Abstract
The measurement of free immunoglobulin light chains is typically performed on serum; however, the use of alternative specimen types has potential benefits. Using the Freelite™ kappa and lambda free light chains assay on a Roche Diagnostics cobas 8000 c502 analyzer, we compared three specimen types (serum, EDTA-plasma and lithium heparin plasma separator gel-plasma) on 100 patients. Using Deming regression and eliminating outliers (limiting data to light chain concentrations below 400 mg/L), the three specimen types showed comparable results for kappa light chain concentration, lambda light chain concentration, and kappa/lambda ratio with slopes close to 1.0 and y-intercepts close to zero. EDTA-plasma showed slightly more positive bias relative to serum than lithium heparin. Analysis using EDTA-plasma and lithium heparin plasma showed comparable linearity, precision, and temperature stability. A single sample showing hook effect (not in the comparison set) gave comparable results using either plasma specimen type. For the Freelite™ kappa and lambda free light chains assay, both EDTA-plasma or lithium heparin-plasma can serve as acceptable substitutes for serum, at least for the Roche cobas 8000 analyzer.
Keywords: Immunoglobulin light chains, Laboratory automation, Nephelometry, Plasma, Serum
Background
Measurement of kappa and lambda free immunoglobulin light chains in serum has been shown to be valuable in the diagnosis and management of a variety of diseases, especially plasma cell disorders such as multiple myeloma, Waldenström’s macroglobulinemia, AL amyloidosis, and light chain deposition diseases (Bradwell et al. 2001; Dimopoulos et al. 2011; Dispenzieri et al. 2009, 2010; Hoedemakers et al. 2011; Katzmann et al. 2005; Lachmann et al. 2003; Morris et al. 2007; Snozek et al. 2008; Tosi et al. 2013). Serum free light chain analysis is often used in conjunction with serum and urine protein electrophoresis (Hoedemakers et al. 2011; Kim et al. 2014; McTaggart et al. 2013). Serum is the mandatory specimen for protein electrophoresis; thus, the same serum specimen is often used for measurement of free light chains. However, analysis of plasma may have potential practical advantages compared to serum. For example, the ability to use plasma as a specimen for free light chain analysis may limit number of blood collection tubes needed during phlebotomy for some patients (e.g., if plasma but not serum is needed for other tests co-ordered for a patient) or to allow add-on orders for free light chain analysis if serum is not available as a pre-existing specimen (Nelson et al. 2015). The ability to run free light chain analysis on automated chemistry instrumentation typically allows for faster turnaround time than protein electrophoresis, which requires more specialized instrumentation and result interpretation.
In this study we compared the differences between serum and plasma for measurement of kappa and lambda free light chains using the Freelite™ serum free light chain assays on a Roche Diagnostics cobas 8000 c502 analyzer. Plasma specimens obtained from ethylenediaminetetraacetic acid (EDTA)-anticoagulated tubes and lithium heparin plasma separator tubes (PST) were used for the comparisons. A previous study has compared plasma versus serum for another marketed free light chain assay (N Latex FLC) and showed similar results using either specimen type (te Velthuis et al. 2011). Another study compared serum versus serum separator gel and lithium heparin plasma samples for the Freelite™ assay on a Dade Behring BNII analyzer (Hansen et al. 2012). However, there is no published study doing the same comparison for the Freelite™ assay on the Roche cobas system, and the manufacturer instructions for the Freelite™ assay on this analytical platform only list serum as the acceptable specimen type (Freelite™ Human Kappa Free and Human Lambda Free Light Chains package insert.).
Experimental
Sample collection and processing for comparison studies
This study had approval from the University of Iowa Institutional Review Board (protocol #201407792). Testing was performed in the University of Iowa Hospitals and Clinics (UIHC) core clinical laboratory. The layout and informatics of this clinical laboratory has been detailed in previous publications (Krasowski et al. 2014, 2015). The inclusion criteria were: (1) patient who had free light chain analysis performed on a serum specimen, (2) EDTA-anticoagulated and lithium heparin PST specimens drawn on patient for clinical testing during same phlebotomy encounter, and (3) sufficient specimen remaining in the EDTA and PST specimens for light chain analysis after performance of provider-ordered clinical testing. The details on the three specimen types were: BD Vacutainer® red top silica clot activator coated tube (BD Diagnostics, Franklin Lakes, NJ), BD Vacutainer® light green top plasma separator tubes (PST™) containing polymer gel and lithium heparin (BD Diagnostics), and BD Vacutainer® pink top spray coated K2EDTA tube (BD Diagnostics). No extra tubes were drawn on any patient for purposes of this study, i.e., all analyses used pre-existing specimens leftover from clinical testing that would otherwise have been discarded.
Upon completion of the clinically ordered tests, the specimens were transferred to a refrigerator for storage using a Roche Diagnostics (Indianapolis, IN) P701 automated archival retrieval system (Nelson et al. 2015). Samples were stored for up to 16 h until they were retrieved for use in the study. When samples were retrieved, they were centrifuged and loaded on to the Roche Diagnostics cobas 8000 Modular Analytics System c502 analyzer and assayed using the Freelite™ kappa and lambda free light chains assay (Freelite™ Human Kappa Free and Human Lambda free light chains package insert 2001).
