Abstract
Purpose
The increasing incidence of thyroid cancer worldwide has drawn attention to the needs for assessing and managing health-related quality of life (HRQoL) of thyroid cancer survivors. We conducted this study to validate the Korean version of the thyroid cancer-specific quality of life (THYCA-QoL) questionnaire.
Methods
Data obtained from 227 thyroid cancer survivors were analyzed using standard validity and reliability analysis techniques. Reliability was assessed by measuring internal consistency via Cronbach α coefficient, and validity was assessed by determining the Pearson correlation coefficient between the THYCA-QoL questionnaire and the following relevant assessment tools: the European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30), the Korean version of Brief Fatigue Inventory (BFI-K), the Korean version of Brief Encounter Psychosocial Instrument (BEPSI-K), Goldberg Short Screening Scale for Anxiety and Depression, and a nine-item Patient Health Questionnaire (PHQ-9). A multitrait scaling analysis was performed to assess each item's convergent and discriminant validity.
Results
The reliability of the THYCA-QoL questionnaire was confirmed by Cronbach α coefficients for multiple-item scales which ranged from 0.54 (sensory) to 0.82 (psychological). Except for a single item (sexual interest), the questionnaire's validity was established by significant correlation observed between scales in the THYCA-QoL questionnaire and scales used in other assessment tools. A multitrait scaling analysis confirmed that all scales met the recommended psychometric standards.
Conclusion
The Korean version of the THYCA-QoL questionnaire is a reliable and valid assessment tool that can be used in combination with the EORTC QLQ-C30 to assess the HRQoL of thyroid cancer survivors in Korea.
Keywords: Thyroid neoplasms, Quality of life, Validity, Korea
INTRODUCTION
An increasing incidence of thyroid cancer has been reported both in Korea and worldwide during the past decades [1,2]. An analysis published in the report "Cancer Incidence in Five Continents" (CI5) [1] compared trends in the incidence of thyroid cancer in 19 different populations during a 30-year period ranging from 1973-2002. The results suggested that increased incidence rates had occurred in most populations worldwide, and were not confined to a particular region of the world. In Korea specifically, thyroid cancer has been reported as the most common type of cancer (18.6% of cases: male, 6.4%; female, 31.1%), and its incidence has the most rapid annual growth rate (23.7%) [2]. Despite its high prevalence and longterm disease-free survival rates (10-year survival in 98.6% of cases) [2], thyroid cancer survivors have been shown to have a decreased health-related quality of life (HRQoL) when compared to the general population [3,4]. This finding demonstrates the need to properly assess and manage the HRQoL of thyroid cancer patients. However, to improve the HRQoL of these patients, it is first necessary to understand the major determinants affecting the HRQoL of thyroid cancer survivors, and then accurately assess that quality. To date, relatively few studies have assessed the HRQoL of thyroid cancer survivors, and no validated questionnaire has been developed that specifically assesses thyroid cancer-specific HRQoL among Korean thyroid cancer survivors.
Thus, we conducted this study to translate and validate the Korean version of the thyroid cancer-specific quality of life (THYCA-QoL) questionnaire that was developed in 2013 [5]. The original version of the THYCA-QoL questionnaire was designed to assess thyroid cancer-specific aspects of HRQoL that were not sufficiently addressed in the more general European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30) questionnaire [6].
METHODS
Study sample
The subjects included in this cross-sectional study were recruited at the outpatient clinic of the thyroid cancer center at a university medical center in Korea from June 2014 to October 2014. All subjects were aged ≥18 years, had received a pathological diagnosis of cancer following surgical treatment, and were not currently receiving anticancer treatment. All enrolled subjects were asked to independently complete written questionnaires at the outpatient clinic. Information regarding each subject's demographic and clinical characteristics (e.g., cancer stage at diagnosis, pathologic type, and type of treatment) was obtained from the hospital cancer registry. This study was conducted according to the recommendations of the Declaration of Helsinki, and was approved by Institutional Review Board at a university medical center. Signed, written informed consent was obtained from each participant.
