Abstract
Purpose
ABT-751 is an antimitotic and vascular disrupting agent with potent preclinical anticancer activity. We conducted a phase I and randomized double-blind phase II study of pemetrexed with ABT-751 or placebo in patients with recurrent advanced or metastatic non–small-cell lung cancer (NSCLC).
Methods
One hundred seventy-one patients received intravenous pemetrexed 500 mg/m2 day 1 and oral ABT-751 or placebo days 1 to 14 of 21-day cycles. The primary end point was progression-free survival (PFS). Secondary end point included overall survival (OS); pharmacokinetic and pharmacodynamic parameters were also analyzed.
Results
The recommended phase II dose of ABT-751 with pemetrexed is 200 mg. Fatigue, constipation, anemia, nausea, and diarrhea were the most common toxicities in both study arms. No pharmacokinetic interactions were observed. Median PFS in the ABT-751 arm was 2.3 months versus 1.9 for placebo (P = .819, log-rank) for the intention-to-treat population. However, differences in PFS (P = .112, log-rank) and OS (P = .034, log-rank; median 3.3 v 8.1 months) favoring ABT-751 were seen in the squamous NSCLC subgroup. Baseline circulating tumor cell concentrations were predictive of improved OS (P = .013). Changes from baseline of greater than 20% in plasma levels of placenta growth factor (P = .056), squamous cell carcinoma antigen (P = .03), and cytokeratin 19 fragment antigen 21-1 (P = .01) were markers best associated with improved OS.
Conclusion
Addition of ABT-751 to pemetrexed is well-tolerated, but does not improve outcome in unselected patients with recurrent NSCLC. ABT-751 may have therapeutic potential in squamous NSCLC. Exploratory cellular and molecular analyses in this study identified biomarkers that may correlate with survival.
INTRODUCTION
Limited therapeutic options exist for recurrent and progressive metastatic non–small-cell lung cancer (NSCLC). Agents approved for the management of NSCLC in this setting include pemetrexed, docetaxel, and erlotinib.1–3 A phase III study of previously treated advanced NSCLC demonstrated that the median overall survival (OS) of pemetrexed-treated patients was comparable to that of docetaxel-treated patients (hazard ratio [HR], 0.99; N = 571), with pemetrexed-treated patients having significantly lower incidences of grade 3 or 4 neutropenia, neutropenia with infections, febrile neutropenia, and hospitalizations for febrile neutropenia.4 When this study was initiated, all three of these agents were approved for use in NSCLC regardless of histologic subtype. Subsequent analyses have suggested that pemetrexed has substantial activity against nonsquamous NSCLC, but minimal activity against squamous cell NSCLC.5–7 This has prompted a change in the US Food and Drug Administration label for pemetrexed, restricting use to nonsquamous NSCLC.8
ABT-751 is an oral antimitotic sulfonamide that binds to the colchicine binding site on β-tubulin, inhibiting polymerization of microtubules.9,10 ABT-751 is not a substrate for the multiple drug resistance transporter.9 In addition to direct cytotoxic effects, ABT-751 acts as a vascular disrupting agent, reducing tumor-associated blood flow.11 ABT-751 exhibits broad-spectrum antitumor activity in vitro, including against cell lines resistant to other antimicrotubule agents.9 ABT-751 is active in multiple human tumor xenograft models, as a single agent and in combination with standard cytotoxics and radiation.12,13 Administration of ABT-751 to adult and pediatric patients in phase I studies resulted in rapid absorption and elimination, and dose proportional, time-independent pharmacokinetics (PK).14,15 In a phase II study, ABT-751–treated patients with taxane-refractory NSCLC had a median OS of 8.4 months and a median progression-free survival (PFS) of 2.1 months (n = 35). ABT-751 was well tolerated, with treatment-related grade 3 or 4 adverse events (AEs) limited to fatigue (17%), constipation (9%), and dehydration (9%).16 Notably, ABT-751 was not associated with myelosuppression, suggesting that it may be safely combined with myelosuppressive chemotherapeutic agents.17
Based on antitumor activity and favorable tolerability profiles as single agents, we hypothesized that the combination of ABT-751 and pemetrexed might have therapeutic potential in patients with advanced NSCLC. To test this hypothesis, we conducted a phase I dose-escalation study of the combination, leading into a placebo-controlled, double-blind, randomized phase II study. Maximum-tolerated dose (MTD) determinations from previous phase II monotherapy studies guided the selection of 200 mg once daily ABT-751 as the initial dose in the phase I portion of this trial.17–20 The objectives of this study were to compare the PFS, OS, safety, PK, and pharmacodynamics of ABT-751 plus pemetrexed versus pemetrexed monotherapy in patients with previously treated advanced-stage NSCLC. Given the recent data demonstrating selective activity of pemetrexed in nonsquamous NSCLC, we have retrospectively analyzed clinical outcome data from this study based on histologic subtype (squamous v nonsquamous).
METHODS
Patient Population
Eligible candidates for this study (NCT00297089) were adults with histologically or cytologically confirmed locally advanced (stage III or IV) NSCLC not amenable to curative surgery or radiotherapy, who had received only one prior antitumor regimen for metastatic disease. One additional regimen in the neoadjuvant or adjuvant setting was allowed. Patients enrolled in phase II were required to have experienced disease progression during or after the previous therapy, and to exhibit measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST).21 Eligible patients had an Eastern Cooperative Oncology Group performance status of 0 to 2 and a life expectancy of ≥ 3 months. Patients were excluded if they had class 3 or 4 cardiovascular disability or a current or previous malignancy at other sites, with certain protocol-defined exceptions.
This study was conducted according to the Declaration of Helsinki and with approval from institutional review boards of all participating study sites. All participants provided written informed consent before participating.
