Abstract
Background
Anisotropic and slow conduction in the atrium underlie the development of atrial fibrillation (AF). This study aimed to investigate the P wave characteristics associated with the development of AF in patients with a biphasic P wave in the inferior leads.
Methods
Digital analysis of retrospectively recorded 12-lead electrocardiograms was performed to select patients with a biphasic P wave (positive/negative) in lead II from a database of 114,334 patients. Characteristics of the P wave in the inferior leads associated with incidence of AF were determined. Receiver operating characteristic curves dichotomized P wave variables were measured in each lead.
Results
A total of 141 patients (77 men; mean age, 64±19 years) were enrolled in this study. Twenty-nine (20.6%) patients developed AF (AF group) vs. 112 (79.6%) who did not (non-AF group) during a follow-up period of 50±62 months. The amplitude of the initial P wave portion in lead II was significantly larger in the AF group when compared with the non-AF group (77.3±77.0 µV vs. 51.0±30.1 µV, p=0.003), while the amplitude of the terminal P wave portion in lead III was significantly decreased in the AF group when compared with the non-AF group (−70.6±41.3 µV vs. −89.1±38.1 µV, p=0.024). The duration of the initial P wave portion in lead III was significantly longer in the AF group when compared with the non-AF group (52.7±34.6 ms vs. 35.8±30.4 ms, p=0.011). Multivariate Cox proportional-hazards analysis confirmed that the increased duration of the initial P wave portion in lead III (≥71 ms) was independently associated with AF development (hazard ratio 2.90, 95% confidence interval 1.16–7.11, p=0.02).
Conclusion
The analyses of the biphasic P wave in the inferior leads suggest that the development of AF could be attributed to increased atrial slow conduction.
Keywords: Biphasic P wave, Atrial fibrillation, Electrocardiography, Conduction, Prognosis
1. Introduction
Atrial fibrillation (AF) occurs when atrial conduction converts from normal signal propagation to multiple reentering wavelets. On the electrocardiogram (ECG), the P wave reflects atrial depolarization and the indices provide information as to whether atrial depolarization usually occurs [1]. We previously reported that marked left atrial overload represented by a negative terminal portion of the P wave in lead V1 was associated with an increased risk of AF development [2]. In contrast, a biphasic P wave in the inferior leads was associated with delayed atrial conduction of Bachmann׳s bundle [3] and circling impulse propagation from the lower right atrium to the left atrium [4]. Therefore, both slow and anisotropic conduction could play a significant role in generating a biphasic P wave in inferior leads. In addition, these electrophysiological abnormalities could increase the tendency of reentry degenerating into AF. However, as to whether a biphasic P wave in the inferior leads is clinically associated with the development of AF is yet unknown. In this study, we investigated the following: (1) the association between a biphasic P wave in lead II with the development of AF and (2) the P wave characteristics in the inferior leads that may be associated with the development of AF.
2. Materials and methods
2.1. Study participants
A database for analyzing resting 12-lead ECGs was created using results recorded at the Shiga University of Medical Science Hospital. A total of 114,334 patients (59,243 men and 55,091 women) who had undergone ECG recordings between January 1983 and July 2010 were included in this database, with a total of 359,737 ECG recordings performed during the study period. Twelve leads were simultaneously acquired, and the ECG was recorded for 10 s at a sweep speed of 25 mm/s, calibrated to 1 mV/cm in the standard leads and recorded at 2-ms intervals (i.e., 500 Hz). Digital data was stored on a server computer with a 12-bit resolution. Patients who exhibited biphasic (positive/negative) P waves in lead II were chosen from the database using the analysis software MUSE 7.1 (GE Marquette Medical Systems, Inc., Milwaukee, WI, USA). AF incidence was determined using an ECG recording exhibiting AF. The ECG was performed either when patients were symptomatic or when they were scheduled to visit the hospital. Underlying diseases were classified according to the International Classification of Diseases codes. This research protocol was approved by the Institutional Review Board of the Shiga University of Medical Science (approval number: 19-75, approval date: Feb. 19, 2008).