Study design
The module validation process included three major phases. The first phase involved translating the original version of the THYCA-QoL [5] questionnaire into Korean using "forward and backward" translation guidelines developed by the EORTC QoL study group [7]. The two initial forward translations were conducted independently by two translators who were native Korean speakers fluent in English. The third independent coordinator then merged the two translated versions into one reconciled forward translation where there was agreement between the two forward translations. Differences existed between the two forward translations on some items, requiring discussion for resolution, but those differences were trivial, and they soon reached agreement without necessitating further backward translation. During the translation process, several questions in the module were identified as being equivalent to items in the previous EORTC Item Bank [8], and were thus adapted for inclusion in the new questionnaire to provide comparability of data. The translated module was then assessed and revised by an expert committee until a consensus was reached. In the second phase, the resultant new questionnaire was pilot-tested with 18 thyroid cancer survivors to perceive and solve potential translation problems related to wording and the clarity of items. This process was done in accordance with QoL questionnaire guidelines developed by the European Organization for Research and Treatment of Cancer [9]. In the final phase, the questionnaire was field tested by being administered to Korean patients for purposes of assessing its psychometric properties and ensuring its reliability and validity. During this final phase, a cohort of thyroid cancer survivors was asked to complete surveys that included the THYCAQoL questionnaire as well as five other previously validated assessment tools. The scoring procedure involved a linear transformation of raw scores to a scale of 0-100, as outlined in the EORTC QLQ C-30 scoring guidelines [10].
Study measures
THYCA-QoL questionnaire
The original version of the THYCA-QoL is a 24-item thyroid cancer-specific questionnaire for assessing the HRQoL of thyroid cancer survivors, developed according to the methodology of the EORTC guideline [9]. The questionnaire incorporates seven multi-item scales (neuromuscular, voice, concentration, sympathetic, throat/mouth, psychological, and sensory problems) and six single items (problems with scar, felt chilly, tingling hands/feet, gained weight, headaches, and interest in sex) [4,5]. The questionnaire has a specific time frame (one week for all items, except for four weeks for the sexuality item), and each item is rated on a four-point Likert scale, ranging from 1 ("not at all") to 4 ("very much"). A higher score on the symptom scale represents more complaints.
Korean version of EORTC QLQ-C30
The EORTC QLQ-C30 is a 30-item core cancer quality of life questionnaire [6,11]. The questionnaire includes both multi-item scales and single-item measures, consisting of five functional scales, three symptom scales, a global health status/QoL scale, and six single items. With the exception of the global QOL scale, all items adapt four-point response scales, ranging from 1 to 4 (response categories: "not at all," "a little," "quite a bit," and "very much"). The global QoL scale adapts a modified seven-point linear analog scale, ranging from 1 "very poor" to 7, "excellent". A high score for the functional scale or the global QoL scale represents a healthy level of functionality or high QoL, whereas a high score for a symptom scale/item represents a high level of symptomatology. The Korean version of the EORTC QLQ-C30 (version 3.0) was validated by the EORTC QoL Study Group [6].
Korean version of Brief Fatigue Inventory
BFI-K, the validated Korean version of Brief Fatigue Inventory, is a nine-item questionnaire assessing fatigue in cancer patients, which measures fatigue severity and interference with life activities over the previous 24 hours [12]. BFI assesses fatigue severity by asking patients to describe their fatigue in three severity categories (worst fatigue, usual fatigue, and current fatigue), with each response scale ranging from "0" ("no fatigue") to "10" ("fatigue as bad as you can imagine"). BFI evaluates fatigue interference by asking patients the extent to which their fatigue interferes with aspects of their lives in six categories (general activities, mood, walking, normal work, relations with other people, and their enjoyment of life), with each response scale ranging from "0" ("does not interfere") to "10" ("completely interferes"). Each composite fatigue severity score and composite fatigue interference score is calculated as the average of the three severity items and the six interference items, respectively.
Korean version of Brief Encounter Psychosocial Instrument
BEPSI-K, the validated Korean version of Brief Encounter Psychosocial Instrument, is a five-item questionnaire designed to measure stress levels [13,14]. The questionnaire is rated on a five-point Likert scale, and BEPSI-K score is calculated as the average of the five items. A BEPSI-K score of more than 2.2 is defined as a high stress level, and a higher score indicates greater stress.
