Abstract
The objective of the study was to examine salivary biomarker response to fluid consumption in exercising athletes. Exercise induces stress on the body and salivary alpha amylase (sAA) and salivary cortisol are useful biomarkers for activity in the sympathoadrenal medullary system and the hypothalamic pituitary adrenal axis which are involved in the stress response. Fifteen college students were given 150 ml and 500 ml of water on different days and blinded to fluid condition. The exercise protocol was identical for both fluid conditions using absolute exercise intensities ranging from moderate to high. Saliva was collected prior to exercise, post moderate and post high intensities and analyzed by Salimetrics assays. Exercise was significant for sAA with values different between pre-exercise (85 ± 10 U · ml−1) and high intensity (284 ± 30 U · ml−1) as well as between moderate intensity (204 ± 32 U · ml−1) and high intensity. There was no difference in sAA values between fluid conditions at either intensity. Exercise intensity and fluid condition were each significant for cortisol. Cortisol values were different between pre-exercise (0.30 ± 0.03 ug · dL−1) and high intensity (0.45 ± 0.05 ug · dL−1) as well as between moderate intensity (0.33 ± 0.04 ug · dL−1) and high intensity. Moderate exercise intensity cortisol was lower in the 500 ml condition (0.33 ± 0.03 ug · dL−1) compared with the 150 ml condition (0.38 ± 0.03 ug · dL−1). This altered physiological response due to fluid consumption could influence sport performance and should be considered. In addition, future sport and exercise studies should control for fluid consumption.
Keywords: saliva, biomarkers, exercise, fluid consumption
INTRODUCTION
Exercise produces a stress response activating the sympathoadrenal medullary system (SAM) and the hypothalamic pituitary adrenal axis (HPA) [1–5]. The former has been traditionally measured via serum catecholamine levels and the latter was commonly measured via serum cortisol levels. Salivary biomarkers are becoming a more commonly used method in human physiological research [6, 7]. Salivary cortisol and salivary alpha amylase are such biomarkers used as tools in the examination of human physiological stress response [7–9]. The non-invasive nature of saliva collection and the ease of multiple sampling make it an ideal method of data collection that eliminates confounding stressors such as needles and blood collection [10].
Salivary cortisol has been shown to be highly correlated to unbound serum cortisol and therefore becomes a useful biomarker for HPA activity [6, 7, 11]. Salivary alpha amylase (sAA) has been proposed as a sensitive biomarker for physiological stress that results from increased sympathetic nervous activity [12, 13]. sAA has been shown to increase in response to acute stress and be correlated to serum norepinephrine [13]. Examination of HPA and SAM activity is becoming more common in research examining human physiological stress responses [1]. Commonality of structures between both HPA and SAM leads to expected symmetry in responses [8].
Researchers have examined the stress response of humans to exercise in various situations including pre-exercise (anticipatory), during exercise, and post-exercise. Salivary cortisol and sAA have been demonstrated to respond to an exercise condition. Presumably this response reflects activation of the HPA and SAM axes [6–8, 13]. Steerenberg [14] reported an increase in salivary alpha amylase of triathletes comparing post-race values to pre-race. The increase was identified despite an overall decrease in total salivary protein. Numerous studies have reported a salivary cortisol response to exercise [15, 16]. Butki [17] found an increase in salivary cortisol in response to treadmill running in comparison with resting values in subjects. In a study by Hill [18], serum cortisol levels increased in response to exercise, however, this response differed with differing exercise intensities. The Hill [18] study found a threshold of exercise intensity that led to significant increase on post-exercise salivary cortisol levels compared to baseline values.
The purpose of this study is to examine the salivary alpha amylase and salivary cortisol response to fluid consumption in athletes exercising at moderate and high intensity exercise (to voluntary exhaustion). The fluid amounts chosen for this study, 150 ml and 500 ml of water are controlled representations of the volume of fluid ejected with the former representing two squeezes of a typical water bottle and the latter amount representing the volume of fluid found in a commercially available bottle of water. The expectation is that the two volumes of fluid would result in different degrees of esophageal and/or gastric distention and may lead to a difference in physiological effect. Given the established use of salivary biomarkers as a measure of stress response to exercise, it would be important to examine whether consuming fluid alters this response.
