochemical properties, especially in substrate specificity (Galuszka et al. 2007). Tsai et al. (2012) suggested that each rice CKX gene has a distinct role in the regulation of the cytokinin level in different tissues, because CKXs exhibit differential expression and regulation patterns in roots and shoots.
CKX proteins are widely distributed in plants (Schmulling et al. 2003) and are generally classified as glycoproteins (Armstrong 1994). Synthesis and accumulation of CKXs are subjected to multiple regulatory mechanisms, depending on endogenous cytokinin contents in plant cells (Kowalska et al. 2010). Interestingly, expression of multiple CKX genes is differentially regulated in roots and shoots of rice in response to treatment with exogenous cytokinin (Tsai et al. 2012). For example, OsCKX2 transcript increases in rice shoots, but the expression of OsCKX6 is down-regulated specifically in the shoots in response to cytokinin treatment (Tsai et al. 2012). CKXs are glycosylated posttranslationally. Glycosylation, the attachment of sugar moieties to proteins, is one of the important post-translational modifications in eukaryotic protein biosynthesis (Varki et al. 1995). Glycosylation of proteins in plants affects protein structure, folding, stability and biological activity (Ceriotti et al. 1998; Severino et al. 2010). It has been demonstrated in tobacco that the cytokinin-induced upregulation of CKX activity is mainly associated with the N-glycosylated form of the enzyme, suggesting that the control of CKX activity is at least in part dependent on glycosylation (Motyka et al. 2003). Despite our understanding of the biochemical properties of CKXs, the specific function and regulation of CKXs in plants have not been fully elucidated.
Most agriculturally important traits in crops, such as yield and quality, are controlled by several genes known as quantitative trait loci (QTLs) (Collard et al. 2005). In the past decade, efforts have been made to characterize QTLs for grain productivity. QTL analysis in rice has suggested that a locus responsible for grain yield, Gn1a, encodes cytokinin oxidase/dehydrogenase (OsCKX2) (Ashikari et al. 2005). Decreased expression of OsCKX2 in transgenic rice harboring antisense OsCKX2 cDNA resulted in increased grain number in the panicle (Ashikari et al. 2005). CKX is therefore regarded as a negative regulator of cytokinin metabolism. Consistent with this view, high-yielding rice cultivars which produce more grains in the panicles were found to have reduced or lost function of OsCKX2 and had higher cytokinins accumulated in the inflorescence meristems. In good agreement, a double mutant of CKX3 and CKX5 in Arabidopsis formed more and larger flowers (Bartrina et al. 2011). Thus, there is an increasing interest in manipulating CKX gene expression in crops as a means to alter their growth and development through enhanced or decreased cytokinin levels. An earlier study showed that overexpression of AtCKX gene in transgenic tobacco plants led to reduced endogenous cytokinin contents (Werner et al. 2001). Suppression or overexpression of GhCKX (Gossypium hirsutum L.) in transgenic tobacco gave rise to a cytokinin-overproducing (e.g., more flowers and capsules) or cytokinin-deficient (e.g., fewer or no flowers) phenotype (Zeng et al. 2012). In addition, silencing of HvCKX1-hpRNAi (hairpin RNA interference) in transgenic barley resulted in a lower CKX activity and a higher grain yield (Zalewski et al. 2010). Taken together, these results suggest that crop yields can be manipulated by altering the expression of CKX genes and cytokinin contents.
Rice (Oryza sativa L.) is one of the most important crops and food sources worldwide. To study the role of CKX2 in relation to rice productivity, we specifically down-regulated the expression of OsCKX2 by RNAi in this study, using stable transformation of short hairpin RNAs (shRNAs) that comprised the N-glycosylation site sequences of OsCKX2 (Brummelkamp et al. 2002). Our results showed that these shRNAs efficiently and specifically suppress OsCKX2 expression in transgenic rice, leading to enhanced growth and productivity via increased tiller numbers and grain weight. Consistently, insertional activation of OsCKX2 in transgenic rice resulted in increased expression of OsCKX2 with the plants showing a decreased tiller number phenotype.
Results
PCR and RT-PCR Analyses of Primary To Transformants
For each transformation vector, a total of 10 primary T0 transformants from five independent transgenic lines were first screened for the presence of CX3 or CX5 transgene by PCR. Eight individual transformants from the CX3- and ten from the CX5-suppression rice lines were detected positive for hptII, GUS and CX3 (or CX5) genes (Fig. 1a). These transgenes were not detected in the wild type (WT) or negative control (H2O as template). Moreover, the PCR products for these transgenes were sequenced, showing the correct sequences (data not shown). Representative T0 transgenic lines containing all of these three transgenes were selected for further analysis.
Fig. 1.
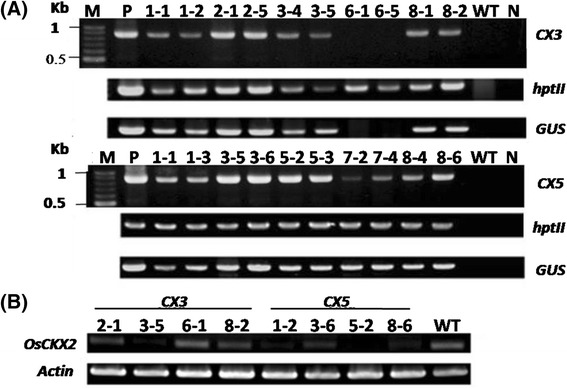
Molecular analysis of selected primary OsCKX2 transformants (T0) and wild-type (WT) plants. a Detection of transgenes in selected primary transformants. The presence of CX3 (or CX5), hptII and GUS from selected primary transformants was detected by PCR. M: DNA markers, P: positive control (plasmid DNA as template), WT: untransformed wild type, N: negative control (H2O as template). b Expression of OsCKX2 in selected primary transformants and wild type (WT), as assayed by RT-PCR using specific primers. Expression of actin was used as a cDNA loading control
The expression of OsCKX2 in the leaves of selected primary transformants was first analyzed by semi-quantitative RT-PCR (RT-PCR) using specific primers. There was variation in the expression level of OsCKX2 among the selected transformants. In general, the OsCKX2 transcript levels were much lower in the transformants than in WT (Fig. 1b), except for line 6–1 of the CX3-suppression line (Fig. 1a). The OsCKX2 transcript level in line 6–1 was the same as that in the control, which could be attributed to the low expression of shRNA-CX3 in this transgenic line. Overall, the results showed that shRNA-CX3 and -CX5 introduced into the rice genome resulted in reduced expression of OsCKX2 in these transgenic lines.
Southern Analysis of To Transgenic Rice
Based on the preliminary results of PCR and RT-PCR analyses, representative T0 transgenic lines were selected for gene integration analysis. Southern blot analysis indicated that both hptII and CX3 genes on the T-DNA were integrated into the rice genome at 2 and 3 copies in the CX3-3 and the CX3-8 transgenic lines, respectively (Fig. 2a, b). Two copies of the hptII and CX5 genes were present in the CX5-1, CX5-3, and CX5-5 transgenic lines (Fig. 2c, d). The same pattern of integration of both hptII and CX3 (or CX5) in the rice genome indicates that the two transgenes on the T-DNA were transferred together.
Fig. 2.