Goldberg short screening scale for Anxiety and Depression
Goldberg short screening scale is an 18-item questionnaire (nine on the anxiety scale and nine on the depression scale) developed for screening anxiety and depression in a general medical setting [15,16]. The validity and reliability of the Korean version has been established.
Nine-item Patient Health Questionnaires
The PHQ-9 is a nine-item questionnaire developed for depression screening in primary care settings, and each item has score ranging from 0 to 3 (leading to a maximum score of 27) [17]. A simple additive score PHQ-9 of 10 or greater identifies mild major depression, a score of 15 or greater identifies moderate major depression, and a score of 20 or greater identifies severe major depression.
Statistical analyses
Demographic and clinical data were analyzed using descriptive statistical methods. The reliability of the THYCA-QoL questionnaire was assessed by determining its Cronbach α coefficient, which measures the internal consistency of responses.[5,6,18,19] A multitrait scaling analysis was performed to confirm the questionnaire's scale structure, and assess its item convergence and discrimination validity [5,6,20]. The convergent validity for each scale was assessed by determining the correlation between each item and its own scale, whereas discriminant validity was assessed by evaluating the correlation between each item and other scales [20]. The validity of the questionnaire was assessed by determining the Pearson correlation coefficient between the THYCA-QoL questionnaire and each of the other five aforementioned questionnaires [5,6,18]. All the statistical analyses were performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). P-values <0.05 (twosided tests) were considered statistically significant.
