Abstract
Purpose
Programmed death 1 is an immune checkpoint that suppresses antitumor immunity. Nivolumab, a fully human immunoglobulin G4 programmed death 1 immune checkpoint inhibitor antibody, was active and generally well tolerated in patients with advanced solid tumors treated in a phase I trial with expansion cohorts. We report overall survival (OS), response durability, and long-term safety in patients with non–small-cell lung cancer (NSCLC) receiving nivolumab in this trial.
Patients and Methods
Patients (N = 129) with heavily pretreated advanced NSCLC received nivolumab 1, 3, or 10 mg/kg intravenously once every 2 weeks in 8-week cycles for up to 96 weeks. Tumor burden was assessed by RECIST (version 1.0) after each cycle.
Results
Median OS across doses was 9.9 months; 1-, 2-, and 3-year OS rates were 42%, 24%, and 18%, respectively, across doses and 56%, 42%, and 27%, respectively, at the 3-mg/kg dose (n = 37) chosen for further clinical development. Among 22 patients (17%) with objective responses, estimated median response duration was 17.0 months. An additional six patients (5%) had unconventional immune-pattern responses. Response rates were similar in squamous and nonsquamous NSCLC. Eighteen responding patients discontinued nivolumab for reasons other than progressive disease; nine (50%) of those had responses lasting > 9 months after their last dose. Grade 3 to 4 treatment-related adverse events occurred in 14% of patients. Three treatment-related deaths (2% of patients) occurred, each associated with pneumonitis.
Conclusion
Nivolumab monotherapy produced durable responses and encouraging survival rates in patients with heavily pretreated NSCLC. Randomized clinical trials with nivolumab in advanced NSCLC are ongoing.
INTRODUCTION
Progress in the treatment of advanced non–small-cell lung cancer (NSCLC) over the last decade has been modest.1 Although molecularly targeted therapies have significantly affected the small proportion of patients whose tumors harbor epidermal growth factor receptor (EGFR) mutations2,3 or anaplastic lymphoma kinase (ALK) gene rearrangements,4 the majority of patients with advanced NSCLC die within 1 year of diagnosis. Clearly, a plateau has been reached with chemotherapy, with some enhancement of benefit from the addition of bevacizumab, an antiangiogenesis agent targeting vascular endothelial growth factor.5 Additional molecularly targeted agents have been evaluated in clinical trials with limited success, and efforts are currently focusing on identifying biomarkers in tumor or blood to predict benefit from such therapies.
Programmed death 1 (PD-1) is an immune checkpoint receptor expressed on activated T cells, which normally serves to dampen the immune response to protect against excessive inflammation and the development of autoimmunity.6,7 However, in the setting of malignancy, PD-1 signaling, driven primarily by adaptive expression of programmed death ligand 1 (PD-L1) within the tumor, inactivates primed T cells that recognize tumor-specific antigens, allowing tumor growth and metastasis.8–10 PD-1 pathway blockade with monoclonal antibodies offers a novel approach to restoring T cell–mediated antitumor immunity, with the potential for application across a broad population of patients with NSCLC.
The tolerability and activity of nivolumab, a fully human immunoglobulin G4 PD-1 immune checkpoint inhibitor antibody,11 were previously reported in patients with NSCLC, melanoma, and renal cell carcinoma treated in a phase I multidose clinical trial.12 We now report overall survival (OS) outcomes in the population of patients with NSCLC, in addition to further characterizing response duration and the long-term safety profile of nivolumab.
PATIENTS AND METHODS
Trial Design
This phase I dose-escalation cohort expansion trial evaluated the safety and clinical activity of nivolumab in patients with advanced NSCLC, melanoma, and kidney, colorectal, and castration-resistant prostate cancer. Detailed methods, including the study protocol and statistical analysis plan, have been previously published.12 Nivolumab was administered intravenously as a 1-hour infusion every 2 weeks in 8-week treatment cycles in an outpatient setting. During dose escalation, patients with all cancer types received 1-, 3-, or 10-mg/kg doses of nivolumab; during cohort expansion, patients with NSCLC were stratified for squamous versus nonsquamous cell histology and randomly assigned to receive 1-, 3-, or 10-mg/kg doses of nivolumab. Patients continued treatment for up to 96 weeks (12 cycles) or until unacceptable toxicity, confirmed complete response, confirmed disease progression, or withdrawal of consent. In the absence of clinical deterioration, patients could continue treatment after initial disease progression to allow for patterns of response consistent with immune-related response criteria (ie, persistent reduction in target lesions in presence of new lesions or regression of target lesions after initial growth).13 Patients with stable disease or ongoing complete or partial responses at the end of the 96-week treatment period could restart nivolumab at the time of confirmed disease progression, if this occurred within 1 year of completing initial therapy, and continue treatment for up to 1 year.
The study protocol was approved by local institutional review boards, and the study was conducted in accordance with international standards of good clinical practice. All patients or their legal representatives provided written informed consent before enrollment.
Patients
Patients eligibility criteria were as follows: pathologically confirmed advanced NSCLC, age ≥ 18 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (before implementation of amendment 4 in October 2010, ECOG performance status of 0 to 2 was allowed), adequate organ function, and one to five prior systemic treatment regimens for advanced NSCLC. Patients also had to have experienced progression through at least one platinum- or taxane-based regimen and have at least one measurable lesion by RECIST (version 1.0).14 Patients with treated brain metastases stable for at least 8 weeks were eligible. Exclusion criteria included autoimmune disease, prior therapy with T cell–modulating antibodies (eg, anti–CTLA-4, anti–PD-1, and anti–PD-L1), conditions requiring immunosuppressive medications, history of infection by HIV, and active infection by hepatitis B or C viruses.
Evaluation
All treated patients were evaluated for safety by laboratory tests, physical examination, and adverse event assessment at screening, every 2 weeks during therapy, and up to 70 days after receiving the last dose of nivolumab. Adverse events were coded using the Medical Dictionary for Regulatory Activities (version 15.1). The severity of adverse events was graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).15 Select adverse events, defined as adverse events with potential immunologic etiologies that require more frequent monitoring or intervention with immune suppression or hormone replacement, were identified based on a sponsor-derived prespecified list of Medical Dictionary for Regulatory Activities terms in seven categories.16
Tumor status was assessed radiographically at screening and after each 8-week treatment cycle. The objective response rate (ORR; percentage of patients with confirmed complete or partial responses among all treated patients) was the primary parameter of clinical activity. The protocol was amended after all patients were enrolled so that survival data could be collected. After completion of the treatment and follow-up periods, survival status was assessed approximately every 3 months either by office visits or telephone calls until study completion. Baseline characteristics of age, sex, ECOG performance status, histology, and number of prior therapies were collected for all patients; EGFR and Kirsten rat sarcoma viral oncogene homolog (KRAS) tumor mutation status were also collected from the study sites when available.