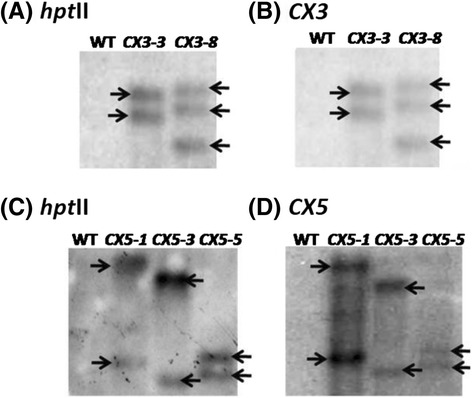
Southern blot analysis of hptII, CX3 and CX5 in selected T0 primary rice transformants. The T0 primary transformants selected included two independent transgenic lines (CX3-3 and CX3-8) from transformation with shRNA-CX3 (a, b) and three independent transgenic lines (CX5-1, CX5-3 and CX5-5) from transformation with shRNA-CX5 (c, d). WT: untransformed wild type. Genomic DNA was digested with SacI (a, b) or HindIII (c, d) and hybridized with a 32P-labelled probe corresponding to hptII (a, c), CX3 (b), or CX5 (d). The number of reactive bands in each lane represents the transgene copies in each transgenic line
OsCKX2 Expression Profile and Silencing of OsCKX2 Expression in Transgenic Rice
The expression profiles of OsCKX2 in different organs (leaf, root, stem, inflorescence and flower) of WT plants were examined by RT-PCR first. As shown in Additional file 1: Figure S1A, low transcript levels of OsCKX2 were detected in leaves and roots, but a high transcript level was detected in the stems of the WT plants. In contrast, the representative transformants of CX3- and CX5-suppression transgenic lines showed a significantly reduced OsCKX2 transcript level in the young stems, as compared to WT (Additional file 1: Figure S1B). Next, the transcript levels of OsCKX2 in the young stems of different transgenic lines were evaluated by northern gel blot analysis, relative to that of WT. The representative plants of selected independent transgenic lines all exhibited low levels of OsCKX2 transcript (Additional file 1: Figure S1C). The expression of OsCKX2 in the CX3- and CX5-supression transgenic lines was further analyzed by quantitative real time PCR (qRT-PCR). The results indicated that both constructs effectively suppressed the expression of OsCKX2 in most T0 plants (Fig. 1b), but shRNA-CX5 construct was more effective in suppressing its expression than shRNA-CX3 construct (Fig. 3).
Fig. 3.
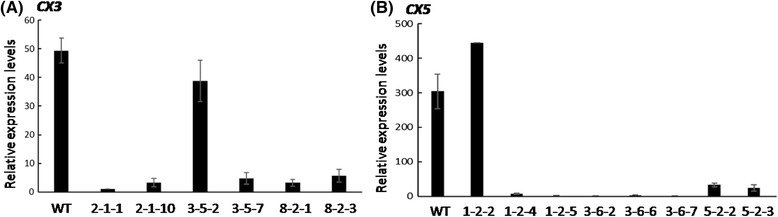
Relative OsCKX2 expression levels in selected T1 CX3- and CX5-suppression lines by qRT-PCR. Total RNA was isolated from young stems of wild type (WT) and selected T1 CX3-suppression lines (2–1–1, 2–1–10, 3–5–2, 3–5–7, 8–2–1, 8–2–3) (a) and CX5-suppression lines (1–2–2, 1–2–4, 1–2–5, 3–6–2, 3–6–6, 3–6–7, 5–2–2, 5–2–3) (b). 17S rRNA was used as an internal control for normalization. The expression level of line 2–1–1 and line 3–6–7 were used as a reference in the comparison of CX3- and CX5-suppression lines, respectively. Values presented were mean +/− SD of 3 replicates of cDNA samples
To test whether the RNAi-OsCKX2 constructs also down-regulated the expression of other OsCKX genes, the expression of CKX1 and CKX3 in the CX3- and CX5-suppression lines was examined by qRT-PCR. Phylogenetic analysis (Matsuo et al. 2012) showed that rice CKX1 is most closely related to OsCKX2 (51.7 % identity in amino acid sequence). Rice CKX3 (OsCKX3) was randomly selected among other OsCKX genes (Matsuo et al. 2012; Li et al. 2013). Our results revealed that expression of both rice CKX1 or CKX3 was not significantly altered in both suppression lines (Additional file 2: Figure S2). These results suggest that the integration of shRNA-CX3 or -CX5 into the genome of these transgenic rice plants has caused sequence-specific interference with homologous OsCKX2 expression withouting affecting the expression of other CKX genes.
Phenotypic Characterization of CX3- or CX5-Suppression Transgenic Rice
For growth analysis, attempts were made first to screen homozygous suppression lines based on hygromycin resistance during seed germination since hptII and CX3 (or CX5) were integrated together (Fig. 2). About 80 self-pollinated T1 seeds from selected T0 lines were germinated on 1/2 MS medium containing 50 mg L−1 hygromycin. For each line, vigorous seedlings from germinated seeds were selected randomly to grow in pots, and self-pollinated T2 seeds were subjected to another round of screening. The T3 seeds from two CX3 and three CX5 T2 lines showed 100 % germination on hygromycin-containing MS medium, which were considered homozygous for the introduced genes. Since both CX3 and CX5 transgenic lines contained 2–3 copies of the transgenes based on Southern analysis (Fig. 2), the possibility that some of the T3 transgenic lines could be heterozygous for some of the transgenes introduced cannot be totally ruled out. Hygromycin resistant T3 seedlings were transferred to soil and grown in the greenhouse for phenotype observation. The phenotypes of CX3- and CX5-suppression rice plants were compared with that of the WT at different developmental stages. All transgenic plants appeared uniformly greener than WT at all stages and showed a delayed senescence. For example, three-month-old transgenic plants had a higher leaf chlorophyll content (3–9 %, significance level at P <0.05) for two of the five lines (data not shown). Morphological comparison showed that all transgenic lines produced more tillers with a high fertility (four-month-old plants shown in Fig. 4a). In comparison to WT, the number of mature tillers increased by 15–43 %, while the plant height was reduced by 3–12 % in the T3 transgenic rice (Fig. 4b).
Fig. 4.
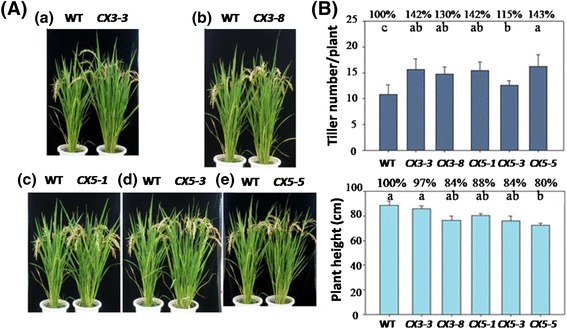
Growth phenotypes of representative T3 CX3- and CX5-Suppression transgenic rice lines grown in the greenhouse. a Mature plants of 4-month-old untransformed wild type (WT) and selected transgenic lines (lines 3 and 8 from transformation with pshRNA-CX3; lines 1, 3 and 5 from transformation with pshRNA-CX5). b Tiller number/plant and plant height analyzed upon harvest. The % data presented are expressed relative to the control (100 %). Values are mean ± SD (n = 7), P <0.01. WT: untransformed wild-type plants; CX3 suppression lines (CX3-3 and CX3-8); CX5 suppression lines (CX5-1, CX5-3 and CX5-5)
Agronomic Traits of T3 Transgenic Rice in the Field
To assess the influence of decreased expression of OsCKX2 on growth and productivity, homozygous T3 seedlings of two CX3 and three CX5 suppression rice lines were grown in the field with one seedling per hill. At maturity, the panicle number per plant increased significantly, by 27–81 %, in these transgenic rice lines, as compared to WT (Fig. 5a). CX3-3 and CX3-8 suppression lines also exhibited an increase in total grain number/plant, by 44 and 67 %, and in total grain weight/plant, by 58 and 75 %, respectively (Fig. 5b, c). Consistently, suppression lines CX5-1, CX5-3 and CX5-5 also showed an increase in total grain number/plant, by 54, 40 and 41 %, and in total grain weight/plant, by 44, 33 and 24 %, respectively, relative to WT (Fig. 5b, c). One thousand grain weight (g) also increased by 5–15 % in these transgenic rice lines (Fig. 5d). The enhancements in these agronomic traits were significant at P <0.01. Taken together, these results suggest that transgenic rice carrying the CX3- or CX5-silencing gene exhibit significant promotion in growth, leading to increased grain productivity, mainly due to a combination of increased panicle number and heavier grain weight.
