Abstract
Purpose
Primary thyroid lymphoma (PTL) is a rare disease and it has been investigated in a limited number of studies. The present multicenter study evaluated the clinical features and treatment outcomes of PTL.
Methods
The medical records of patients diagnosed with PTL between 2000 and 2013 in three centers were retrospectively reviewed.
Results
The study included 11 men and 27 women with a median age of 63.3 years (range, 42-83 years). The median follow-up was 56.0 months (range, 3-156 months). Of the 38 patients included, 16 had mucosa-associated lymphoid tissue (MALT) lymphoma, six had mixed MALT and diffuse large B-cell lymphoma (DLBCL), and 16 had DLBCL. Thirty-five patients (92.1%) had early stage (stage I/II) disease. Of the 16 MALT lymphoma patients, 14 were treated by surgery, and radiotherapy (RT) or chemotherapy was combined in five patients. Two patients received RT or chemotherapy alone. Of the six mixed MALT and DLBCL patients, three underwent surgery with chemotherapy and three underwent chemotherapy alone, RT alone, or surgery with RT. All of the 16 DLBCL patients received chemotherapy, and surgery and RT was combined in 4 and 1 patients, respectively. The 5-year survival was 100% for MALT lymphoma (7 of 7) and mixed MALT and DLBCL patients (5 of 5) and 87.5% for DLBCL patients (7 of 8).
Conclusion
Early stage PTL has an excellent prognosis when managed by single or combined treatment modalities. Clinicians should consider PTL in patients with underlying Hashimoto's thyroiditis presenting with an enlarging thyroid mass.
Keywords: Lymphoma, Thyroid lymphoma, Primary thyroid lymphoma, Marginal zone B-cell lymphoma, Diffuse Large B-cell lymphoma
INTRODUCTION
Primary thyroid lymphoma (PTL) is defined as a lymphoma developing in the thyroid gland with or without involvement of regional lymph nodes or other visceral tissues [1]. It is a rare disease accounting for 1%-5% of thyroid malignancies [2,3], and few studies on PTL have been published because of its rarity. Hashimoto's thyroiditis is a risk factor for PTL, and patients with Hashimoto's thyroiditis have a 67- to 80-fold greater risk of developing PTL than those without thyroiditis [4,5]. PTL generally affects women aged 50-80 years, and its incidence is low in patients younger than 40 years [1,6]. B-cell type non-Hodgkin's lymphoma is the most common type of PTL, whereas Hodgkin's and T-cell lymphoma are rare [7]. B-cell type non-Hodgkin's lymphoma is classified into three categories, namely mucosa-associated lymphoid tissue (MALT) lymphoma, diffuse large B-cell lymphoma (DLBCL), and mixed MALT and DLBCL. The most common symptom of PTL is an enlarging anterior neck mass with or without cervical lymphadenopathy that is often associated with obstructive symptoms [8]. Approximately 10% of patients present with the classic B-type symptoms such as fever, night sweating, and weight loss [9]. In the diagnosis of PTL, fine needle aspiration (FNA) cytology is not a reliable method, and the primary diagnostic modality is ultrasound-guided needle biopsy or surgical biopsy [7,10].
Despite controversy regarding the optimal modality for the management of PTL, the mainstay is combination chemotherapy and locoregional radiotherapy. The role of surgery is limited to intrathyroidal MALT lymphoma, which has a benign biological behavior [9,11]. The conventional chemotherapeutic regimen consists of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), and RT is used for local disease control. Patient survival is dependent on the histologic subtype, and DLBCL is associated with a worse prognosis than MALT lymphoma [1]. Stage is also an important prognostic factor; the 5-year disease-specific survival for stage I patients is as high as 86%-100% [1,6,12]. However, no randomized controlled trials assessing the treatment of PTL have been conducted because of the rarity of the disease, and a standardized treatment protocol has not been established. In the present study, we evaluated the clinicopathological characteristics and treatment outcomes of PTL patients.
METHODS
Patients
Patients treated for PTL between 2000 and 2013 at three hospitals (Seoul National University Hospital, Seoul National University Boramae Medical Center, Seoul National University Bundang Hospital) were retrospectively analyzed. Clinicopathological data were reviewed, including age, gender, diagnostic modality, presence of Hashimoto's thyroiditis, treatment protocol, pathologic results, and survival status. Hashimoto's thyroiditis was diagnosed when there was lymphocytic infiltration in the thyroid glands according to the pathologic report or the presence of antithyroglobulin or anti-microsomal antibody regardless of thyroid function, as suggested by Watanabe et al. [13] The Institutional Review Board of each hospital approved the study protocol.
