Abstract
Nucleotide-binding oligomerization domain 2 (Nod2) is an intracellular receptor that can sense the bacterial peptidoglycan component, muramyl dipeptide. Upon activation, Nod2 induces the production of various inflammatory molecules such as cytokines and chemokines. Genetic linkage analysis identified and revealed three major mutations in Nod2 that are associated with the development of Crohn's disease. The objective of this study is to further characterize this protein by determining whether Nod2 is posttranslationally modified by O-N-acetylglucosamine (O-GlcNAc). O-GlcNAcylation is one type of posttranslational modification in which the O-GlcNAc transferase transfers GlcNAc from UDP-GlcNAc to selected serine and threonine residues of intracellular proteins. We found that wild-type Nod2 and a Nod2 Crohn's-associated variant are O-GlcNAcylated and this modification affects Nod2's ability to signal via the nuclear factor kappa B pathway.
Keywords: Crohn's disease, NF-κB, Nod2, O-GlcNAcylation, OGT
Introduction
The human innate immune system is the first line of defense against pathogens. This system is critical in distinguishing the “good” vs. “bad” bacteria that populate the human body (Janeway and Medzhitov 2002). The misrecognition of bacteria by the innate immune system is one factor that causes inflammatory disorders, including Crohn's disease (Macia et al. 2012). The innate immune receptors or pattern recognition receptors (PRRs) play major roles in recognizing bacterial components (Tosi 2005). Membrane-bound Toll-like receptors and cytoplasmic NOD-like receptors (NLR) are two major forms of PRRs (Martinon and Tschopp 2005). Nucleotide-binding oligomerization domain 2 (Nod2), a human NLR cytoplasmic receptor senses bacterial components (Magalhaes et al. 2011). Upon binding to its bacterial-derived ligand, Nod2 can interact with receptor-interacting protein 2 to activate nuclear factor kappa B (NF-κB), a transcription factor that can induce the production of various inflammatory molecules (Abbott et al. 2004; Grimes et al. 2012; Mo et al. 2012). Mutations in Nod2 are associated with Crohn's disease and with a decreased ability to activate the NF-κB pathway (Hugot et al. 2001).
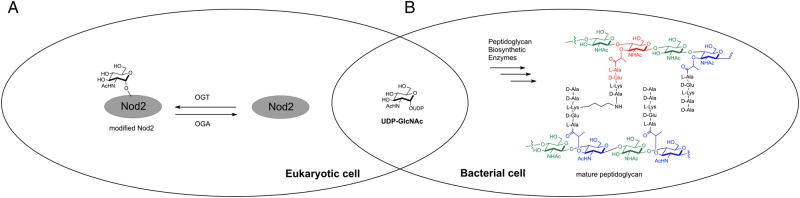
Bacterial cell wall, a peptidoglycan, is a highly conserved structure found in all bacteria. Peptidoglycan contains a repetition of carbohydrates, N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (Figure 1) cross-linked by short chains of peptides to form a lattice surrounding the entire cell (van Heijenoort 2001; Lovering et al. 2012). Large amounts of GlcNAc and other bacterial cell wall building blocks, such as UDP-GlcNAc, are released during bacterial growth/division and bacterial host invasion (Park and Uehara 2008; Naseem et al. 2012). GlcNAc is not only important in bacterial cell wall but also serves as a metabolite of the hexosamine biosynthetic pathway, one endpoint of which is the modification and regulation of proteins by O-GlcNAcylation (Groves et al. 2013).
Fig. 1.
UDP-GlcNAc is used a substrate for (A) the human enzyme, OGT and (B) the bacterial cell wall biosynthetic enzymes. MDP structure is highlighted in red.