Study Design
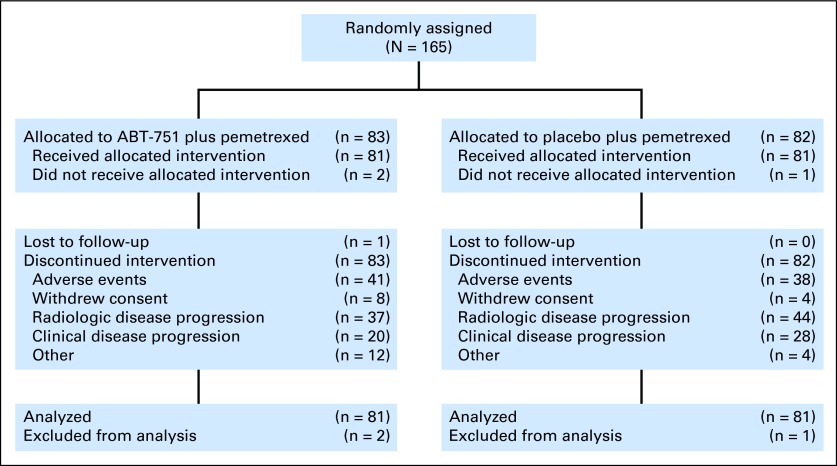
The phase I component of this clinical trial was an open-label, dose-escalation study using a standard 3+3 patient cohort design to determine the MTD and the recommended phase II dose of ABT-751 in combination with pemetrexed. Phase II was a double-blind, randomized (1:1), placebo-controlled study (Fig 1) to determine whether addition of ABT-751 to standard pemetrexed prolonged PFS compared to pemetrexed monotherapy. All patients received pemetrexed (500 mg/m2) on day 1 of each 21-day cycle via intravenous infusion over 10 minutes; folic acid and vitamin B12 supplementation was initiated ≥ 5 days before the first dose of pemetrexed to reduce treatment-related toxicities. ABT-751 (or placebo, phase II only) was administered orally once daily on days 1 to 14 of each 21-day cycle. During phase 1, ABT-751 was initially dosed at 200 mg once daily and subsequently at 250 mg once daily. During phase II, ABT-751 was administered at the recommended phase 2 dose of 200 mg once daily.
Fig 1.
CONSORT diagram.
Assessments
Safety.
Safety assessments included history and physical examinations, vital signs, Eastern Cooperative Oncology Group performance status, AEs, ECG, blood chemistry, hematology, and urinalysis. In general, safety assessments were performed at screening, weekly during cycles 1 and 2, on day 1 of subsequent cycles, and at the final study visit. Adverse event severity was graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0. AEs judged possibly or probably related to ABT-751 or pemetrexed administration were considered dose-limiting toxicities (DLTs) if they satisfied any of the following criteria: grade 4 absolute neutrophil count (ANC; < 0.5 × 109/L) longer than 7 days; grade 4 thrombocytopenia (platelets < 25.0 × 109/L); grade ≥ 3 ANC with fever (ANC < 1.0 × 109/L; fever ≥ 38.5°C); grade ≥ 3 decreased hemoglobin (< 65-80 g/L); any other grade ≥ 3 toxicity representing ≥ 2 grade increase from baseline (excluding inadequately treated nausea and vomiting). The MTD was defined as the dose at which ≥ 30% of patients experienced DLTs during cycle 1.
PK.
Plasma concentrations and PK parameters of pemetrexed, ABT-751, and its primary metabolites (ABT-751 glucuronide and ABT-751 sulfate) were evaluated during phase I. Blood samples were collected before dosing on day 1 of cycle 1, immediately after pemetrexed infusion, and after ABT-751 administration at 0.5, 1, 2, 4, 6, 8, and 24 hours. Plasma concentrations were measured using liquid chromatography/tandem mass spectrometry.22 Pharmacokinetic parameters of ABT-751 assessed included maximum plasma concentration, time to maximum plasma concentration, area under the plasma concentration-time curve, and terminal phase elimination half-life.
Efficacy.
Efficacy variables including PFS and OS were evaluated during phase II. Tumor response was assessed using RECIST after every 2 cycles of therapy.21
Pharmacodynamic correlates.
Blood specimens for analyzing circulating tumor cells (CTC) were collected at screening, before cycle 2, and at the final visit from patients in the United States only. CTC detection was performed as previously described,18 using the CellSearch system (Veridex, Raritan, NJ). Plasma samples were taken at screening, before cycles 2 and 3, and at the final visit and stored at −70°C or colder until analyzed for quantitative assessment of tumor markers. Progastrin-releasing peptide, carcinoembryonic antigen (CEA), squamous cell carcinoma antigen (SCC), CA15-3, and CA125, were assessed in the laboratory of Petra Stieber (Institute of Clinical Chemistry, University Hospital Munich, Germany); placenta growth factor (PlGF) was measured at Abbott Diagnostics (Abbott Park, IL) using ARCHITECT kits (Abbott Diagnostics, Abbott Park, IL). Cytokeratin 19 fragment antigen 21-1 (CYFRA 21-1) and neuron-specific enolase were measured using automated electrochemoluminescent assays on Elecsys 2010 (Roche Diagnostics, Germany) in the laboratory of Petra Stieber. These determinants have been previously explored as diagnostic, prognostic, or predictive biomarkers in patients with cancer, including NSCLC.23–26
Statistical Analysis
All tests of hypotheses were conducted at two-sided α = .05 level. Stratified log-rank test and stratified Cox proportional hazard model were used to compare PFS and OS time between the two treatment arms in the phase II, with stratification for performance status, number of prior antitumor regimens, smoking history, and time since last antitumor treatment. The distributions of PFS and OS were estimated using Kaplan-Meier method. In addition for patients with squamous and nonsquamous histology separately, log-rank test and Cox proportional hazard model was used to compare PFS and OS between the two treatment arms. Statistical comparisons of the incidence of AEs between the phase II treatment groups were performed using Fisher's exact test. Mean changes from baseline in hematology, chemistry, urinalysis, and vital sign parameters were compared using an analysis of covariance with treatment as the factor and baseline measurement as a covariate. Noncompartmental methods were used to determine plasma PK parameters of pemetrexed and ABT-751 and its metabolites. A 20% decrease from baseline in CYFRA 21-1 and CEA has been previously reported to correlate with clinical outcome in patients treated with chemotherapy27; this threshold was chosen for analysis of all putative biomarkers. The association of a marker decrease of 20% and OS was examined for each marker individually. A composite signature was generated by clustering patients by changes in the three most informative individual markers: PlGF, SCC, and CYFRA 21-1 (Spotfire DecisionSite software, TIBCO, Palo Alto, CA).
RESULTS
Patient Characteristics and Study Drug Dosing
Nine patients participated in the phase I portion of this study; three patients received ABT-751 200 mg and six received ABT-751 250 mg. In total, 165 patients were randomly assigned in the phase II portion of the study; 81 of 83 randomly assigned patients received ABT-751 200 mg plus pemetrexed and 81 of 82 randomly assigned patients received placebo plus pemetrexed. The two arms in the phase II portion of this study were well balanced for demographic and stratification factors (Table 1).
Table 1.