Following the package insert procedure, kappa light chain measurements for serum specimens are linear to 56.25 mg/L. Values that exceed 56.25 mg/L are treated with ×10 dilution with saline. Values that still exceed linearity require a manual ×21 dilution with saline. Lambda light chain measurements are linear to 93.33 mg/L. Similar to the procedure with kappa light chains, lambda light chain values that exceed 93.33 mg/L are treated with ×10 dilution with saline. Values that still exceed linearity require a manual ×21 dilution with saline.
Linearity studies
Linearity was determined according to CLSI guideline EP6A (Clinical and Laboratory Standards Institute 2003) using plasma samples just above the upper measuring range for serum, i.e., 56.25 mg/L for kappa and 93.33 mg/L for lambda. At least 10 dilutions of 90–2.5 % were measured for both EDTA-plasma and lithium heparin PST matrices. Five replicates for each dilution were measured. The mean result was analyzed by linear and cubic analysis. Fits were evaluated using the Microsoft Excel add-in Analyze-it®.
Method imprecision
The precision study was performed according to CLSI EP5-A2 guideline (Clinical and Laboratory Standards Institute 2004). Plasma pools were made from routine patient samples that had no detectable monoclonal bands with serum electrophoresis and immunotyping.
Reference ranges and medical decision levels
The normal (reference) ranges for the free light chains following manufacturer recommendations in the package insert are: kappa (3.30–19.40 mg/L), lambda (5.71–26.30 mg/L), kappa/lambda ratio (0.26–1.65). The lower and upper limits of the reference ranges for serum in the assay package insert were considered the medical decision levels (MDL). Assay measurement using serum (specimen type recommended in package insert) was considered the gold standard.
Statistical analysis
Linear regression and statistical analysis was performed using EP Evaluator release 11 (Data Innovations, Inc., South Burlington, VT). Deming linear regression was performed. Identification of outliers used an algorithm in EP Evaluator that identifies points whose distance from the regression line exceeds 10 times the standard error of estimate (SEE), where SEE is computed from the data set with outliers excluded.
Results
Precision studies
The precision of the kappa and lambda light chain assays at different levels of control material are summarized in Table 1. The coefficient of variation (CV) was less than 5 % for the within- and between-run precision studies. The results of the precision studies for plasma pools are summarized in Fig. 1. The % CV was generally 5–7 % or less across most of the measuring range except for kappa and lambda concentrations less than 5 mg/L. At concentrations near 1 mg/L, the % CV values approach 20 %.
Table 1.
Precision using two different levels of control material
| Light chain assay | Mean (mg/L) | Within-run imprecision % CV (n = 10) | Between-run imprecision % CV (n = 20) |
|---|---|---|---|
| Kappa | 17.4 | 2.9 | 4.8 |
| Kappa | 34.1 | 3.5 | 4.6 |
| Lambda | 30.0 | 3.4 | 4.9 |
| Lamdba | 63.3 | 2.6 | 4.5 |
CV coefficient of variation
Fig. 1.
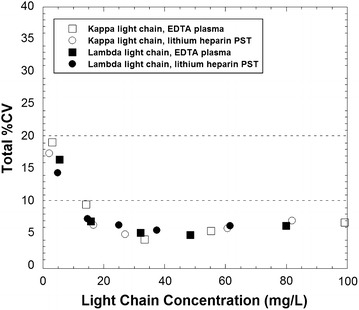
Precision profile of the total coefficient of variation (CV) estimated according to CLSI EP5-A2. Squares (open and filled) indicate kappa and lambda concentrations, respectively, using EDTA-plasma. Circles (open and filled) indicate kappa and lambda concentrations, respectively, using lithium heparin plasma separator tubes
Linearity studies
Serial dilutions of samples with a concentration just above the measuring range (56.25 mg/L for kappa and 93.33 mg/L for lambda) were prepared. For kappa, linearity was confirmed between 1.0 and 56.25 mg/L for both EDTA-plasma and lithium heparin PST (maximum difference between linear and cubic fit of 18.8 %). For lambda, linearity was confirmed between 0.8 and 93.33 mg/L (maximum difference between linear and cubic fit of 17.4 %).
Method comparison
Samples from a total of 100 patients met the inclusion criteria for this study with sufficient serum, EDTA-plasma, and lithium heparin-plasma for free light chain analysis. Scatterplots for kappa, lambda and kappa/lambda ratio are shown for all 100 samples in Fig. 2 and for the subset of kappa and lambda values less than 400 mg/L in Fig. 3. By classifying the specimens on the basis of the reference intervals, there was 84 and 86 % agreement, respectively, between serum and either EDTA-plasma or lithium heparin PST specimens for the kappa light chain assay (Table 2). The agreement rate was 95 % for the lambda light chain assays (serum versus either EDTA-plasma and lithium heparin plasma) and 98 % for the kappa/lambda ratio (serum versus either EDTA-plasma and lithium heparin plasma) (Table 2). All of the discrepancies with respect to reference interval were the result of the plasma result being higher than the value obtained from serum (Table 2). A summary of the specimens showing discrepancies is in Table 3, with clinical history and age/gender of patient described.