MATERIALS AND METHODS
Fifteen 18-25 year old (20.1 ± 1.4 years, 173.2 ± 7.4 cm, 69.0 ± 8.2 kg) State University of New York at Fredonia male and female college students (7 women, 8 men) were subjects for this study. Subjects were recruited via campus flyer and informational recruitment email sent to campus athletic coaches. The protocol was approved for human subjects by the State University of New York at Fredonia Institutional Human Subject Review Board. The experiments were performed in accordance with the ethical standards of the Helsinki Declaration and participants signed an informed consent form. All subjects were screened by the principle investigator. Subjects were disqualified at the screening for not being on birth control and/ or inability to attend morning only laboratory visits. Ultimately 20 subjects were screened in person and 5 were removed for noncompliance. After the screening visit, participation in the study required three laboratory visits; all visits were completed between 10:00 am and 12:00 pm. The day before each laboratory visit, subjects were instructed to refrain from strenuous exercise, not drink alcoholic beverages, and not eat after midnight.
Screening Laboratory Visit
All female subjects were required to undergo a pregnancy screening (hCG ACON laboratories Inc., San Diego, CA) to assure that only non-pregnant women participated. After ACSM low CVD risk was assessed [19], maximal oxygen consumption was determined. A Modified Bruce Protocol was performed on a Pacer Treadmill. A bonnet style headpiece and mask was used. The mask was attached to the turbine of the Vacumed CPX Mini system (Ventura, California). The Vacumed CPX mini uses a breath by breath analysis and gas volume measurement. Heart rate and RPE was recorded throughout the test. Blood pressure was also recorded until speed became a limiting factor. Heart rate was monitored with a Polar heart rate monitor (Woodbury, NY). Borg Scale RPE was recorded at the end of each stage.
Fluid Ingestion
Subjects arrived at the laboratory between 10:00am and 12:00pm on testing days. This was done to eliminate any potential confounding fluctuations related to circadian rhythm [20, 21]. Fluid conditions were assigned prior to the subjects’ arrival and subjects were blinded to the condition. The bottle preparation was done without the principal investigator in the room and the principal investigator left the room while the subjects drank their bottle of water. Before consuming any fluids a urine sample was collected, naked dry weight was measured as well as the first saliva collection taken. After saliva and urine collection and body mass recording, each subject was instructed to stand on the treadmill and drink from an opaque water bottle. The urine sample was used to determine specific gravity. If the subject's urine specific gravity was 1.020 or higher they were excluded from continuing the study protocol for that day. After meeting the specific gravity requirement, subjects were weighed naked.
Exercise Protocol
The exercise protocol was identical for both fluid conditions and split into two sessions based on absolute intensity. The moderate treadmill session consisted of three 6min stages for a total duration of 18 min in length. The intensities (all at 2% grade) of the stages were: stage 1 at 4.83 km · h−1; stage 2 at 8.05 km · h−1; stage 3 at 9.66 km · h−1.
Heart rates and RPE (Borg Scale 6-20) were recorded at two minutes elapsed and at the end of each stage. Immediately after the last stage the subject left the treadmill and saliva was collected (described later). The subject then started the high intensity to voluntary exhaustion treadmill session. This session consisted of a two minute stage followed by progressively higher intensity 30 s stages. The intensities were 12.07 km · h−1 for 2 min followed by a 0.8 km · h−1 increase for every successive 30 s interval. The intensities of this portion were absolute; however the subject was instructed to complete as many stages as possible, therefore, ending speeds and durations varied. Heart rates and RPE were recorded twice for the initial two minute stage and once for each 30 s stage. Elapsed time and ending speed were recorded for this exercise portion. Subjects were verbally encouraged, additionally, they were encouraged to complete a 30 s stage once it was started. Immediately upon reaching voluntary exhaustion the subject was instructed to leave the treadmill and saliva was collected. It should be noted that under either condition if a subject asked for water during the test they were denied it. This study was conducted as part of a larger study that included 3 cognitive test batteries. The cognitive tests were administered before starting exercise and after both moderate and high intensity time points.