Patient Demographics and Baseline Characteristics
| Demographic or Characteristic | Phase I |
Phase II (ITT population) |
||||||
|---|---|---|---|---|---|---|---|---|
| ABT-751 200 mg + Pemetrexed(n = 3) |
ABT-751 250 mg + Pemetrexed(n = 6) |
ABT-751 200 mg + Pemetrexed(n = 83) |
Placebo + Pemetrexed(n = 82) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Sex | ||||||||
| Male | 1 | 33.3 | 2 | 33.3 | 58 | 69.9 | 54 | 65.9 |
| Female | 2 | 66.7 | 4 | 66.7 | 25 | 30.1 | 28 | 34.1 |
| Race | ||||||||
| White | 3 | 100.0 | 4 | 66.7 | 81 | 97.6 | 73 | 89.0 |
| Black | 0 | 2 | 33.3 | 2 | 2.4 | 7 | 8.5 | |
| Asian | 0 | 0 | 0 | 2 | 2.4 | |||
| Ethnicity | ||||||||
| Hispanic | 0 | 0 | 1 | 1.2 | 1 | 1.2 | ||
| Non-Hispanic | 3 | 100.0 | 6 | 100.0 | 82 | 98.8 | 81 | 98.8 |
| Age, years | ||||||||
| Median | 60 | 59.5 | 64 | 60.5 | ||||
| Range | 58-62 | 50-73 | 31-83 | 42-87 | ||||
| Smoking history | ||||||||
| Current or former | 3 | 100.0 | 6 | 100.0 | 72 | 86.7 | 71 | 86.6 |
| Never-smoker | 0 | 0 | 11 | 13.3 | 11 | 13.4 | ||
| Performance status | ||||||||
| 0-1 | 3 | 100.0 | 6 | 100.0 | 76 | 1.6 | 72 | 87.8 |
| ≥ 2 | 0 | 0 | 7 | 8.4 | 10 | 12.2 | ||
| Prior antitumor regimens* | ||||||||
| 1 | 2 | 66.7 | 5 | 83.3 | 75 | 90.4 | 68 | 82.9 |
| 2 | 1 | 33.3 | 1 | 16.7 | 8 | 9.6 | 14 | 17.1 |
| Time since last anti-tumor treatment, months | ||||||||
| < 3 | 1 | 33.3 | 3 | 50.0 | 42 | 50.6 | 43 | 52.4 |
| ≥ 3 | 2 | 66.7 | 3 | 50.0 | 41 | 49.4 | 39 | 47.6 |
| Disease stage | ||||||||
| 3 | 0 | 2 | 33.3 | 21 | 25.3 | 14 | 17.1 | |
| 4 | 3 | 100.0 | 4 | 66.7 | 62 | 74.7 | 67 | 81.7 |
| Unknown | 0 | 0 | 0 | 1 | 1.2 | |||
| Histology | ||||||||
| Squamous cell | 1 | 33.3 | 2 | 33.3 | 25 | 30.1 | 20 | 24.4 |
| Nonsquamous | 2 | 66.7 | 4 | 66.7 | 58 | 69.9 | 62 | 75.6 |
Abbreviation: ITT, intention to treat.
Only one prior chemotherapy regimen was allowed as per eligibility criteria, but prior adjuvant therapy was permitted.
During phase II, the major reasons for study discontinuation included disease progression (ABT-751, 44.6%; placebo 53.7%) and AEs (ABT-751, 49.4%; placebo, 46.3%). The median number of treatment cycles in the ABT-751 group was three (range, one to 20) and the median treatment duration was 36 days (range, 4 to 281). The median number of treatment cycles in the placebo group was three (range, one to 19) and the median treatment duration was 42 days (range, 4 to 258).
Safety
DLTs.
None of the three patients in the ABT-751 200 mg cohort experienced DLTs. One patient in the ABT-751 250 mg cohort developed grade 3 peripheral neuropathy. The 250-mg cohort was expanded to three additional patients, one of whom experienced grade 5 intestinal infarction. Because two patients experienced DLTs, it was concluded that the MTD had been exceeded. This was further supported by the higher incidence of grade 3 or 4 neutropenia in the ABT-751 250-mg group (50%) compared with the 200-mg group (0%). The MTD and recommended phase II dose were therefore defined as ABT-751 200 mg plus pemetrexed 500 mg/m2.
AEs.
During phase II, 97.5% of patients in each treatment group developed ≥ 1 treatment-emergent AE. Toxicity attributions were assigned by the principal investigator at each participating site. The most common treatment-related AEs included fatigue, constipation, anemia, nausea, and diarrhea. Treatment-related constipation occurred in a significantly greater proportion of patients in the ABT-751 group versus the placebo group (ABT-751 or placebo related, P < .001; pemetrexed related, P = .027; Table 2). Nearly two thirds (61.7%) of patients in each treatment group experienced ≥ 1 grade 3 or 4 AE. The frequencies of treatment-related grade 3 or 4 AEs were similar in the ABT-751 and placebo groups. The proportion of patients discontinuing therapy because of gastrointestinal events was significantly higher in the ABT-751 group (13.6%) versus the placebo group (2.5%; P = .018). However, there were no significant differences in treatment discontinuation rates between the two groups based on the individual gastrointestinal events of nausea, vomiting, constipation, diarrhea, or ileus. Observed rates of grade 3 or 4 hematologic toxicity in the combination and control arms were within the expected range for pemetrexed alone (Table 2, and data not shown).
Table 2.