Fig. 2.
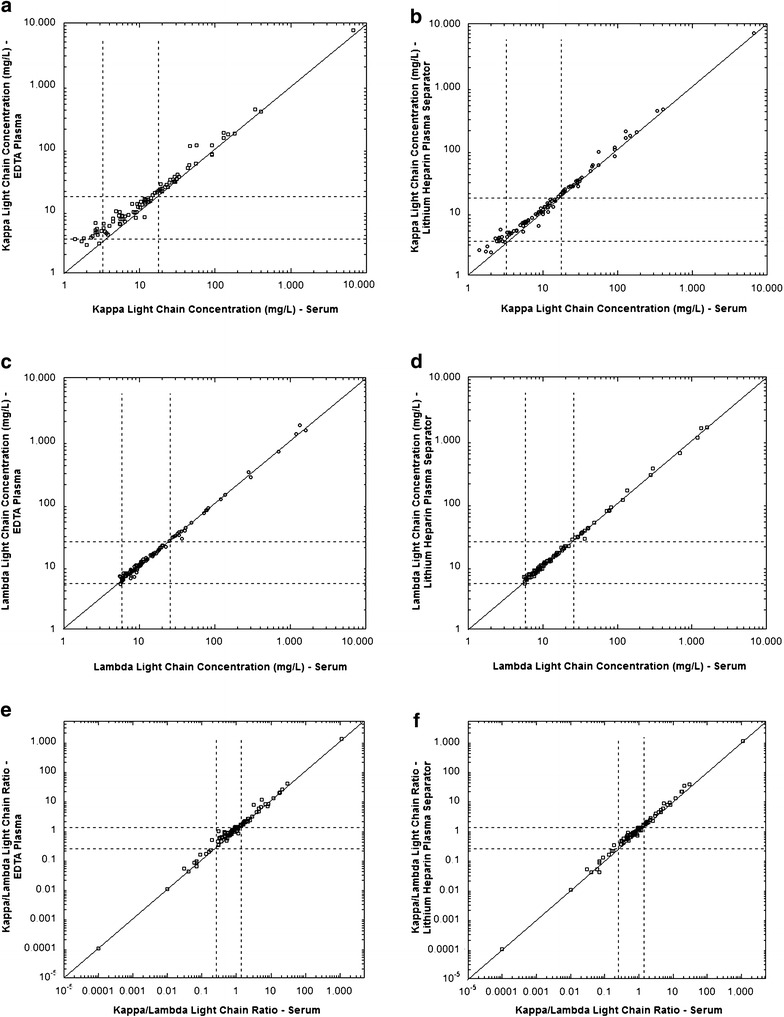
Comparisons of serum versus EDTA-plasma or lithium heparin-plasma separator tube as specimens for measurement of free light chains (n = 100). The graphs are scatter plots of data for kappa concentration (a, b), lambda concentration (c, d), and kappa/lambda ratio (e, f). The line is the line of identity
Fig. 3.
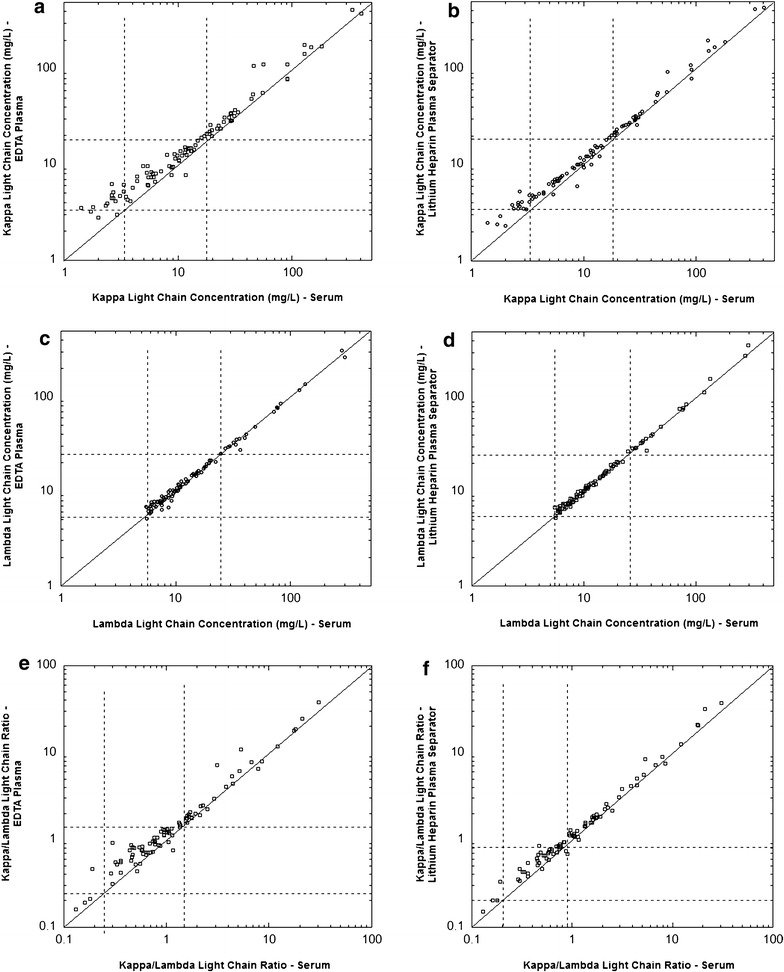
Comparisons of serum versus EDTA-plasma or lithium heparin-plasma separator tube as specimens for measurement of free light chains showing subset of data. The graphs are scatter plots of data for kappa concentration (a, b; restricted to 400 mg/L or less), lambda concentration (c, d; restricted to 400 mg/L or less), and kappa/lambda ratio (e, f; restricted to 100 or less). The line is the line of identity
Table 2.