RESULTS
Patient demographic and clinical characteristics
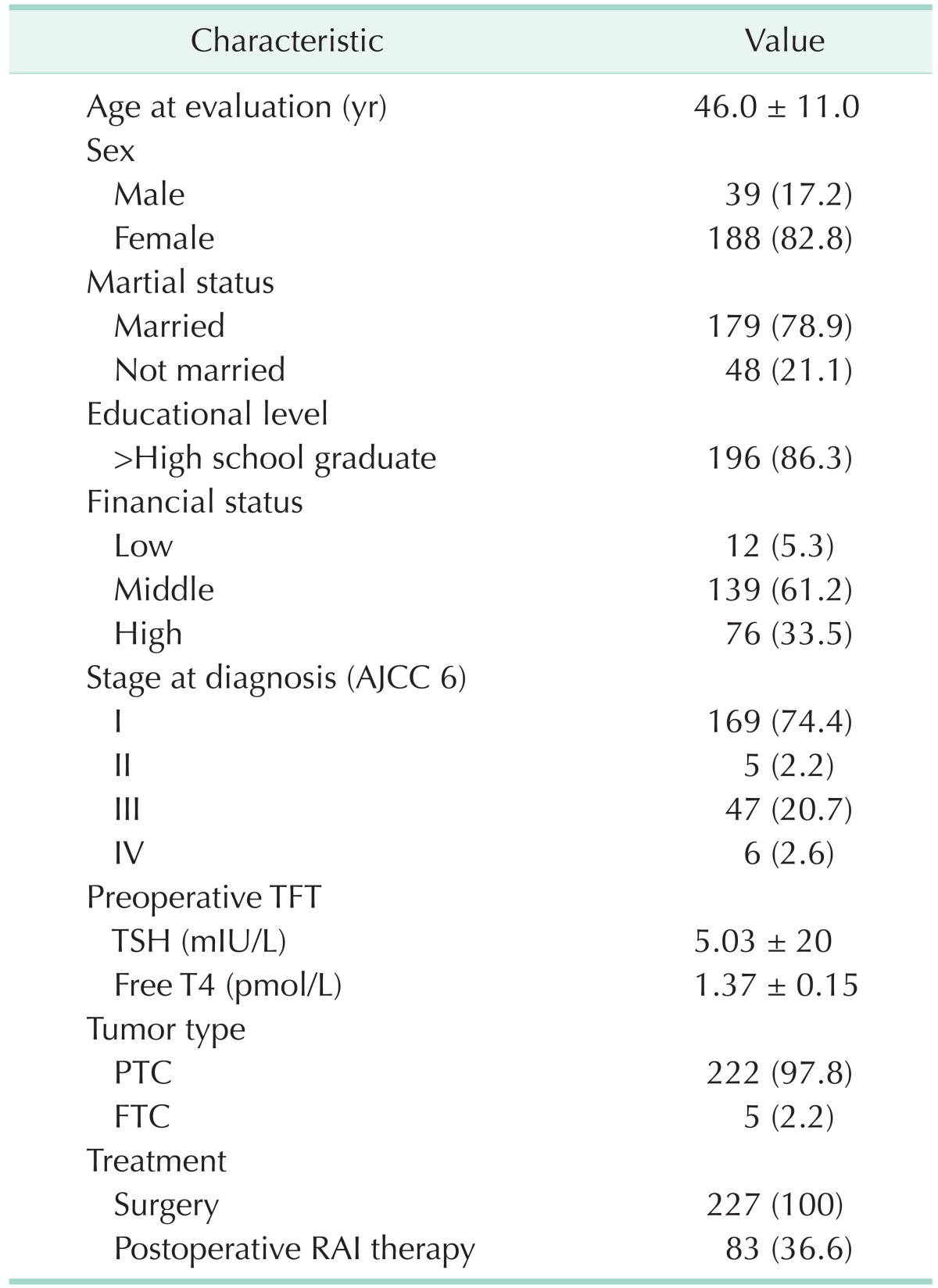
A total of 227 thyroid cancer survivors completed the questionnaire, and all of the requested information was provided. Baseline characteristics for the study participants are described in Table 1. The thyroid cancer patients had a mean age of 45.0 years (range, 18-77 years), and 82.8% were female. The study participants had received a pathological diagnosis of cancer. The most common stage of cancer at diagnosis was stage I (74.4%), and all patients had undergone surgical treatment.
Table 1. Patient demographic and clinical characteristics (n = 227).
Values are presented as mean ± standard deviation or number (%).
AJCC, American Joint Committee on Cancer; TFT, thyroid function test; TSH, thyroid-stimulating hormone; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; RAI, radioactive iodine.
Reliability
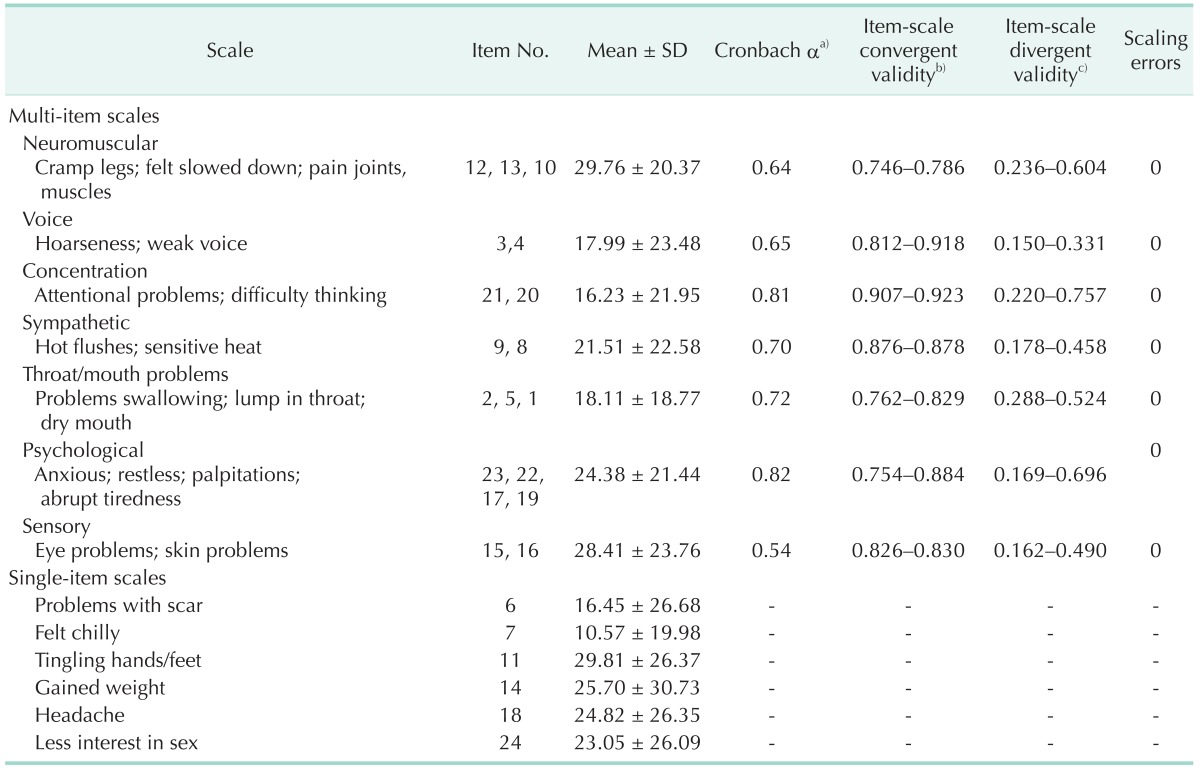
The Cronbach α coefficients for multi-item scales ranged from 0.54 (sensory) to 0.82 (psychological) (Table 2). Four of the seven multi-item scales ("concentration," "sympathetic," "throat/mouth," and "psychological") scored higher than 0.70, which is the commonly accepted minimum standard level of reliability [5,6,18,19]. However, the coefficients of three other multi-item scales ("neuromuscular," "voice," and "sensory") were relatively low (0.64, 0.65, and 0.54, respectively).