Treatment-Related Adverse Events in ≥ 10% of Patients
| Adverse Event | Phase 1 |
Phase 2 (all treated patients) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABT-751 + Pemetrexed(n = 9) |
ABT-751 + Pemetrexed(n = 81) |
Placebo + Pemetrexed(n = 81) |
||||||||||
| ABT-751 Related |
Pemetrexed Related |
ABT-751 Related |
Pemetrexed Related |
Placebo Related |
Pemetrexed Related |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Blood and lymphatic system disorders | 4 | 44.4 | 4 | 44.4 | 29 | 35.8 | 41 | 50.6 | 23 | 28.4 | 31 | 38.3 |
| Anemia | 3 | 33.3 | 3 | 33.3 | 18 | 22.2 | 30 | 37.0 | 13 | 16.0 | 18 | 22.2 |
| Leukopenia | 0 | 0 | 8 | 9.9 | 9 | 11.1 | 6 | 7.4 | 5 | 6.2 | ||
| Neutropenia | 1 | 11.1 | 1 | 11.1 | 10 | 12.3 | 16 | 9.8 | 7 | 8.6 | 11 | 13.6 |
| Thrombocytopenia | 0 | 0 | 7 | 8.6 | 12 | 14.8 | 5 | 6.2 | 8 | 9.9 | ||
| GI disorders | 8 | 88.9 | 7 | 77.8 | 41 | 50.6 | 41 | 50.6 | 32 | 39.5 | 36 | 44.4 |
| Constipation | 4 | 44.4 | 3 | 33.3 | 22* | 27.2 | 12† | 14.8 | 5* | 6.2 | 3† | 3.7 |
| Diarrhea | 1 | 11.1 | 1 | 11.1 | 13 | 16.0 | 15 | 18.5 | 8 | 9.9 | 7 | 8.6 |
| Intestinal infarction | 1 | 11.1 | 1 | 11.1 | 0 | 0 | 0 | 0 | ||||
| Nausea | 6 | 66.7 | 6 | 66.7 | 17 | 21.0 | 23 | 28.4 | 21 | 25.9 | 23 | 28.4 |
| Vomiting | 2 | 22.2 | 2 | 22.2 | 11 | 13.6 | 13 | 16.0 | 4 | 4.9 | 6 | 7.4 |
| General disorders and administration site conditions | 8 | 88.9 | 8 | 88.9 | 26 | 32.1 | 27 | 33.3 | 22 | 27.2 | 27 | 33.3 |
| Fatigue | 8 | 88.9 | 8 | 88.9 | 23 | 28.4 | 24 | 29.6 | 17 | 21.0 | 22 | 27.2 |
| Peripheral edema | 1 | 11.1 | 1 | 11.1 | 0 | 0 | 2 | 2.5 | 3 | 3.7 | ||
| Pyrexia | 0 | 0 | 2 | 2.5 | 4 | 4.9 | 3 | 3.7 | 4 | 4.9 | ||
| Metabolism and nutrition disorders | 4 | 44.4 | 4 | 44.4 | 20 | 24.7 | 26 | 32.1 | 11 | 13.6 | 14 | 17.3 |
| Anorexia | 4 | 44.4 | 4 | 44.4 | 17 | 21.0 | 20 | 24.7 | 8 | 9.9 | 8 | 9.9 |
| Musculoskeletal and connective tissue disorders | 2 | 22.2 | 1 | 11.1 | 6 | 7.4 | 5 | 6.2 | 7 | 8.6 | 5 | 6.2 |
| Back pain | 0 | 0 | 0 | 0 | 1 | 1.2 | 1 | 1.2 | ||||
| Neoplasms | 0 | 0 | 1 | 1.2 | 0 | 0 | 1 | 1.2 | ||||
| Non–small-cell lung cancer | 0 | 0 | 1 | 1.2 | 0 | 0 | 1 | 1.2 | ||||
| Nervous system disorders | 8 | 88.9 | 7 | 77.8 | 12 | 14.8 | 9 | 11.1 | 9 | 11.1 | 9 | 11.1 |
| Peripheral neuropathy | 3 | 33.3 | 2 | 22.2 | 3 | 3.7 | 1 | 1.2 | 3 | 3.7 | 3 | 3.7 |
| Psychiatric disorders | 0 | 0 | 5 | 6.2 | 5 | 6.2 | 2 | 2.5 | 3 | 3.7 | ||
| Insomnia | 0 | 0 | 2 | 2.5 | 2 | 2.5 | 1 | 1.2 | 1 | 1.2 | ||
| Respiratory, thoracic, and mediastinal disorders | 3 | 33.3 | 3 | 33.3 | 5 | 6.2 | 8 | 9.9 | 1 | 1.2 | 2 | 2.5 |
| Cough | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Dyspnea | 2 | 22.2 | 2 | 22.2 | 3 | 3.7 | 3 | 3.7 | 0 | 0 | ||
| Skin and subcutaneous tissue disorders | 0 | 0 | 9 | 11.1 | 12 | 14.8 | 9 | 11.1 | 18 | 22.2 | ||