Fig. 5.
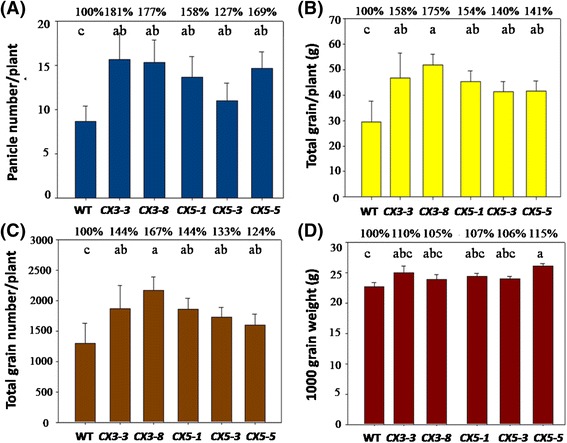
Agronomic traits of wild-type (WT) and T3 OsCKX2 suppression lines grown in the field. a Panicle number/plant, (b) total grain weight/plant, (c) total grain number/plant and (d) 1000 grain weight. Abbreviations for transgenic lines are the same as described in Fig. 5. Values are mean ± SD (eight replicates with 5 plants/replicate); different letters denoted significance level at P <0.01. The % data presented are expressed relative to the control (100 %)
Growth Traits of Transgenic Rice with Insertional Activation of CKX2
In a separate experiment, we have obtained many transgenic rice lines overexpressing maize cytosolic carbonic anhydrase (CA) (Additional file 3: Figure S3A) and one of the lines exhibited reduced tiller number whereas other lines showed a normal tiller phenotype. Southern hybridization indicated the maize gene was inserted into the rice genome in a single copy (Additional file 3: Figure S3B). Using primers designed for the T-DNA LB and RB sequences we performed tail-PCR analysis and determined the insertion location of the gene on chromosome 1 at 5269546 bp or 98 bp of the 3′-UTR of CKX2 (LOC_Os01g10110) (Additional file 3: Figure S3C). To further test the influence of this insertion on expression of CKX2, genotyping using primers based on the neighboring boarding sequences of the maize gene was used to determine the homozygosity among the T1 plants of this transgenic line. Among the T1 plants analyzed, two homozygous, 2 heterozygous and 2 null lines were selected for growth analysis in the greenhouse. As expected, western immunoblot analysis using specific antibodies against the maize CA showed that the homozygous plants expressed a high level of maize CA and the heterozygous plants expressed a lower level of the protein, while the WT and null lines did not express the maize protein at all (Additional file 4: Figure S4). As reported previously (Jeong et al. 2002; Wang 2008), insertion of a transgene under the control of a strong promoter in the 3′-UTR of another gene can lead to its activation, we found that the expression levels of OsCKX2 in the T1 homozygous (Lines 1–6, 1–9) and heterozygous plants (1–28, 1–41) were about 13–13.5 and 3.5–6.5 fold higher than that of WT, respectively, whereas the null lines and WT had low levels of expression (Fig. 6a). High expression level of CKX2 in the homozygous plants is expected to reduce cytokinin accumulation through increased oxidative degradation by the enzyme. Consistent with this hypothesis, agronomic trait analysis showed insertional activation or overexpression of OsCKX2 led to reduced panicle number and total biomass in the heterozygous and homozygous plants, compared to WT and null lines (Fig. 6c, d). The homozygous plants showed low tiller numbers (7–11 tillers/plant), as compared to null lines (18–20) and WT (17–22 tillers/plant) (Fig. 6b). The heterozygous plants also exhibited reduced tiller numbers (13–15 tillers/plant) though not as low as homozygous plants. Among these T1 plants, the OsCKX2 transcript levels were highly correlated with panicle number (r = 0.88) and total biomass (r = 0.89). Thus, our results revealed a clear gene-dosage effect on panicle number and biomass. To test if the changes in tiller number among these plants were related to the expression of rice SPL14 (Luo et al. 2012), D53 (Zhou et al. 2013) and TB1 (Choi et al. 2012) that are known to be involved in regulation of tillering, we conducted qRT-PCR to examine the expression levels of these genes in the null, heterozygous and homozygous plants derived from the OsCKX2 overexpression line. Our results showed clearly the expression of these genes was not significantly different from that of WT (Additional file 5: Figure S5A). Also, the expression levels of both rice CKX1 and CKX3 were similar to that of WT (Additional file 5: Figure S5B). Taken together, these results strongly support our hypothesis that insertional activation leads to increased expression and accumulation of OsCKX2, which in turn led to reduced tiller number/biomass, presumably due to a lower level of cytokinin accumulated in the shoots.
Fig. 6.
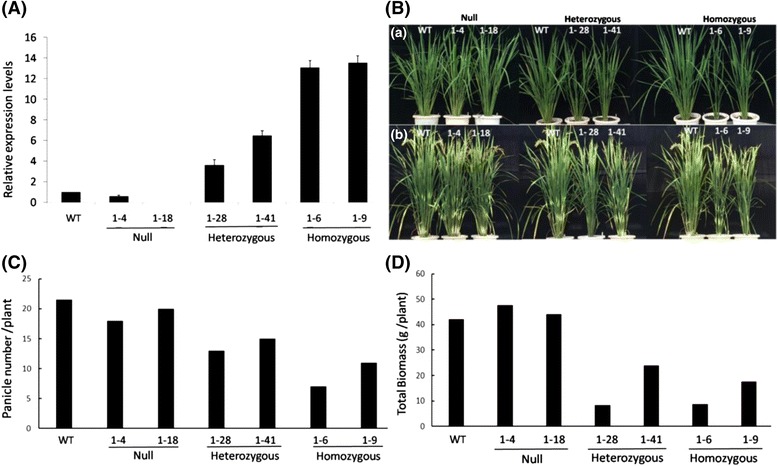
Relative expression levels of OsCKX2 (a) and growth traits (b, c, d) of representative null, heterozygous and homozygous T1 plants derived from OsCKX2-overexpression line, as analyzed by qRT-PCR. a Total RNA was isolated from the leaves of wild type (WT), null lines (1–4, 1–18), CKX2-overexpression heterozygous (1–28, 1–41) and homozygous (1–6, 1–9) lines. 17S rRNA was used as an internal control for normalization. The expression level of WT was used as a reference. Values presented were mean +/− SD of 3 replicates of cDNA samples. b Growth phenotypes of WT, null lines, CKX2-overexpression heterozygous and homozygous lines. (a) Two-month-old plants. b Three-month-old plants. Agronomic traits analyzed upon harvest were (c) panicle number/plant and (d) total biomass/plant
Discussion
The goal of this study was to specifically down regulate the expression of rice CKX2 using shRNA-mediated gene silencing to examine its influence on rice growth and productivity. Introduction of dsRNA- or shRNA-producing transgenes through transformation has been successively demonstrated in plants (Wang et al. 2000; Zalewski et al. 2010; Zhao et al. 2006), leading to a long-term gene silencing of the target mRNAs (Sliva and Schnierle 2010; Kim et al. 2012). Similarly, here we showed that CX3- or CX5-suppression transgenic rice lines expressed the shRNAs efficiently and specifically reduced the expression of homologous OsCKX2 (Fig. 1b, Additional file 1: Figure S1B-C, Fig. 3) withouting affecting the expression of other CKX genes, such as CKX1 and CKX3 (Additional file 2; Figure S2). Our results are consistent with those of transgenic barley plants that harbored hairpin (hp) RNA-expression constructs containing barley yellow dwarf virus-PAV (BYDV-PAV) sequences (Wang et al. 2000) and partial cDNA fragments of HvCKX1 (Zalewski et al. 2010), resulting in blockage of the translation of target mRNAs into proteins. Earlier studies also demonstrated that gene silencing by stable integration can be triggered by the presence of multiple transgene copies in transgenic plants with varying degrees of target reduction (Waterhouse et al. 2001; Kerschen et al. 2004). The CX3- or CX5-suppression transgenic rice lines in this study contained two or three copies of the silencing genes in the genome (Fig. 2), conferring an efficient reduction of OsCKX2 transcript in most lines (Additional file 1: Figure S1B, 1C, Fig. 3). Our data also tend to suggest that CX5 may be more efficient than CX3 in suppression of OsCKX2 expression (Fig. 3). Most importantly, the specific suppression of OsCKX2 expression by CX3- or CX5 was demonstrated in the progenies, suggesting that the silencing transgenes were stably inherited in the following generations. Kerschen et al. (2004) also reported a similar result on constitutive transcriptional transgene silencing in the T4 homozygous RNAi lines of Arabidopsis.