2.2. ECG analysis
ECG analysis was performed retrospectively using MUSE 7.1 software for identifying identical P waves using a template matching technique. Points with an area ≥160 μV ms from baseline were considered P wave onset, while points with an area ≤160 μV ms from baseline were considered P wave offset. Computer-processed analysis of ECG recordings defined the selection criteria of the biphasic P wave in lead II as follows: (1) ECGs displaying a positive initial P wave portion with an amplitude ≥1 μV and (2) a negative terminal P wave portion with an amplitude of ≤−20 μV (Fig. 1A and B). The amplitudes of the initial and terminal P wave portions were measured with respect to baseline levels interpolated from P wave onset to offset. Patients with atrial pacing rhythm, AF, atrial flutter, ectopic atrial rhythm, or WPW syndrome were excluded from the study. The duration, amplitude, and the areas of initial and terminal P waves in leads II, III, and aVF were measured using the matrix parameters available in MUSE 7.1. The P wave area was constructed by integrating measurements of duration and amplitude. The PQ interval was determined using the time between P wave onset (the earliest P wave deflection in any lead) and QRS complex onset (the earliest QRS complex deflection in any lead). The QRS complex itself was determined from a point with an area ≤160 μV ms from baseline. The P/PQ interval ratio was defined as the ratio of total P wave duration to the PQ interval and was determined in leads II, III, and aVF. All variables were prepared using the average value of each ECG measurement during 10 s of recording time. As all measurements of the 12-lead ECGs were performed digitally using MUSE 7.1, neither intra- nor inter-observer variability was a factor in this study.
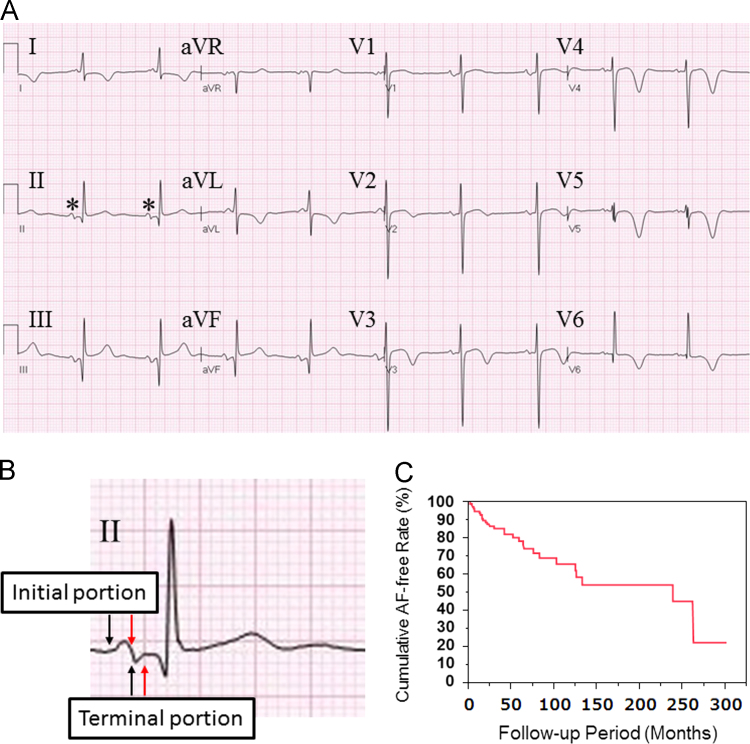
Fig. 1.
(A) Twelve-lead ECG showing a typical pattern of the biphasic P wave in lead II. Asterisks indicate biphasic P waves with identical morphology detected by template matching. (B) Magnified ECG trace of lead II. Black arrows indicate the onset of the initial and terminal portions; and red arrows, the offset. (C) Kaplan–Meier estimates of atrial fibrillation (AF)-free event rate in patients with a biphasic P wave in lead II.