Concordance tables between plasma and serum for light chain analysis
| Assay | Comparison | Identical % | Serum below ref. range/plasma within ref. range | Serum within ref. range/plasma above ref. range |
|---|---|---|---|---|
| Kappa | EDTA vs. serum | 84 | 11 | 5 |
| Kappa | Lithium heparin PST vs. serum | 86 | 10 | 4 |
| Lambda | EDTA vs. serum | 98 | 2 | 0 |
| Lambda | Lithium heparin PST vs. serum | 98 | 1 | 1 |
| Kappa/lambda ratio | EDTA vs. serum | 95 | 1 | 4 |
| Kappa/lambda ratio | Lithium heparin PST vs. serum | 95 | 1 | 4 |
Table 3.
Samples with discrepancy with respect to reference intervals
| Patient age, gender, and clinical history | Serum | EDTA-plasma | Lithium heparin PST | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Kappa (mg/L) | Lambda (mg/L) | K/L | Kappa (mg/L) | Lambda (mg/L) | K/L | Kappa (mg/L) | Lambda (mg/L) | K/L | |
| Discrepancy involving kappa | |||||||||
| 60 Y M, multiple myeloma, IgG kappa | 2.6 | 5.9 | 0.44 | 4.4 | 5.8 | 0.76 | 3.8 | 6.2 | 0.61 |
| 66 Y F, multiple myeloma, IgG lambda | 2.7 | 5.8 | 0.47 | 4.4 | 6.6 | 0.67 | 3.7 | 6.8 | 0.54 |
| 44 Y F, multiple myeloma, IgG lambda | 17.8 | 40.0 | 0.45 | 20.8 | 36.8 | 0.57 | 20.2 | 39.5 | 0.51 |
| 60 Y F, multiple myeloma, IgG lambda | 18.8 | 10.7 | 1.76 | 20.8 | 11.6 | 1.79 | 20.1 | 11.1 | 1.81 |
| 61 Y M, multiple myeloma, IgG kappa | 2.8 | 6.1 | 0.46 | 4.1 | 6.5 | 0.63 | 4.1 | 6.1 | 0.67 |
| 51 Y M, multiple myeloma, IgG kappa | 2.7 | 5.8 | 0.47 | 5.1 | 6.2 | 0.82 | 5.3 | 6.2 | 0.85 |
| 86 Y F, multiple myeloma, lambda light chain | 2.3 | 76 | 0.03 | 3.7 | 77.5 | 0.05 | 3.8 | 75.1 | 0.05 |
| 60 Y F, multiple myeloma, IgA lambda | 2.4 | 6.7 | 0.36 | 3.9 | 6.9 | 0.57 | 3.5 | 6.5 | 0.54 |
| 70 Y F, multiple myeloma, IgA lambda | 2.6 | 8.1 | 0.32 | 4.8 | 8.7 | 0.55 | 3.5 | 8.2 | 0.43 |
| 39 Y M, multiple myeloma, IgG lambda | 2.6 | 8.6 | 0.30 | 6.3 | 6.8 | 0.93 | 4 | 8.7 | 0.46 |
| 60 Y M, hairy cell leukemia | 19.1 | 18.7 | 1.02 | 26.1 | 19.6 | 1.33 | 21.8 | 19.7 | 1.11 |
| 62 Y M, biclonal IgG kappa + lambda light chaina | 3.1 | 1195 | <0.01 | 4.7 | 1275 | <0.01 | 3.4 | 1103 | <0.01 |
| 66 Y F, multiple myeloma, lambda light chaina | 17.0 | 296 | 0.06 | 19.7 | 263 | 0.08 | 19.3 | 359 | 0.05 |
| Discrepancy involving kappa and lambda | |||||||||
| 53 Y F, multiple myeloma, IgG kappa | 18.9 | 24.7 | 0.77 | 21.9 | 25.0 | 0.88 | 21.9 | 26.6 | 0.82 |
| 84 Y M, multiple myeloma, lamba light chain | 2.9 | 5.6 | 0.52 | 3.0 | 6.8 | 0.44 | 3.5 | 5.3 | 0.66 |
| 51 Y M, multiple myeloma, IgG kappa | 1.8 | 5.5 | 0.33 | 3.6 | 6.9 | 0.52 | 2.9 | 6.8 | 0.43 |
| Discrepancy involving kappa and kappa/lambda ratio | |||||||||
| 54 Y F, multiple myeloma, kappa light chain | 1.4 | 7.2 | 0.19 | 3.5 | 7.6 | 0.46 | 2.5 | 7.6 | 0.33 |
| Discrepancy involving kappa/lambda ratio | |||||||||
| 67 Y M, multiple myeloma, IgG kappa | 29.1 | 18.3 | 1.59 | 34.4 | 18.4 | 1.87 | 29.3 | 18.5 | 1.58 |
| 64 Y M, multiple myeloma, kappa light chain | 21.9 | 13.8 | 1.59 | 23.7 | 14.9 | 1.59 | 25.1 | 13.9 | 1.81 |