Cytokinins are known to play an important role in plant developmental and physiological processes (Mok 1994; Letham 1994). Consequently, they regulate crop yield by modifying the growth and development of both vegetative and reproductive tissues. An earlier study showed that exogenous application of cytokinins promotes chlorophyll accumulation in cucumber cotyledons (Fletcher and McCullagh 1971). Similarly, our CX3- and CX5-suppression rice lines also have elevated leaf chlorophyll contents at all stages (e.g. 3–9 % for 3-month-old plants), with a delayed leaf senescence (data not shown). This may be attributed to a higher cytokinin level in the plants, conferring the transgenic plants a higher photosynthetic capacity over a longer duration. In addition, an increase of endosperm cell number and grain weight of rice by treating roots, leaves and panicles with exogenous kinetin provides direct evidence for its role in regulating endosperm development (Yang et al. 2002). Consistently, maize kernels exposed to high temperature (35 °C) exhibit a decline in endogenous cytokinin levels, leading to disrupted endosperm development and starch biosynthesis (Cheikh and Jones 1994). Development of heat-stressed kernels can be recovered after treatment with exogenous BA (Benzyladenine) (Cheikh and Jones 1994). These results strongly suggest that development of reproductive organs is tightly regulated by cytokinins and high temperature inhibition of seed development is related to reduced cytokinins. Therefore, higher levels of cytokinins in the reproductive organs could lead to bigger or heavier grains, consistent with the observation in the present study (Fig. 5).
As CKX is the only known enzyme responsible for irreversible degradation of cytokinins in plants, mutant or transgenic plants expressing low levels of CKX genes have been shown to possess a clear cytokinin-overproducing phenotype. For example, rice cultivars with more grains in the panicle have a higher level of cytokinins in the inflorescence meristems (Ashikari et al. 2005). In Arabidopsis, a CKX3/CKX5 double mutant forms more and larger flowers (Bartrina et al. 2011). Recent genetic studies through transformation suggest that CKX expression can be manipulated for enhanced crop productivity. In rice, decreased expression of OsCKX2 in transgenic by antisense also leads to an increase in grain number in the panicles (Ashikari et al. 2005). There were more capsules produced in CKX-suppression tobacco plants caused by ihpRNA (intron-containing hairpin RNA) (Zeng et al. 2012). Also, downregulation of CKX2 in both rice and barley by antisense and hpRNAi suppression resulted in enhanced grain yields (Ashikari et al. 2005; Zalewski et al. 2010). In the present study, the T3 homozygous CX3- and CX5-suppression lines produced significantly more tillers/panicles and grain yields on a per plant basis, and had a heavier 1000 grain weight, as compared to the wild type in both green house (Fig. 4) and field conditions (Fig. 5). Thus, our results suggest that specific down regulation of OsCKX2 leads to enhanced tillering and thus a higher productivity in rice, presumably due to enhanced cytokinin levels. The higher chlorophyll contents and delayed leaf senescence in combination may have contributed to heavier grains.
A good understanding of the expression pattern of various CKX genes in different cultivars or in different organs of a given cultivar would be useful for identifying a specific target gene for manipulation of cytokinin contents. In this work, we showed that OsCKX2 was highly expressed in the stem and inflorescence of TNG67, but negligible in the leaf and root (Additional file 1: Figure S1). The expression pattern of OsCKX2 in TNG67 was generally consistent with another cultivar Taichung 65, except that a high level of OsCKX2 was detected in the leaf of the cultivar Taichung 65 (Ashikari et al. 2005). In contrast, barley HvCKX1 transcript accumulates highly in developing spikes (0 to 14 DAP) and seedling roots (Zalewski et al. 2010); the expression pattern of other HvCKXs has not been characterized. Moreover, the expression of OsCKXs is subjected to cytokinin feed forward regulation. Multiple OsCKX genes are induced in response to cytokinin in rice root and/or in shoot, and OsCKX2 is up-regulated in both root and shoot (Tsai et al. 2012). The maize ZmCKX1 transcript accumulates in developing kernels (0 to 34 DAP), which is correlated with different levels of cytokinin oxidase activity and cytokinin contents (Brugiere et al. 2003). These results demonstrate the complexity in the expression and regulation of cytokinin degradation genes in plants and their importance as a key regulatory factor in crop productivity. Ashikari et al. (2005) suggested that the expression level of OsCKX2 in different rice cultivars is inversely related to the content of cytokinins in inflorescence meristems, which in turn controls the rice grain number in main panicles. Although reduced levels of OsCKX2 transcript were detected in the young stems of our CX3- and CX5-suppression plants, there were no significant increases in grain number in the panicles, except an increased tiller number and grain weight (Fig. 5). Consistently, our transgenic rice lines with insertional activation of CKX2 had elevated expression of CKX2 (Fig. 6a) and exhibited a reduced tiller phenotype and growth in a gene-dosage dependent manner (Fig. 6b). And the changes in tiller number are not related to the expression of three tiller related genes in rice, SPL14, D53 and TB1 (Additional file 5: Figure S5A). Thus, the results from both gain-of-function and loss-of-function experiments support our conclusion that specific down-regulation of cytokinin oxidase 2 expression enhances rice yield by increasing tiller number and grain weight. The lack of increased grain number by inhibiting CKX2 expression, as observed in this study, may be caused by a lower expression of shRNAs in the inflorescence (i.e. a lower amount of cytokinins accumulated) to trigger differentiation of more florets in the panicles. Alternatively, the difference could be accounted for by the different cultivars used between the studies, as a certain QTL could be only applicable in certain varieties (Miura et al. 2011).
Taken together, our data demonstrated that the expression of the endogenous OsCKX2 gene can be effectively suppressed by shRNA-CX3 and -CX5 in transgenic rice, resulting in increased chlorophyll content, growth, grain weight and yields. The increases in grain yield are mainly due to increased tillering capability and heavier grains. Most importantly, the cytokinin-overproducing phenotypes in the transgenic plants are stably inherited over the next generations. To the best of our knowledge, this is the first study in rice where efficient suppression of a specific target gene is successfully achieved by shRNAi. Thus, the shRNA-based gene silencing technique provides a simple new approach for manipulating rice productivity. Our results support the idea that manipulation of the CKX gene expression and the cytokinin level in crop plants can result in increased growth and productivity (Ashikari et al. 2005; Zalewski et al. 2010; Zeng et al. 2012). Furthermore, a cytokinin overproducing genotype may confer an adaptive significance at high temperatures, which is known to inhibit cytokinin accumulation in the seeds (Cheikh and Jones 1994).