2.3. Statistical analysis
In the present study, occurrence of AF was set as the study endpoint and the prognostic factors for development of AF were explored in the analysis. Patients whose ECG exhibited AF during the follow-up period were assigned to the AF group and were compared to those with no sign of AF (the non-AF group). In the AF group, the follow-up period was defined as the interval between the first day of biphasic P wave recording in lead II and the first day of AF recording. In the non-AF group, this period was defined as the interval between the first day of biphasic P wave recording in lead II and the final day on which an ECG was performed. Death by any cause during the follow-up period was assessed using the patient׳s medical records. Data is presented as the mean±standard deviation (SD) or percentage, and group comparisons were performed using a Student׳s t-test as appropriate. Categorical variables were compared using the chi-square test. A receiver operating characteristic curve was used to determine the cutoff point for prognostic factors that optimized the sensitivity and specificity of ECG variables for the end-point. A Kaplan–Meier curve was used to describe the AF-free survival rate and differences between groups were compared using the log-rank test. Cox proportional-hazards models were used to estimate multivariate adjusted hazard ratios (HRs) accounting for confounders (age, sex, and ECG variables). Variables included in the Cox models were selected using a backward stepwise procedure with a criterion of p<0.1 for inclusion. All statistical tests were two-tailed and a p-value<0.05 was considered statistically significant. Data analyses were conducted using JMP® 10 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Clinical and ECG characteristics
A total of 280 patients were chosen from the database based on the presence of a biphasic P wave in lead II using MUSE 7.1 software. Patients who had undergone an ECG recording only once were excluded and 141 patients (77 men, mean age: 64±19 years; 64 women, mean age: 63±21 years) presenting with a biphasic P wave in lead II were enrolled in the study. Of these patients, 29 developed AF (AF group, 20.6%) and the remainder were designated to the non-AF group. The mean follow-up period was 50±62 months. The number of ECG recordings averaged 19.2±2.0 per patient. Kaplan–Meier estimates of the probability of freedom from AF in patients with a biphasic P wave in lead II are presented in Fig. 1C.
3.2. Comparison of clinical characteristics
The clinical characteristics of patients in the AF and non-AF groups are presented in Table 1. No significant difference in age or sex was observed between the two groups. The mean follow-up period of the AF group averaged 56.8±66.5 months and 48.4±61.3 months for the non-AF group (p=0.52). The average age at ECG documentation of AF was 70.0±8.9 years.
Table 1.
Comparison of clinical characteristics between the AF and non-AF groups.
| AF group (n=29) | Non-AF group (n=112) | p value | |
|---|---|---|---|
| Age (years) | 68.1±8.8 | 63.1±20.3 | 0.20 |
| Men | 16 (55) | 61 (55) | 0.95 |
| Follow-up period (months) | 56.8±66.5 | 48.4±61.3 | 0.52 |
| Hypertension | 17 (59) | 53 (47) | 0.28 |
| Angina pectoris | 4 (14) | 2 (2) | 0.01 |
| Myocardial infarction | 2 (7) | 3 (3) | 0.31 |
| Valvular heart disease | 7 (24) | 23 (21) | 0.68 |
| Dilated cardiomyopathy | 1 (3) | 1 (1) | 0.35 |
| Hypertrophic cardiomyopathy | 2 (7) | 0 (0) | 0.01 |
| COPD | 3 (10) | 9 (8) | 0.71 |
| Diabetes mellitus | 8 (28) | 31 (28) | 0.99 |
| Congenital heart disease | 2 (7) | 2 (2) | 0.18 |
| Sarcoidosis | 0 (0) | 1 (1) | 0.50 |
AF=atrial fibrillation, COPD=chronic obstructive pulmonary disease. Patients with congenital heart disease were in a postoperative state.
Values are given as the mean±SD or n (%).