Statistical Analysis
Efficacy analysis, including OS results for all patients, is reported as of September 2014. Baseline characteristics and the safety analysis are reported as of March 2013. Associations between clinical activity and various baseline characteristics were explored by estimating the ORR by baseline characteristic subgroups and by plots of changes in tumor burden for selected characteristics (eg, by prior therapy or mutation status).
RESULTS
Patient Characteristics
From November 2008 through January 2012, 129 patients with NSCLC were enrolled across 12 sites in the United States. Patients had a median age of 65 years, and 98% had an ECOG performance status of 0 or 1; 42% and 57% had squamous and nonsquamous NSCLC, respectively (Table 1). NSCLC histology was unknown in one patient. Patients were heavily pretreated; 54% had received three or more prior systemic treatments for advanced NSCLC. As of September 2014, 129 patients were evaluated for clinical activity, with a median follow-up of 39 months (range, 32 to 66 months).
Table 1.
Baseline Characteristics and Prior Therapy of All Treated Patients With NSCLC
| Characteristic | All Treated Patients (N = 129) |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 65 | |
| Range | 38-85 | |
| Sex | ||
| Male | 79 | 61.2 |
| Female | 50 | 38.8 |
| Tumor cell histology | ||
| Squamous | 54 | 41.9 |
| Nonsquamous | 74 | 57.4 |
| Unknown | 1 | 0.8 |
| ECOG performance status17 | ||
| 0 or 1 | 127 | 98.4 |
| 2* | 2 | 1.6 |
| No. of prior systemic treatment regimens | ||
| 1-2 | 59 | 45.7 |
| ≥ 3 | 70 | 54.3 |
| Nature of prior therapy | ||
| Platinum-based chemotherapy | 128 | 99.2 |
| Tyrosine kinase inhibitor | 36 | 27.9 |
| Surgery† | 85 | 65.9 |
| Radiotherapy† | 75 | 58.1 |
| Hormonal, immunologic, or biologic therapy | 16 | 12.4 |
| Other | 9 | 7.0 |
| EGFR tumor mutation status | ||
| Mutant | 12 | 9.3 |
| Wild type | 56 | 43.4 |
| Unknown‡ | 61 | 47.3 |
| KRAS tumor mutation status | ||
| Mutant | 21 | 16.3 |
| Wild type | 36 | 27.9 |
| Unknown‡ | 72 | 55.8 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NSCLC, non–small-cell cancer.
One patient was enrolled before protocol amendment 4, which changed eligibility requirements from ECOG performance status of 0-2 to 0-1. Second patient was enrolled without evaluation of ECOG performance status at screening and had ECOG performance status of 2 at time of first nivolumab treatment.
Surgery and radiotherapy were not considered to be systemic therapies.
EGFR or KRAS mutational testing were not required for entry into this trial; 41 (67%) of 61 patients with unknown EGFR tumor status and 43 (60%) of 72 patients with unknown KRAS tumor status had squamous cell histology; these patients likely were not tested for EGFR or KRAS mutations, because squamous non–small-cell lung cancer rarely harbors EGFR or KRAS mutations.18–20
Clinical Activity
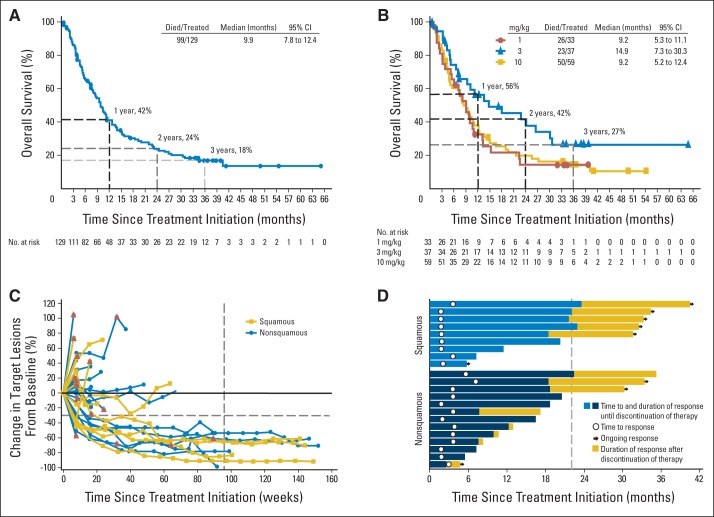
Median OS was 9.9 months (95% CI, 7.8 to 12.4) for all 129 patients with NSCLC (Table 2; Fig 1A). In 37 patients receiving nivolumab 3 mg/kg, the dose currently being used for phase III trials, median OS was 14.9 months (95% CI, 7.3 to 30.3). Median OS was 9.2 months in both the 1- and 10-mg/kg cohorts (Table 2; Fig 1B). In the total population of patients with NSCLC, across all dose levels, 1-, 2-, and 3-year survival rates were 42% (95% CI, 33 to 50), 24% (95% CI, 17 to 33), and 18% (95% CI, 11 to 25), respectively. At the 3-mg/kg dose, 1-, 2-, and 3-year OS rates were 56% (95% CI, 38 to 71), 42% (95% CI, 24 to 58), and 27% (95% CI, 12 to 43), respectively. Median OS and survival rates were similar in patients with squamous and nonsquamous histologies (Table 2; Data Supplement). Median progression-free survival (PFS) across doses was 2.3 months (95% CI, 1.8 to 3.7), with PFS rates at 6 months, 1 year, and 2 years of 33%, 22%, and 9%, respectively (Data Supplement).
Table 2.