Conclusions
Rice is a staple food for more than half of the world population. To meet the expanding food demands for the rapidly growing world population, increase in rice yield and production is of utmost importance. Results from both gain-of-function and loss-of-function experiments in this study support the notion that specific down-regulation of cytokinin oxidase 2 expression enhances rice yield by increasing tiller number and grain weight. Thus, efficient suppression of OsCKX2 expression through shRNA-mediated gene silencing provides a simple means to enhance rice growth and productivity.
Methods
Selection of siRNA Target Sites
The N-glycosylation sites of rice OsCKX2 (cytokinin oxidase/dehydrogenase; GenBank: AB205193) were identified using the PROSITE database (http://prosite.expasy.org/prosite.html) and NetNglyc 1.0 server (http://www.cbs.dtu.dk/services/NetNGlyc/). The analysis showed that N-glycosylation sites are present at amino acid residues 64–67 and 464–467 (Additional file 6: Table S1 and Additional file 7: Figure S6). Thus, nucleotides 182–202 and 1389–1409, which code for the N-glycosylation sites, were used for the construction of shRNAs as specific siRNAs against OsCKX2 mRNA and designated CX3 and CX5, respectively. Also, the 21 nt target sequences (CX3, CX5) were submitted to the NCBI/BLAST database to confirm their specificity for targeting OsCKX2 mRNAs in rice.
Construction of Transformation Vectors
To prepare vectors for shRNA-mediated gene silencing in rice, an oligonucleotide sequence (Additional file 8: Table S2) was designed for the loop linking sense and antisense strands, as described by Elbashir et al. (2001). shRNAs containing the loop sequence (TTCAAGAGA) have been shown to be highly efficient in silencing target genes (more than 90 %) (Brummelkamp et al. 2002). Thus, this loop sequence was adopted, while the siRNA target sequences (CX3 and CX5) were designed in the sense and antisense orientations. Oligonucleotide sequences of CX3-Sense and -Antisense (or CX5-Sense and -Antisense) with BamHI and SalI restriction sequences at the 5′ and 3′ ends were synthesized and phosphorylated at the 5′ ends (Fig. 7).
Fig. 7.
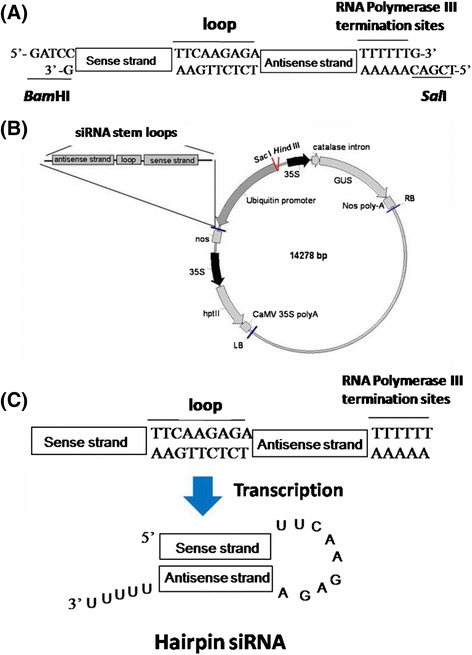
Construct of pC1301-CX3 or -CX5 containing a shRNA stem loop for rice transformation. a Diagram of a short hairpin RNA (shRNA) cassette. This cassette comprises inverted repeat sequences (CX3 or CX5) against the target gene (OsCKX2), a spacer fragment (loop) and RNA polymerase III termination sites. b Schematic representation of the transformation constructs (not drawn to scale). shRNA stem loop: 21 nt sense and 21 nt antisense sequences linked by an unpaired loop sequence; Ubiquitin promoter: maize ubiquitin promoter; 35S: CaMV 35S promoter; nos: nopaline synthase terminator; GUS: β-glucuronidase gene; hptII: hygromycin phosphotransferase II gene, LB: left border; RB: right border. c Diagram of self-complementary hairpin siRNA
To generate double-stranded DNA, equal molar concentration of both complementary oligos were mixed in an annealing buffer [10 mM Tris–HCl (pH 8.0), 50 mM NaCl, 1 mM EDTA], followed by heating at 94 °C for 5 min and slow cooling to room temperature for 45–60 min. After annealing, oligonucleotide sequences of CX3-Sense and CX3-Antisense (or CX5-Sense and CX5-Antisense) formed double-stranded DNAs with BamHI and SalI sites at the 5′ and 3′ ends, respectively (Fig. 7a). These vectors were designated shRNA-CX3 and shRNA-CX5, accordingly.
shRNA-CX3 or shRNA-CX5 was subsequently cloned into a BamHI-SalI pBluescribe M13+ vector to produce an intermediate pshRNA-CX3 or -CX5. Positive clones were identified by sequencing; the pshRNA-CX3 or -CX5 vector was digested with XbaI, treated with Klenow polymerase to generate blunt ends, and ligated to a blunt-ended maize ubiquitin promoter fragment to produce pUbi-CX3 or -CX5. The orientation of the ubiquitin promoter in the positive clones was confirmed by restriction enzyme digestion. Finally, a SacI-KpnI shRNA-CX3 or -CX5 fragment carrying the ubiquitin promoter formed pUbi-CX3 or -CX5, and a KpnI-EcoRI nos terminator fragment was ligated to a SacI-EcoRI binary vector pCAMBIA1301 to produce the final plasmid pC1301-CX3 or pC1301-CX5 (Fig. 7c). The final constructs were transfected via electroporation into the Agrobacterium tumefaciens strain AGL1.
Expression of siRNA with Stem Loop Structure in Transgenic Rice
The pC1301-CX3 or pC1301-CX5 vector containing shRNA-CX3 or -CX5 with loop sequences was used to silence homologous OsCKX2 expression in rice after transformation. This shRNA stem loop structure with target DNA sequences of CX3 (ACTTTGGCAACCTCTCCGTCGTTCAAGAGACGACGGAGAGGTTGCCAAAGT) or CX5 (TAACATGTCGGCAGTGATCACTTCAAGAGAGTGATCACTGCCGACATGTTA) was inserted in the sense-loop-antisense orientation under the control of the maize ubiquitin promoter (Fig. 7b). The underlined nucleotides represent the loop sequence.
The basic transcriptional unit of hairpin siRNA was produced as follows. The inverted repeat sequences of CX3 or CX5 (also called the stem) and a spacer loop formed a self-complementary hairpin structure after transcription in plants (Fig. 7c). The stem region of hairpin RNAs was then processed into 21-nt siRNAs by RNase III enzymes Dicer (Sliva and Schnierle 2010; Kim et al. 2012). In addition, the pC1301-CX3 or pC1301-CX5 vector contained β-glucuronidase (GUS) and hygromycin phosphotransferase II (hptII) genes driven by CaMV 35S promoters (Fig. 7b). These two genes were used as selectable markers for screening primary transformants.