Saliva Collection and Analysis
All subjects arrived at the laboratory between 10:00 am and 12:00 pm EST for their first visit and within 30 min of their first visit time for subsequent visits in order to control for circadian rhythm influences on salivary measures. Saliva samples were collected at pre, intermediate and post exercise. The saliva was ultimately collected into 5 mL polypropylene cryovial. The subjects were instructed to let saliva collect in their mouth and gently push the saliva into a short section of common drinking straw into the cryovial. The saliva collection was timed for two minutes. The saliva was immediately weighed (on a scale tared for weight of an empty cryovial). Salivary samples were then aliquoted into 2 mL polypropylene cryovials, with color coded lids. The samples were stored at −80 degrees Celsius. Each collection would yield four to five aliquots. The aliquots were not equally distributed; the first three aliquots were intended to be sent out for biochemical analysis.
Following Granger et al. [7], alpha amylase assay was completed using a commercially available assay (Salimetrics LLC, State College PA). The enzymatic action of alpha amylase can be spectrophotometrically measured at 405 nm using a micro plate reader. The amount of alpha amylase activity present in the sample is directly proportional to the increase in absorbance at 405 nm.
Duplicate salivary cortisol assays were performed using an enzyme immunoassay (Salimetrics, PA). The test uses 25 ul of saliva per determination, has a lower limit of sensitivity of 0.003 ug · dL−1, standard curve range from 0.012 to 3.0 ug · dL−1, and average intra- and inter-assay coefficients of variation 3.5% and 5.1% respectively. Method accuracy, determine by spike and recovery, and linearity, determined by serial dilution are 100.8% and 91.7%. Values from matched serum and saliva samples show the expected strong linear relationship, r (63) = 0.89, p < 0.0001.
Statistics
Repeated measures general linear model ANOVAs were performed on mean alpha-amylase (U · ml−1) and mean cortisol (ug · dL−1). The within-subject factors were exercise (pre, moderate intensity and high intensity) and fluid condition (150 ml and 500 ml) and the between-subject factor was gender. All interaction effects were evaluated by the models. If significant in the repeated measures general linear model, post-hoc tests were completed for exercise and condition overall and within genders and adjusted for multiple testing. Values are expressed as means ± SE, except for subject characteristics which are mean ± SD. Significance of tests was compared with a significance level of 0.05 except for correction for multiple testing.
RESULTS
Subjects
For the alpha-amylase and cortisol test subjects the overall averages were 20.2 ± 1.5 years old, 172.5 ± 7.4 cm tall, 69.6 ± 7.5 kilograms, 13.1 ± 4.9 percent body fat, and an average VO2max of 57.2 ± 7.8 ml · kg−1 · min−1 (Table 1). The averages for the female subjects were 20.3 ± 1.0 years old, 168.9 ± 4.1 cm tall, 65.0 ± 5.3 kilograms, 15.3 ± 3.7 percent body fat, and an average VO2max of 53.7 ± 5.2 ml · kg−1 · min−1 (Table 1). The averages for the male subjects were 20.0 ± 2.0 years old, 176.8 ± 8.6 cm tall, 75.2 ± 6.0 kilograms, 10.4 ± 5.1 percent body fat, and an average VO2max of 61.4 ± 8.7 ml · kg−1 · min−1 (Table 1).
TABLE 1.