Clinical Activity of Nivolumab in Patients With NSCLC*
| Dose (mg/kg) | ORR† |
Duration of Response (months)ठ|
OS (months)§‖ |
OS Rate§ |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Year |
2 Years |
3 Years |
||||||||||||||
| No. of Patients | % | 95% CI | Median | Range | Median | 95% CI | % | 95% CI | No. at Risk | % | 95% CI | No. at Risk | % | 95% CI | No. at Risk | |
| NSCLC¶ | ||||||||||||||||
| All doses | 22 of 129 | 17.1 | 11.0 to 24.7 | 17.0 | 1.4+ to 36.8+ | 9.9 | 7.8 to 12.4 | 42 | 33 to 50 | 48 | 24 | 17 to 33 | 26 | 18 | 11 to 25 | 12 |
| 1 | 1 of 33 | 3.0 | 0.1 to 15.8 | 14.7 | 14.7 to 14.7 | 9.2 | 5.3 to 11.1 | 33 | 17 to 49 | 9 | 15 | 5 to 30 | 4 | 15 | 5 to 30 | 1 |
| 3 | 9 of 37 | 24.3 | 11.8 to 41.2 | 17.0 | 3.7+ to 32.6+ | 14.9 | 7.3 to 30.3 | 56 | 38 to 71 | 17 | 42 | 24 to 58 | 11 | 27 | 12 to 43 | 5 |
| 10 | 12 of 59 | 20.3 | 11.0 to 32.8 | 19.1 | 1.4+ to 36.8+ | 9.2 | 5.2 to 12.4 | 38 | 26 to 50 | 22 | 20 | 11 to 31 | 11 | 14 | 7 to 25 | 6 |
| Squamous NSCLC | ||||||||||||||||
| All doses | 9 of 54 | 16.7 | 7.9 to 29.3 | NR# | 3.7 to 36.8+ | 9.2 | 7.3 to 12.5 | 41 | 27 to 54 | 20 | 24 | 14 to 37 | 12 | 19 | 9 to 32 | 6 |
| 1 | 0 of 15 | 0 | 0 | 0 | 0 | 8.0 | 2.4 to 13.3 | 29 | 9 to 52 | 4 | 14 | 2 to 37 | 2 | 0 | 0 | 0 |
| 3 | 4 of 18 | 22.2 | 6.4 to 47.6 | NR# | 3.7+ to 32.6+ | 9.5 | 5.3 to NE | 49 | 23 to 71 | 7 | 35 | 13 to 58 | 5 | 28 | 9 to 51 | 3 |
| 10 | 5 of 21 | 23.8 | 8.2 to 47.2 | 19.1 | 3.7 to 36.8+ | 10.5 | 4.9 to 16.7 | 43 | 22 to 62 | 9 | 24 | 9 to 43 | 5 | 18 | 5 to 37 | 3 |
| Nonsquamous NSCLC | ||||||||||||||||
| All doses | 13 of 74 | 17.6 | 9.7 to 28.2 | 14.2 | 1.4+ to 29.9 | 10.1 | 5.7 to 13.7 | 42 | 30 to 53 | 27 | 23 | 14 to 34 | 13 | 16 | 8 to 26 | 6 |
| 1 | 1 of 18 | 5.6 | 0.1 to 27.3 | 14.7 | 14.7 to 14.7 | 9.9 | 5.3 to 22.5 | 36 | 15 to 58 | 5 | 15 | 3 to 36 | 2 | 15 | 3 to 36 | 1 |
| 3 | 5 of 19 | 26.3 | 9.1 to 51.2 | 13.6 | 5.6 to 17.0 | 18.2 | 5.2 to 30.8 | 62 | 37 to 80 | 10 | 48 | 22 to 69 | 6 | 24 | 6 to 48 | 2 |
| 10 | 7 of 37 | 18.9 | 8.0 to 35.2 | 18.3 | 1.4+ to 29.9 | 7.4 | 4.5 to 11.0 | 34 | 19 to 49 | 12 | 16 | 6 to 30 | 5 | 12 | 4 to 26 | 3 |
Abbreviations: NE, not estimable; NR, not reached; NSCLC, non–small-cell cancer; ORR, objective response rate; OS, overall survival.
September 2014 data analysis.
Percentage of patients with confirmed complete or partial responses among all treated patients. CIs were calculated using Clopper-Pearson method. Individual patient responses were adjudicated per RECIST (version 1.0).
Time from date of first documented complete or partial response to time of documented progression, death, or last tumor assessment for censored data (denoted by +).
Time-to-event end points (duration of response, OS, and OS rate) were estimated using Kaplan-Meier method. CIs for medians were calculated using Brookmeyer and Crowley method, and CIs for survival rates were calculated using Greenwood formula.
Time from date of first treatment to date of death resulting from any cause or last tumor assessment for those patients who were alive at date of data analysis.
One patient with unknown histology.
Time point at which probability that responder's progress drops below 50% not reached because of insufficient No. of events and/or follow-up.
Fig 1.
Clinical activity in patients with non–small-cell lung cancer (NSCLC) receiving nivolumab. Kaplan-Meier curves of overall survival (OS) for (A) total patient population (N = 129) and (B) patients who received nivolumab 1 (n = 33), 3 (n = 37), or 10 mg/kg (n = 59). Symbols indicate censored events, defined for OS as time to last known date alive before date of data analysis, for patients without death. (C) Tumor burden kinetics in patients with NSCLC treated with nivolumab 3 mg/kg (n = 37). Baseline tumor measurements are standardized to zero. Tumor burden was measured as sum of longest diameters of target lesions compared with baseline. Red triangles indicate first occurrence of new lesion. Horizontal dashed line at −30% indicates threshold for defining objective response (partial tumor regression) in absence of new lesions or nontarget disease progression, according to RECIST (version 1.0); vertical dashed line at 96 weeks indicates protocol-defined maximum duration of continuous nivolumab therapy. (D) Characteristics of objective responses in patients with squamous cell histology (n = 9) and nonsquamous cell histology (n = 13) treated with nivolumab. Vertical dashed line at 22 months indicates maximum planned duration of continuous nivolumab therapy. Eighteen responders discontinued nivolumab therapy for reasons other than disease progression, including: completion of maximum cycles (n = 7), adverse events (n = 8), withdrawal of consent (n = 2), and other (n = 1).
An ORR of 17% was observed across all doses (Table 2). Patients with squamous and nonsquamous histologies had similar ORRs (17% and 18%, respectively). ORRs by dose were 3% (1 mg/kg), 24% (3 mg/kg), and 20% (10 mg/kg). For the 110 patients randomly assigned to the 1-, 3-, and 10-mg/kg doses, ORR was 3%, 22.2%, and 19.5%, respectively, and OS was 9.2, 14.9, and 8.6 months, respectively, similar to the overall population. Six (5%) of 129 patients had unconventional immune-pattern responses (Data Supplement) and were not considered responders in the calculation of ORR. OS for these patients was 7.3, 11.2, 16.7, 26.7, 34.5+ (ongoing), and 54.3+ months as of the September 2014 data analysis. Stable disease lasting at least 24 weeks was observed in an additional 10% of patients.