Rice Transformation and Regeneration
Rice transformation through A. tumefaciens was carried out according to the methods described previously (Ku et al. 1999; Toki 1997). Calli induced from immature seeds of rice (Oryza sativa cv. TNG67) were used for rice transformation. The yellow compact embryogenic calli were co-cultured with A. tumefaciens strain AGL1 carrying the plasmid pC1301-CX3 or pC1301-CX5 and incubated at 28 °C in dark for 3–4 days. After co-cultivation, calli were thoroughly washed with 250 mg L−1 cefotaxime in sterile distilled water and transferred to a selection medium containing 50 mg L−1 hygromycin B (Invitrogen) and 250 mg L−1 cefotaxime at 28 °C in light for one month. Healthy hygromycin-resistant calli were subsequently transferred to a MS regeneration medium supplemented with 2,5 mg L−1 kinetin, 1 mg L−1 NAA and 50 mg L−1 hygromycin. Transgenic plantlets were transferred to a hormone-free 1/2 MS medium without hygromycin in magenta boxes for 10–14 days to promote root growth. Calli selection and plant regeneration were conducted in a growth chamber at 28 °C and 60 % relative humidity under a 16 h/8 h light/dark photoperiod. Transgenic seedlings were transplanted in soil, cultured in the greenhouse, and self-pollinated for seed production.
PCR, Semi-Quantitative RT-PCR (RT-PCR) and Quantitative Real-Time PCR (qRT-PCR) Analyses
To detect transgenes and to evaluate the OsCKX2 transcript level in transgenic lines, specific primers were used in PCR, RT-PCR and qRT-PCR analyses (Table 1). For PCR analysis, genomic DNA was isolated from leaves of transgenic and WT (wild type) plants, as described by Sheu et al. (1996). Amplification of GUS and CX3 or CX5 transgenes was performed in a thermal cycler under the following conditions: 94 °C/5 min, 30 cycles of 94 °C/1 min, 56 °C/1 min and 72 °C/1 min, and a final extension at 72 °C/5 min. The conditions for amplification of hptII were 94 °C/5 min, 28 cycles of 94 °C/1 min, 65 °C/1 min and 72 °C/1 min, and a final extension at 72 °C/5 min.
Table 1.
Specific primers used in this study
| Primer name | Sequence (5′ to 3′) | Target gene |
|---|---|---|
| Hygromycin-F | GACCTGATGCAGCTCTCGGAG | hptII |
| Hygromycin-R | TGCTCCATACAAGCCAACCACG | |
| GUS-F | AAAAAACTCGACGGCCTGTGGG | GUS |
| GUS-R | GCATCTTCATGACGACCAAAGC | |
| Ubiquitin pro.-F | CTGATGCATATACAGAGATGC | a CX3 or CX5 |
| Nos ter.-R | TGACAGCTTATCATCGGATC | |
| CKX2-F | GTCCACGACGGCGAGCTCAA | OsCKX2 |
| CKX2-R | TCCATCTTGGCATCTCTCAG | |
| Actin-F | GGTAATGTGTTGGACTCTGG | Actin |
| Actin-R | GCAGTGATCTTCCTTGCTCA | |
| 3′UTR-F | CGGTGACGAGGTGTTCTACAC | OsCKX2 3′ UTR |
| 3′UTR -R | CCAAGATCTCGTCGTTCTGC | |
| rRNA-F | GATAACTCGACGGATCGCACGG | 17S rRNA |
| rRNA-R | GCCTGCTGCCTTCCTTGGATGTG | |
| OsCKX1-F | GCACCCGTGGCTCAACCTG | CKX1 |
| OsCKX1-R | GATGTCGGTGGCCGTCTGG | |
| OsCKX3-F | TTTCTTATGCTGATGTGGGTG | CKX3 |
| OsCKX3-R | GAACATTGCTAATCTGAGGTCC | |
| OsSPL14-F | TGAATTTGACCAAGGAAAAC | SPL14 |
| OsSPL14-R | ATCCAACGTAAAGCTTCTGA | |
| D53-F | CCAAGCAGTTTGAAGCGAC | D53 |
| D53-R | CCGCAAGTTTATCAAAGTCAA | |
| OsTB1-F | CAAGAAATCTCGGCGGCTAG | TB1 |
| OsTB1-R | CGAATTGGCGTAGACGAC |
ashRNA cannot be amplified directly by PCR. Thus, a 867 bp fragment containing partial promoter, shRNA and partial terminator were amplified for detecting the CX3 or CX5 gene
For RT-PCR and qRT-PCR analyses, total RNA was isolated from different tissues, as described by Wang and Vodkin (1994). Total RNA was first treated with RNase-free DNase I to remove genomic DNA and the resulting RNA was used for first-strand cDNA synthesis with an Oligo (dT) primer and M-MLV reverse transcriptase (Promega) (Chen et al. 2006). Two μg cDNA was used for gene expression assay by RT-PCR, conducted at 94 °C/5 min, 28 cycles of 94 °C/1 min, 58 °C/1 min and 72 °C/1 min, and a final extension at 72 °C/5 min. For qRT-PCR, the protocols of Livak and Schmittgen (2001) and Yeh et al. (2014) were followed using 3′URT specific primers for OsCKX2 (Table 1). Expression of 17S rRNA (accession number X00755) (Table 1) was used as an internal control for normalization of expression levels.
Southern Blot Analysis
Southern gel blot analysis was performed according to the method of Ku et al. (1999). Genomic DNA was digested with SacI or HindIII, electrophoresed on 1 % agarose gel and transferred to a nylon membrane for probe hybridization. The hptII, CX3, and CX5 fragments, either amplified by PCR or excised from the plasmids pC1301-CX3 and pC1301-CX5 by restriction enzyme digestion, were used as probes. Probes were radioactively labeled via random primer labeling, and hybridization was performed at 65 °C for 8–12 h. After hybridization membrane was stripped off non-specific probes successively in washing solution containing 2× SSC/0.1 % SDS and then in solution containing 0.1× SSC/0.1 % SDS at 45 °C for 10–20 min.
Northern Blot Analysis
Total RNA was isolated from different organs, as described by Wang and Vodkin (1994). RNA gel blot analysis was performed according to the method described by Chen et al. (2006). Total RNA was denatured with formaldehyde and formamide, electrophoresed on 1.2 % agarose gel and transferred to nylon membrane. Hybridization probes were radioactively labeled using a random primer labeling method. Hybridization and washing procedures were the same as described in Southern analysis.
Antibodies and Western Immunoblotting
Antibodies against the maize carbonic anhydrase (CA) were produced against the synthetic peptide (KKKEGPAKEKPSTDTP), specific to the maize protein, in rabbits and purified by affinity chromatography (Yao-Hong Biotechnology Inc., Taiwan). The protocol of Dai et al. (1994) was used for SDS-PAGE and western immunodetection.
Field Experiments
Field tests were carried out in the transgenic field of Taiwan Agricultural Research Institute in random block design, with each block containing 60 transgenic or WT seedlings (1 seedling/hill). Row spacing was 30 cm and plant spacing was 15 cm. For each line, after excluding the boarding plants 5 randomly chosen plants were harvested and pooled as one replicate and a total of 8 replicates of plant samples were harvested for analysis. Total biomass, panicle number, total grain weight and total grain number per plant, and 1000 grain weight were analyzed after drying (43 °C/2 days for seeds and 60 °C/2 days for stems and leaves). Data presented were mean ± SD with a significance level at P < 0.05.
Acknowledgments
This work was supported in part by a grant from National Science Council of Taiwan (NSC 99-2324-B-415-001) and by Academia Sinica, Taiwan (AS-102-SS-A13) and by the Innovative Translational Agricultural Research Program, Taiwan.