3.3. Comparison of ECG characteristics
The ECG measurements of the AF and non-AF groups are presented in Table 2. Heart rate, P wave duration in lead II, frontal plane P wave axis, and PQ interval were not significantly different between the two groups. In lead II, the amplitude and area of the initial P wave portion was significantly larger in the AF group when compared with the non-AF group, while the terminal P wave portion did not differ between the two groups. The P/PQ interval ratio between the two cohorts was not significantly different. In lead III, the duration and area of P wave initial portion were significantly greater in the AF group when compared with the non-AF group, but the amplitude of P wave terminal portion was significantly decreased in the AF group when compared with the non-AF group. The P/PQ interval ratio was not significantly different between the two cohorts. In lead aVF, the amplitude, duration, and area of the initial P wave portion were all significantly greater in the AF group when compared with the non-AF group. Furthermore, no significant differences were observed when comparing P wave terminal portion variables or P/PQ intervals between the two cohorts.
Table 2.
Comparison of ECG measurements between the AF and non-AF groups.
| AF group (n=29) | Non-AF group (n=112) | p Value | |
|---|---|---|---|
| Heart rate (beats/min) | 64.4±7.5 | 69.5±14.5 | 0.07 |
| P wave duration in lead II (ms) | 112.4±36.3 | 101.8±26.0 | 0.07 |
| P wave axis (deg) | 3.1±57.1 | 1.3±68.8 | 0.90 |
| PR interval (ms) | 184.1±40.3 | 170.8±44.5 | 0.15 |
| P wave measures in lead II | |||
| Amplitude (μV) | |||
| Initial portion | 77.3±77.0 | 51.0±30.1 | <0.01 |
| Terminal portion | −70.6±50.2 | −59.5±39.1 | 0.20 |
| Duration (ms) | |||
| Initial portion | 62.1±28.7 | 53.2±21.2 | 0.07 |
| Terminal portion | 50.4±23.8 | 48.5±21.1 | 0.68 |
| Area (μV ms) | |||
| Initial portion | 139.0±123.0 | 89.4±72.9 | <0.01 |
| Terminal portion | 95.0±100.4 | 84.4±84.2 | 0.56 |
| P/PQ interval ratio | 0.62±0.19 | 0.61±0.15 | 0.77 |
| P wave measures in lead III | |||
| Amplitude (μV) | |||
| Initial portion | 50.2±49.8 | 35.7±37.2 | 0.09 |
| Terminal portion | −70.6±41.3 | −89.1±38.1 | 0.02 |
| Duration (ms) | |||
| Initial portion | 52.7±34.6 | 35.8±30.4 | 0.01 |
| Terminal portion | 60.7±34.1 | 70.2±31.0 | 0.15 |
| Area (μV ms) | |||
| Initial portion | 106.4±133.4 | 57.4±75.3 | 0.01 |
| Terminal portion | 134.4±109.2 | 166.8±104.5 | 0.14 |
| P/PQ interval ratio | 0.62±0.17 | 0.63±0.17 | 0.91 |
| P wave measures in lead aVF | |||
| Amplitude (μV) | |||
| Initial portion | 60.4±50.2 | 41.7±32.2 | 0.02 |
| Terminal portion | −57.7±36.6 | −67.3±40.9 | 0.25 |
| Duration (ms) | |||
| Initial portion | 61.8±36.9 | 43.7±28.4 | <0.01 |
| Terminal portion | 50.7±35.3 | 62.1±28.2 | 0.07 |
| Area (μV ms) | |||
| Initial portion | 138.3±143.8 | 70.9±72.8 | <0.001 |
| Terminal portion | 88.2±91.7 | 114.6±82.6 | 0.14 |
| P/PQ interval ratio | 0.62±0.19 | 0.62±0.16 | 0.96 |
AF=atrial fibrillation.
Values are given as the mean±SD.
P/PQ interval ratio indicates a relative P wave duration to PQ interval.
