1. Case history
An 82-year-old frail woman with a prior history of hypertension, complete heart block, and dual permanent pacemaker (Sensia DR SEDR01, Medtronic Inc., Minneapolis, MN) presented to pacemaker clinic with symptoms of shortness of breath (SOB), dizziness, and “low pulse rate”. Pacemaker interrogation showed underlying normal sinus rhythm, sinus rate of around 74 beats/min with atrial sensed events, and ventricular paced rhythm with frequent symptomatic premature ventricular complexes (PVC). Pacemaker parameters were DDD, lower rate limit (LRL) of 60 beats/min, and maximum tracking rate of 120 beats/min, along with paced/sensed AV delay of 300/250 ms. Also noted was high pacing threshold of 3.5 V at 1 ms in the atrial lead with normal sensing parameters. In view of these, the pacemaker mode was reprogrammed to VDD mode and the patient was given beta-blocker therapy for frequent PVCs.
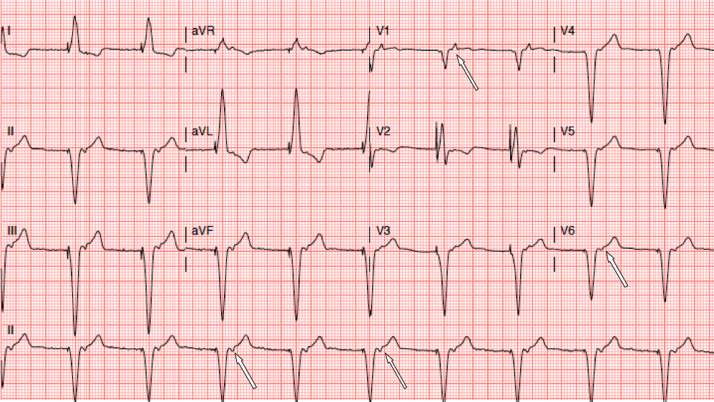
She presented to arrhythmia clinic a few weeks later because of worsening SOB, light-headedness, and transient ischemic attack-like symptoms. Electrocardiography (ECG) performed in the clinic showed ventricular paced rhythm with retrograde P waves (Fig. 1). What was the underlying arrhythmia mechanism? What treatment options are available?
Fig. 1.
Surface 12-lead electrocardiogram obtained on initial presentation showing retrograde P waves (arrows) associated with ventricular paced rhythm at a basal rate of 60 beats/min.
2. Discussion
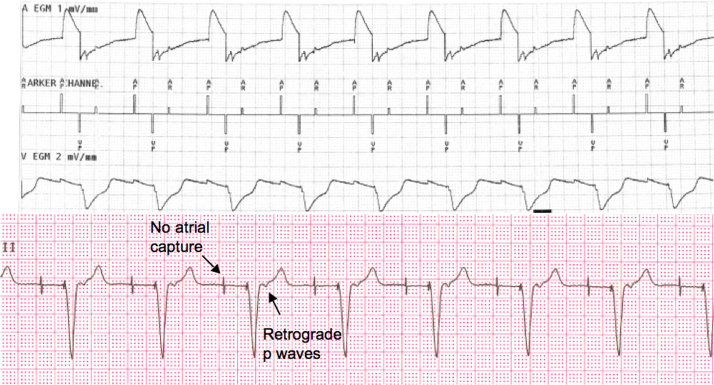
This case highlights the importance of understanding different modes of pacemaker modes and its role in different patient populations. In our patient, ECG revealed retrograde atrial depolarizations that were occurring in the post-ventricular atrial refractory (PVAR) period, and therefore were not tracked in the VDD mode. With no subsequent atrial pacing, tracking of the subsequent ventricular beat requires a sinus beat prior to timing out of ventricular escape cycle length of 1000 ms, which was suppressed by beta-blockers. Based on physiological and ECG findings, this condition is similar to repetitive non-reentrant ventricular atrial synchrony (RNRVAS), with both of these conditions leading to ventricular pacing at a LRL with retrograde p waves falling during PVAR period and therefore not tracked (functional undersensing) [1]. Both of these conditions can result in palpitations, typical pacemaker syndrome symptoms, congestive heart failure, light-headedness, and possibly syncope. RNRVAS is, however, observed in DDD mode with lack of atrial capture with subsequent atrial pacing due to refractoriness of atrial myocardium due to prior retrograde atrial depolarization (functional non-capture). As atrial sensed events during the PVAR period do not reset the atrial pacing interval and both atrial senses and atrial paced events are counted toward mode switching, these patients may have pseudo-mode switch episodes on pacemaker interrogation. Fig. 2 illustrates an example of RNRVAS in our patient in DDD mode that was triggered by lack of atrial capture (subthreshold output) with underlying high pacing thresholds.