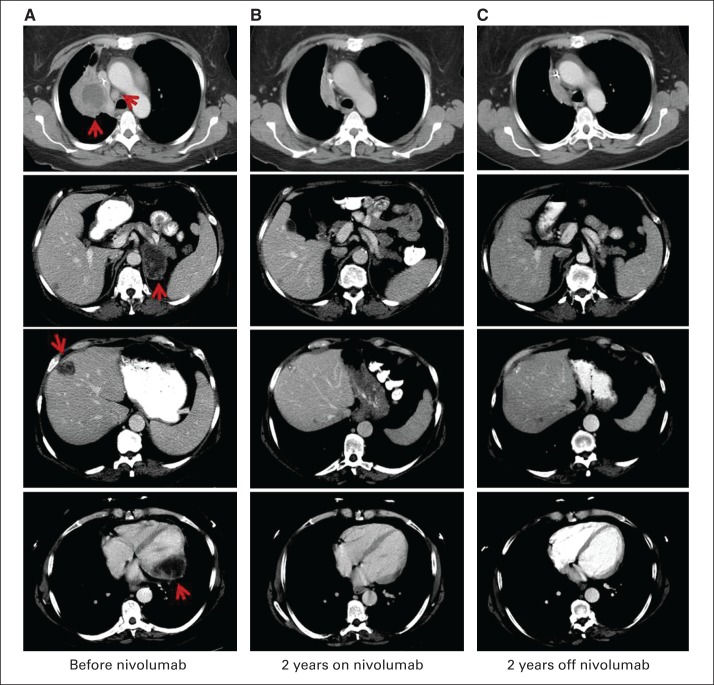
Among the 22 patients with objective responses, the Kaplan-Meier estimated median duration of response was 17.0 months (range, 1.4+ to 36.8+ months; Table 2 and Data Supplement). Eleven (50%) responses were documented at the first 8-week tumor assessment (Figs 1C and 1D). Median PFS of the 22 responders was 20.6 months (95% CI, 11.4 to not reached; range, 4.7+ to 40.3+ months; Fig 1D). At the time of data analysis, responses were ongoing in 41% (nine of 22) of the responders. Among 18 responders who discontinued nivolumab therapy for reasons other than disease progression (completion of maximum cycles, n = 7; adverse events, n = 8; withdrawal of consent, n = 2; other, n = 1), 50% (nine) had responses for more than 9 months after the end of therapy (range, 9.2 to 16.4+ months). Figure 2 shows an example of an ongoing response in a patient with widely metastatic chemotherapy-refractory squamous NSCLC.
Fig 2.
Partial response of patient with metastatic squamous non–small-cell lung cancer (NSCLC) treated with nivolumab, with sustained response after drug discontinuation. Female former smoker age 57 years had widely metastatic chemotherapy-refractory squamous NSCLC after four lines of systemic therapy for stage IV disease. She received 10-mg/kg dose of nivolumab every 2 weeks and achieved partial response after 16 weeks of treatment (two cycles). Response was sustained through 96-week course of nivolumab and, as of September 2014, was ongoing 50 months after initiation of nivolumab treatment and 26 months after drug discontinuation. Computer tomography imaging shows NSCLC metastases (A) before nivolumab, (B) after 2 years with nivolumab, and (C) 2 years after stopping nivolumab therapy, involving lung and mediastinal lymph node (upper row), adrenal gland (second row), liver (third row), and myocardium (bottom row). Arrows indicate locations of metastatic disease.
Analysis of predefined patient subgroups revealed similar ORRs (Table 3). Best changes in tumor burden presented by number of prior therapies and by EGFR and KRAS tumor mutation status are shown in the Data Supplement. Exploratory analysis by tumor PD-L1 expression, using an automated immunohistochemistry assay (Dako North America, Carpinteria, CA), on archived tumor samples from 68 patients found no clear association between PD-L1 expression and response or survival (Data Supplement).21 An additional exploratory analysis conducted retrospectively by select sites of response by smoking exposure in 80 evaluable patients found ORR was higher in patients with a smoking history of more than 5 pack-years (30%; n = 66) than in those with a history of 5 pack-years or less (no responses; n = 14).22
Table 3.
ORRs by Baseline Characteristic Subgroups*
| Subgroup | ORR† |
||
|---|---|---|---|
| No. of Patients | % | 95% CI | |
| Age, years | |||
| < 70 | 15 of 90 | 16.7 | 9.6 to 26.0 |
| ≥ 70 | 7 of 39 | 17.9 | 7.5 to 33.5 |
| Sex | |||
| Male | 13 of 79 | 16.5 | 9.1 to 26.5 |
| Female | 9 of 50 | 18.0 | 8.6 to 31.4 |
| ECOG performance status17 | |||
| 0 | 3 of 27 | 11.1 | 2.4 to 29.2 |
| 1-2‡ | 19 of 102 | 18.6 | 11.6 to 27.6 |
| Tumor cell histology | |||
| Squamous | 9 of 54 | 16.7 | 7.9 to 29.3 |
| Nonsquamous | 13 of 74 | 17.6 | 9.7 to 28.2 |
| No. of prior therapies | |||
| 1-2 | 7 of 59 | 11.9 | 4.9 to 22.9 |
| ≥ 3 | 15 of 70 | 21.4 | 12.5 to 32.9 |
| Prior TKI therapy | |||
| Yes | 4 of 36 | 11.1 | 3.1 to 26.1 |
| No | 18 of 93 | 19.4 | 11.9 to 28.9 |
| EGFR tumor status | |||
| Mutant | 2 of 12 | 16.7 | 2.1 to 48.4 |
| Wild type | 11 of 56 | 19.6 | 10.2 to 32.4 |
| Unknown | 9 of 61 | 14.8 | 7.0 to 26.2 |
| KRAS tumor status | |||
| Mutant | 3 of 21 | 14.3 | 3.0 to 36.3 |
| Wild type | 9 of 36 | 25.0 | 12.1 to 42.2 |
| Unknown | 10 of 72 | 13.9 | 6.9 to 24.1 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ORR, objective response rate; TKI, tyrosine kinase inhibitor.
September 2013 data analysis.
Percentage of patients with confirmed complete or partial responses compared with total No. of treated patients. CIs were calculated using Clopper-Pearson method. Individual patient responses were adjudicated per RECIST (version 1.0).
Only two patients had ECOG performance status of 2.