Table 2. Multitrait scaling analyses of the Korean THYCA-QoL questionnaire.
THYCA-QoL, thyroid cancer-specific quality of life; SD, standard devation.
a)Cronbach α value ≥0.70 indicates adequate scale reliability. b)Pearson correlation between items and hypothesized scale (corrected for overlap). c)Pearson correlation between items and other scales.
Validity
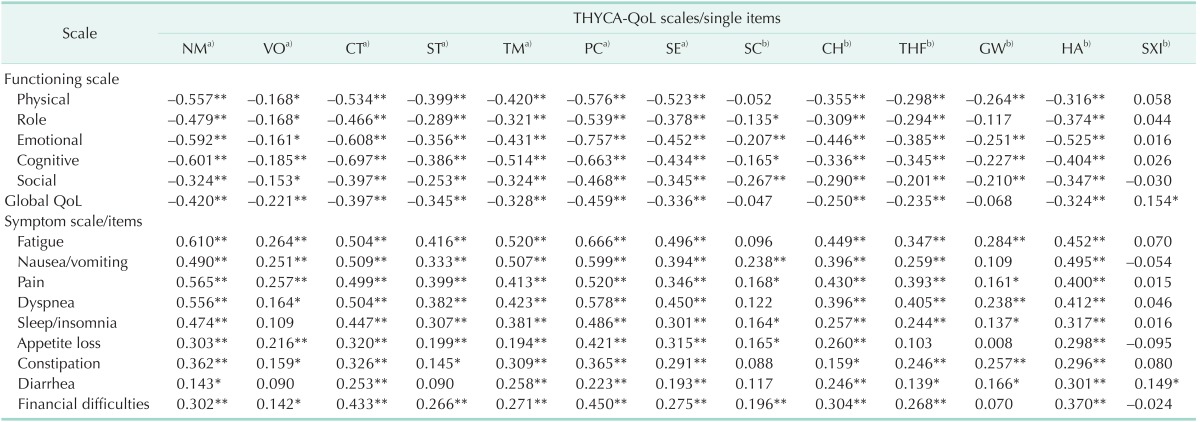
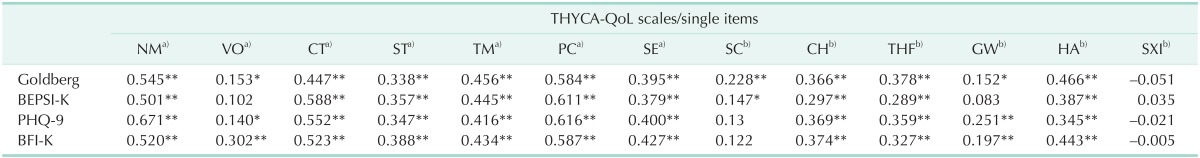
A multitrait scaling analysis performed to assess item convergence and discrimination validity showed that all itemscale correlations were >0.40, and the correlation of each item with its own scale was greater than its correlation with other scales, with no scaling errors (Table 2). Validity was assessed by determining the Pearson correlation coefficients for scales in the THYCA-QoL questionnaire with scales in the EORTC QLQ C30, and the results are shown in Table 3. With the exception of one item (sexual interest), all scales in the THYCA-QoL questionnaire showed statistically significant correlations with all subscales in the EORTC QLQ-C30. The "psychological" and "concentration" scales in the THYCA-QoL questionnaire were highly correlated with the "emotional functioning" and "cognitive functioning" scales in the EORTC QLQ C-30 (correlation coefficients: r > 0.60; P < 0.01) [5,21,22]. The "neuromuscular," "concentration," and "psychological" scales in the THYCA-QoL questionnaire were all moderately correlated with the "physical functioning," "role functioning," "fatigue," "nausea/vomiting," "pain," "dyspnea," and "sleep/insomnia" scales in the EORTC QLC C-30 (correlation coefficients: 0.40 < r < 0.60, P < 0.01) [5,21,22]. The Pearson correlation coefficients for scales in the THYCA-QoL questionnaire with scales in the BFI-K, BEPSI-K, Goldberg scale, and PHQ-9 are shown in Table 4. Similar to their correlations with the EORTC QLQ-C30, most of the scales in the THYCA-QoL questionnaire (with the exception of the sexual interest scale) were significantly correlated with scales in the BFI-K, BEPSI-K, Goldberg scale, and the PHQ [21,22]. The "psychological" and "neuromuscular" scales in the THYCAQoL questionnaire showed moderate to high correlations with scales in the BEPSI and PHQ-9, and the "concentration" and "throat/mouth" scales showed moderate correlations with scales in those assessment tools.
Table 3. Pearson correlation coefficients between EORTC QLQ-C30 and scales/items in the THYCA-QoL questionnaire.