3.4. Follow-up period and predictors of AF development
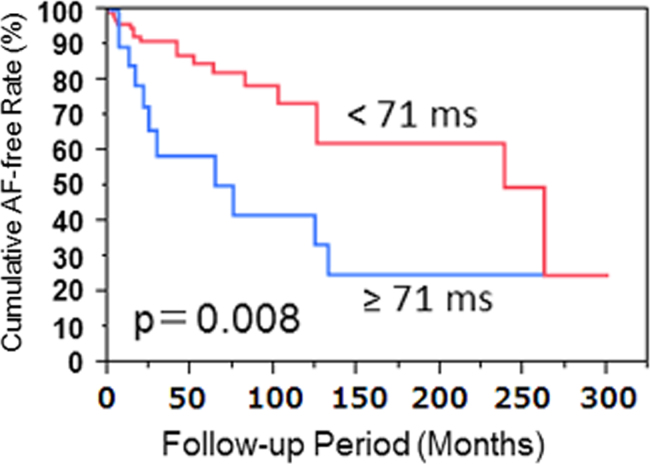
Univariate and multivariate analyses showing associations with the development of AF are presented in Table 3. By univariate analysis, age and sex were not found to be associated with the development of AF. Multivariate analysis confirmed that the duration of the initial portion of the P wave in lead III ≥71 ms was significantly and independently associated with an increased propensity for the development of AF in patients with a biphasic P wave in lead II. To avoid collinearity, significant correlations between P wave measurements were not included in the multivariate analysis. These included the area of the initial P wave portion in lead II vs. the amplitude of the same (r=0.83), the area of the initial P wave portion in lead III vs. the duration of the same (r=0.81), the amplitude of the initial P wave portion in lead aVF vs. the amplitude of the initial P wave portion in lead II (r=0.79), the duration of the initial P wave portion in lead aVF vs. the duration of the initial P wave portion in lead III (r=0.83), and the area of the initial P wave portion in lead aVF vs. the amplitude of the initial P wave portion in lead II (r=0.85). Fig. 2 shows the Kaplan–Meier estimates of the probability of freedom from AF in patients with a biphasic P wave in lead II, according to the results observed for P wave characteristics. The cumulative AF rate of development was associated with the duration of the initial P wave portion in lead III.
Table 3.
Probability of AF development during follow-up based on clinical and ECG variables.
| HR | 95% CI | p Value | |
|---|---|---|---|
| A. Univariate analysis | |||
| Age≥68 years | 1.12 | 0.51–2.23 | 0.87 |
| Sex (men=1) | 1.29 | 0.62–2.75 | 0.49 |
| Amplitude of initial P wave portion in lead II≥73 (μV) | 1.93 | 0.90–4.02 | 0.09 |
| Amplitude of terminal P wave portion in lead III≥−48(μV) | 2.07 | 0.94–4.36 | 0.07 |
| Duration of initial P wave portion in lead III≥71 (ms) | 3.00 | 1.35–6.36 | <0.01 |
| B. Multivariate analysis | |||
| Model 1 | |||
| Age≥68 years | 1.15 | 0.55–2.47 | 0.71 |
| Sex (men=1) | 1.25 | 0.59–2.66 | 0.56 |
| Model 2 | |||
| Age≥68 years | 0.94 | 0.43–2.07 | 0.88 |
| Sex (men=1) | 1.25 | 0.58–2.80 | 0.57 |
| Amplitude of initial P wave portion in lead II≥73 (μV) | 1.22 | 0.50–2.88 | 0.65 |
| Amplitude of terminal P wave portion in lead III≥−48 (μV) | 1.60 | 0.68–3.72 | 0.28 |
| Duration of initial P wave portion in lead III≥71 (ms) | 2.90 | 1.16–7.11 | 0.02 |
AF=atrial fibrillation, ECG=electrocardiograms, HR=hazard ratio, CI=confidence interval.
Model 1: adjusted for age and sex.
Model 2: model 1 plus adjustment for amplitude of initial P wave portion in lead II, amplitude of terminal P wave portion in lead III, and duration of initial P wave portion in lead III.
Fig. 2.
Kaplan–Meier estimates of atrial fibrillation (AF)-free event rate in patients with a biphasic P wave in lead II depending on the duration of the initial P wave portion in lead III (≥71 ms vs. <71 ms).