Subject demographic data for alpha-amylase and cortisol tests. Values expressed as means ± SD.
| Age (years) | Height (cm) | Weight (kg) | Body Fat (%) | VO2max (ml/kg/min) | |
|---|---|---|---|---|---|
| Combined | 20.2 ± 1.5 | 172.5 ± 7.4 | 69.6 ± 7.5 | 13.1 ± 4.9 | 57.2 ± 7.8 |
| Female (n = 7) | 20.3 ± 1.0 | 168.9 ± 4.1 | 65.0 ± 5.3 | 15.3 ± 3.7 | 53.7 ± 5.2 |
| Male (n = 8) | 20.0 ± 2.0 | 176.8 ± 8.6 | 75.2 ± 6.0 | 10.4 ± 5.1 | 61.4 ± 8.7 |
Alpha amylase
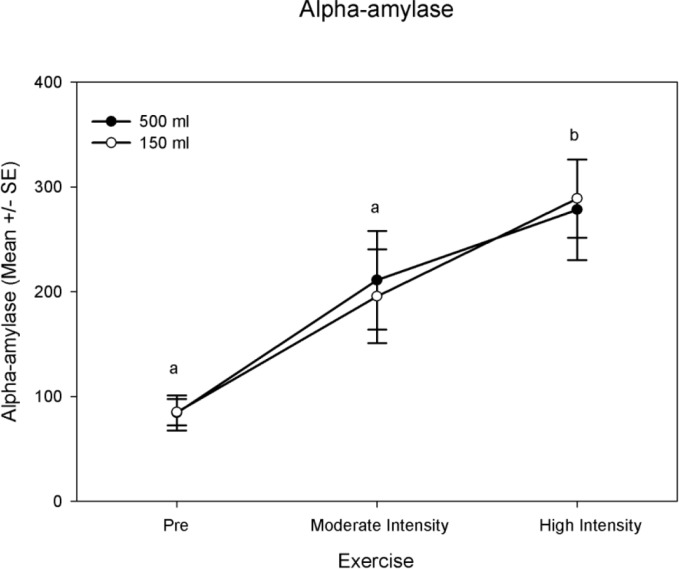
The exercise factor (P = 0.001) was significant for alpha amylase (Table 2, Fig. 1). A post-hoc examination of the exercise factor revealed pre-exercise and high intensity exercise as well as moderate intensity and high intensity exercise to be significantly different in alpha amylase (Table 2, Fig. 1). The alpha amylase mean value for pre-exercise was 85 ± 10 U · ml−1, for moderate intensity exercise was 204 ± 32 U · ml−1, and for high intensity exercise was 284 ± 30 U · ml−1 (Fig. 1). There was a significant interaction effect between gender and fluid condition for alpha amylase (P = 0.001, Table 2). For males, the 500 ml fluid condition mean was greater than the 150 ml condition mean (235 ± 48 U · ml−1 and 198 ± 42 U · ml−1respectively). For females, the 500 ml condition mean was less than the 150 ml condition mean (145 ± 22 U · ml−1 and 177 ± 24 U · ml−1 respectively).
TABLE 2.
Significant results from repeated measures general linear models for salivary alpha-amylase and salivary cortisol.
| Salivary Variable | F | Df | Sig |
|---|---|---|---|
| Alpha-amylase | |||
| Exercise | 15.45 | 1.519* | P = 0.001 |
| Fluid Condition*Gender | 27.34 | 1 | P = 0.001 |
| Cortisol | |||
| Exercise | 19.87 | 2 | P < 0.0005 |
| Fluid Condition | 5.57 | 1 | P = 0.043 |
Note: Within-subject factors were exercise (pre, moderate intensity and high intensity exercise) and condition (fluid intervention). Between-subject factor was gender. All interaction effects were evaluated by the models. The post-hoc tests are compared with a corrected α level of 0.016 to account for multiple testing.
df is based on Huynh-Feldt correction for lack of sphericity
FIG. 1.
Alpha-amylase mean values (U · ml−1) at pre-exercise, moderate intensity exercise, and high intensity exercise between fluid condition.
Exercise intensity not sharing a common letter are different (P< 0.05). Fluid condition not sharing a common symbol is different (P< 0.05). Significance is based on P < 0.05 unless corrected for multiple testing.