Abbreviations
- CA
carbonic anhydrase
- CKX
cytokinin oxidase/dehydrogenase
- GUS
β-glucuronidase
- hpRNAi
hairpin RNA interference
- hptII
hygromycin phosphotransferase II
- qRT-PCR
quantitative real-time PCR
- QTLs
quantitative trait loci
- RT-PCR
semi-quantitative PCR
- shRNA
short hairpin RNA
Additional files
Expression of OsCKX2 transcript in different rice organs of untransfomed wild type (WT) and in the young stems of CX3- and CX5-suppression lines, as assayed by RT-PCR (A, B) and by northern blot analysis (C). (A) Total RNA was isolated from leaf (L), root (R), stem (S), inflorescence (I), and green floret (F) of untransformed WT. (B) Total RNA was isolated from the young stems of untransformed WT and selected T1 transgenic lines. Expression of actin was included as a cDNA loading control. (C) RNA gel blot analysis of OsCKX2 transcript in the young stems of untransformed WT and selected T1 transgenic lines. Total RNAs isolated from transgenic lines harboring shRNA-CX3 (C-a), and shRNA-CX5 (C-b) were hybridized with a 32P-labelled OsCKX2 probe. Ethidium bromide staining of rRNA indicated an equal loading of RNA in each sample. (DOC 241 kb)
Relative expression levels of rice CKX1 and CKX3 in WT and selected T1 CX3- and CX5-suppression lines, as assayed by qRT-PCR. Total RNA was isolated from the young stems of wild type (WT) and selected T1 CX3-suppression lines (3–5–2, 3–5–7) and CX5-suppression lines (1–2–2, 1–2–4). Water (NC) was included as a negative control and 17S rRNA was used as an internal control for normalization. The expression level of WT was used as a reference. Values presented were mean +/− SD of 3 replicates of cDNA samples. (DOC 70 kb)
(A) pCAMBIA-1300 based construct used for overexpressing maize cytosolic carbonic anhydrase (CA) gene in rice. Maize PEPC gene promoter was used to drive the expression of CA and 35S prmomter driven bar, which encodes DPAT (demethylphosphinothricin acetyltransferase), was used for selection of transgenic plants by the herbicide bialophos. (B) Southern blot analysis of bar in wild type (WT) and selected T0 CKX2-overexpressiom transgenic lines (1, 2, 3, 5, 9, 10) containing the maize carbonic anhydrase gene. Plasmid DNA (P) was used as a positive control. Genomic DNA was digested with HindIII, resolved by electrophoresis and hybridized with DIG labeled bar probe. (C) Location of insert fragment on rice chromosome 1. (DOC 108 kb)
Western blot analysis of carbonic anhydrase (49.5 kDa) in wild type (WT) and T1 null (1–4, 1–18) and CKX2-overexpression heterozygous (1–28, 1–41) and homozygous (1–6, 1–9) lines. Maize was used as a positive control. (DOC 61 kb)
Relative expression levels of three rice tiller related genes, SPL14, D53 and TB1 (A), and CKX1 and CKX3 (B) in representative T2 CKX2-overexpression lines by qRT-PCR. Total RNA was isolated from the shoot apex of wild type (WT) and null lines (1–4–1, 1–18–1), T2 CKX2-overexpression heterozygous (1–28–16, 1–41–6) and homozygous (1–6–2, 1–9–5) lines. 17S rRNA was used as an internal control for normalization. The expression level of WT was used as a reference. Values presented were mean +/− SD of 3 replicates of cDNA samples. (DOC 146 kb)
Significant putative sites present in the amino acid sequence of OsCKX2. (DOC 30 kb)
Predicted N-glycosylation sites in the amino acid sequence of OsCKX2. (DOC 52 kb)
Oligonucleotides used in the study. (DOC 27 kb)
Footnotes
Su-Ying Yeh, Hau-Wen Chen and Chun-Yeung Ng contributed equally to this work.
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
S-YY carried out selection of siRNA target sites, construction of transformation vectors, rice transformation and quantitative real-time PCR. H-WC performed rice transformation and molecular analyses (PCR, RT-PCR, Southern blot and northern blot) and agronomic evaluation of suppression transgenic rice. C-YN characterized insertional activation of OsCKX2 and performed western immunoblotting, qRT-PCR and growth analysis. C-YL prepared antibodies. T-HT performed field experiments. S-YY, W-HL and MSBK wrote manuscript together. All authors approved the final manuscript.
References
- Armstrong DJ. Cytokinin oxidase and the regulation of cytokinin degradation. In: Mok DWS, Mok MC, editors. Cytokinins: chemistry, activity, and function. Boca Raton: CRC; 1994. pp. 139–154. [Google Scholar]
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M. Cytokinin oxidase regulates rice grain production. Science. 2005;309:741–745. doi: 10.1126/science.1113373. [DOI] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmulling T. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus, seed yield in Arabidopsis thaliana. Plant Cell. 2011;23:69–80. doi: 10.1105/tpc.110.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugiere N, Jiao SP, Hantke S, Zinselmeier C, Roessler JA, Niu XM, Jones RJ, Habben JE. Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress. Plant Physiol. 2003;132:1228–1240. doi: 10.1104/pp.102.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Ceriotti A, Duranti M, Bollini R. Effects of N-glycosylation on the folding and structure of plant proteins. J Exp Bot. 1998;49:1091–1103. doi: 10.1093/jxb/49.324.1091. [DOI] [Google Scholar]
- Cheikh N, Jones RJ. Disruption of maize kernel growth and development by heat stress. Plant Physiol. 1994;106:45–51. doi: 10.1104/pp.106.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PW, Chiang CM, Tseng TH, Yu SM. Interaction between rice MYBGA and the gibberellin response element controls tissue-specific sugar sensitivity of α-amylase genes. Plant Cell. 2006;18:2326–2340. doi: 10.1105/tpc.105.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MS, Woo MO, Koh EB, Lee J, Ham TH, Seo HS, Koh HJ. Teosinte Branched 1 modulates tillering in rice plants. Plant Cell Rep. 2012;31:57–65. doi: 10.1007/s00299-011-1139-2. [DOI] [PubMed] [Google Scholar]
- Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica. 2005;142:169–196. doi: 10.1007/s10681-005-1681-5. [DOI] [Google Scholar]
- Dai Z, Ku MSB, Zhang D, Edwards GE. Effects of growth regulators on the induction of Crassulacean acid metabolism in the facultative halophyte Mesembryanthemum crystallinum L. Planta. 1994;192:287–294. doi: 10.1007/BF00198562. [DOI] [Google Scholar]
- Elbashir SM, Martinez J, Patkaniowska A, Lendeckel W, Tuschl T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 2001;20:6877–6888. doi: 10.1093/emboj/20.23.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RA, McCullagh D. Cytokinin-induced chlorophyll formation in cucumber cotyledons. Planta. 1971;101:88–90. doi: 10.1007/BF00387693. [DOI] [PubMed] [Google Scholar]
- Galuszka P, Frébort I, Šebela M, Sauer P, Jacobsen S, Peé P. Cytokinin oxidase or dehydrogenase? Eur J Biochem. 2001;268:450–461. doi: 10.1046/j.1432-1033.2001.01910.x. [DOI] [PubMed] [Google Scholar]
- Galuszka P, Popelkova H, Werner T, Frebortova J, Pospisilova H, Mik V, Kollmer I, Schmülling T, Frebort I. Biochemical characterization of cytokinin oxidases/dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L. J Plant Growth Regul. 2007;26:255–267. doi: 10.1007/s00344-007-9008-5. [DOI] [Google Scholar]