4. Discussion
The major findings of this study were as follows: (1) patients with a biphasic P wave in lead II had an increased propensity toward the occurrence of AF and a subsequent high rate of incidence and (2) the duration of the initial P wave portion in lead III was independently associated with the development of AF. The incidence of AF observed in the present study was much higher than that of a previous study in the general Japanese population [5] (234.7/1000 patient-years vs. 9.3/1000 patient-years).
4.1. Pathophysiology of the biphasic P wave
Bachmann [6] reported that the conduction time from the right to the left atrium increased when a muscle band overlaying both auricles was crushed. Waldo et al. [4] demonstrated the alteration of P wave morphology by dissecting the specialized atrial tissue from the canine heart. A biphasic P wave in the inferior leads results from interference of the atrial conduction of Bachmann׳s bundle, which in turn results in delayed activation of the left atrium as the impulse propagated from the lower right atrium to the left atrium occurs in a caudo-cranial direction. These findings indicate that the terminal portion of the biphasic P wave in lead II is caused by inter-atrial anisotropic conduction during sinus rhythm. Therefore, in the present study, patients may present with diseased lesions in Bachmann׳s bundle and anisotropic conduction in the atrium could occur.
4.2. The biphasic P wave and atrial vulnerability
Leier et al. [10] reported that intra- and inter-atrial conduction delays were associated with spontaneous occurrence of atrial flutter in patients with normal sized atria. Luna et al. [11] further reported that patients with a prolonged P wave ≥120 ms and a biphasic P wave in the inferior leads represented inter-atrial block and retrograde activation of the left atrium and that these patients had a higher incidence of atrial flutter and/or AF when compared with control subjects. Ariyarajah et al. [12] provided further evidence and reported that prolonged duration of the P wave was associated with AF. From these reports, slow and anisotropic conduction is thought to play a significant role in the generation of atrial tachyarrhythmia. In this study, the amplitude of the initial P wave portion in leads II and aVF was higher in the AF group when compared with the non-AF group. Furthermore, the amplitude of the terminal P wave portion in lead III was decreased in the AF group when compared with the non-AF group. These findings suggest that impulse propagation circles with a larger arc in the right atrium in the AF group when compared with the non-AF group. In addition, the duration of the initial P wave portion in leads III and aVF was associated with the development of AF. When impulse propagation circles in the right atrium and then proceeds to the left atrium in a caudal–cranial direction, the initial and terminal P wave portions in leads III and aVF could reflect the depolarization of the right atrium and/or the atrial septum in conjunction with the left atrium, respectively. Therefore, increased duration of the initial P wave portion in these leads suggests that slow conduction in the right atrium and/or the atrial septum may be primarily responsible for the development of AF. Only the duration of the initial P wave portion in lead III was shown to be independently associated with the development of AF, indicating that slow conduction increases the propensity for development of AF.
4.3. Biphasic P wave and slow conduction
Macruz et al. [13] investigated the ratio of P wave duration to PQ interval (P/PQ interval ratio). A conduction delay in the right atrium did not prolong the P wave duration but did increase the PQ interval due to increased transit time from the sinus node to the atrioventricular node, thus resulting in a decreased P/PQ interval ratio. In contrast, a conduction delay in the left atrium caused the terminal portion of the P wave to be delayed as a result of the prolonged transit time while the PQ interval remained unchanged, thus rendering an increased P/PQ interval ratio. In the present study, the P/PQ interval ratio was not associated with the development of AF, suggesting that the P/PQ interval was not sufficient to demonstrate the relationship between slow atrial conduction and the development of AF. However, when detailed P wave measurements were performed, the increased duration of the initial P wave portion in lead III manifested as an independent factor for the development of AF. This finding suggests that atrial vulnerability to fibrillation is likely to increase when slow and anisotropic conductions coexist in the right atrium and/or the atrial septum.