Cortisol
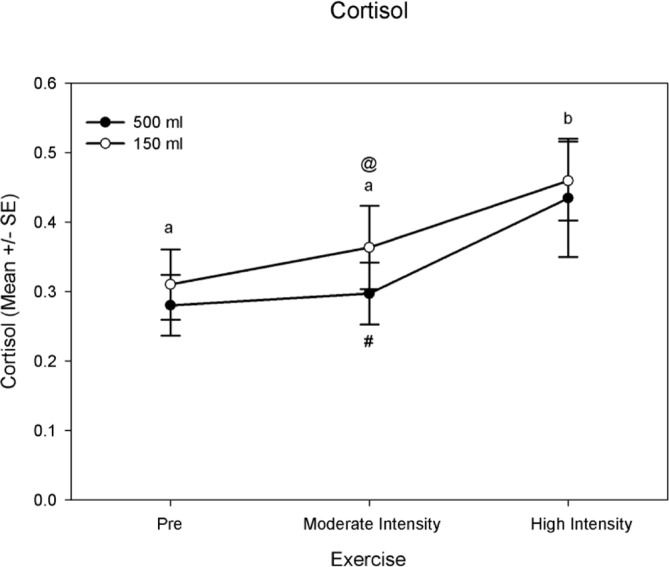
The exercise (P < 0.0005) and fluid condition factor (P = 0.043) were each significant for cortisol (Table 2, Fig. 2). A post-hoc examination of the exercise factor revealed pre-exercise and high intensity exercise as well as moderate intensity and high intensity exercise to be significantly different in cortisol. The cortisol mean
FIG. 2.
Cortisol mean values (ug · dL−1) at pre-exercise, moderate intensity exercise, and high intensity exercise between fluid condition. Exercise intensity not sharing a common letter are different (P< 0.05) Fluid condition not sharing a common symbol are different (P< 0.05) Significance is based on P < 0.05 unless corrected for multiple testing
value for pre-exercise was 0.30 ± 0.03 ug · dL−1, for moderate intensity exercise was 0.33 ± 0.04 ug · dL−1, and for high intensity exercise was 0.45 ± 0.05 ug · dL−1 (Fig. 2). Moderate intensity exercise revealed a significant difference in fluid condition. (Table 2, Fig. 2). The cortisol mean value for the 500 ml fluid condition was 0.33 ± 0.03 ug · dL−1, and for the 150 ml condition was 0.38 ± 0.03 ug · dL−1.
DISCUSSION
Salivary alpha amylase and salivary cortisol have been investigated and identified as biomarkers of physiological stress [1]. Increased activity of, or stress on, the sympathetic adrenomedullary system (SAM) has been demonstrated by increased levels of salivary alpha-amylase [9]. The glucocorticoid hormone cortisol appears in saliva and indicates stress on, or increased activity of, the hypothalamus pituitary adrenocortical axis [20].
Physiological stressors are correlated well with increased salivary alpha amylase appearance [1, 7]. There was a significant difference in salivary alpha amylase between pre-exercise and high intensity exercise (mean difference of 119 U · ml−1). There was also a significant difference in salivary alpha amylase at moderate intensity exercise and high intensity exercise (mean difference of 199 U · ml−1). This significant rise was irrespective of fluid amount. This supports previous work that demonstrated an increase in salivary alpha amylase with increased physiological stress [7, 22, 23]; furthermore, exercise (moderate and high intensity) elicited heart rates over 100 bpm. This increase in heart rate is largely due to increased sympathetic drive which could account for the increased production and appearance of alpha amylase in saliva [24, 25].
A previous study by Longhurst [26] found that gastric distension in cats elicited a sympathoadrenal hemodynamic response. The lack of difference of a salivary alpha amylase response between fluid amounts and the clear effect of exercise on salivary alpha amylase may be accounted for by several different reasons. The two fluid amounts may not have been different enough to elicit a different sAA response. Or the absolute fluid volume may not have been enough to produce a stimulatory amount of gastric distention. It is also possible that the SAM axis response to the exercise is greater in magnitude therefore would mask any response caused by esophageal and/ or gastric distention. There may be a gender difference in salivary alpha amylase response to fluid (as indicated by our significant fluid and gender interaction) which could influence an overall fluid response.