EORTC, European Organization for Research and Treatment of Cancer; THYCA-QoL, thyroid cancer-specific quality of life; NM, neuromuscular; VO, voice; CT, concentration; ST, sympathetic; TM, throat/mouth; PC, psychological; SE, sensory; SC, problem with scar; CH, felt chilly; THF, tingling hands/feet; GW, gained weight; HA, headachs; SXI, less interest in sex.
*P < 0.05. **P < 0.01. a)Multi-item scales. b)Single-item scales.
Table 4. Pearson correlation coefficients for Goldberg scale, BEPSI-K, PHQ-9, and BFI-K when compared with the Korean version of the THYCA-QoL questionnaire.
BEPSI-K, Korean version of brief encounter psychosocial instrument; PHQ-9, nine-item patient health questionnaire; BFI-K, Korean version of brief fatigue inventory; THYCA-QoL, thyroid cancer-specific quality of life; NM, neuromuscular; VO, voice; CT, concentration; ST, sympathetic; TM, throat/mouth; PC, psychological; SE, sensory; SC, problem with scar; CH, felt chilly; THF, tingling hands/feet; GW, gained weight; HA, headachs; SXI, less interest in sex.
*P < 0.05. **P < 0.01. a)Multi-item scales. b)Single-item scales.
The resulting questionnaire, consisting of 24 items, was named the Korean version of the THYCA-QoL (Supplementary material).
DISCUSSION
This study was designed to assess psychometric properties for the validation of the Korean version of the THYCA-QoL questionnaire. Our statistical analyses confirmed that the internal consistencies of its four multi-item scales were sufficiently high to satisfy the recommended psychometric criterion for reliability [18,19]. Considering that internal consistency is defined as a measure of the extent to which items in a questionnaire subscale are correlated [19], the relatively low reliability scores of the three other multi-item scales ("neuromuscular," "voice," and "sensory") may reflect the limited variability of the items in those scales.
Each item's convergent and discriminant validity was confirmed in the multitrait scaling analysis with no scaling errors, and these results confirmed the hypothesized scale structure of the Korean version of the THYCA-QoL questionnaire. The validity of the new questionnaire was proven in comparisons conducted with other known assessment tools. With the single exception of the sexual interest scale, the majority of THYCA-QoL scales showed moderately strong correlations with other measures. Additionally, all of the observed correlations were in the expected direction, suggesting that the various questionnaires broadly measured the same traits.