Safety
In the dose-escalation portion of this trial, the maximum-tolerated dose was not reached at the highest planned dose of 10 mg/kg. Subsequently, the 1-, 3-, and 10-mg/kg cohorts were expanded in patients with NSCLC. At the time of the March 2013 safety analysis, the median duration of therapy was 13.6 weeks (range, 2 to 104 weeks). Among the treated patients with NSCLC, 71% had experienced treatment-related adverse events of any grade (Data Supplement). The most common were fatigue (24%), decreased appetite (12%), and diarrhea (10%). Eighteen patients (14%) experienced grade 3 to 4 treatment-related adverse events, and the most common was fatigue (3%; Data Supplement). The spectrum, incidence, and severity of the treatment-related adverse events were similar for the NSCLC population (N = 129) and the total patient population (N = 306).23
Treatment-related select adverse events of any grade were observed in 41% of 129 patients with NSCLC, and the most common included skin, GI, and pulmonary events (16%, 12%, and 7%, respectively; Table 4). Four patients (3%) had treatment-related grade ≥ 3 pneumonitis, including one with grade 5 pneumonitis (Data Supplement). Three treatment-related deaths occurred among patients with NSCLC, each associated with pneumonitis (two with unresolved grade 4 pneumonitis, and one with grade 5 pneumonitis). Two of the deaths occurred early in the trial, and the third occurred after the March 2013 safety analysis (descriptions provided in Data Supplement). No clear relationships between the occurrence of pneumonitis and dose level or treatment duration were noted.
Table 4.
Treatment-Related Select AEs Occurring in All Treated Patients in NSCLC Population*
| Select AE | All Patients (N = 129) |
|||
|---|---|---|---|---|
| Any Grade† |
Grades 3 to 4 |
|||
| No. | % | No. | % | |
| Any AE | 53 | 41.1 | 6 | 4.7 |
| Skin | 20 | 15.5 | 0 | 0 |
| GI | 15 | 11.6 | 1 | 0.8 |
| Pulmonary | 9‡§ | 7.0§ | 3‡ | 2.3 |
| Endocrinopathies | 8 | 6.2 | 0 | 0 |
| Hepatic | 6 | 4.7 | 1 | 0.8 |
| Infusion reaction | 5 | 3.9 | 1 | 0.8 |
| Renal | 4 | 3.1 | 0 | 0 |
Abbreviations: AE, adverse event; NSCLC, non–small-cell cancer.
Select AEs were those requiring more frequent monitoring or intervention with immune suppression or hormone replacement, based on prespecified list of Medical Dictionary for Regulatory Activities terms.16 March 2013 data analysis.
Grades 1 to 5.
Eight patients had pneumonitis (grades 1 to 2, n = 5; grades 3 to 4, n = 3), and one patient had grade 2 interstitial lung disease.
Two additional patients had treatment-related grade 2 pneumonitis, which occurred before date of safety analysis, but they were not included, because these data were not available until after this analysis. Third patient had treatment-related grade 5 pneumonitis (detailed in Data Supplement) but was not included because event occurred after date of safety analysis.
DISCUSSION
Current second-line therapies for advanced NSCLC generate ORRs of 7% to 9%, with median OS of approximately 8 months and 1-year survival rates of 30%.24–26 The only third-line therapy approved for use in NSCLC is erlotinib, an EGFR tyrosine kinase inhibitor, with an ORR of 9% in a pooled population of patients, unselected for EGFR-mutant NSCLC, who had received one or two prior therapies for advanced NSCLC.26 Limited data exist supporting the use of chemotherapy after progression through two lines of chemotherapy for advanced NSCLC.27,28 Guidelines of the American Society of Clinical Oncology and the National Clinical Cancer Network currently do not recommend more than two lines of chemotherapy for advanced NSCLC, after which erlotinib, best supportive care alone, or clinical trial consideration is recommended.29,30
Unlike chemotherapy or tyrosine kinase inhibitors, immune checkpoint inhibitors aim to restore antitumor immunity, allowing the destruction of malignant cells with the potential for durable clinical benefit persisting long after cessation of therapy. By blocking the PD-1 inhibitory receptor, nivolumab releases immune suppression of primed tumor-specific T cells, enabling such cells to carry out their cytotoxic functions. The PD-1 receptor seems to be a clinically relevant target in NSCLC, demonstrated here by an ORR to nivolumab monotherapy of 17%, lasting for a median of 17.0 months among patients with heavily pretreated advanced NSCLC. The ORR in the subgroup of patients receiving three or more prior therapies for advanced NSCLC was 21%, similar to the ORR for the entire population of patients with NSCLC. This differs from chemotherapy, where response rates decrease with subsequent lines of therapy.31 Median OS of 9.9 months and 1-, 2-, and 3-year survival rates of 42%, 24%, and 18% with nivolumab surpass expectations of second- and third-line chemotherapies, taking into account the caveats of a phase I dose-escalation/expansion trial design. This effect seemed to vary by dose, with an ORR of 24%, median OS of 14.9 months, and 1-, 2-, and 3-year survival rates of 56%, 45%, and 27%, respectively, in the 3-mg/kg dose cohort.
Response rates to nivolumab were similar across most patient subgroups despite dose, including those with squamous and nonsquamous NSCLC. Activity in squamous cell carcinoma was consistent with a recent report from a trial evaluating nivolumab in 117 patients who received at least two prior lines of chemotherapy for advanced squamous NSCLC, where ORR was 15%, with a 1-year survival rate of 41%.32 Responses were seen in patients with EGFR– and KRAS–wild type and EGFR- and KRAS-mutant NSCLC; however, low patient numbers in each subset preclude association of mutation status with clinical outcomes after nivolumab therapy. Tumor PD-L1 status did not seem to predict response or survival; however, evaluable specimens were available for only half of the patients and were archival (most collected before systemic therapies received before nivolumab; updated data to be reported separately). Because tumor PD-L1 expression may vary over time and be induced by other anticancer therapy, results here may not be truly reflective of immediate prenivolumab tumor PD-L1 status. Analysis by smoking history suggested higher responses rates to nivolumab in former and current smokers, which is consistent with prior reports from trials evaluating nivolumab and other anti–PD-1 and anti–PD-L1 antibodies.31,33–35 One potential explanation for this finding is the expected higher mutational load in smoking-associated lung cancer, leading to more tumor neoantigens and increased immunogenicity.36
The durability of response after discontinuation of nivolumab, coupled with the unconventional responses observed in some patients in this trial, underscores the unique mechanisms of action of immunotherapy compared with standard therapies for NSCLC. Among 18 RECIST responders who discontinued nivolumab for reasons other than disease progression, nine (50%) had responses lasting more than 9 months after their last dose at the time of data analysis. In addition to objective responses by RECIST, 5% of patients experienced immune-related radiographic responses—that is, persistent reduction in target lesions in the presence of new lesions or regression of target lesions after initial growth. Unconventional response patterns such as these will require clinicians to alter their approach to and assessment of patients receiving immunotherapy (eg, potentially continuing therapy in face of radiographic progression in patient who is medically stable without decline of performance status).