Similar to salivary alpha amylase, there was an effect of exercise intensity on the appearance of cortisol in saliva when comparing high intensity exercise point to the pre-exercise values (mean difference of 0.15 ug · dL−1). This difference was irrespective of fluid condition. This result is supported by previous work that reported an increase in salivary cortisol levels to physiological stress [16, 27]. Our data suggest agreement with Hill's threshold effect [18] as evidenced by the increased rate of change of salivary cortisol between the moderate and high exercise intensity time points (mean difference of 0.12 ug · dL−1).
Fluid amount had an effect on salivary cortisol at the moderate exercise time point. The 150 ml fluid amount elicited a significantly higher salivary cortisol response than the 500 ml fluid amount (mean difference of 0.05 ug · dL−1). This difference in cortisol between fluid conditions was no longer seen at the high exercise intensity time point. Since at the termination of exercise there was no longer a difference we concluded that the higher intensity exercise had a greater effect on cortisol than the fluid condition effect. Our data suggests a greater rate of change in salivary cortisol between the moderate and high exercise intensity time points within the 500 ml fluid condition.
It is meaningful to examine the lack of cortisol response in the 500 ml fluid condition at the moderate exercise intensity time point. The exact mechanism the fluid amount may have played is unclear. It is possible that the difference between fluid amounts (350 ml) was enough to blunt the salivary cortisol response to the moderate intensity exercise. Alternatively, the 500 ml of fluid consumed (especially a hypotonic fluid such as water) disrupted fluid and electrolyte homeostasis. Noakes et al. [28] reported hyponatremia in healthy subjects who consumed volumes of hypotonic fluid in excess of urine production rates (approximately 900ml · hr−1). Our fluid amount of 500 ml given prior to exercise may have been large enough to disrupt fluid homeostasis. It is also possible that esophageal or gastric distention caused by the volume stimulated a mechanism in anticipation of a fluid and/or electrolyte disruption.
Our study employed a computer based cognitive test (data not shown) that was administered immediately prior to starting exercise and immediately after each exercise time point. It has been demonstrated, as seen with the Kirschbaum [20] study, that the anticipation of cognitive test batteries can present a psychological stress that could result in salivary cortisol response. In our study however, both of our fluid conditions included the same cognitive test batteries administered in an identical fashion. Therefore, any difference in salivary measures could not be attributed to the cognitive tests thus removing it as a confounding factor.
The impact of gender on salivary alpha amylase should be further examined with larger sample sizes than our study. Future research should examine the role of enteric nervous system monitoring mechanical, osmotic, and thermal changes of the gastrointestinal system in exercising humans and humans anticipating exercise. In our study there was a significant impairment to exercise performance (unpublished data) in the 500 ml condition. Seemingly it would be important to investigate how the consumption of a small amount of fluid may impact the stress response. This work would also call into question adherence to following pre-exercise hydration guidelines.
CONCLUSIONS
In conclusion we demonstrated a stress response in salivary alpha amylase and salivary cortisol to exercise. This salivary cortisol response was influenced by the consumption of 500 ml of fluid. The altered salivary cortisol response could indicate the impact of fluid consumption on physiological responses to exercise and presumably influence sport performance. Additionally given our results, future studies should control for fluid consumption in their subjects. This study is novel in revealing the differing impact of fluid consumption prior to exercise on the human stress biomarker of salivary cortisol.
Acknowledgements
The authors thank the following Fredonia Exercise Science students for their research efforts: Timothy Carrow, Lauren Fial, Robert Newton, and Amanda Richards.