Classification of PTL
B-cell type non-Hodgkin's lymphoma was classified into MALT lymphoma, DLBCL, and mixed type. Stage was determined according to the Ann Arbor staging system as follows: stage I, disease localized to the thyroid; stage II, disease localized to the thyroid and regional lymph node basins; stage III, disease involvement on both sides of the diaphragm; and stage IV, disseminated disease [6].
Statistical analysis
Data were analyzed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). The chi-square test and Fisher exact test were used to compare categorical variables. The unpaired two sample t-test was performed to compare continuous variables. A P-value of <0.05 was considered significant.
RESULTS
Clinicopathological characteristics
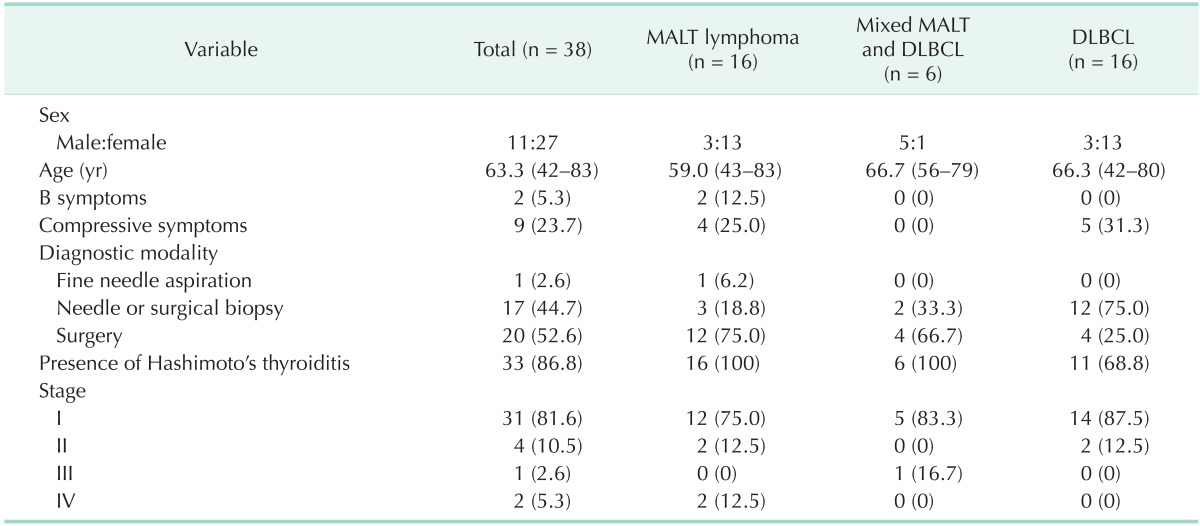
Patient demographics are summarized in Table 1. The study included 11 men and 27 women, and the male to female ratio was 1:2.5. The mean age at diagnosis was 63.3 years (range, 42-83 years). There were 16 MALT lymphoma, six mixed MALT and DLBCL, and 16 DLBCL patients. B symptoms and compressive symptoms were present in 2 (2 MALT lymphoma) and 9 (4 MALT lymphoma and 5 DLBCL) patients, respectively. Most patients were diagnosed by biopsy (44.7%) or surgery (52.6%), and 1 patient (2.6%) with MALT lymphoma was diagnosed by FNA. Hashimoto's thyroiditis was present in 33 patients (86.8%). Thirty-five patients (92.1%) were early stage (stage I or stage II).
Table 1. Patient demographics.
Values are presented as mean (range) or number (%).
MALT, mucosa-associated lymphoid tissue; DLBCL, diffuse large B-cell lymphoma.