Fig. 2.
Surface single-lead electrocardiogram and corresponding pacemaker electrocardiogram showing repetitive non-reentrant ventricular atrial synchrony triggered by subthreshold atrial pacing output.
There are few options to resolve this pacemaker-induced arrhythmia in our patient. The most obvious would be right atrial lead revision and reprogramming the device back to the DDD mode. However, this may still result in RNRVAS triggered by PVC. In addition, in an elderly, frail woman with significant kyphosis and other comorbidities, surgical option was not favored. Shortening of the PVAR period is another consideration but would have led to pacemaker-mediated tachycardia. While stopping beta-blocker therapy may help, it would worsen the PVC. We changed the pacemaker mode to DDD, accepting high thresholds, but decreased the base rate to 50 beats/min and shortened the AV delay to 150/180 ms in the sensed/paced configuration. With lowering of the base rate, atrial pacing would be minimized to preserve battery life. Moreover, decreasing basal rate and shortening of the AV interval would provide longer time for atrial repolarization and would either allow return of sinus activity and/or ensure atrial capture with the subsequent atrial pacing. Finally, noncompetitive atrial pacing (NCAP) was turned on to prevent atrial pacing with atrial sensed events in refractory in order to prevent RNRVAS. A repeat ECG after these programmings revealed a normal sinus rhythm, with a sinus rate of 56 beats/min and normal tracking of sinus beats. During follow-up, the patient had complete resolution of her symptoms without requiring lead revision.
There are few learning points that can be derived from this case. While VDD is an acceptable mode in young patients, one should be cautious of using this mode in elderly patients with sick sinus syndrome, unless retrograde VA conduction is absent. A single PVC can trigger a ventricular paced rhythm with retrograde p waves that will persist until the sinus rate increases, which may be limited in patients with sinus node dysfunction. This is especially true in patients with intact but slow VA conduction and those with slow intra-atrial conduction due to longer time taken from retrograde atrial depolarization to reach atrial lead electrode. Potential for RNRVAS can be unmasked during atrial lead threshold evaluation in patients with complete heart block. Here, pacing the atrium at a faster rate in DDD mode may initiate RNRVAS once atria loose capture. In addition, subsequent atrial capture will not resume even at suprathreshold levels due to RNRVAS, and it may lead to a false diagnosis of atrial lead dysfunction. To minimize the risk of VA synchrony, lower basal rate and shorter AV delay should be programmed for patients with VDD pacer. Similar programming is recommended for patients with complete heart block and those with a biventricular implantable cardioverter defibrillator (ICD) to prevent RNRVAS. NCAP is an algorithm available in DDD mode (pacemaker or ICD) and is primarily intended to prevent triggering of atrial tachyarrhythmia by delaying atrial pacing within the atrial myocardial refractory period. With NCAP, a sensed atrial event occurring in the PVAR period starts NCAP period, typically 300 ms, during which atrial pacing is inhibited. If lower rate pacing is scheduled to occur during this period, the VA interval is extended until the NCAP expires. Furthermore, when an atrial pacing is delayed by NCAP, subsequent paced AV delay is shortened to maintain a stable ventricular rate. This delay allows the atrial myocardium to recover and ensure capture. Occasionally, other algorithms such as extension of atrial escape interval after PVC and synchronous atrial pacing upon detection of PVC can be utilized to prevent this type of arrhythmia.
Conflicts of interests
None.
Financial support
None.
Reference
- 1.Barold S.S., Levine P.A. Pacemaker repetitive nonreentrant ventriculoatrial synchronous rhythm. A review. J Interv Card Electrophysiol. 2001;5:45–58. doi: 10.1023/a:1009853723766. [DOI] [PubMed] [Google Scholar]