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Frankenhaeuser M, Lundberg U. Sympathetic-adrenal and pituitaryadrenal response to challenge. In: Pichot P, editor. Biological Psychiatry, Higher Nervous Activity. New York: Springer; 1985. pp. 699–704. [Google Scholar]
- 2.Hackney AC. Exercise as a stressor to the human neuroendocrine system. Medicina-Kaunas. 2006;42(10):788–97. [PubMed] [Google Scholar]
- 3.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 4.McCarty R, Horwatt K, Konarska M. Chronic stress and sympathetic-adrenal medullary responsiveness. Soc Sci Med. 1988;26(3):333–341. doi: 10.1016/0277-9536(88)90398-x. [DOI] [PubMed] [Google Scholar]
- 5.Steadman RT, Sharkey BJ. Exercise as a stressor. J Sport Med Phys Fit. 1969;9(4):230. [PubMed] [Google Scholar]
- 6.Chiappin S, Antonelli G, Gatti R, De Palo I. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007;383:30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary α-amylase in biobehavioral research: recent developments and applications. Ann NY Acad Sci. 2007;1098(1):122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- 8.Chicharro JL, Lucia A, Perez M, Vaquero AF, Urena R. Saliva composition and exercise. Sports Med. 1998;26:17–27. doi: 10.2165/00007256-199826010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Riad-Fahmy D, Read GF, Walker RF. Salivary steroid assays for assessing variation in endocrine activity. J Steroid Biochem. 1983;19(1):265–272. [PubMed] [Google Scholar]
- 10.Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrino. 2009;34(4):486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Aryeh H, Roll N, Lahav M, Dlin R, Hanne-Paparo N, Szargel R, et al. Effect of exercise on salivary composition and cortisol in serum and saliva in man. J Dent Res. 1989;68:1495–1497. doi: 10.1177/00220345890680110501. [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy B, Dillon E, Mills PJ, Ziegler MG. Catecholamines in human saliva. Life Sci. 2001;69(1):87–99. doi: 10.1016/s0024-3205(01)01111-0. [DOI] [PubMed] [Google Scholar]
- 14.Steerenberg PA, Asperen IA, Amerongen AN, Biewenga J, Mol D, Medema G. Salivary levels of immunoglobulin A in triathletes. Eur J Oral Sci. 1997;105(4):305–309. doi: 10.1111/j.1600-0722.1997.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 15.Azarbayjani MA, Dalvand H, Fatolahi H, Hoseini SA, Farzanegi P, Stannard SR. Responses of salivary cortisol and α-amylase to official competition. J Hum Sport Exer. 2011;6(2):385–391. [Google Scholar]
- 16.Rojas Vega S, Strüder HK, Wahrmann BV, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121(1):59–65. doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- 17.Butki BD, Rudolph DL, Jacobsen H. Self-efficacy, state anxiety, and cortisol responses to treadmill running. Percept Motor Skill. 2001;92(3):1129–1138. doi: 10.2466/pms.2001.92.3c.1129. [DOI] [PubMed] [Google Scholar]
- 18.Hill EE, Zach E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008;31(7):587–591. doi: 10.1007/BF03345606. [DOI] [PubMed] [Google Scholar]
- 19.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 480. [Google Scholar]
- 20.Kirschbaum C, Wüst S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54(6):648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: A better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20(6):329–335. doi: 10.1177/000456328302000601. [DOI] [PubMed] [Google Scholar]
- 22.Kivlighan KT, Granger DA. Salivary α-amylase response to competition: Relation to gender, previous experience, and attitudes. Psychoneuroendocrino. 2006;31(6):703–714. doi: 10.1016/j.psyneuen.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Li T, Gleeson M. The effect of single and repeated bouts of prolonged cycling and circadian variation on saliva flow rate, immunoglobulin A and alpha-amylase responses. J Sport Sci. 2003;22(1112):1015–1024. doi: 10.1080/02640410410001716733. [DOI] [PubMed] [Google Scholar]
- 24.Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, et al. Stress-induced changes in human salivary alpha-amylase activity— associations with adrenergic activity. Psychoneuroendocrino. 2006;31(1):49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Walsh NP. The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J Sport Sci. 1999;17(2):129–134. doi: 10.1080/026404199366226. [DOI] [PubMed] [Google Scholar]
- 26.Longhurst JC, