Abstract
A 45-year-old woman with dilated cardiomyopathy was admitted for the upgrade of a previously implanted pacemaker. Echocardiography showed intraventricular dyssynchrony and a low ejection fraction (0.35). Treatment with a cardiac resynchronization therapy defibrillator (CRT-D) was selected and the device was implanted. CRT-D interrogation revealed proper function. Following procedure termination, the patient went into cardiac arrest and died despite resuscitation attempts. An autopsy revealed that the medial aspect of the right atrium was pierced by an active lead and that the aorta had a deep lesion, 2 mm in length, on its lateral aspect. We explain the probable pathogenesis of this patient׳s death.
Keywords: Aortic perforation, Cardiac resynchronization therapy defibrillator, Implantable cardioverter defibrillator, Active lead
1. Introduction
Cardiac perforation is a dreaded complication of transvenous pacemaker, cardiac resynchronization therapy defibrillator (CRT-D), and implantable cardioverter-defibrillator (ICD) lead placement because of the potential for critical morbidity and mortality.
2. Case report
We report the case of a 45-year-old woman affected by severe dilated cardiomyopathy who was admitted to our Cardiology Department for an upgrade of a previously implanted pacemaker. The patient received a VVI pacemaker implant for complete atrioventricular block 22 years earlier and an upgrade (DDD mode) 11 years later. Echocardiography revealed intraventricular dyssynchrony and a low ejection fraction (0.35). A CRT-D was selected for this patient and the device was implanted.
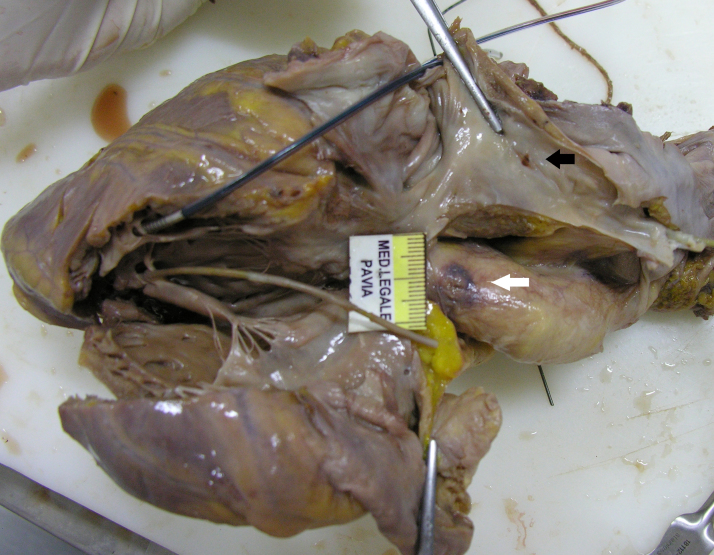
The procedure was performed under local anesthesia. Leads were inserted through the left subclavian vein: (a) an active fixation double catheter in the right ventricle, (b) a bipolar active fixation lead in the right auricle (Fig. 1), and (c) a bipolar lead introduced from the coronary sinus into the antero-lateral vein.
Fig. 1.
The explanted heart: a small hematoma is evident on the antero-lateral aspect of the aorta (white arrow); the medial aspect of the right atrium shows the lesion where the lead metal extremity was implanted (black arrow).
The previously implanted pacemaker and one atrial lead were removed (the old unipolar lead was left in the right ventricle) and a CRT device was connected to the new implanted leads. The atrial lead removal was uneventful. After satisfactory R-wave sensing (>5 mV) and pacing thresholds (<1.0 V at 0.5-ms pulse width) had been demonstrated, the patient underwent defibrillation threshold testing (DFT) to ensure proper device function. Sustained ventricular tachycardia and ventricular fibrillation were induced to make certain that the device was able to constantly sense, detect, and terminate arrhythmias with a shock at 25 J. During skin closure, the patient went into cardiac arrest with pulseless electrical activity. Cardiopulmonary resuscitation maneuvers were performed immediately and echocardiography showed intrapericardial effusion that was partially drained. After 1 h, pulseless electrical activity persisted, the patient was declared dead, and resuscitation attempts were halted.
The unexpected fatal outcome resulted in an allegation of medical negligence against the operating cardiologists. A board of physicians consisting of a forensic doctor and a heart surgeon conducted a post-mortem examination and analysis of the medical records.
At autopsy, approximately 180 cm3 of blood and clots were found inside the pericardial cavity. Careful inspection of the aorta showed a deep lesion, 2 mm in length, on the antero-lateral aspect of the vessel (Fig. 1) 2 cm above the valvular plane (Fig. 2). On the medial aspect of the right atrium, the lead metal extremity was implanted at the base of the auricle on the medial wall. The right atrium was very thin, with an average thickness of 2 mm. Histology showed abundant adipose tissue in its context, endocardial fibrosis, and numerous foci of inflammatory infiltration by various elements including lymphocytes and monocytes as well numerous plasma cells and eosinophils, clearly indicating myocarditis that was not recent.