O-GlcNAcylation is a dynamic posttranslational modification in which the O-GlcNAc transferase (OGT) transfers GlcNAc from UDP-GlcNAc to selected serine and threonine residues, whereas O-GlcNAcase (OGA) removes O-GlcNAc from target proteins (Torres and Hart 1984; Dong and Hart 1994; Wells et al. 2001) (Figure 1). This modification is found on nuclear, mitochondrial and cytoplasmic proteins (Holt et al. 1987; Hart et al. 2007). Further, misregulation of O-GlcNAcylation has been linked to a number of human diseases (Groves et al. 2013; Yang and Suh 2014). O-GlcNAc is thought to regulate proteins in a manner analogous to protein phosphorylation, and like phosphorylation the levels of O-GlcNAc responds to numerous extra- and intracellular signals, including those that simulate an immune response in lymphocytes such as lipopolysaccharide (Raetz and Whitfield 2002). However, the relationship between GlcNAc, bacterial cell wall fragments and cytoplasmic innate immune receptors, such as Nod2 is still unclear. Here, we define the role of O-GlcNAcylation on regulating the function of wild type and Crohn's-associated variants of Nod2.
Results
O-GlcNAcylation of Nod2
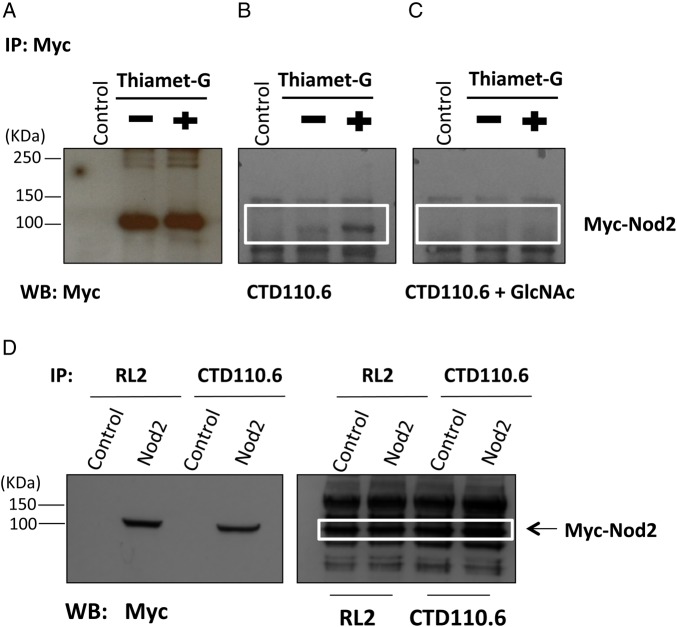
Nod2 was assayed for the O-GlcNAc modification using the GlcNAc-specific antibody, CTD110.6. Briefly, HEK293T cells that stably express Nod2 with a Myc epitope tag on the N-terminus (Nod2Myc) (Mohanan and Grimes 2014) were treated with or without the O-GlcNAcase inhibitor, Thiamet-G (1 µM, 4 h) (Yuzwa et al. 2008). Nod2 was immunoprecipitated from cell lysates with an anti-Myc antibody and detected with CTD110.6 (Figure 2A and B). In order to ensure that the antibody, CTD110.6, was truly detecting the O-GlcNAcylation modification, the CTD110.6 antibody was preincubated with free GlcNAc and then used in the western blot analysis (Figure 2B and C) (Zachara et al. 2011). When Thiamet-G is present, the level of O-GlcNAcylation increases (Figure 2B). When the free GlcNAc is used in the experiment, the band for O-GlcNAcylation is no longer present (Figure 2C). Next, the reverse assay was performed using the antibodies for the O-GlcNAc modification and assayed for the presence of Nod2. Nod2 was immunoprecipitated (Figure 2D). Together, these data demonstrate that Nod2 is posttranslationally modified by OGT.
Fig. 2.
OGT modifies Nod2. (A) Cell lysates were immunopurified with Myc antibody and blotted with Myc antibody; Control cells do not express Myc-Nod2. Thiamet-G inhibits OGA, elevating O-GlcNAcylation. (B) Cell lysates were immunopurified with Nod2 and blotted using CTD110.6. (C) Alternatively, the CTD110.6 was pre-incubated with free GlcNAc. (D) Co-IP were performed using 1 µl of anti-O-GlcNAc (RL2 or CTD110.6) antibody per 200 µL of lysate (HEK293T/Nod2Myc cells) and probed for Myc using rabbit anti-Myc antibody (1:1000), anti-RL2 antibody (1:2000) and anti-CTD110.6 (1:1000).