Treatment modalities
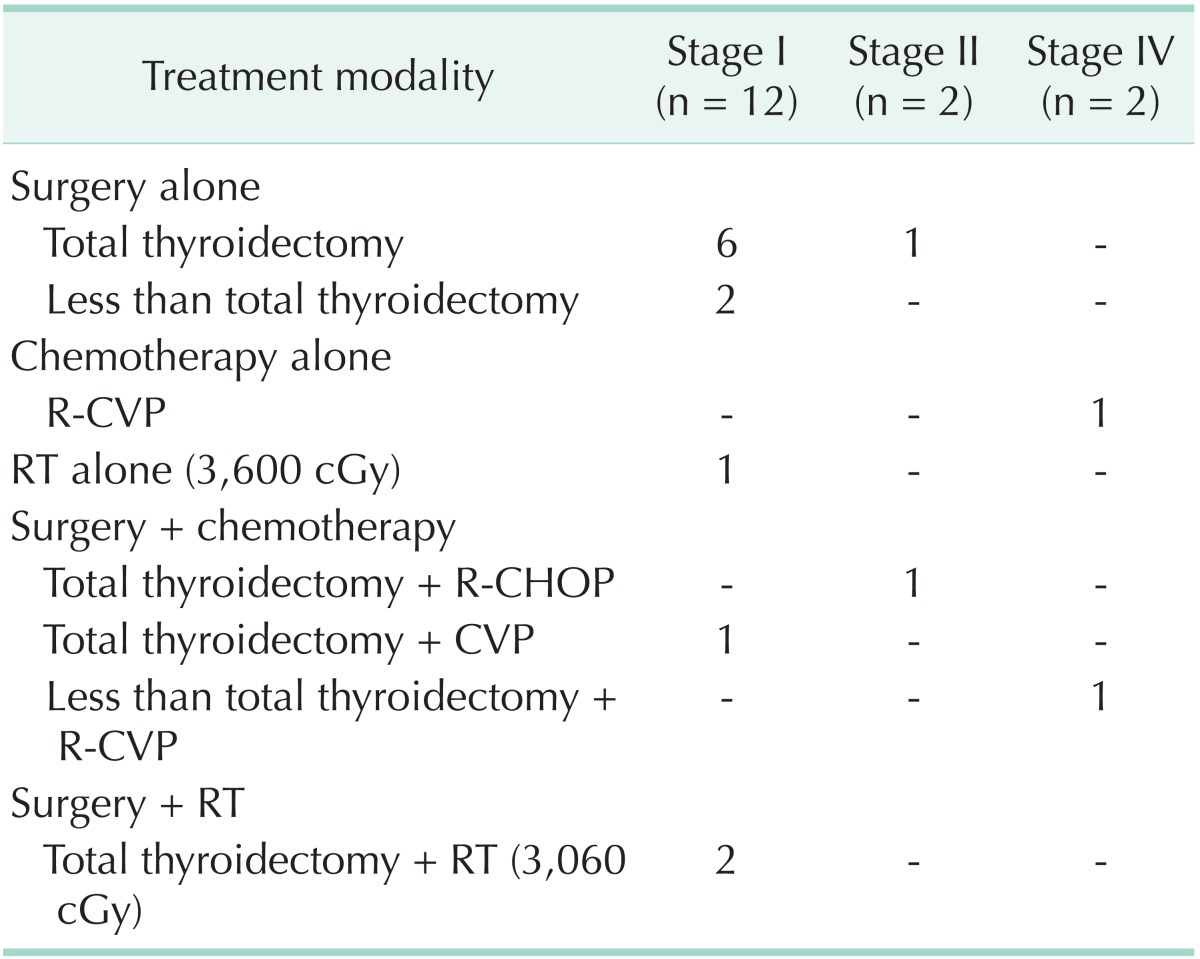
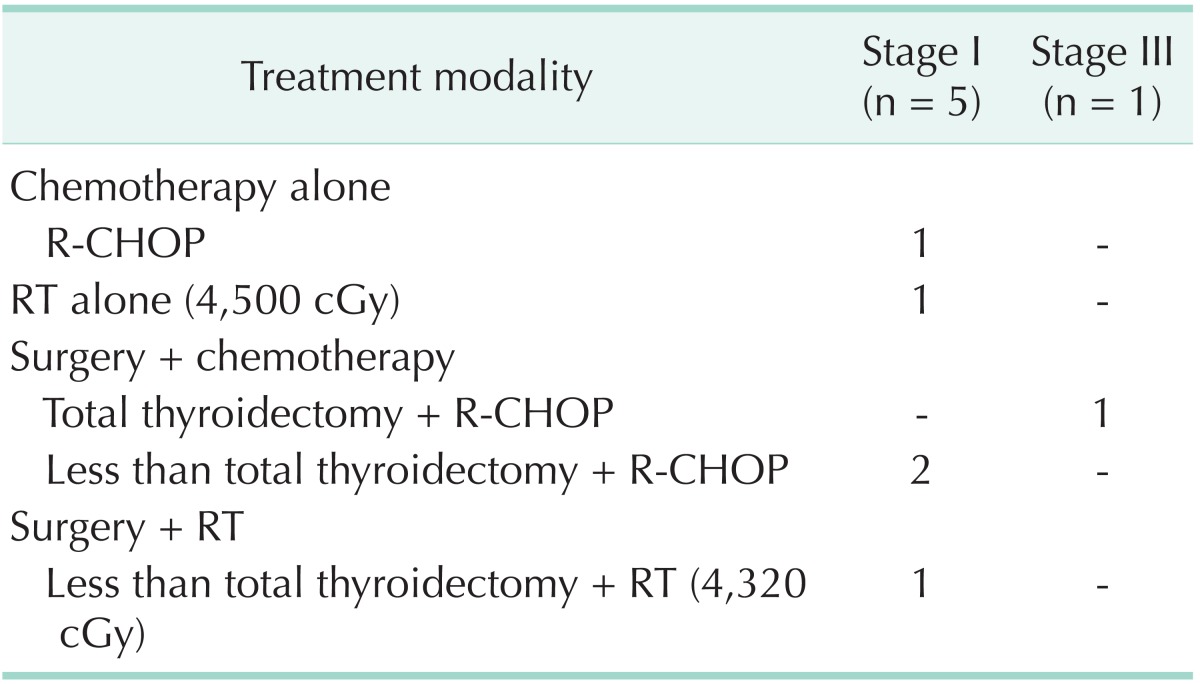
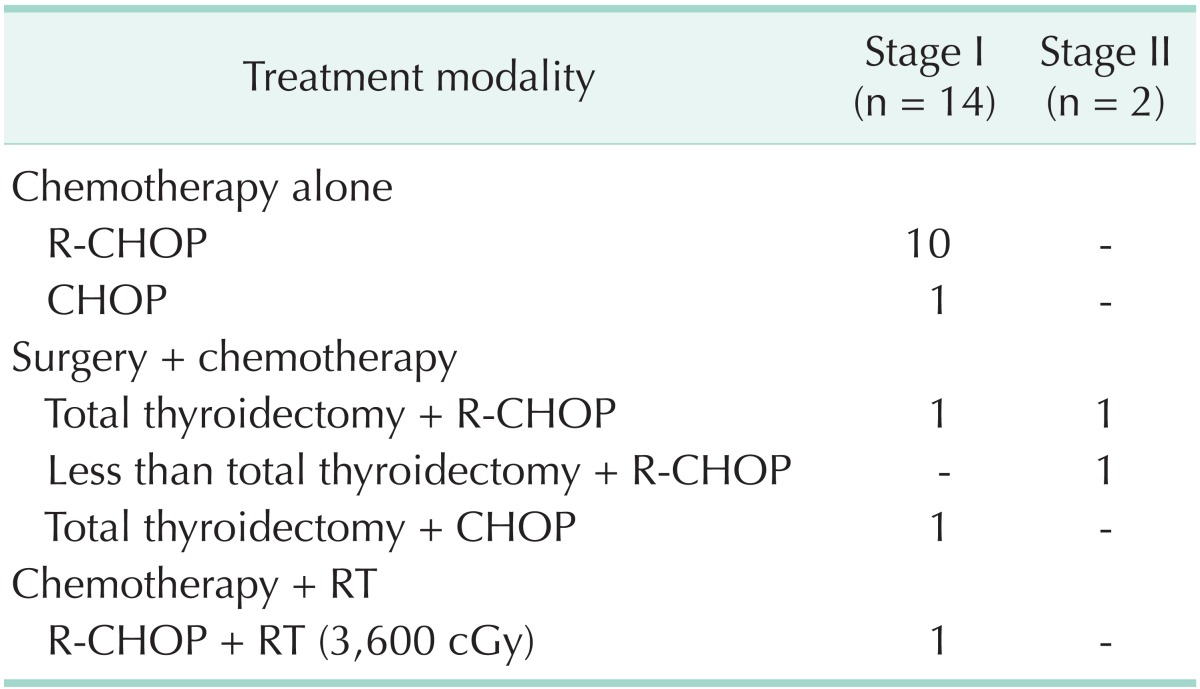
Table 2 shows the treatment modalities for MALT lymphoma patients according to stage. Of the 12 patients with stage I disease, 11 were treated by surgery, and RT or chemotherapy was combined in 3 patients. One patient received RT alone. Among stage II patients, one underwent surgery alone, and the other patient was treated with surgery and chemotherapy. Of the two patients with stage IV, one received chemotherapy alone and one had surgery with chemotherapy. Table 3 describes the treatment modalities used in patients with mixed MALT and DLBCL. Of the five patients with stage I, two underwent surgery with chemotherapy and three underwent chemotherapy alone, RT alone, or surgery with RT. There was 1 patient with stage III who was treated with surgery and chemotherapy. Table 4 shows the treatment modalities used for DLBCL patients. All of the 14 patients with stage I received chemotherapy. Surgery and RT was combined in 2 and 1 patients, respectively. Two patients with stage II were treated with surgery and chemotherapy.