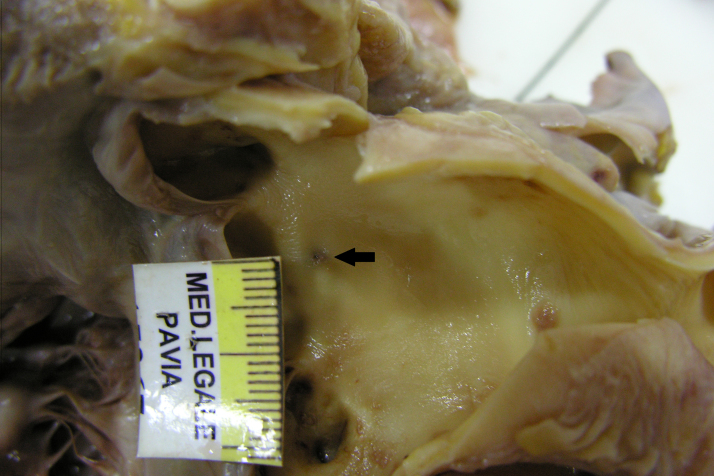
Fig. 2.
The aorta opened: a pinpoint injury was noted 2 cm above the noncoronary cusp (black arrow). This was related to the lesion described on the adventitial portion of the aorta.
The ascending aorta was incised and approximately 2 cm above the noncoronary cusp, a pinpoint injury related to the lesion described on the aortic adventia (Fig. 2) was noted.
3. Discussion
The pathogenetic mechanism underlying this patient׳s death can be explained in the following manner: the active lead (Fig. 1), positioned in the right atrium medial aspect, perforated the thin atrial wall and the lateral aspect of the aortic wall. After aortic perforation, the lead initially remained inside its wall, which explains why the device interrogation revealed satisfactory R-wave sensing (>5 mV) and pacing thresholds (<1.0 V at 0.5-ms pulse width). The patient eventually underwent DFT to guarantee proper device function. At this point, the active lead, positioned inside the aorta like a cork, slipped out, causing cardiac tamponade. In a review of relevant literature, we identified only five cases [1], [2], [3], [4], [5] of atrial damage by a pacemaker lead with concomitant aortic wall perforation, as shown in Table 1. However, not all reports indicate whether the lead responsible for the cardiac laceration was active or passive.
Table 1.
Review of the literature: only five cases of atrial damage by a pacemaker lead with concomitant aortic wall perforation have been published.
| Author | Device | Time after implant | Symptoms | Procedure | Outcome |
|---|---|---|---|---|---|
| Kalijusto [1] | P-M | 14 days | Chest pain | Sternotomy | Alive |
| Kashani [2] | P-M active lead | 1 day | Pain, dyspnea | Thorac/Sternot | Alive |
| Sticco [3] | P-M | 14 days | Syncope | Thorac/Sternot | Alive |
| deRoux [4] | P-M active lead | End procedure | Hypotension | Thoracotomy | Dead |
| Di Marco [5] | P-M active lead | 6 h | Ch. pain, hypot. | Sternotomy | Alive |
A board of physicians conducted a post-mortem examination and their findings did not support a direct allegation of medical negligence against the operating cardiologists who performed the ICD implant. The board analyzed the guidelines for cardiac pacing and cardiac resynchronization therapy and considered the indications for the procedure performed on the patient to be correct. The increased heart size shifted the right atrial appendage closer to the adjacent lateral aspect of the aorta. At the same time, the remarkable thinness of the right atrium was conducive to wall perforation. The perforation was not necessarily a result of malpractice in electrode positioning, but was also caused by other factors such as the pressure exerted during cardiac contraction. Moreover, since she was bearing a pacemaker, the patient could not undergo magnetic resonance imaging before the procedure to highlight the thicknesses of the cardiac wall. In addition, her pre-existing myocarditis undoubtedly reduced the chances of successful resuscitation.
Conflict of interest
The corresponding author, Dr. Antonino M. Grande, affirms on behalf of all co-authors that we have no conflicts of interest in connection with this article.
References
- 1.Kalijusto M.L., Tønnessen T. Aortic perforation with cardiac tamponade two weeks after pacemaker implantation. J Thorac Cardiovasc Surg. 2007;134:502–503. doi: 10.1016/j.jtcvs.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 2.Kashani A., Mehdirad A., Fredman C., Barold S. Aortic perforation by active-fixation atrial pacing lead. Pac Clin Electrophysiol. 2004;27:417–418. doi: 10.1111/j.1540-8159.2004.00456.x. [DOI] [PubMed] [Google Scholar]
- 3.Sticco C.C., Barrett L.O. Delayed cardiac tamponade by iatrogenic aortic perforation with pacemaker implantation. J Thorac Cardiovasc Surg. 2006;131:480–481. doi: 10.1016/j.jtcvs.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 4.deRoux S. Fatality following right atrial perforation by a screw-in pacemaker electrode. J Forensic Sci. 2005;50:1191–1193. [PubMed] [Google Scholar]
- 5.Di Marco A., Nuňez E., Osorio K. Atrial pacing lead: an unusual but serious complication. Tex Heart Inst J. 2014;41:327–328. doi: 10.14503/THIJ-13-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]