O-GlcNAcylation level regulates Nod2 half-life in cells
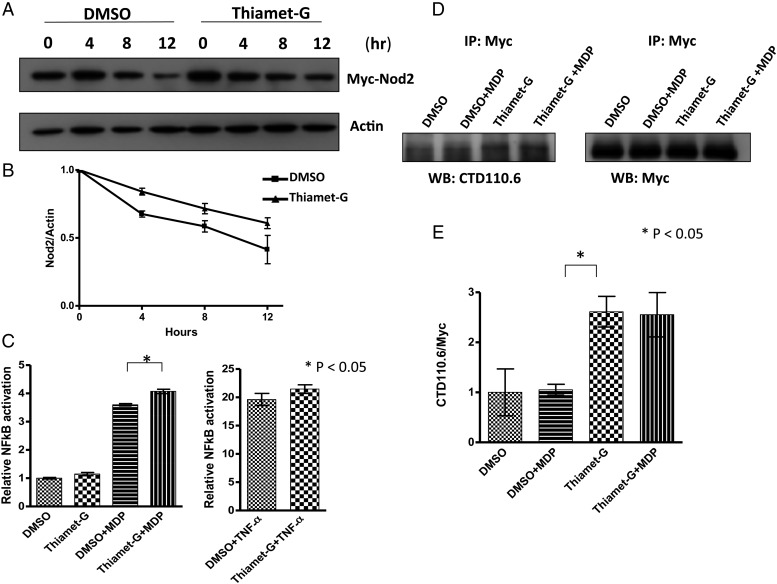
Previous studies demonstrated that O-GlcNAcylation can regulate protein stability and function (Chu et al. 2014). We hypothesized that this modification could affect Nod2 stability, as it has previously been shown that Nod2 is inheritably unstable. In order to test this, the classical protein half-life experiment using cycloheximide (100 µg/mL), a translational inhibitor, was performed (Schneider-Poetsch et al. 2010). When cells are not treated with Thiamet-G, the half-life is 10.6 ± 2.4 h. However, when cells are treated with Thiamet-G (1 µM, 4 h), the half-life is 17.3 ± 4.5 h (Figure 3A and B), suggesting that the modification alters the stability of Nod2, via either direct or indirect mechanisms. Additional experiments would need to be conducted to show that O-GlcNAc on Nod2 is directly impacting its stability.
Fig. 3.
O-GlcNAcylation regulates the half-life of Nod2 and its NF-κB activity in cells. (A) HEK293T-Nod2Myc/Tet-op cells were incubated with 1 µM Thiamet-G or DMSO for 4 h, prior to cycloheximide treatment (100 µg/mL) at the indicated time intervals. Nod2 was detected by anti-Myc western blotting. (B) Relative amount of Nod2 Myc to actin was plotted from three independent experiments as the means + SD (C) Dual-luciferase assay performed on HEK293T-Nod2Myc/Tet-op cells in the presence of 100 nM Thiamet-G or DMSO for 4 h. After 4 h, cells were incubated with 20 µM MDP or 200 nM TNF-α for 5 h, harvested and tested for luciferase activity. *P < 0.05 was considered as significant. (D) HEK293T-Nod2Myc/Tet-op cell lysates (±Thiamet-G (1 µM, 4 h), ±MDP (20 µM, 5 h)) were immunopurified with Myc antibody and blotted with anti-CTD110.6 antibody; Control cells do not express Myc-Nod2. (E) Relative amount of CTD110.6 to Nod2 Myc was plotted from three independent experiments as the means + SD.
O-GlcNAcylation regulates Nod2-induced NF-κB activity
Nod2 is known to activate the NF-κB pathway upon stimulation by muramyl dipeptide (MDP, Figure 1), triggering an inflammatory response. In order to determine the impact of O-GlcNAcylation on Nod2-dependent NF-κB signaling pathway, an established NF-κB luciferase reporter assay was employed (Mohanan and Grimes 2014). The data show that Nod2 increased NF-κB activation when O-GlcNAcylation levels were increased in HEK293T-Nod2Myc/tet-op cells and increased O-GlcNAcylation does not generally activate NF-κB (Figure 3C). In order to determine if this was an effect primarily on Nod2 or other downstream NF-κB proteins, TNF-α, which is not dependent on Nod2, was used. Thiamet-G did not potentiate TNF-α-dependent activation of NF-κB suggesting that in the context of MDP activation O-GlcNAc is acting on Nod2 (Figure 3C). As MDP is structurally similar to GlcNAc (Figure 1), we assayed the ability of Nod2 GlcNAcylation levels to increase in the presence of this molecule; there was no difference in O-GlcNAcylation levels upon MDP treatment in the presence or absence of Thiamet-G (Figure 3D and E). Suggesting that the increase in NF-κB activity when treated with Thiamet-G is a result of increased stability of Nod2, either by modification with GlcNAc or other mechanisms.
Nod2 variant is O-GlcNAcylated and increasing O-GlcNAcylation increases its stability and activity
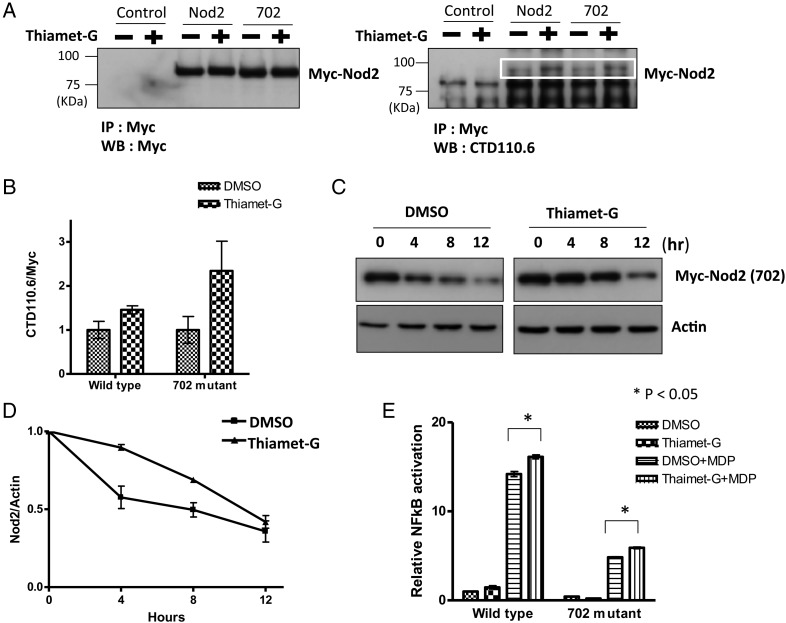
It has been shown that the Nod2 Crohn's mutants are defective in their ability to signal the presence of bacterial cell wall fragments. Moreover, it has been shown that stabilization of Nod2 via overexpression of Hsp70 restores the ability for the mutants to signal (Mohanan and Grimes 2014). Therefore, the Nod2 Crohn's mutants are less stable, leading to decreased protein levels and ability to activate NF-κB pathway. We considered whether the Nod2 Crohn's mutants could be modified by OGT and whether the O-GlcNAc modification of Nod2 could affect the ability of the mutant's stability and ability to signal. A HEK293T cell line stably expressing a Nod2 mutant (702) was treated with Thiamet-G (1 µM, 4 h). Nod2 was immunoprecipitated from the resulting cell lysates and the levels of O-GlcNAc were assessed by immunoblotting (Figure 4A and B). When Thiamet-G is present, the level of O-GlcNAcylation increases (Figure 4A and B). The data show that the Nod2 Crohn's-associated mutant is modified with O-GlcNAc. In addition, increasing the levels of the O-GlcNAcylation increased the half-life of the mutant Nod2 from 8.1 ± 1.7 to 15.0 ± 1.8 h (Figure 4C and D). Finally, if the level of the O-GlcNAc modification is increased with Thiamet-G, cells had a significant increase in MDP-induced NF-κB activity (Figure 4E).
Fig. 4.