Table 2. Treatment modalities for MALT lymphoma patients.
MALT, mucosa-associated lymphoid tissue; R-CVP, rituximab with cyclophosphamide, vincristine, and prednisone; RT, radiotherapy; R-CHOP, rituximab with cyclophosphamide, doxorubicin, cyclophosphamide and prednisone; CVP, cyclophosphamide, vincristine, and prednisone.
Table 3. Treatment modalities for mixed MALT and DLBCL patients.
MALT, mucosa-associated lymphoid tissue; DLBCL, diffuse large B-cell lymphoma; R-CHOP, rituximab with cyclophosphamide, doxorubicin, cyclophosphamide and prednisone; RT, radiotherapy.
Table 4. Treatment modalities for DLBCL patients.
DLBCL, diffuse large B-cell lymphoma; R-CHOP, rituximab with cyclophosphamide, doxorubicin, cyclophosphamide and prednisone; CHOP, cyclophosphamide, doxorubicin, cyclophosphamide and prednisone; RT, radiotherapy.
Treatment outcomes
The median follow-up was 56.0 months (range, 3-156 months). During the follow-up period, there was one case of disease-specific mortality in a 61-year-old woman diagnosed with stage II DLBCL. The patient underwent total thyroidectomy and received adjuvant CHOP with rituximab. Liver and lung metastases developed 49 months after treatment and the patient died of the disease. No other cases of recurrence were reported. The 5-year survival was 100% for MALT lymphoma (7 of 7) and mixed MALT and DLBCL patients (5 of 5), and 87.5% for DLBCL patients (7 of 8).
DISCUSSION
The prognosis of PTL patients is largely dependent on the histologic subtype. MALT lymphoma is considered low grade with an indolent natural history. Most patients with MALT lymphoma present with early stage disease confined to the thyroid, and localized treatment such as surgery or RT is often sufficient to achieve a complete cure [1,14,15]. In the present study, patients with MALT lymphoma had an excellent prognosis, without any disease related mortality, including the two patients with advanced stage disease. Previous studies reported 5-year disease-specific survival rates ranging from 96% to 100% [1,6,16]. DLBCL is considered as a high grade lymphoma with a more aggressive clinical course than that of MALT lymphoma [7], and the 5-year disease-specific survival rate for DLBCL is 71%-75% [1,6]. DLBCL can develop from MALT lymphoma, and these two subtypes can be detected in the same gland [7]. The mixed MALT and DLBCL subtype shows the same clinical behavior as that of DLBCL [2]. In the present study, there was 1 case (4.5%) of disease-specific mortality among 22 patients with DLBCL and mixed MALT and DLBCL. This low mortality rate could be attributed to the fact that there was only 1 patient with advanced stage in the mixed MALT and DLBCL cohort, and none in the DLBCL group.
The prognosis of PTL is also affected by disease stage. In the only population-based study using the Surveillance, Epidemiology, and End Results database of the National Cancer Institute, Graff-Baker et al. [6] reported that the disease-specific survival of PTL patients decreased with increasing stage. The study evaluated 1,408 PTL patients and reported 5-year disease-specific survival rates of 86% for stage I, 81% for stage II, and 64% for stage III/IV patients. In the second largest study on PTL performed by Derringer et al. [1], no patients with stage I died during the follow-up period, which is consistent with the present results. However, the present study was a retrospective study with a short follow-up period (56.0 months) and unstandardized treatment protocols, which limited the analysis of treatment outcomes. Nevertheless, our results showed that patients with stage I disease have excellent survival outcomes.