Nivolumab therapy was generally well tolerated, with 14% of patients experiencing grade 3 to 4 treatment-related adverse events. Toxicities did not seem to be cumulative.16 Select adverse events with immune-based etiologies were generally low grade, and those at higher grades were manageable in most cases with drug discontinuation, immune suppressive agents (steroids or, rarely, infliximab), and/or hormone replacement. Three treatment-related deaths occurred among patients with NSCLC, all associated with pneumonitis, including one that occurred after the date of the last safety analysis. It should be noted that two of the fatal cases occurred early in the trial, before pneumonitis was recognized as a toxicity of treatment with nivolumab. Currently, guidelines are in place to facilitate early identification and management of pneumonitis.37 For grade 1 to 2 pneumonitis, nivolumab therapy is delayed, with administration of steroids for grade 2 pneumonitis. In select cases, rechallenge with nivolumab can be considered after resolution of pneumonitis. For grade 3 to 4 pneumonitis, nivolumab therapy is discontinued, with administration of steroids and additional immune suppressive agents as needed.
On the basis of the encouraging results seen in this large phase I trial, phase III clinical trials are further evaluating nivolumab in patients with NSCLC. The dose of nivolumab 3 mg/kg administered intravenously every 2 weeks was chosen for these phase III trials based on pharmacokinetic exposure, safety, and efficacy data from all patients enrolled onto this study. Two phase III clinical trials comparing standard-of-care docetaxel with nivolumab in patients with previously treated advanced NSCLC completed accrual in late 2013. One trial enrolled patients with nonsquamous NSCLC,38 whereas the other enrolled patients with squamous NSCLC39; both are powered to detect a difference in OS. In each of these trials, secondary end points include OS and ORR by PD-L1 tumor status. Another phase III trial was recently initiated, comparing standard first-line platinum-based doublet chemotherapy with nivolumab in patients with treatment-naive advanced PD-L1–positive NSCLC. This trial is supported by results in a PD-L1–positive NSCLC subset from an ongoing phase I trial evaluating nivolumab as first-line therapy for advanced NSCLC.40,41
Additional phase I, II, and III clinical trials are currently evaluating other anti–PD-1 or anti–PD-L1 antibodies and have shown encouragingly consistent activity in patients with advanced NSCLC.36,42–46 Efforts are now focusing on evaluating potential predictive biomarkers, such as tumor expression of PD-L1, to select populations most likely to benefit from antibodies targeting the PD-1 axis. Clinical trials evaluating combinations of nivolumab and other PD-1 axis inhibitors with chemotherapy, targeted therapy, epigenetic therapy, and other immunotherapies are also underway or being planned and hold promise for fully realizing the potential of immunotherapy in NSCLC.
Supplementary Material
Acknowledgment
We thank the patients who participated in this study; clinical faculty and personnel, including Miriam Akita, Marianne Davies, Emily Duffield, Christina Lakomski, Daniel Morgensztern, Isabel Oliva, Von Potter, Elin Rowen, Rebecca Sipples, and Danielle Wanik of Yale Cancer Center; William Pao and Serena Rucker of Vanderbilt University Medical Center; Jeffrey Infante of Sarah Cannon Research Institute/Tennessee Oncology; Jahleen Byers, Bryan Greene, Jill Hinson, Eric Keller, Lori Lipocky, and Vanica Pharoah of Carolina BioOncology Institute; Nana S. Akom, Jerrold B. Teitcher, Jennifer L. Winkelmann, and Jedd D. Wolchok of Memorial Sloan Kettering Cancer Center; Suzanne Burke, Kim Feldhaus, Elaine Granch, Nabeela Iqbal, Prathima Koppolu, and Terri O'Neill of the University of Michigan; Sarah Bonerigo, Cherylann Carr, Tianna Dauses, Charles Drake, David Ettinger, Robert Gray, Hans Hammers, Christine Hann, Pritish John, Ronan Kelly, Marina Laiko, Dung Le, Evan Lipson, Christian Meyer, Alice Pons, Joanne Reimer, Charles Rudin, and William Sharfman of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; John Gerks, Nils Lonberg, Alan Korman, Susan Vigen, and Ye Zhou of Bristol-Myers Squibb; and Susan Leinbach, medical writer, Clinical Solutions Group, and Lou Passador, medical writer, StemScientific.
Footnotes
Processed as a Rapid Communication manuscript.
Supported by Bristol-Myers Squibb, which also funded medical writing assistance, and by Ono Pharmaceutical.
Presented in part at the 49th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, May 31-June 4, 2013; the 15th World Conference on Lung Cancer, Sydney, New South Wales, Australia, October 27-30, 2013; the 50th ASCO Annual Meeting, Chicago, IL, May 30-June 3, 2014; and the Chicago Multidisciplinary Symposium in Thoracic Oncology, Chicago, IL, October 30-November 1, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00730639.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Scott N. Gettinger, Scott J. Antonia, Suzanne L. Topalian, Drew M. Pardoll, Jon M. Wigginton, Ashok K. Gupta, Julie R. Brahmer
Administrative support: Mark Salvati
Provision of study materials or patients: Scott N. Gettinger, Leora Horn, David R. Spigel, Naiyer A. Rizvi, John D. Powderly, Richard D. Carvajal, David M. Jackman, Lecia V. Sequist, Philip Leming, Mary C. Pinder-Schenck, F. Stephen Hodi, Jeffrey A. Sosman, Julie R. Brahmer
Collection and assembly of data: Scott N. Gettinger, Leora Horn, Leena Gandhi, Scott J. Antonia, Naiyer A. Rizvi, John D. Powderly, Rebecca S. Heist, Richard D. Carvajal, David M. Jackman, David C. Smith, F. Stephen Hodi, Jeffrey A. Sosman, Mario Sznol, David F. McDermott, Vindira Sankar, Christoph M. Ahlers, Matthew D. Hellmann, Ashok K. Gupta, Julie R. Brahmer
Data analysis and interpretation: Scott N. Gettinger, Leena Gandhi, David R. Spigel, Scott J. Antonia, Naiyer A. Rizvi, Rebecca S. Heist, Richard D. Carvajal, Lecia V. Sequist, David C. Smith, Philip Leming, David P. Carbone, Mary C. Pinder-Schenck, Suzanne L. Topalian, F. Stephen Hodi, Jeffrey A. Sosman, Mario Sznol, David F. McDermott, Drew M. Pardoll, Mark Salvati, Jon M. Wigginton, Matthew D. Hellmann, Georgia D. Kollia, Ashok K. Gupta, Julie R. Brahmer
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Overall Survival and Long-Term Safety of Nivolumab (Anti–Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Scott N. Gettinger