Although the present study demonstrates the validity and reliability of the translated version of the THYCA-QoL questionnaire, certain limitations should be acknowledged. First, the results of the reliability analysis revealed suboptimal values for the "neuromuscular," "voice," and "sensory" subscales (0.64, 0.65, and 0.54, respectively). Those values are considered to be close to the recommended psychometric criterion for reliability [18,19], as some references consider a Cronbach α value of >0.6 acceptable [23]. Given that Cronbach α coefficient is a measure of the internal consistency of items in a composite scale, potential item irrelevance may have led to lower scale reliability [24,25]. The "sensory" scale yielded the lowest reliability estimate (0.54), and two items from the "sensory" scale, each assessing skin and eye problems, were likely internally inconsistent, because they were not necessarily highly correlated. These two items did examine important aspects of sensory symptoms, however, and the low reliability estimate therefore does not necessarily indicate poor scale construction from this aspect. This was also noted in the original version of the questionnaire [5]. The researchers agreed to preserve the scale structure of the original version despite the suboptimal reliability of these scales, because those items were considered clinically relevant by thyroid cancer survivors and healthcare providers during the development of the new questionnaire. Clinical relevance needs to be considered along with psychometric properties when developing a health measurement scale [26], and therefore, the questionnaire's construction is justifiable from this perspective. Moreover, the study population was not representative of most thyroid cancer survivors, as the majority of participants were female (82.8%) and pathologically diagnosed as early stage (stage I for 74.4% of the cases) papillary thyroid cancer (97.8%) or follicular thyroid cancer (2.2%). It is unknown whether the biased study sample affected our findings regarding the reliability and validity of the questionnaire. Therefore, further studies should be conducted with patients having symptoms of advanced-stage thyroid cancer or with a different tumor type, such as anaplastic or medullary thyroid cancer.
The present study is not without limitations, but it is worthwhile to address the value of the Korean version of the questionnaire as a measurement tool. In addition to its satisfactory measurement properties, the questionnaire proved to be very accessible. The pilot study confirmed that the translated questionnaire contained no ambiguous or difficult items, and the resultant questionnaire was easily understood by participants, resulting in a high response rate and low levels of missing data in this clinical study. These outcomes imply that despite the cultural and language differences, the translated questionnaire was well adapted, and may be applicable for use with Korean-speaking thyroid cancer survivors. Another strength of this study is its use of various relevant measures in the known-measure comparisons, as it increased the comparability of data when assessing the questionnaire's validity.
As the original THYCA-QoL questionnaire was developed in Europe, this study was designed to determine whether the newly translated Korean version is appropriate for use with Korean cancer survivors. In conclusion, the new questionnaire was found to be reliable, valid, and suitable for use in primary care settings for measuring the HRQoL of Korean-speaking thyroid cancer survivors.
ACKNOWLEDGEMENTS
This paper was supported by Konkuk University in 2015.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIAL
Supplementary questionnaire can be found via http://astr.or.kr/src/sm/astr-89-287-s001.pdf.
The Korean version of the THYCA-QoL questionnaire
References
- 1.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2011. Goyang: National Cancer Center; 2013. [Google Scholar]
- 3.Lee JI, Kim SH, Tan AH, Kim HK, Jang HW, Hur KY, et al. Decreased healthrelated quality of life in disease-free survivors of differentiated thyroid cancer in Korea. Health Qual Life Outcomes. 2010;8:101. doi: 10.1186/1477-7525-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Husson O, Haak HR, Buffart LM, Nieuwlaat WA, Oranje WA, Mols F, et al. Health-related quality of life and disease specific symptoms in long-term thyroid cancer survivors: a study from the population-based PROFILES registry. Acta Oncol. 2013;52:249–258. doi: 10.3109/0284186X.2012.741326. [DOI] [PubMed] [Google Scholar]