| Rash | 0 | 0 | 5 | 6.2 | 8 | 9.9 | 6 | 7.4 | 12 | 14.8 | ||
Study drug-related constipation occurred in a significantly greater proportion of patients in the ABT-751 group v the placebo group (P < .001).
Pemetrexed-related constipation occurred in a significantly greater proportion of patients in the ABT-751 group v the placebo group (P = .027).
PK.
Because of the small number of patients participating in phase I (n = 9), only a limited PK analysis was performed. The PK parameters of ABT-751 coadministered with pemetrexed were similar to those of ABT-751 monotherapy. Similarly, the PK parameters of pemetrexed coadministered with ABT-751 were similar to those of pemetrexed monotherapy (Appendix Table A1, online only).
Efficacy
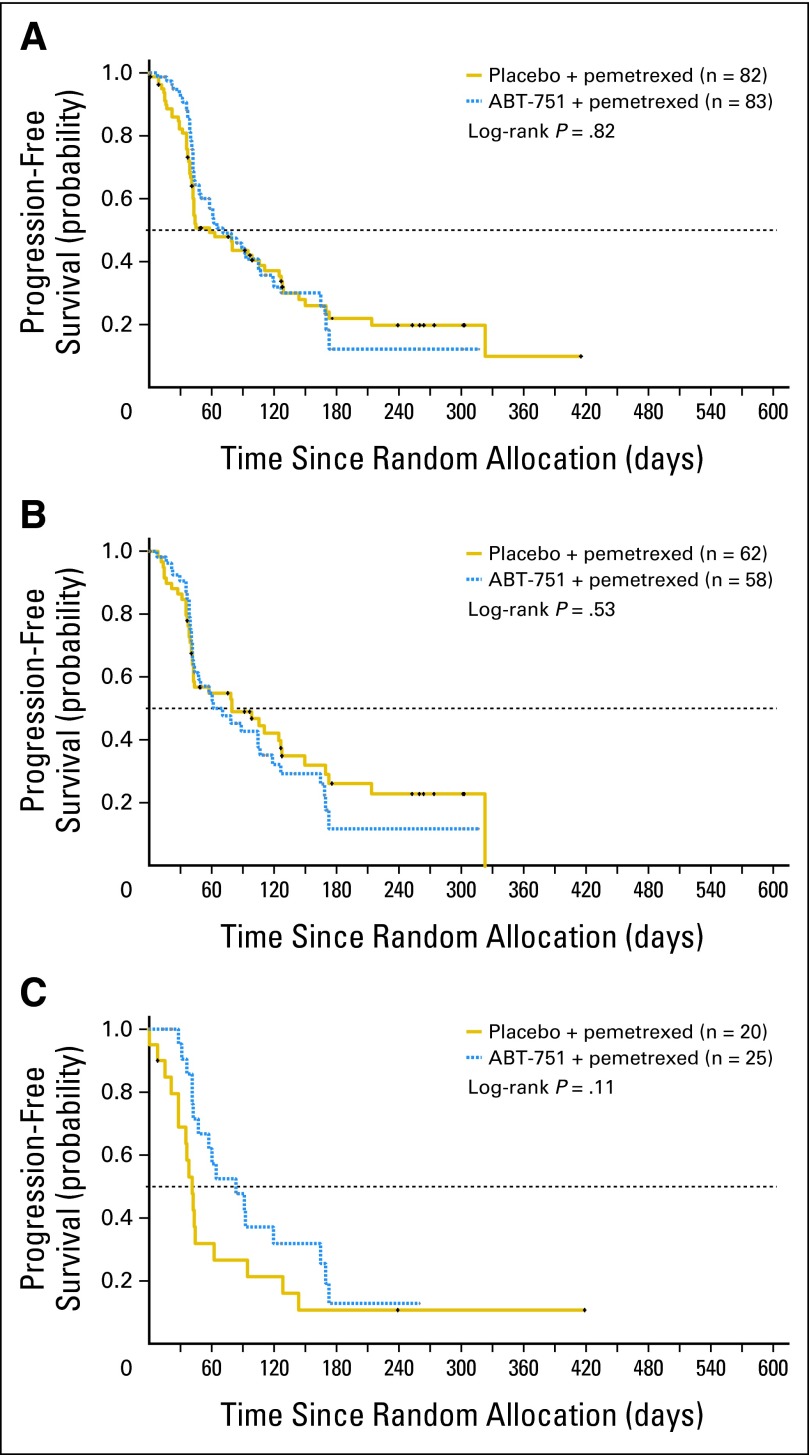
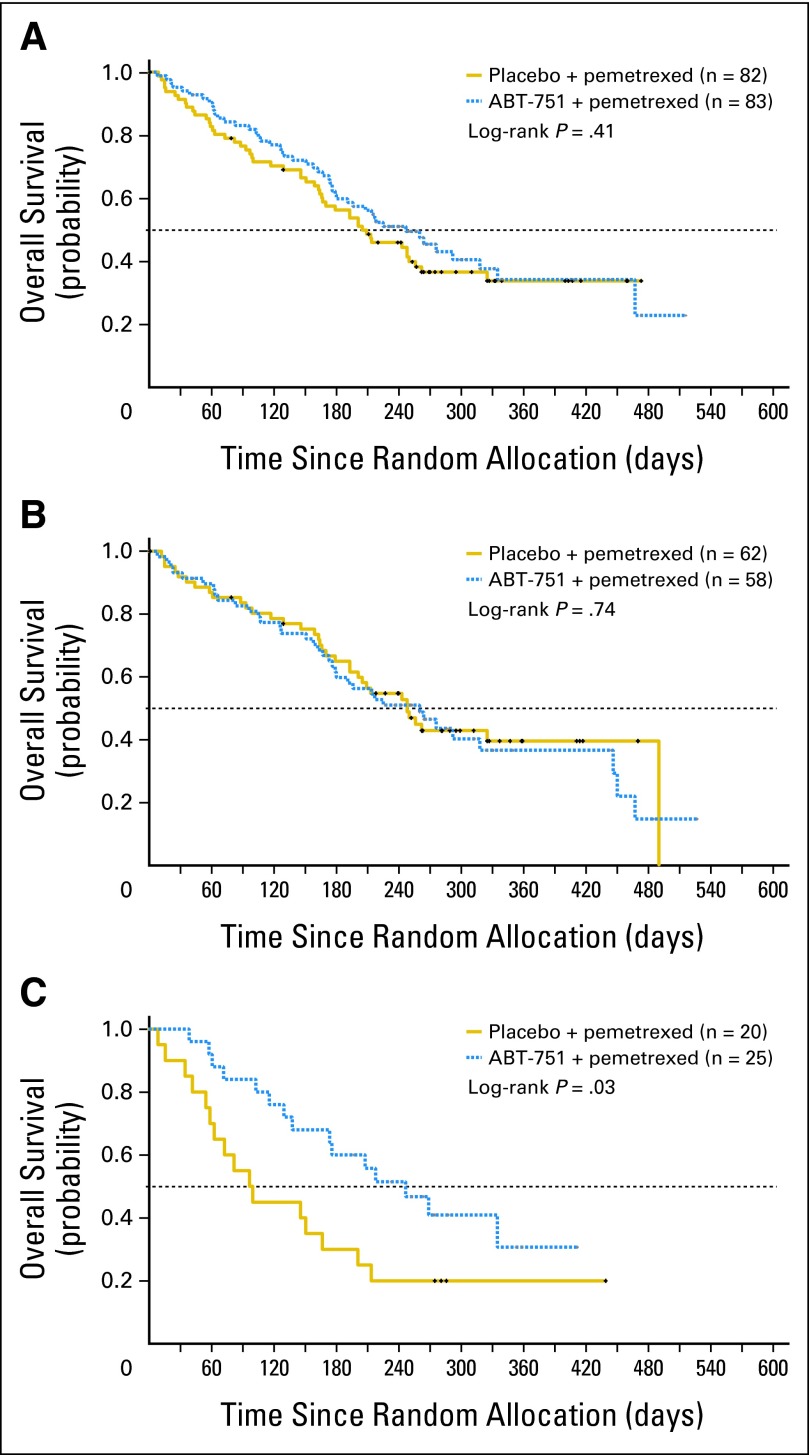
No statistically significant differences in PFS or OS were observed between the treatment groups in the full intent-to-treat population (Table 3; Figs 2A and 3A). Median PFS for all randomly assigned patients (n = 165) was 1.9 months versus 2.3 months (HR, 0.975; 95% CI, 0.670 to 1.420) and median OS was 6.7 versus 8.1 months (HR, 0.851; 95% CI, 0.573 to 1.266), for pemetrexed plus placebo versus pemetrexed plus ABT-751, respectively. Similarly, PFS and OS were comparable between study arms for patients with tumors of nonsquamous histology (Table 3; Figs 2B and 3B). However, in patients with squamous cell NSCLC (n = 45), median PFS was 1.4 versus 2.8 months (HR, 0.58; 95% CI, 0.30 to 1.15), and median OS was 3.3 versus 8.1 months (HR, 0.47; 95% CI, 0.23 to 0.96) favoring pemetrexed plus ABT-751 (Table 3; Figs 2C and 3C). Objective response rates (95% CI) were surprisingly low at 1.23% (95%, 0.03% to 6.69%) and 6.02% (95% CI, 1.98% to 13.5%) for pemetrexed plus placebo and pemetrexed plus ABT-751 groups, respectively.
Table 3.
Comparison of Antitumor Activity of ABT-751 Plus Pemetrexed Versus Placebo Plus Pemetrexed
| Parameter | All Patients |
Nonsquamous NSCLC |
Squamous Cell NSCLC |
|||
|---|---|---|---|---|---|---|
| ABT-751 + Pemetrexed (n = 83) | Placebo + Pemetrexed (n = 82) | ABT-751 + Pemetrexed (n = 58) | Placebo + Pemetrexed (n = 62) | ABT-751 + Pemetrexed (n = 25) | Placebo + Pemetrexed (n = 20) | |
| Median PFS, months | 2.3 | 1.9 | 2.3 | 2.6 | 2.7 | 1.4 |
| HR* | 0.97 | 1.16 | 0.58 | |||
| 95% CI | 0.65 to 1.46 | 0.73 to 1.82 | 0.30 to 1.15 | |||
| P by log-rank test† | .819 | .534 | .112 | |||
| Median OS, months | 8.1 | 6.7 | 8.5 | 8.2 | 8.1 | 3.3 |
| HR* | 0.84 | 1.08 | 0.47 | |||
| 95% CI | 0.55 to 1.27 | 0.68 to 1.72 | 0.23 to 0.96 | |||
| P by log-rank test† | .410 | .742 | .034 | |||
Abbreviations: NSCLC, non–small-cell lung cancer; PFS, progression-free survival; HR, hazard ratio; OS, overall survival.