Honoraria: Bristol-Myers Squibb, ARIAD Pharmaceuticals
Consulting or Advisory Role: Bristol-Myers Squibb, ARIAD Pharmaceuticals
Research Funding: Bristol-Myers Squibb (Inst), ARIAD Pharmaceuticals (Inst), Genentech (Inst), AstraZeneca (Inst), Bayer Pharmaceuticals (Inst), Pfizer (Inst)
Leora Horn
Consulting or Advisory Role: Helix Bio, Clovis Oncology, PUMA, Merck, Bayer, Xcovery, Genentech
Research Funding: Astellas Pharma (Inst)
Leena Gandhi
Honoraria: Merck
Consulting or Advisory Role: Merck, Genentech/Roche
Travel, Accommodations, Expenses: Genentech/Roche (investigator meeting)
David R. Spigel
Consulting or Advisory Role: Bristol-Myers Squibb, Roche/Genentech, Merck, AstraZeneca/MedImmune
Research Funding: Bristol-Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Roche/Genentech (Inst), Pfizer (Inst)
Scott J. Antonia
Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Bristol-Myers Squibb
Research Funding: MedImmune (Inst)
Naiyer A. Rizvi
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech/Roche, Novartis, Merck Sharp & Dohme, AstraZeneca/MedImmune
Research Funding: Bristol-Myers Squibb (Inst)
John D. Powderly
Employment: BioCytics
Leadership: BioCytics
Stock or Other Ownership: BioCytics
Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, Amplimmune
Speakers' Bureau: Bristol-Myers Squibb, Merck, Dendreon
Research Funding: Bristol-Myers Squibb, Genentech, Amplimmune, EMD Serono, AstraZeneca, Eli Lilly/ImClone, Incyte, Macrogenics
Rebecca S. Heist
Consulting or Advisory Role: Boehringer Ingelheim, Momenta
Research Funding: Genentech (Inst), GlaxoSmithKline (Inst), sanofi-aventis (Inst), Celgene (Inst), Debiopharm (Inst), Peregrine (Inst), Exelixis (Inst)
Richard D. Carvajal
No relationship to disclose
David M. Jackman
Consulting or Advisory Role: Genentech, Cowen Group, Gerson Lehrman Group
Lecia V. Sequist
Consulting or Advisory Role: Clovis Oncology, Novartis, Merrimack Pharmaceuticals, AstraZeneca, Genentech, Taiho Pharmaceutical, GlaxoSmithKline
Research Funding: Boehringer Ingelheim (Inst), Clovis Oncology (Inst), Genentech (Inst), Merrimack Pharmaceuticals (Inst), GlaxoSmithKline (Inst), ArQule (Inst), Daiichi Sankyo (Inst), Novartis (Inst), AstraZeneca (Inst), Johnson & Johnson (Inst), Eli Lilly (Inst), Merck (Inst), Taiho Pharmaceutical (Inst)
David C. Smith
Research Funding: AstraZeneca (Inst), OncoMed (Inst), Exelixis (Inst), MedImmune (Inst), Tekmira (Inst), Atterocor (Inst), ImClone Systems (Inst), Incyte (Inst), Celgene (Inst), Aragon Pharmaceuticals (Inst), Bristol-Myers Squibb/Medarex (Inst), Teva (Inst), Bayer (Inst), Eisai (Inst), Millennium Pharmaceuticals (Inst), Debiopharm Group (Inst), Boston Biomedical (Inst), Eli Lilly (Inst), Oncogenex (Inst), PSMA Development (Inst), Abraxis BioScience (Inst), Novartis (Inst)
Philip Leming
No relationship to disclose
David P. Carbone
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Roche, BioDesix, Boehringer Ingelheim, Amgen, Pfizer, Novartis, GlaxoSmithKline
Research Funding: Bristol-Myers Squibb (Inst)
Mary C. Pinder-Schenck
Employment: GlaxoSmithKline
Stock or Other Ownership: GlaxoSmithKline
Honoraria: Genentech
Consulting or Advisory Role: Myriad Genetics
Speakers' Bureau: Genentech, Celgene
Research Funding: Pfizer
Suzanne L. Topalian
Stock or Other Ownership: Aduro, Amplimmune, Compugen, NexImmune, Jounce Therapeutics, Potenza Therapeutics (I)
Consulting or Advisory Role: Jounce Therapeutics, sanofi-aventis, Amplimmune (I), GlaxoSmithKline, Five Prime Therapeutics
Research Funding: Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Amplimmune (Inst), Bristol-Myers Squibb (Inst), Aduro (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, sanofi-aventis, Five Prime Therapeutics
F. Stephen Hodi
Consulting or Advisory Role: Merck Sharp & Dohme, Novartis, Genentech
Research Funding: Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech/Roche (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending as per institutional policy (Inst)
Jeffrey A. Sosman
Honoraria: GlaxoSmithKline, Amgen
Consulting or Advisory Role: GlaxoSmithKline, Amgen
Research Funding: Bristol-Myers Squibb (Inst), GlaxoSmithKline (Inst)
Mario Sznol
Stock or Other Ownership: Amphivena, Adaptive Biotechnologies
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech/Roche, Amgen, AstraZeneca/MedImmune, Symphogen, Merus, Immune Design, Anaeropharma, Kyowa-Hakko Kirin, Lion Biotechnologies, Nektar, Pfizer, Novartis, Prometheus, Astellas Pharma
Other Relationship: Haymarket Media
David F. McDermott
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech/Roche, Pfizer
Research Funding: Prometheus
Drew M. Pardoll
Stock or Other Ownership: Compugen, Aduro, Potenza, Jounce, Neximmune
Consulting or Advisory Role: Pfizer, Medimmune, sanofi-aventis, Merck, Amgen
Research Funding: Aduro, Bristol-Myers Squibb, Compugen, Potenza
Patents, Royalties, Other Intellectual Property: Royalties on patents licensed to Aduro, Bristol-Myers Squibb, and Potenza
Vindira Sankar
Employment: Bristol-Myers Squibb
Christoph M. Ahlers
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Mark Salvati
Employment: Bristol-Myers Squibb, Janssen Pharmaceuticals, Novartis (I)
Stock or Other Ownership: Bristol-Myers Squibb, Janssen Pharmaceuticals, Novartis (I)
Patents, Royalties, Other Intellectual Property: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Jon M. Wigginton
Employment: Macrogenics (current), Bristol-Myers Squibb (former)
Stock or Other Ownership: Macrogenics (current), Bristol-Myers Squibb (former)
Matthew D. Hellmann
Consulting or Advisory Role: Third Rock Ventures
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Genentech, Bristol-Myers Squibb
Georgia D. Kollia
Employment: Bristol-Myers Squibb, Bristol-Myers Squibb (I)
Stock or Other Ownership: Bristol-Myers Squibb, Bristol-Myers Squibb (I)
Ashok K. Gupta
Employment: Bristol-Myers Squibb, AstraZeneca/MedImmune
Stock or Other Ownership: Bristol-Myers Squibb, AstraZeneca/MedImmune
Patents, Royalties, Other Intellectual Property: Use of PD-1 inhibitors in treatment of cancer
Julie R. Brahmer
Consulting or Advisory Role: Merck, Bristol-Myers Squibb, Eli Lilly
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst), Celgene (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck
Other Relationship: Bristol-Myers Squibb
REFERENCES