- 5.Husson O, Haak HR, Mols F, Nieuwenhuijzen GA, Nieuwlaat WA, Reemst PH, et al. Development of a disease-specific health-related quality of life questionnaire (THYCA-QoL) for thyroid cancer survivors. Acta Oncol. 2013;52:447–454. doi: 10.3109/0284186X.2012.718445. [DOI] [PubMed] [Google Scholar]
- 6.Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004;13:863–868. doi: 10.1023/B:QURE.0000021692.81214.70. [DOI] [PubMed] [Google Scholar]
- 7.Dewolf L, Koller M, Velikova G, Johnson C, Scott N, Botomley A on behalf of the EORTC Quality of Life Study Group. Quality of Life study group translation procedure. 3rd ed. Brussels: EORTC; 2009. [Google Scholar]
- 8.Vachalec S, Bjordal K, Bottomley A, Blazeby J, Flechtner H, Ruyskart P on behalf of the EORTC Quality of Life Study Group. EORTC item bank guidelines. Brussels: EORTC; 2001. [Google Scholar]
- 9.Johnson C, Aaronson N, Blazeby JM, Bottomley A, Fayers P, Koller M, et al. Guidelines for developing questionnaire modules. 4th ed. Brussels: EORTC; 2011. [Google Scholar]
- 10.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, et al. EORTC QLQ-C30 scoring manual. 3rd ed. Brussels: EORTC; 2001. [Google Scholar]
- 11.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a qualityof-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 12.Yun YH, Wang XS, Lee JS, Roh JW, Lee CG, Lee WS, et al. Validation study of the korean version of the brief fatigue inventory. J Pain Symptom Manage. 2005;29:165–172. doi: 10.1016/j.jpainsymman.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Im JH, Bae JM, Choi SS, Kim SW, Hwang HS, Hur BR. The validity of modified Korean-translated BEPSI (brief encounter psychosocial instrument) as instrument of stress measurement in outpatient clinic. J Korean Acad Fam Med. 1996;17:42–49. [Google Scholar]
- 14.Frank SH, Zyzanski SJ. Stress in the clinical setting: the Brief Encounter Psychosocial Instrument. J Fam Pract. 1988;26:533–539. [PubMed] [Google Scholar]
- 15.Kim JS, Kim YS, Lee GY, Park TJ, Lee YH, Kong BK, et al. The standardization of Korean-translated Goldberg's shart screening scale for Anxiety and Depression. J Korean Acad Fam Med. 1997;18:1452–1460. [Google Scholar]
- 16.Goldberg D, Bridges K, Duncan-Jones P, Grayson D. Detecting anxiety and depression in general medical settings. BMJ. 1988;297:897–899. doi: 10.1136/bmj.297.6653.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arroll B, Goodyear-Smith F, Crengle S, Gunn J, Kerse N, Fishman T, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8:348–353. doi: 10.1370/afm.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aaronson N, Alonso J, Burnam A, Lohr KN, Patrick DL, Perrin E, et al. Assessing health status and quality-oflife instruments: attributes and review criteria. Qual Life Res. 2002;11:193–205. doi: 10.1023/a:1015291021312. [DOI] [PubMed] [Google Scholar]
- 19.Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Yun YH, Mendoza TR, Kang IO, You CH, Roh JW, Lee CG, et al. Validation study of the Korean version of the M.D. Anderson Symptom Inventory. J Pain Symptom Manage. 2006;31:345–352. doi: 10.1016/j.jpainsymman.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Nunnally J. Psychometric theory. New York: McGraw-Hill; 1994. [Google Scholar]
- 22.Shin YS, Kim JH. Validation of the Korean version of the European Organization for Research and Treatment of Cancer brain cancer module (EORTC QLQ-BN20) in patients with brain tumors. Health Qual Life Outcomes. 2013;11:145. doi: 10.1186/1477-7525-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle GJ. Does item homogeneity indicate internal consistency or item redundancy in psychometric scales? Personal Individ Differ. 1991;12:291–294. [Google Scholar]
- 24.Jenkins GD, Taber TD. A Monte Carlo study of factors affecting three indices of composite scale reliability. J Appl Psychol. 1976;62:392–398. [Google Scholar]
- 25.McCrae RR, Kurtz JE, Yamagata S, Terracciano A. Internal consistency, retest reliability, and their implications for personality scale validity. Pers Soc Psychol Rev. 2011;15:28–50. doi: 10.1177/1088868310366253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marx RG, Bombardier C, Hogg-Johnson S, Wright JG. Clinimetric and psychometric strategies for development of a health measurement scale. J Clin Epidemiol. 1999;52:105–111. doi: 10.1016/s0895-4356(98)00148-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Korean version of the THYCA-QoL questionnaire