| 57 Y F, multiple myeloma, IgG lambda | 25.1 | 15.7 | 1.60 | 27.7 | 15.6 | 1.78 | 26.2 | 15.5 | 1.69 |
| 55 Y M, multiple myeloma, lambda light chain | 12.1 | 7.4 | 1.64 | 14.8 | 7.5 | 1.97 | 13.9 | 7.4 | 1.88 |
| 59 Y M, Waldenstroms, IgM lambda | 19.8 | 12.6 | 1.57 | 22.7 | 12.7 | 1.79 | 23.5 | 12.9 | 1.82 |
PST plasma separator tube
aAlso identified as outlier for lambda light chain analysis by Deming regression (Table 4)
Comparison between serum and the two plasma specimen types was done by Deming linear regression. Outliers identified in the analysis were predominantly due to specimens with kappa and lambda concentrations exceeding 400 mg/L (thus Fig. 3 is restricted to kappa and lambda concentrations less than 400 mg/L). The outliers are summarized in Table 4 (some of these overlapped with the specimens described in Table 3).
Table 4.
Outliers identified by Deming regression analysis
| Patient age, gender, and clinical history | Serum | EDTA-plasma | Lithium heparin PST | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Kappa (mg/L) | Lambda (mg/L) | K/L | Kappa (mg/L) | Lambda (mg/L) | K/L | Kappa (mg/L) | Lambda (mg/L) | K/L | |
| Kappa outliers | |||||||||
| 60 Y M, multiple myeloma, kappa light chain | 46.1 | 14.7 | 3.14 | 107 | 15.0 | 7.16 | 55.8 | 14.7 | 3.80 |
| 59 Y F, multiple myeloma, IgG kappa | 6684 | 6.1 | 1096 | 7764 | 6.1 | 1273 | 7125 | 6.7 | 1063 |
| 52 Y M, multiple myeloma, IgG kappa | 337 | 11.0 | 30.6 | 419 | 11.1 | 37.8 | 415 | 11.2 | 37.1 |
| 60 Y M, multiple myeloma, IgG kappa | 56.2 | 10.5 | 5.35 | 112 | 10.3 | 10.87 | 91.9 | 10.8 | 8.51 |
| 59 Y M, multiple myeloma, IgG kappa | 128 | 6.1 | 21.03 | 177 | 7.2 | 24.6 | 197 | 6.2 | 31.8 |
| Lambda outliers | |||||||||
| 60 Y M, plasma cell leukemia, IgG lambda | 4.0 | 693 | 0.01 | 5.7 | 672 | 0.01 | 5.0 | 621 | 0.01 |
| 74 Y F, multiple myeloma, IgG lambda | 4.4 | 1590 | 0.00 | 6.7 | 1457 | 0.00 | 5.2 | 1571 | 0.00 |
| 55 Y F, multiple myeloma, lambda light chain | 11.8 | 1334 | 0.01 | 12.7 | 1747 | 0.01 | 12.0 | 1562 | 0.01 |
| 65 Y M, multiple myeloma, lambda light chain | 9.9 | 278 | 0.04 | 12.5 | 311 | 0.04 | 10.1 | 278 | 0.04 |
| 59 Y F, acute renal failure, seropositive rheumatoid arthritis | 29.8 | 36.4 | 0.82 | 28.8 | 27.2 | 1.06 | 25.9 | 27.4 | 0.95 |
| 62 Y M, biclonal IgG kappa + lambda light chain | 3.1 | 1195 | <0.01 | 4.7 | 1275 | <0.01 | 3.4 | 1103 | <0.01 |
| 66 Y F, multiple myeloma, lambda light chain | 17.0 | 296 | 0.06 | 19.7 | 263 | 0.08 | 19.3 | 359 | 0.05 |
PST plasma separator tube
Linear regression parameters are summarized in Table 5. For all comparisons, slopes are close to 1.0. A slightly more noticeable positive bias was noted with EDTA-plasma versus serum. For lithium heparin PST versus serum, the confidence intervals for the slope and y-intercept for kappa light chain, lambda light chain, and kappa/lambda ratio overlapped with 1.000 and 0.0, respectively. At the MDL, the confidence intervals overlapped with that for serum.
Table 5.