O-GlcNAcylation of Crohn's-associated Nod2 variant regulates its half-life and NF-κB activity in cells. (A) Control cells do not express Myc-Nod2. 702 cells express a Nod2 variant associated with Crohn's disease. Cell lysates treated with and without Thiamet-G (1 µM, 4 h) were immunopurified with Myc antibody and blotted with anti-Myc or anti-O-GlcNAc antibodies. (B) Relative amount of CTD110.6 to Nod2 Myc was plotted from three independent experiments as the means + SD. (C) HEK293T-702 cells were incubated with 1 µM Thiamet-G or DMSO for 4 h, and before cycloheximide treatment (100 µg/mL) at the indicated time intervals. Nod2 was detected by anti-Myc western blotting. (D) Relative amount of Nod2 Myc to actin was plotted from three independent experiments as the means + SD. (E) Dual-luciferase assay performed on HEK293T transient-transfected with 0.1 ng Nod2 and 702 plasmids in the presence of 100 nM Thiamet-G or DMSO for 4 h. After 4 h cells were incubated with 20 µM MDP for 5 h, harvested, and tested for luciferase activity. *P < 0.05 was considered as significant.
Thus, increasing the levels of O-GlcNAc in the cell leads to changes in stability and activity of both the wild-type and Crohn's-mutant Nod2.
Discussion
Nod2 is an innate immune receptor that recognizes bacterial cell wall fragments and triggers an inflammatory response. In 2001, genetic linkage analysis showed that Nod2 mutations are associated with an incidence of occurrence for Crohn's disease (Ogura, Bonen, et al. 2001; Ogura, Inohara, et al. 2001). Crohn's disease is a chronic inflammatory disorder that affects 750,000 people in the United States. Further studies showed that Nod2 mutations linked to Crohn's disease is a loss-of-function mutation that lacks the ability to respond to bacterial invasion, which is predicted to be the cause for the chronic inflammation associated with the disease. Currently, there is no cure for this disease.
Recently, we have shown that Nod2 is an unstable protein and the Crohn's-associated mutants are even more unstable. We have shown that overexpression of the molecular chaperone Hsp70 restores the stability and the proper signaling to the Crohn's mutants. We proposed that targeting the stabilization of Nod2 mutants might rescue their activity by allowing the proper protein level to be maintained. In this work, we show a novel method that could be implemented to stabilize Nod2 mutants thus rescuing their activity.
Biochemically, the Nod2 protein remains to be fully characterized due to the lack of tools to study this inherently unstable protein. For the first time, we show that Nod2 is posttranslationally modified by a small carbohydrate, GlcNAc. We identified this by using antibodies that specifically detect O-GlcNAc linkage, by perform co-immunoprecipitation using GlcNAc antibodies and probing for Nod2 and vice versa. In addition, in order to initially probe the role of O-GlcNAcylation on the Crohn's-associated mutants, we performed experiments with one of the Crohn's-associated mutants; we demonstrated the 702 Crohn's-associated Nod2 mutant is O-GlcNAcylated.
Further characterization of this modification revealed that increasing the level of O-GlcNAc in cells increases the half-life of Nod2. Maintaining the stability of proteins is one of the primary effects of the GlcNAc modification on proteins (Chu et al. 2014; Zhu et al. 2015). We have previously shown that Crohn's-associated Nod2 mutants have a decreased half-life compared with the wild type (Mohanan and Grimes 2014). 702 Nod2 Crohn's mutant expressed in cells incubated with Thiamet-G displayed an increased half-life compared with control. Further, we determined that increasing O-GlcNAc levels using a small molecular inhibitor of OGA, Thiamet-G could affect the MDP-induced NF-κB activity via Nod2. These data demonstrate that increasing cellular O-GlcNAcylation levels not only stabilize Nod2 and its mutant but also increase its ability to activate the NF-κB pathway. Thus, this finding could have an impact on the development of novel treatments of Crohn's disease where the mutants lack their stability and fail to respond to bacterial cell wall fragments. We note that Thiamet-G is a synthetic inhibitor of the glycosidase, OGA, which removes the carbohydrate modification from Nod2. By implementing Thiamet-G in this study, we have shown that Nod2 is posttranslationally modified with the carbohydrate, GlcNAc and this modification has the ability to alter its stability and cellular response either by direct or indirect mechanisms (i.e. by increasing its ability to bind ligand or interact with other proteins).