4.4. Study limitations
This study had several limitations. First, development of AF was identified by the review of previously recorded ECGs. If AF terminated spontaneously before a hospital visit, a recording may have been missed. When no AF of a patient with transient AF was documented during the follow-up period, the patient was classified in the non-AF group. As the AF-free duration would then appear to be longer than the true duration, an underestimation of AF occurrence could result. Second, we did not investigate the morphological characteristics of the atria. Accordingly, echocardiography should be performed to study the detailed risk stratification. Third, intrinsic selection bias in a cohort must be taken into account. Finally, the present study included patients who had undergone an ECG recording in our hospital and so the risk of AF identified in this study population is undoubtedly higher than that of the general population. This should be considered when these results are extrapolated to a broader population.
5. Conclusions
The risk of mortality increases with the incidence of AF because AF causes both thromboembolism and heart failure. However, the identification of those patients at greatest risk for developing AF using ECG remains ill defined. The results of the present study show that P wave analysis using standard 12-lead ECG recordings could successfully detect the risk factor for AF. In addition, the quantitative relationship between P waves and incidence of AF presented here could provide useful information as to which patients with palpitation are likely to benefit from preventive anticoagulant therapy. An automatic algorithm for analysis of the P wave is required in order to care for patients in a best-predictive manner.
Funding sources
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
We thank Seiichi Fujisaki and Tatsumi Uchiyama (GE Yokokawa Medical System Co.) for their technical assistance. We also thank Shoko Shimizu, Yoshihisa Fujisawa, Takako Ishigaki, Yumi Izumi, Yumi Izumi, Chihiro Okuni, and Tatsuya Nishikawa (Department of Central Clinical Laboratory, Shiga University of Medical Science Hospital) for the ECG recordings. We greatly appreciate the assistance of Richard John Hodge for his work on the final editing of this manuscript.
Contributor Information
Hideki Hayashi, Email: hayashih@belle.shiga-med.ac.jp.
Minoru Horie, Email: horie@belle.shiga-med.ac.jp.
References
- 1.Magnani J.W., Williamson M.A., Ellinor P.T. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol. 2009;2:72–79. doi: 10.1161/CIRCEP.108.806828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishida K., Hayashi H., Miyamoto A. P wave and the development of atrial fibrillation. Heart Rhythm. 2010;7:289–294. doi: 10.1016/j.hrthm.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Kitkungvan D., Spodick D.H. Interatrial block: Is it time for more attention? J Electrocardiol. 2009;42:687–692. doi: 10.1016/j.jelectrocard.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Waldo A.L., Bush H.L., Jr., Gelband H. Effects on the canine P wave of discrete lesions in the specialized atrial tracts. Circ Res. 1971;29:452–457. doi: 10.1161/01.res.29.5.452. [DOI] [PubMed] [Google Scholar]
- 5.Iguchi Y., Kimura K., Shibazaki K. Annual incidence of atrial fibrillation and related factors in adults. Am J Cardiol. 2010;106:1129–1133. doi: 10.1016/j.amjcard.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann G. The inter-auricular time interval. Am J Physiol. 1961;41:309–320. [Google Scholar]
- 10.Leier C.V., Meacham J.A., Schaal S.F. Prolonged atrial conduction. A major predisposing factor for the development of atrial flutter. Circulation. 1978;57:213–216. doi: 10.1161/01.cir.57.2.213. [DOI] [PubMed] [Google Scholar]
- 11.Bayes de Luna A., Cladellas M., Oter R. Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur Heart J. 1988;9:1112–1118. doi: 10.1093/oxfordjournals.eurheartj.a062407. [DOI] [PubMed] [Google Scholar]
- 12.Ariyarajah V., Apiyasawat S., Fernandes J. Association of atrial fibrillation in patients with interatrial block over prospectively followed controls with comparable echocardiographic parameters. Am J Cardiol. 2007;99:390–392. doi: 10.1016/j.amjcard.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 13.Macruz R., Perloff J.K., Case R.B. A method for the electrocardiographic recognition of atrial enlargement. Circulation. 1958;17:882–889. doi: 10.1161/01.cir.17.5.882. [DOI] [PubMed] [Google Scholar]