Linear regression summary statistics of specimen comparisonsa
| Slope (95 % CI)b | Y-intercept (95 % CI)b (mg/L) | Correlation coefficient | 95 % CI at lower MDLc (mg/L) | 95 % CI at upper MDLc (mg/L) | |
|---|---|---|---|---|---|
| EDTA vs. serum | |||||
| Kappa | 0.969 (0.950–0.988) | 2.58 (1.54–3.64) | 0.9954 | 4.8–6.8 | 20.4–22.4 |
| Lambda | 0.995 (0.986–1.003) | 0.367 (0.127–0.608) | 0.9992 | 5.8–6.3 | 26.3–26.7 |
| κ/λ ratio | 1.001 (0.982–1.021) | 0.133 (0.066–0.199) | 0.9957 | 0.33–0.46 | 1.73–1.84 |
| Li-heparin plasma separator tube vs. serum | |||||
| Kappa | 1.081 (1.063–1.100) | 0.64 (−0.38 to 1.67) | 0.9964 | 3.2–5.2 | 19.4–22.5 |
| Lambda | 0.988 (0.981–1.000) | 0.279 (−0.101 to 0.557) | 1.0000 | 5.7–6.3 | 26.1–26.6 |
| κ/λ ratio | 1.175 (1.154–1.196) | −0.091 (−0.188 to 0.007) | 0.9961 | 0.12–0.31 | 1.65–1.83 |
aAnalysis uses Deming regression excluding outliers (see Table 4)
b CI confidence interval
c MDL medical decision limit: kappa, 3.3 and 19.4 mg/L; lambda, 5.7 and 26.3 mg/L; κ/λ ratio, 0.26 and 1.65
Stability studies
Stability studies were performed for pooled plasma samples stored either refrigerated (4 °C) or frozen at −20 °C. Results were generally within 10 % of those obtained at initial measurement (Fig. 4). The highest variability was seen at kappa and lambda concentrations less than 10 mg/L, similar to that described above for the precision studies (Fig. 1).
Fig. 4.
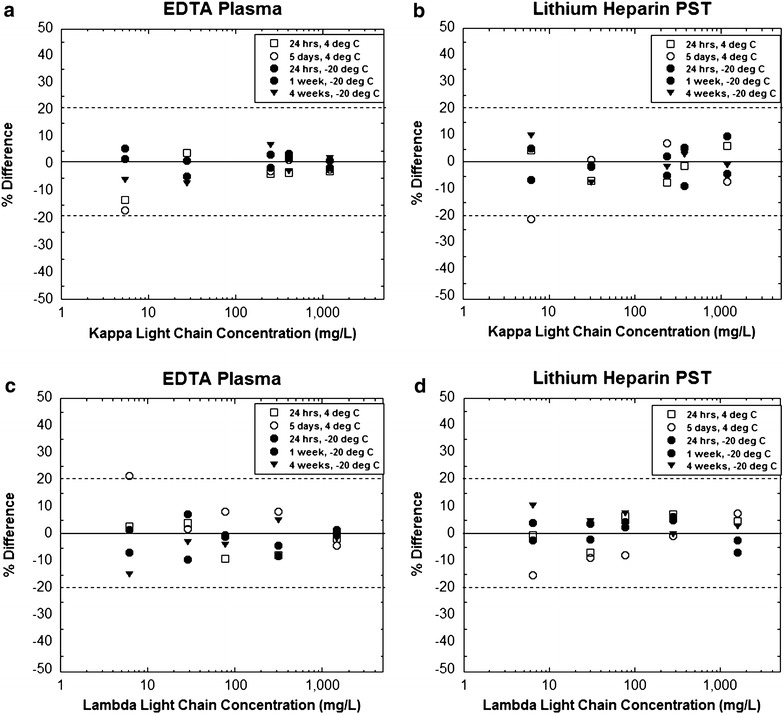
Stability of free light chain measurements at different storage conditions. The plot shows the percent change relative to initial analysis for kappa (a, b) and lambda (c, d) measurements using EDTA-plasma and lithium heparin plasma separator tube (PST) specimens
Antigen excess
During the time period of study, a single specimen was analyzed that showed marked hook effect for lambda light chain. This specimen was from a patient whose specimens had previously shown hook effect during multiple occasions. Specimens from this patient were not in the comparison studies (occurred after those studies completed). The hook effect was comparable in EDTA-plasma and lithium heparin PST specimen. In particular, the apparent lambda light chain concentration in undiluted specimens was 89.3 and 89.8 mg/L, respectively, for EDTA-plasma and lithium heparin PST specimens. Dilution analysis shown the actual lambda concentrations to be 4053 and 3862 mg/L, respectively, in these two sample types.
Discussion
Overall, EDTA-plasma and lithium heparin-plasma gave comparable results to serum for kappa light chain concentration, lambda light chain concentration, and kappa/lambda ratio for the Freelite™ assays performed on the cobas 8000 c502 analyzer. Similar to previous reports (Hansen et al. 2012; te Velthuis et al. 2011), these data suggest that plasma is an acceptable specimen for free light chain analysis. The precision, linearity, and analyte stability in plasma is also comparable to that described for serum in previous publications (Altinier et al. 2013; Briand et al. 2010; Cha et al. 2014; Hansen et al. 2012; Pretorius et al. 2012; Tate et al. 2003, 2009; Vercammen et al. 2015).