Materials and methods
Materials
The mouse monoclonal anti-O-GlcNAc antibody, CTD110.6 and RL-2 were produced and purified by Core C4 (Department of Biological Chemistry, JHU). All other antibodies were purchased from Cell Signaling Technology. Thiamet-G was purchased from Sigma-Aldrich. MDP was purchased from Bachem.
Cell culture
HEK293T and HCT116 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). HEK293T-Nod2Myc/tet-op and HEK293T-Nod2Myc cells are previously described (Mohanan and Grimes 2014). Cells were cultured as described (Mohanan and Grimes 2014). HEK293T-702 Myc and HEK293T-CONTROL cell lines were created by using K2605 lentiviral vector having the Nod2-702 insert or empty vector, respectively (Mohanan et al. 2013; Mohanan and Grimes 2014).
Western blot analysis
Analysis was performed as described in Mohanan and Grimes (2014).
Co-immunoprecipitation
Cells were rinsed with 1× PBS, and cells were harvested and lysed as described in Mohanan and Grimes (2014). Four milligrams of lysate were mixed with the appropriate amount of primary antibody and incubated overnight at 4°C. Lysate/antibody mixture was incubated with 40 µL of protein A Dynabeads (Invitrogen) for 3 h at 4°C. After 3 h, lysate/antibody bead mixture was washed four times as described in Mohanan and Grimes (2014). Proteins were eluted at 100°C for 5 min with 2× loading buffer and analyzed by western blot.
Half-life determination
Cycloheximide was used at a concentration of 100 µg/mL, and the lysates were collected every 4 h. Thiamet-G was incubated 4 h prior to the addition of cycloheximide. Cells were treated, lysed, quantified for protein content and analyzed by protein gel as described in Mohanan and Grimes (2014).
The protein bands for Nod2 and Actin were quantified using Image Lab 5.0. Actin is used as the loading control and the ratio of the intensity of Nod2 to actin bands (Ir) were used to analyze the half-life values as previously described (Belle et al. 2006). Briefly, relative Nod2 band intensities were plotted against time assuming first-order decay (ln(Ir) vs. time). The rate constant was calculated using the negative slope of the line (k =− slope), and the corresponding half-life was calculated (T1/2 = ln(2)/k). Each condition was performed in triplicate and the Student's t-tests were used to determine statistical significance. A P-value of ≤0.05 was considered to be statistically significant.
Luciferase reporter assay
Ten nanograms of pGL4.32 (luc2P/NF-κB-RE/Hygro), 1 ng pRL Renilla luciferase reporter vector, 0.1 ng of Nod2-CMV vector were transfected to cells (seeding density = 7 × 104) in a 24-well dish using Lipofectamine LTX. Cells were pre-incubated with Thiamet-G for 4 h and then incubated with stimuli for 5 h, and the lysates were collected to perform the Dual-Luciferase Reporter Assay (Promega) and were normalized to Renilla activity. Relative luciferase activity of firefly to Renilla is plotted. Results shown are the means ± SD of triplicate experiments.
Funding
This work was supported by the Mizutani Glycosciences Foundation; C.L.G. is a Pew Biomedical Scholar; N.E.Z. receives funding from the NHLBI (P01HL107153).
Conflict of interest statement
None declared.
Abbreviations
GlcNAc, N-acetylglucosamine; ; MDP, muramyl dipeptide; NLR, NOD-like receptors; Nod2, nucleotide-binding oligomerization domain 2; OGT, O-GlcNAc transferase; OGA, O-GlcNAcase; PRRs, pattern recognition receptors.
Acknowledgments
We thank Prof. John Koh for use of the tissue culture facility. CTD110.6 and RL2 were a gift from Core C4 (Johns Hopkins University School of Medicine, P01HL107153).