- 1.Gettinger S, Lynch T. A decade of advances in treatment for advanced non-small cell lung cancer. Clin Chest Med. 2011;32:839–851. doi: 10.1016/j.ccm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Socinski MA, Evans T, Gettinger S, et al. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e341S–e368S. doi: 10.1378/chest.12-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: A phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3342–3350. doi: 10.1200/JCO.2012.46.1764. [DOI] [PubMed] [Google Scholar]
- 4.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:20–30. doi: 10.1093/annonc/mds590. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 7.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 9.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates Cancer Immunol Res. 2014;2:1–11. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (version 3.0) http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 16.Topalian S, Sznol M, McDermott D, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 18.Marchetti A, Martella C, Felicioni L, et al. EGFR mutations in non–small-cell lung cancer: Analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23:857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Rekhtman N, Paik PK, Arcila ME, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: Lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res. 2012;18:1167–1176. doi: 10.1158/1078-0432.CCR-11-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap) Ann Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gettinger SN, Horn L, Gandhi L, et al. Long-term survival, clinical activity, and safety of nivolumab (Anti-PD-1; BMS-936558, ONO-4538) in patients (pts) with advanced non-small cell lung cancer (NSCLC) Intl J Radiat Oncol Biol Phys. 2014;90(suppl):5s. abstr 170. [Google Scholar]
- 22.Hellmann MD, Creelan BC, Woo K, et al. Smoking history and response to nivolumab in patients with advanced NSCLC. Ann Oncol. 2014;25(suppl):4s. abstr 1229PD. [Google Scholar]
- 23.Topalian SL, Sznol M, Brahmer JR, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with advanced solid tumors: Survival and long-term safety in a phase I trial. J Clin Oncol. 2013;31(suppl 15s):173s. abstr 3002. [Google Scholar]
- 24.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non–small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 25.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non–small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 27.Girard N, Jacoulet P, Gainet M, et al. Third-line chemotherapy in advanced non-small cell lung cancer: Identifying the candidates for routine practice. J Thorac Oncol. 2009;4:1544–1549. doi: 10.1097/JTO.0b013e3181bbf223. [DOI] [PubMed] [Google Scholar]
- 28.Penrod JR, Korytowsky B, Petrilla A, et al. Survival of U.S. Medicare patients with advanced non-small cell lung cancer by line of therapy. J Clin Oncol. 2014;32(suppl 15s):430s. abstr 6582. [Google Scholar]
- 29.American Society of Clinical Oncology. Institute for Quality: Practice guidelines. http://www.asco.org/quality-guidelines/guidelines.
- 30.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Non-small cell lung cancer (version 3.2014) http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 31.Asahina H, Sekine I, Horinouchi H, et al. Retrospective analysis of third-line and fourth-line chemotherapy for advanced non-small-cell lung cancer. Clin Lung Cancer. 2012;13:39–43. doi: 10.1016/j.cllc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Ramalingam S, Mazieres J, Planchard D, et al. Phase II study of nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients with advanced, refractory squamous non-small cell lung cancer. Intl J Rad Oncol Biol Physics. 2014;90(suppl):5s. abstr LB2. [Google Scholar]
- 33.Garon EB, Gandhi L, Rizvi N, et al. Antitumor activity of pembrolizumab (pembro; MK-3475) and correlation with programmed death ligand 1 (PD-L1) expression in a pooled analysis of patients (pts) with advanced non-small cell lung. Ann Oncol. 2014;25(suppl):5s. abstr LBA43. [Google Scholar]
- 34.Rizvi N, Shepherd FA, Antonia SJ, et al. First-line monotherapy with nivolumab (Anti-PD-1; BMS-936558, ONO-4538) in advanced non-small cell lung cancer (NSCLC): Safety, efficacy, and correlation of outcomes with PD-L1 status. Intl J Rad Oncol Biol Biophys. 2014;90(suppl):5s. abstr 165. [Google Scholar]
- 35.Horn L, Herbst R, Spiegel D, et al. An analysis of the relationship of clinical activity to baseline EGFR status, PD-L1 expression and prior treatment history in patients with non-small cell lung cancer (NSCLC) following PD-L1 blockade with MPDL3280A (anti-PDL1). Presented at the International Association for the Study of Lung Cancer 14th World Conference on Lung Cancer; July 3-7, 2011; Amsterdam, the Netherlands. [Google Scholar]
- 36.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without braf mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 38.ClinicalTrials.gov. Study of BMS-936558 (nivolumab) compared to docetaxel in previously treated metastatic non-squamous NSCLC. http://clinicaltrials.gov/show/NCT01673867.
- 39.ClinicalTrials.gov. Study of BMS-936558 (nivolumab) compared to docetaxel in previously treated advanced or metastatic squamous cell non-small cell lung cancer (NSCLC) http://clinicaltrials.gov/show/NCT01642004.
- 40.ClinicalTrials.gov. Study of nivolumab (BMS-936558) in subjects with advanced or metastatic squamous cell non-small cell lung cancer who have received at least two prior systemic regimens. http://clinicaltrials.gov/show/NCT01721759.
- 41.Gettinger S, Shepherd F, Antonia S, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol. 2014;32(suppl 15s):512s. abstr 8024. [Google Scholar]
- 42.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ClinicalTrials.gov. An open-label, randomized, phase 3 trial of nivolumab versus investigator's choice chemotherapy as first-line therapy for stage IV or recurrent PD-L1+ non-small cell lung cancer. http://clinicaltrials.gov/show/NCT02041533.
- 44.Brahmer J, Rizvi N, Lutzky J, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol. 2014;32(suppl 15s):511s. abstr 8021. [Google Scholar]
- 45.Garon E, Leighl N, Rizvi N, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;32(suppl 15s):511s. abstr 8020. [Google Scholar]
- 46.Rizvi N, Garon E, Patnaik A, et al. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;32(suppl 15s):507s. abstr 8007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.