Based on stratified Cox proportional hazards regression model comparing two treatment groups for all patients analysis and unstratified Cox proportional hazards model comparing two treatment groups in the subgroups of patients with nonsquamous NSCLC and squamous NSCLC.
Based on stratified log-rank test comparing two treatment groups for all patients analysis and unstratified log-rank test comparing two treatment groups in the subgroups on patients with nonsquamous NSCLC and squamous NSCLC.
Fig 2.
Progression-free survival. Kaplan-Meier curves for intent-to-treat patient population, and within histologic subsets, by treatment arm. (A) Intent-to-treat patients. (B) Patients with nonsquamous non–small-cell lung cancer (NSCLC). (C) Patients with squamous NSCLC.
Fig 3.
Overall survival. Kaplan-Meier curves for intent-to-treat population, and within histologic subsets, by treatment arm. (A) Intent-to-treat patients. (B) Patients with nonsquamous non–small-cell lung cancer (NSCLC). (C) Patients with squamous NSCLC.
Pharmacodynamic Correlates
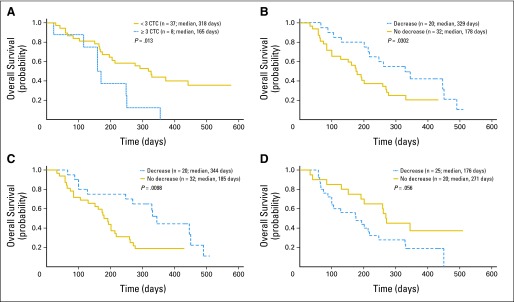
Baseline CTC values were predictive of improved OS among treated patients. Median OS among eight patients with ≥ 3 CTC at baseline was 5.4 months, whereas median OS among 37 patients with fewer than 3 CTC at baseline was 10.5 months (P = .013; Fig 4A). In contrast, no significant difference in median OS was observed comparing patients with ≥ 3 CTC versus fewer than 3 CTC at week 4 (P = .44).
Fig 4.
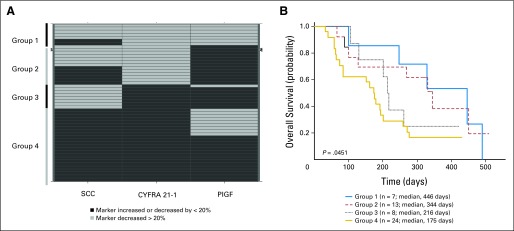
Relationship between overall survival and biomarkers in all patients who provided samples for analysis. (A) Association of baseline concentration of circulating tumor cells (CTC) with overall survival. (B-D) Biomarker decreases from baseline to end of cycle 1 of greater than 20% decrease were segregated from patients without a similar decrease. (B) Squamous cell carcinoma antigen (C) cytokeratin 19 fragment antigen 21-1, and (D) placenta growth factor showed strongest association with overall survival. P value was log-rank.
Reductions of more than 20% in the plasma concentration of SCC (P = .03) or CYFRA 21-1 (P = .01) between baseline and the end of cycle 1 were associated with improved median OS (Figs 3B and 3C). A decrease of more than 20% in PlGF (P = .056) showed a trend toward being associated with decreased median OS (Fig 3D). Similar changes in neuron-specific enolase, CEA, progastrin-releasing peptide, CA15-3, or CA125 expression were not associated with OS. Greater than 20% reductions of plasma SCC and CYFRA 21-1, and lower than 20% change of PlGF could be combined to find composite patient subtypes with improved median OS (P = .05; Appendix Fig A1, online only).
DISCUSSION
This phase I/II study demonstrated that ABT-751 plus pemetrexed combination therapy is safe and well-tolerated in patients with previously treated advanced or metastatic NSCLC. The most common treatment-related toxicities included fatigue, constipation, anemia, nausea, and diarrhea, which are consistent with the AE profile reported in previous studies of ABT-751 monotherapy and combination therapy.17,28 No clinically relevant differences in AE severity, serious AEs, treatment discontinuations, or laboratory parameters were observed between the treatment arms. Notably, myelosuppression was not significantly increased among patients receiving ABT-751 plus pemetrexed combination therapy. Forty-one patients (n = 83) randomly assigned to the ABT-751 arm and 38 (n = 82) on the placebo arm discontinued treatment due to adverse events, including 20 patients on the ABT-751 arm and 28 on the placebo arm who discontinued due to clinical disease progression. Discontinuation without definitive radiologic progression may have impacted analyses of PFS.
The MTD of the combination regimen was ABT-751 200 mg administered 14 of every 21 days in combination with standard pemetrexed (500 mg/m2). This MTD is consistent with results from phase II studies of ABT-751 monotherapy.17–20 The DLTs observed in this study included grade 3 peripheral neuropathy and grade 5 intestinal infarction. Peripheral neuropathy was also reported as a DLT of ABT-751 monotherapy.14,29,30
No PK interaction was observed in this study between ABT-751 and pemetrexed in patients with previously treated advanced or metastatic NSCLC. The PK parameters of ABT-751 coadministered with pemetrexed are similar to those previously reported for ABT-751 monotherapy.15 Similarly, the PK parameters of pemetrexed coadministered with ABT-751 are consistent with those of pemetrexed monotherapy.28
No significant therapeutic advantage was observed when ABT-751 was administered in combination with pemetrexed to unselected patients with previously treated advanced or metastatic NSCLC; median PFS and median OS were similar among patients treated with ABT-751 plus pemetrexed or placebo plus pemetrexed. ABT-751 did not enhance pemetrexed efficacy in nonsquamous NSCLC, the subset of patients for whom pemetrexed therapy is indicated.
The outcome data in patients with squamous NSCLC are surprising, and suggest that this agent may have significant activity in this subtype of NSCLC. Clinical outcome with pemetrexed plus placebo was poor, consistent with prior studies demonstrating that pemetrexed is ineffective in squamous NSCLC—median survival for patients with squamous NSCLC in the placebo arm of this study was 3.3 months. In contrast, median survival for patients with squamous NSCLC was 8.1 months for those treated on the ABT-751 arm. Survival among patients with squamous NSCLC treated with ABT-751 was as good as that of patients with nonsquamous NSCLC treated with pemetrexed. While these results are promising, in view of the modest 1.4 months difference in PFS versus the 4.8 months difference in OS both in favor of the ABT-751 arm, an imbalance in impact of subsequent therapies cannot be excluded. Nonetheless, these data suggest that exploration of ABT-751 together with cytotoxic therapy of proven benefit in squamous NSCLC may be of interest. This question is currently the focus of active study in preclinical models.
Pharmacodynamic correlative analyses incorporated into this study have defined hypothesis-generating correlations that will be explored for validation in subsequent clinical trials. Improved median OS was observed among patients with reduced CTC concentrations at baseline (the number of samples was too small to allow treatment group comparisons). Improved median OS among patients with reduced baseline CTC concentrations is consistent with previous studies in patients with metastatic breast cancer.29 Improved median OS was observed among patients with a higher than 20% decrease from baseline in plasma SCC and/or CYFRA 21-1 after one cycle of therapy. A trend toward a decrease in OS was observed in patients with less than 20% decrease in PlGF after one cycle of therapy. Previous studies have also implicated SCC and/or CYFRA 21-1 as predictors of disease progression and response to therapy in NSCLC.30,31 The analysis of multiple pharmacodynamic factors for correlation with PFS and OS increases the probability of spurious associations, and these associations should be considered hypothesis generating. Ongoing studies continue to assess the clinical utility of tumor biomarkers in predicting response to ABT-751 therapy.
Acknowledgment
We thank Ann Marie Fitzmaurice, PhD, ProEd Communications, for editorial assistance, and Petra Stieber and Katja Krocker for assay analysis and scientific advice.
Appendix
Fig A1.
Relationship between overall survival and > 20% reduction in the combination of placenta growth factor (PIGF), squamous cell carcinoma antigen (SCC), and cytokeratin 19 fragment antigen 21-1 (CYFRA 21-1) level between baseline and the end of cycle 1 in patients with advanced or metastatic non–small-cell lung cancer.
Table A1.
Pharmacokinetic Parameters of ABT-751, ABT-751 Glucuronide, ABT-751 Sulfate, and Pemetrexed
| Parameter | Pharmacokinetic Parameter (mg) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABT-751 |
ABT-751 Glucuronide |
ABT-751 Sulfate |
Pemetrexed |
|||||||||||||
| 200 |
250 |
200 |
250 |
200 |
250 |
200 |
250 |
|||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| No. of patients | 3 | 6 | 2 | 6 | 2 | 6 | 3 | 6 | ||||||||
| Tmax, hr | 0.8 | 0.3 | 2.8 | 1.8 | 4.0 | 0.0 | 5.7 | 1.5 | 3.0 | 1.4 | 4.0 | 2.2 | 0.2 | 0.1 | 0.2 | 0.1 |
| Cmax, μg/mL | 9.94 | 9.85 | 9.70 | 5.19 | 4.37 | 1.74 | 7.54 | 3.41 | 7.98 | 4.96 | 7.40 | 2.28 | 134 | 39.8 | 130 | 36.9 |
| AUCt, μg × hr/mL | 38.3 | 12.9 | 60.9 | 24.2 | 56.5 | 18.1 | 114 | 50.5 | 88.1 | 43.8 | 96.4 | 25.4 | 215 | 26.6 | 192 | 51.9 |
| AUC∞, μg × hr/mL | 44.1 | 10.3 | 66.6 | 25.0 | 102 | 2.89 | 215 | 77.5 | 133 | 19.1 | 141 | 51.8 | 215 | 26.6 | 192 | 51.9 |
| t1/2, hr | 8.1 | 2.6 | 6.6 | 1.5 | 18.4 | 9.0 | 12.4 | 6.5 | 14.4 | 9.2 | 10.5 | 4.1 | 2.6 | 0.2 | 2.7 | 0.2 |
| CL/F, L/hr | 4.74 | 1.26 | 4.56 | 2.79 | — | — | — | — | — | — | — | — | — | — | — | — |
| CL, L/hr × m2 | — | — | — | — | — | — | — | — | — | — | 2.35 | 0.29 | 2.75 | 0.64 | ||
| Vdβ/F, L | 59.4 | 26.2 | 45.5 | 27.0 | — | — | — | — | — | — | — | — | — | — | — | — |
| Vdβ, L/m2 | — | — | — | — | — | — | — | — | — | — | — | — | 8.77 | 0.94 | 10.4 | 2.02 |
Abbreviations: SD, standard deviation; Tmax, time to Cmax; Cmax, maximum observed plasma concentration; AUCt area under the concentration-time curve from time 0 to time of last measurable concentration; AUC∞, AUC from 0 to infinite time; t1/2, terminal phase elimination half-life; CL, clearance; CL/F, apparent oral clearance where F is the bioavailability of ABT-751; Vdβ, apparent volume of distribution in the β phase; Vdβ/F, apparent oral volume of distribution in the β phase.
Footnotes
Supported by Abbott Laboratories.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00297089.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Andrew Coates, Abbott Laboratories (C); Evelyn McKeegan, Abbott Laboratories (C); Peter Ansell, Abbott Laboratories (C); Xiangdong Zhou, Abbott Laboratories (C); Jane Qian, Abbott Laboratories (C); Rajendra Pradhan, Abbott Laboratories (C); Barry Dowell, Abbott Laboratories (C); Andrew Krivoshik, Abbott Laboratories (C); Gary Gordon, Abbott Laboratories (C) Consultant or Advisory Role: Charles M. Rudin, Syndax Pharmaceuticals (C), Genentech (C), OSI Pharmaceuticals (C) Stock Ownership: Andrew Coates, Abbott Laboratories; Evelyn McKeegan, Abbott Laboratories; Peter Ansell, Abbott Laboratories; Jane Qian, Abbott Laboratories; Rajendra Pradhan, Abbott Laboratories; Barry Dowell, Abbott Laboratories; Andrew Krivoshik, Abbott Laboratories; Gary Gordon, Abbott Laboratories Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Charles M. Rudin, Ann Mauer, Evelyn McKeegan, Peter Ansell, Jane Qian, Barry Dowell, Andrew Krivoshik, Gary Gordon
Provision of study materials or patients: Charles M. Rudin, Martin Smakal, Rosalyn Juergens, Stanislav Spelda, Michael Wertheim
Collection and assembly of data: Charles M. Rudin, Martin Smakal, Rosalyn Juergens, Stanislav Spelda, Michael Wertheim, Andrew Coates, Evelyn McKeegan, Peter Ansell, Xiangdong Zhou, Barry Dowell, Andrew Krivoshik, Gary Gordon
Data analysis and interpretation: Charles M. Rudin, Andrew Coates, Evelyn McKeegan, Peter Ansell, Xiangdong Zhou, Jane Qian, Rajendra Pradhan, Barry Dowell, Andrew Krivoshik, Gary Gordon
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Indianapolis, IN: Eli Lilly and Company; ALIMTA (pemetrexed) package insert. [Google Scholar]
- 2.Bridgewater, NJ: sanofi-aventis; TAXOTERE (docetaxel) package insert. [Google Scholar]
- 3.Melville, NY: OSI Pharmaceuticals; TARCEVA (erlotinib) package insert. [Google Scholar]