References
- Abbott DW, Wilkins A, Asara JM, Cantley LC. 2004. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 14:2217–2227. [DOI] [PubMed] [Google Scholar]
- Belle A, Tanay A, Bitincka L, Shamir R, O'Shea EK. 2006. Quantification of protein half-lives in the budding yeast proteome. Proc Natl Acad Sci USA. 103:13004–13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CS, Lo PW, Yeh YH, Hsu PH, Peng SH, Teng YC, Kang ML, Wong CH, Juan LJ. 2014. O-GlcNAcylation regulates EZH2 protein stability and function. Proc Natl Acad Sci USA. 111:1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong DL, Hart GW. 1994. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. J Biol Chem. 269:19321–19330. [PubMed] [Google Scholar]
- Grimes CL, Ariyananda Lde Z, Melnyk JE, O'Shea EK. 2012. The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc. 134:13535–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves JA, Lee A, Yildirir G, Zachara NE. 2013. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperon. 18:535–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. 2007. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 446:1017–1022. [DOI] [PubMed] [Google Scholar]
- Holt GD, Haltiwanger RS, Torres CR, Hart GW. 1987. Erythrocytes contain cytoplasmic glycoproteins. O-linked GlcNAc on Band 4.1. J Biol Chem. 262:14847–14850. [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M et al. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 411:599–603. [DOI] [PubMed] [Google Scholar]
- Janeway CA Jr, Medzhitov R. 2002. Innate immune recognition. Annu Rev Immunol. 20:197–216. [DOI] [PubMed] [Google Scholar]
- Lovering AL, Safadi SS, Strynadka NC. 2012. Structural perspective of peptidoglycan biosynthesis and assembly. Annu Rev Biochem. 81:451–478. [DOI] [PubMed] [Google Scholar]
- Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, Vieira AT, Kranich J, Mackay CR. 2012. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev. 245:164–176. [DOI] [PubMed] [Google Scholar]
- Magalhaes JG, Sorbara MT, Girardin SE, Philpott DJ. 2011. What is new with Nods? Curr Opin Immunol. 23:29–34. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. 2005. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 26:447–454. [DOI] [PubMed] [Google Scholar]
- Mo J, Boyle JP, Howard CB, Monie TP, Davis BK, Duncan JA. 2012. Pathogen sensing by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is mediated by direct binding to muramyl dipeptide and ATP. J Biol Chem. 287:23057–23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanan V, Grimes CL. 2014. The molecular chaperone HSP70 binds to and stabilizes NOD2, an important protein involved in Crohn disease. J Biol Chem. 289:18987–18998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanan V, Temburni MK, Kappes JC, Galileo DS. 2013. L1CAM stimulates glioma cell motility and proliferation through the fibroblast growth factor receptor. Clin Exp Metastasis. 30:507–520. [DOI] [PubMed] [Google Scholar]
- Naseem S, Parrino SM, Buenten DM, Konopka JB. 2012. Novel roles for GlcNAc in cell signaling. Commun Integr Biol. 5:156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH et al. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 411:603–606. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 276:4812–4818. [DOI] [PubMed] [Google Scholar]
- Park JT, Uehara T. 2008. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan). Microbiol Mol Biol Rev. 72:211–227, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu Rev Biochem. 71:635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Poetsch T, Ju J, Eyler DE, Dang Y, Bhat S, Merrick WC, Green R, Shen B, Liu JO. 2010. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 6:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres CR, Hart GW. 1984. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 259:3308–3317. [PubMed] [Google Scholar]
- Tosi MF. 2005. Innate immune responses to infection. J Allergy Clin Immunol. 116:241–249; quiz 250. [DOI] [PubMed] [Google Scholar]
- van Heijenoort J. 2001. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology. 11:25R–36R. [DOI] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW. 2001. Glycosylation of nucleocytoplasmic proteins: Signal transduction and O-GlcNAc. Science. 291:2376–2378. [DOI] [PubMed] [Google Scholar]
- Yang YR, Suh PG. 2014. O-GlcNAcylation in cellular functions and human diseases. Adv Biol Regul. 54:68–73. [DOI] [PubMed] [Google Scholar]
- Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ et al. 2008. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 4:483–490. [DOI] [PubMed] [Google Scholar]
- Zachara NE, Vosseller K, Hart GW. 2011. Detection and analysis of proteins modified by O-linked N-acetylglucosamine. Cur Protoc Mol Biol . Chapter 12:Unit12 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liu TW, Cecioni S, Eskandari R, Zandberg WF, Vocadlo DJ. 2015. O-GlcNAc occurs cotranslationally to stabilize nascent polypeptide chains. Nat Chem Biol. 11:319–325. [DOI] [PubMed] [Google Scholar]