- Jeong D-H, An S, Kang H-G, Moon S, Han J-J, Park S, Lee HS, An K, An G. T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiol. 2002;130:1636–1644. doi: 10.1104/pp.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RJ, Schreiber BMN. Role and function of cytokinin oxidase in plants. J Plant Growth Regul. 1997;23:123–134. doi: 10.1023/A:1005913311266. [DOI] [Google Scholar]
- Kerschen A, Napoli CA, Jorgensen RA, Muller AE. Effectiveness of RNA interference in transgenic plants. FEBS Lett. 2004;566:223–228. doi: 10.1016/j.febslet.2004.04.043. [DOI] [PubMed] [Google Scholar]
- Kim NY, Baek JY, Choi HS, Chung IS, Shin S, Lee JI, Choi JY, Yang JM. Short-hairpin RNA-mediated gene expression interference in Trichoplusiani cells. J Microbiol Biotechnol. 2012;22:190–198. doi: 10.4014/jmb.1108.08045. [DOI] [PubMed] [Google Scholar]
- Kowalska M, Galuszka P, Frebortova J, Sebela M, Beres T, Hluska T, Smehilova M, Bilyeu KD, Frebort I. Vacuolar and cytosolic cytokinin dehydrogenases of Arabidopsis thaliana heterologous expression, purification and properties. Phytochemistry. 2010;71:1970–1978. doi: 10.1016/j.phytochem.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Ku MSB, Agarie S, Nomura M, Fukayama H, Tsuchida H, Ono K, Hirose S, Toki S, Miyao M, Matsuoka M. High-level expression of maize phosphoenolpyruvate carboxylase in transgenic rice plants. Nature Biotech. 1999;17:70–80. doi: 10.1038/5256. [DOI] [PubMed] [Google Scholar]
- Letham DS. Cytokinins as phytohormones – sites of biosynthesis, translocation, and function of translocated cytokinin. In: Mok DWS, Mok MC, editors. Cytokinins: Chemistry, Activity, and Function. Boca Raton: CRC; 1994. pp. 57–80. [Google Scholar]
- Li S, Zhao B, Yuan D, Duan M, Qian Q, Tang L, Wang B, Liu X, Zhang J, Wang J, Sun J, Liu Z, Feng YQ, Yuan L, Li C. Rice Zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc Natl Acad Sci U S A. 2013;110:3167–3172. doi: 10.1073/pnas.1300359110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−DeltaC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo L, Li W, Miura K, Ashikari M, Kyozuka J. Control of tiller growth of rice by OsSPL14 and Strigolactones, which work in two independent pathways. Plant Cell Physiol. 2012;53:1793–1801. doi: 10.1093/pcp/pcs122. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Kikuchi K, Fukuda M, Honda I, Imanishi S. Roles and regulation of cytokinins in tomato fruit development. J Exp Bot. 2012;63:5569–5579. doi: 10.1093/jxb/ers207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Ashikari M, Matsuoka M. The role of QTLs in the breeding of high yielding rice. Trends Plant Sci. 2011;16:319–326. doi: 10.1016/j.tplants.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Mok MC. Cytokinins and plant development: an overview. In: Mok DW, Mok MC, editors. Cytokinins: chemistry, activity, and function. Boca Raton: CRC; 1994. pp. 155–166. [Google Scholar]
- Motyka V, Vanková R, Čapková V, Petrášek J, Kamínek M, Schmülling T. Cytokinin-induced upregulation of cytokinin oxidase activity in tobacco includes changes in enzyme glycosylation and secretion. Physiol Plant. 2003;117:11–21. doi: 10.1034/j.1399-3054.2003.1170102.x. [DOI] [Google Scholar]
- Schmulling T, Werner T, Riefler M, Krupkova E, Bartrina y Manns I. Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J Plant Res. 2003;116:241–252. doi: 10.1007/s10265-003-0096-4. [DOI] [PubMed] [Google Scholar]
- Severino V, Chambery A, Di Maro A, Marasco D, Ruggiero A, Berisio R, Giansanti F, Ippoliti R, Parente A. The role of the glycan moiety on the structure-function relationships of PD-L1, type 1 ribosome-inactivating protein from Phytolacca dioica leaves. Mol Biosyst. 2010;6:570–579. doi: 10.1039/b919801f. [DOI] [PubMed] [Google Scholar]
- Sheu J-J, Yu T-S, Tong W-F, Yu S-M. Carbohydrate starvation stimulates differential expression of rice α-amylase genes that is modulated through complicated transcriptional and posttranscriptional processes. J Biol Chem. 1996;271:26998–27004. doi: 10.1074/jbc.271.43.26998. [DOI] [PubMed] [Google Scholar]
- Sliva K, Schnierle BS. Selective gene silencing by viral delivery of short hairpin RNA. Virol J. 2010;7:248–259. doi: 10.1186/1743-422X-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S. Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Mol Biol Rep. 1997;15:16–21. doi: 10.1007/BF02772109. [DOI] [Google Scholar]
- Tsai YC, Weir NR, Hill K, Zhang W, Kim HJ, Shiu SH, Schaller GE, Kieber JJ. Characterization of genes involved in cytokinin signaling and metabolism from rice. Plant Physiol. 2012;158:1666–1684. doi: 10.1104/pp.111.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Cummings R, Fsko J, Freeze H, Hart G, Marth J. Essentials of glycobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1995. [PubMed] [Google Scholar]
- Wang YH. How effective is T-DNA insertional mutagenesis in Arabidopsis. J Biochem Tech. 2008;1:11–20. [Google Scholar]
- Wang CS, Vodkin LO. Extraction of RNA from tissues containing high levels of procyanidins that bind RNA. Plant Mol Biol Rep. 1994;12:132–145. doi: 10.1007/BF02668374. [DOI] [Google Scholar]
- Wang MB, Abbott DC, Waterhouse PM. A single copy of a virus-derived transgene encoding hairpin RNA gives immunity to barley yellow dwarf virus. Mol Plant Pathol. 2000;1:347–356. doi: 10.1046/j.1364-3703.2000.00038.x. [DOI] [PubMed] [Google Scholar]
- Waterhouse PM, Wang M-B, Lough T. Gene silencing as an adaptive defence against viruses. Nature. 2001;411:834–842. doi: 10.1038/35081168. [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proc Natl Acad Sci U S A. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang J, Huang Z, Wang Z, Zhu Q, Liu L. Correlation of cytokinin levels in the endosperms and roots with cell number and cell division activity during endosperm development in rice. Ann Bot. 2002;90:369–377. doi: 10.1093/aob/mcf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SY, Huang FC, Hoffmann T, Mayershofer M, Schwab W. FaPOD27 functions in the metabolism of polyphenols in strawberry fruit (Fragaria sp.) Front Plant Sci. 2014;5:1–18. doi: 10.3389/fpls.2014.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski W, Galuszka P, Gasparis S, Orczyk W, Nadolska-Orczyk A. Silencing of the HvCKX1 gene decreases the cytokinin oxidase/dehydrogenase level in barley and leads to higher plant productivity. J Exp Bot. 2010;61:1839–1851. doi: 10.1093/jxb/erq052. [DOI] [PubMed] [Google Scholar]
- Zeng Q-w, Qin S, S-q S, Zhang M, Y-h X, Luo M, Hou L, Pei Y. Molecular cloning and characterization of a cytokinin dehydrogenase gene from upland cotton (Gossypium hirsutum L.) Plant Mol Biol Rep. 2012;30:1–9. doi: 10.1007/s11105-011-0308-3. [DOI] [Google Scholar]
- Zhao MM, An DR, Zhao J, Huang GH, He ZH, Chen JY. Transiently expressed short hairpin RNA targeting 126 kDa protein of tobacco mosaic virus interferes with virus infection. Acta Biochim Biophys Sin. 2006;38:22–28. doi: 10.1111/j.1745-7270.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L, Ma W, Gao H, Chen J, Yang C, Wang D, Tan J, Zhang X, Guo X, Wang J, Jiang L, Liu X, Chen W, Chu J, Yan C, Ueno K, Ito S, Asami T, Cheng Z, Wang J, Lei C, Zhai H, Wu C, Wang H, Zheng N, Wan J. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature. 2013;504:406–410. doi: 10.1038/nature12878. [DOI] [PMC free article] [PubMed] [Google Scholar]