- 4.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 5.Kubota K, Niho S, Enatsu S, et al. Efficacy differences of pemetrexed by histology in pretreated patients with stage IIIB/IV non-small cell lung cancer: Review of results from an open-label randomized phase II study. J Thorac Oncol. 2009;4:1530–1536. doi: 10.1097/JTO.0b013e3181b9e608. [DOI] [PubMed] [Google Scholar]
- 6.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: A review of two phase III studies. Oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 7.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MH, Justice R, Pazdur R. Approval summary: Pemetrexed in the initial treatment of advanced/metastatic non-small cell lung cancer. Oncologist. 2009;14:930–935. doi: 10.1634/theoncologist.2009-0092. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimatsu K, Yamaguchi A, Yoshino H, et al. Mechanism of action of E7010, an orally active sulfonamide antitumor agent: Inhibition of mitosis by binding to the colchicine site of tubulin. Cancer Res. 1997;57:3208–3213. [PubMed] [Google Scholar]
- 10.Galmarini CM. ABT-751 (Abbott) Curr Opin Investig Drugs. 2005;6:623–630. [PubMed] [Google Scholar]
- 11.Segreti JA, Polakowski JS, Koch KA, et al. Tumor selective antivascular effects of the novel antimitotic compound ABT-751: An in vivo rat regional hemodynamic study. Cancer Chemother Pharmacol. 2004;54:273–281. doi: 10.1007/s00280-004-0807-0. [DOI] [PubMed] [Google Scholar]
- 12.Koyanagi N, Nagasu T, Fujita F, et al. In vivo tumor growth inhibition produced by a novel sulfonamide, E7010, against rodent and human tumors. Cancer Res. 1994;54:1702–1706. [PubMed] [Google Scholar]
- 13.Jorgensen TJ, Tian H, Joseph IB, et al. Chemosensitization and radiosensitization of human lung and colon cancers by antimitotic agent, ABT-751, in athymic murine xenograft models of subcutaneous tumor growth. Cancer Chemother Pharmacol. 2007;59:725–732. doi: 10.1007/s00280-006-0326-2. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Noda K, Yoshimura A, et al. Phase I study of E7010. Cancer Chemother Pharmacol. 1998;42:127–134. doi: 10.1007/s002800050795. [DOI] [PubMed] [Google Scholar]
- 15.Hande KR, Hagey A, Berlin J, et al. The pharmacokinetics and safety of ABT-751, a novel, orally bioavailable sulfonamide antimitotic agent: Results of a phase I study. Clin Cancer Res. 2006;12:2834–2840. doi: 10.1158/1078-0432.CCR-05-2159. [DOI] [PubMed] [Google Scholar]
- 16.Mauer AM, Szeto L, Belt RJ, et al. Preliminary results of a phase 2 study of ABT-751 in patients (pts) with taxane-refractory non-small cell lung carcinoma (NSCLC) J Clin Oncol. 2005;23(suppl):654s. abstr 7137. [Google Scholar]
- 17.Mauer AM, Cohen EE, Ma PC, et al. A phase II study of ABT-751 in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:631–636. doi: 10.1097/JTO.0b013e318174e01f. [DOI] [PubMed] [Google Scholar]
- 18.Benson AB, Kindler HL, Jodrell D, et al. Phase 2 study of ABT-751 in patients with refractory metastatic colorectal carcinoma (CRC) J Clin Oncol. 2005;23(suppl):255s. abstr 3537. [Google Scholar]
- 19.Hagey AE, Figlin RA, Moldawer N, et al. Preliminary phase 2 results of ABT-751 in subjects with advanced renal cell carcinoma (RCC) J Clin Oncol. 2005;23(suppl):403s. abstr 4603. [Google Scholar]
- 20.Washington DK, Storniolo AV, Saleh M, et al. Phase 2 results of ABT-751 in subjects with taxane-refractory breast cancer: Interim analysis. J Clin Oncol. 2005;23(suppl):59s. abstr 724. [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Rudek MA, Zhao M, He P, et al. Validation and implementation of a liquid chromatography/tandem mass spectrometry assay to quantitate ABT-751, ABT-751 glucuronide, and ABT-751 sulfate in human plasma for clinical pharmacology studies. J Pharm Biomed Anal. 2006;42:253–260. doi: 10.1016/j.jpba.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Haas M, Laubender RP, Stieber P, et al. Prognostic relevance of CA 19-9, CEA, CRP, and LDH kinetics in patients treated with palliative second-line therapy for advanced pancreatic cancer. Tumour Biol. 2010;31:351–357. doi: 10.1007/s13277-010-0044-6. [DOI] [PubMed] [Google Scholar]
- 24.Holdenrieder S, von Pawel J, Dankelmann E, et al. Nucleosomes, ProGRP, NSE, CYFRA 21-1, and CEA in monitoring first-line chemotherapy of small cell lung cancer. Clin Cancer Res. 2008;14:7813–7821. doi: 10.1158/1078-0432.CCR-08-0678. [DOI] [PubMed] [Google Scholar]
- 25.Molina R, Auge JM, Bosch X, et al. Usefulness of serum tumor markers, including progastrin-releasing peptide, in patients with lung cancer: Correlation with histology. Tumour Biol. 2009;30:121–129. doi: 10.1159/000224628. [DOI] [PubMed] [Google Scholar]
- 26.Molina R, Filella X, Auge JM, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis: Comparison with the main clinical and pathological prognostic factors. Tumour Biol. 2003;24:209–218. doi: 10.1159/000074432. [DOI] [PubMed] [Google Scholar]
- 27.Ardizzoni A, Cafferata MA, Tiseo M, et al. Decline in serum carcinoembryonic antigen and cytokeratin 19 fragment during chemotherapy predicts objective response and survival in patients with advanced nonsmall cell lung cancer. Cancer. 2006;107:2842–2849. doi: 10.1002/cncr.22330. [DOI] [PubMed] [Google Scholar]
- 28.Boulder, CO: OSI Pharmaceuticals; 2001. OSI-774 Investigators Brochure. [Google Scholar]
- 29.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 30.Molina R, Auge JM, Filella X, et al. Pro-gastrin-releasing peptide (proGRP) in patients with benign and malignant diseases: Comparison with CEA, SCC, CYFRA 21-1 and NSE in patients with lung cancer. Anticancer Res. 2005;25:1773–1778. [PubMed] [Google Scholar]
- 31.Zhang L, Chen J, Ke Y, et al. Expression of placenta growth factor (PlGF) in non-small cell lung cancer (NSCLC) and the clinical and prognostic significance. World J Surg Oncol. 2005;3:68. doi: 10.1186/1477-7819-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]