In the present study, we confirmed that the treatment outcome of PTL is excellent. There was only one case of disease-specific mortality in the study population. There are two possible explanations for this result. Firstly, most of the patients included were enrolled during a relatively recent period. Thirtytwo patients (84.2%) were diagnosed after 2006, and the rate of diagnosis of PTL at an earlier stage has increased over time [6]. The proportion of early stage (stage I/II) patients was 92.1% in this study. The development of diagnostic modalities and early surveillance may contribute to the early detection of the disease. Moreover, rituximab was approved by the U.S. Food and Drug Administration in 2006 as a first-line drug for PTL, and most of the DLBCL and mixed MALT and DLBCL patients who were expected to have poor prognosis were eligible for a combination rituximab and CHOP treatment regimen in this study. The combination of rituximab with CHOP is associated with improved survival rates among elderly patients with B-cell lymphoma and is a promising treatment for elderly patients with PTL [17,18]. Secondly, the proportion of patients with MALT lymphoma, which has better prognosis than DLBCL, was greater in the present study (42.1%) than in previous studies in the literature, which reported rates ranging from 10.0% to 28.0% [1,6,7,19].
Hashimoto's thyroiditis is not only the risk factor for PTL but it can also evolve into MALT lymphoma [20]. Furthermore, Hashimoto's thyroiditis is often accompanied by PTL (86.8% in this study). Currently, FNA is the procedure of choice for the diagnosis of thyroid nodules [21]. However, distinguishing PTL from Hashimoto's thyroiditis by FNA is challenging, although the presence of Hashimoto's thyroiditis combined with suspicious sonographic findings might indicate a diagnosis of PTL [22,23,24]. Therefore, clinicians should consider PTL in patients presenting with an enlarging thyroid mass even when the FNA cytology suggests thyroiditis.
In summary, PTL has excellent prognosis when it is confined to the regional neck area and treated properly according to histologic subtype and stage. To improve the early diagnosis of PTL and achieve complete remission, clinicians should consider PTL in patients with underlying Hashimoto's thyroiditis who present with an enlarging thyroid mass.
ACKNOWLEDGEMENTS
This study was supported by Korean Foundation for Cancer Research (Grant No.: CB-2011-03-01).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Derringer GA, Thompson LD, Frommelt RA, Bijwaard KE, Heffess CS, Abbondanzo SL. Malignant lymphoma of the thyroid gland: a clinicopathologic study of 108 cases. Am J Surg Pathol. 2000;24:623–639. doi: 10.1097/00000478-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Widder S, Pasieka JL. Primary thyroid lymphomas. Curr Treat Options Oncol. 2004;5:307–313. doi: 10.1007/s11864-004-0021-7. [DOI] [PubMed] [Google Scholar]
- 3.Ansell SM, Grant CS, Habermann TM. Primary thyroid lymphoma. Semin Oncol. 1999;26:316–323. [PubMed] [Google Scholar]
- 4.Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601–604. doi: 10.1056/NEJM198503073121001. [DOI] [PubMed] [Google Scholar]
- 5.Kato I, Tajima K, Suchi T, Aozasa K, Matsuzuka F, Kuma K, et al. Chronic thyroiditis as a risk factor of B-cell lymphoma in the thyroid gland. Jpn J Cancer Res. 1985;76:1085–1090. [PubMed] [Google Scholar]