The highest variability seen in our study was with very low or very high kappa and lambda concentrations. This was evident in the regression outlier analysis. The light chain analysis procedure in the package insert for the Freelite™ assay on the cobas 8000 analyzers requires two dilutions (one on-line and one manual) for the highest concentrations (kappa greater than 563 mg/L and lambda greater than 933 mg/L). These dilutions offer potential for error. At high concentrations of free light chains, antigen excess is also possible (Bosmann et al. 2010; Vercammen et al. 2015). We did not study antigen excess in detail but did observe comparable results with both plasma specimen types in a patient previously observed to have hook effect with lambda concentrations. Specimens with very low values of kappa or lambda can also lead to higher variability in the kappa/lambda ratio, especially with the high imprecision typical of analyses in these low concentration ranges (Altinier et al. 2013; Briand et al. 2010; Cha et al. 2014; Hansen et al. 2012; Pretorius et al. 2012; Tate et al. 2003, 2009; Vercammen et al. 2015).
While plasma gave comparable results to serum in our study, it is probably prudent to use a single specimen type and analyzer platform for patient analysis. Reference intervals and medical decision levels should be reassessed carefully for any specimen type other than serum. The trending of light chain values is used for treatment decisions and consistency in analysis is important. Future studies can focus on different instrument platforms and assay formats. A larger set of samples can also facilitate detailed studies on how plasma compares with serum with respect to antigen excess.
Author’s contributions
LSN and CSM performed the data collection. LSN and BS performed data analysis and drafted the manuscript. MDK was involved in the study design, interpretation, and data analysis and also completed the final draft of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
MDK thanks the Department of Pathology (Dr. Nitin Karandikar, Department Executive Officer) for providing research funding. The authors thank Priyal Patel for assistance with the study of the sample showing hook effect.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Louis S. Nelson, Email: louisstevennelson@gmail.com
Bryan Steussy, Email: bryan.steussy@gmail.com.
Cory S. Morris, Email: cory-morris@uiowa.edu
Matthew D. Krasowski, Phone: 1-319-384-9380, Email: matthew-krasowski@uiowa.edu
References
- Altinier S, Seguso M, Zaninotto M, Varagnolo M, Adami F, Angeli P, Plebani M. Serum free light chain reference values: a critical approach. Clin Biochem. 2013;46:691–693. doi: 10.1016/j.clinbiochem.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Bosmann M, Kossler J, Stolz H, Walter U, Knop S, Steigerwald U. Detection of serum free light chains: the problem with antigen excess. Clin Chem Lab Med. 2010;48:1419–1422. doi: 10.1515/CCLM.2010.283. [DOI] [PubMed] [Google Scholar]
- Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, Drew R. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47:673–680. [PubMed] [Google Scholar]
- Briand PY, Decaux O, Caillon H, Grosbois B, Le Treut A, Guenet L. Analytical performance of the serum free light chain assay. Clin Chem Lab Med. 2010;48:73–79. doi: 10.1515/CCLM.2010.012. [DOI] [PubMed] [Google Scholar]
- Cha KH, Sim YB, Chae H, Park HI, Kim M, Kim Y. The analytical performance evaluation of Freelite Human Kappa Free and Human Lambda Free on the SPAPLUS immunoturbidimetric analyzer. J Clin Lab Anal. 2014;28:229–236. doi: 10.1002/jcla.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute . Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. Wayne, PA: CLSI document EP06-A, CLSI; 2003. [Google Scholar]
- Clinical and Laboratory Standards Institute . Evaluation of precision performance of quantitative measurement methods. Wayne, PA: CLSI document EP05-A2, CLSI; 2004. [Google Scholar]
- Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, Ludwig H, Joshua D, Mehta J, Gertz M, Avet-Loiseau H, Beksac M, Anderson KC, Moreau P, Singhal S, Goldschmidt H, Boccadoro M, Kumar S, Giralt S, Munshi NC, Jagannath S. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701–4705. doi: 10.1182/blood-2010-10-299529. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A, Kyle R, Merlini G, Miguel JS, Ludwig H, Hajek R, Palumbo A, Jagannath S, Blade J, Lonial S, Dimopoulos M, Comenzo R, Einsele H, Barlogie B, Anderson K, Gertz M, Harousseau JL, Attal M, Tosi P, Sonneveld P, Boccadoro M, Morgan G, Richardson P, Sezer O, Mateos MV, Cavo M, Joshua D, Turesson I, Chen W, Shimizu K, Powles R, Rajkumar SV, Durie BG. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ, 3rd, Colby CL, Therneau TM, Clark R, Kumar SK, Bradwell A, Fonseca R, Jelinek DF, Rajkumar SV. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–1728. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freelite™ Human Kappa Free and Human Lambda free light chains package insert (2001) The Binding Site Group Ltd., Birmhingham, UK