- 6.Graff-Baker A, Roman SA, Thomas DC, Udelsman R, Sosa JA. Prognosis of primary thyroid lymphoma: demographic, clinical, and pathologic predictors of survival in 1,408 cases. Surgery. 2009;146:1105–1115. doi: 10.1016/j.surg.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Alzouebi M, Goepel JR, Horsman JM, Hancock BW. Primary thyroid lymphoma: the 40 year experience of a UK lymphoma treatment centre. Int J Oncol. 2012;40:2075–2080. doi: 10.3892/ijo.2012.1387. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Khosla D, Kumar N, Ghoshal S, Bera A, Das A, et al. Survival and failure outcomes in primary thyroid lymphomas: a single centre experience of combined modality approach. J Thyroid Res. 2013;2013:269034. doi: 10.1155/2013/269034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green LD, Mack L, Pasieka JL. Anaplastic thyroid cancer and primary thyroid lymphoma: a review of these rare thyroid malignancies. J Surg Oncol. 2006;94:725–736. doi: 10.1002/jso.20691. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M, Sone H, Koyama H, Ishiguro S. Fine-needle aspiration cytology of malignant lymphoma of the thyroid. Diagn Cytopathol. 1987;3:244–249. doi: 10.1002/dc.2840030314. [DOI] [PubMed] [Google Scholar]
- 11.Sakorafas GH, Kokkoris P, Farley DR. Primary thyroid lymphoma (correction of lympoma): diagnostic and therapeutic dilemmas. Surg Oncol. 2010;19:e124–e129. doi: 10.1016/j.suronc.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Belal AA, Allam A, Kandil A, El Husseiny G, Khafaga Y, Al Rajhi N, et al. Primary thyroid lymphoma: a retrospective analysis of prognostic factors and treatment outcome for localized intermediate and high grade lymphoma. Am J Clin Oncol. 2001;24:299–305. doi: 10.1097/00000421-200106000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe N, Noh JY, Narimatsu H, Takeuchi K, Yamaguchi T, Kameyama K, et al. Clinicopathological features of 171 cases of primary thyroid lymphoma: a long-term study involving 24553 patients with Hashimoto\'s disease. Br J Haematol. 2011;153:236–243. doi: 10.1111/j.1365-2141.2011.08606.x. [DOI] [PubMed] [Google Scholar]
- 14.Tsang RW, Gospodarowicz MK, Pintilie M, Wells W, Hodgson DC, Sun A, et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003;21:4157–4164. doi: 10.1200/JCO.2003.06.085. [DOI] [PubMed] [Google Scholar]
- 15.Thieblemont C, Mayer A, Dumontet C, Barbier Y, Callet-Bauchu E, Felman P, et al. Primary thyroid lymphoma is a heterogeneous disease. J Clin Endocrinol Metab. 2002;87:105–111. doi: 10.1210/jcem.87.1.8156. [DOI] [PubMed] [Google Scholar]
- 16.Oh SY, Kim WS, Kim JS, Kim SJ, Lee S, Lee DH, et al. Primary thyroid marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: clinical manifestation and outcome of a rare disease - consortium for improving survival of lymphoma study. Acta Haematol. 2012;127:100–104. doi: 10.1159/000333113. [DOI] [PubMed] [Google Scholar]
- 17.Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe N, Narimatsu H, Noh JY, Kunii Y, Mukasa K, Matsumoto M, et al. Rituximab-including combined modality treatment for primary thyroid lymphoma: an effective regimen for elderly patients. Thyroid. 2014;24:994–999. doi: 10.1089/thy.2013.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cha H, Kim JW, Suh CO, Kim JS, Cheong JW, Lee J, et al. Patterns of care and treatment outcomes for primary thyroid lymphoma: a single institution study. Radiat Oncol J. 2013;31:177–184. doi: 10.3857/roj.2013.31.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyjek E, Isaacson PG. Primary B cell lymphoma of the thyroid and its relationship to Hashimoto\'s thyroiditis. Hum Pathol. 1988;19:1315–1326. doi: 10.1016/s0046-8177(88)80287-9. [DOI] [PubMed] [Google Scholar]
- 21.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 22.Mack LA, Pasieka JL. An evidence-based approach to the treatment of thyroid lymphoma. World J Surg. 2007;31:978–986. doi: 10.1007/s00268-005-0768-z. [DOI] [PubMed] [Google Scholar]
- 23.Stein SA, Wartofsky L. Primary thyroid lymphoma: a clinical review. J Clin Endocrinol Metab. 2013;98:3131–3138. doi: 10.1210/jc.2013-1428. [DOI] [PubMed] [Google Scholar]
- 24.Nam M, Shin JH, Han BK, Ko EY, Ko ES, Hahn SY, et al. Thyroid lymphoma: correlation of radiologic and pathologic features. J Ultrasound Med. 2012;31:589–594. doi: 10.7863/jum.2012.31.4.589. [DOI] [PubMed] [Google Scholar]