- Hansen CT, Munster AM, Nielsen L, Pedersen P, Abildgaard N. Clinical and preclinical validation of the serum free light chain assay: identification of the critical difference for optimized clinical use. Eur J Haematol. 2012;89:458–468. doi: 10.1111/ejh.12013. [DOI] [PubMed] [Google Scholar]
- Hoedemakers RM, Pruijt JF, Hol S, Teunissen E, Martens H, Stam P, Melsert R, Te Velthuis H. Clinical comparison of new monoclonal antibody-based nephelometric assays for free light chain kappa and lambda to polyclonal antibody-based assays and immunofixation electrophoresis. Clin Chem Lab Med. 2011;50:489–495. doi: 10.1515/CCLM.2011.793. [DOI] [PubMed] [Google Scholar]
- Katzmann JA, Abraham RS, Dispenzieri A, Lust JA, Kyle RA. Diagnostic performance of quantitative kappa and lambda free light chain assays in clinical practice. Clin Chem. 2005;51:878–881. doi: 10.1373/clinchem.2004.046870. [DOI] [PubMed] [Google Scholar]
- Kim HS, Shin KS, Song W, Kim HJ, Park MJ. Clinical comparisons of two free light chain assays to immunofixation electrophoresis for detecting monoclonal gammopathy. Biomed Res Int. 2014;2014:647238. doi: 10.1155/2014/647238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowski MD, Davis SR, Drees D, Morris C, Kulhavy J, Crone C, Bebber T, Clark I, Nelson DL, Teul S, Voss D, Aman D, Fahnle J, Blau JL. Autoverification in a core clinical chemistry laboratory at an academic medical center. J Pathol Inform. 2014;5:13. doi: 10.4103/2153-3539.129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowski MD, Chudzik D, Dolezal A, Steussy B, Gailey MP, Koch B, Kilborn SB, Darbro BW, Rysgaard CD, Klesney-Tait JA. Promoting improved utilization of laboratory testing through changes in an electronic medical record: experience at an academic medical center. BMC Med Inform Decis Mak. 2015;15:11. doi: 10.1186/s12911-015-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann HJ, Gallimore R, Gillmore JD, Carr-Smith HD, Bradwell AR, Pepys MB, Hawkins PN. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 2003;122:78–84. doi: 10.1046/j.1365-2141.2003.04433.x. [DOI] [PubMed] [Google Scholar]
- McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis with serum free light chain analysis as a first-line test for detecting plasma cell disorders offers increased diagnostic accuracy and potential health benefit to patients. Am J Clin Pathol. 2013;140:890–897. doi: 10.1309/AJCP25IHYLEWCAHJ. [DOI] [PubMed] [Google Scholar]
- Morris KL, Tate JR, Gill D, Kennedy G, Wellwood J, Marlton P, Bird R, Mills AK, Mollee P. Diagnostic and prognostic utility of the serum free light chain assay in patients with AL amyloidosis. Intern Med J. 2007;37:456–463. doi: 10.1111/j.1445-5994.2007.01368.x. [DOI] [PubMed] [Google Scholar]
- Nelson LS, Davis SR, Humble RM, Kulhavy J, Aman DR, Krasowski MD. Impact of add-on laboratory testing at an academic medical center: a 5 year retrospective study. BMC Clin Pathol. 2015;15:11. doi: 10.1186/s12907-015-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius CJ, Klingberg S, Tate J, Wilgen U, Ungerer JP. Evaluation of the N Latex FLC free light chain assay on the Siemens BN analyser: precision, agreement, linearity and variation between reagent lots. Ann Clin Biochem. 2012;49:450–455. doi: 10.1258/acb.2012.011264. [DOI] [PubMed] [Google Scholar]
- Snozek CL, Katzmann JA, Kyle RA, Dispenzieri A, Larson DR, Therneau TM, Melton LJ, 3rd, Kumar S, Greipp PR, Clark RJ, Rajkumar SV. Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: proposed incorporation into the international staging system. Leukemia. 2008;22:1933–1937. doi: 10.1038/leu.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JR, Gill D, Cobcroft R, Hickman PE. Practical considerations for the measurement of free light chains in serum. Clin Chem. 2003;49:1252–1257. doi: 10.1373/49.8.1252. [DOI] [PubMed] [Google Scholar]
- Tate J, Bazeley S, Sykes S, Mollee P. Quantitative serum free light chain assay–analytical issues. Clin Biochem Rev. 2009;30:131–140. [PMC free article] [PubMed] [Google Scholar]
- te Velthuis H, Knop I, Stam P, van den Broek M, Bos HK, Hol S, Teunissen E, Fischedick KS, Althaus H, Schmidt B, Wagner C, Melsert R. N Latex FLC—new monoclonal high-performance assays for the determination of free light chain kappa and lambda. Clin Chem Lab Med. 2011;49:1323–1332. doi: 10.1515/CCLM.2011.624. [DOI] [PubMed] [Google Scholar]
- Tosi P, Tomassetti S, Merli A, Polli V. Serum free light-chain assay for the detection and monitoring of multiple myeloma and related conditions. Ther Adv Hematol. 2013;4:37–41. doi: 10.1177/2040620712466863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen MJ, Broodtaerts L, Meirlaen P, Bossuyt X. Overestimation of free light chain antigen excess rate. Clin Chim Acta. 2015;444:297–302. doi: 10.1016/j.cca.2015.02.047. [DOI] [PubMed] [Google Scholar]


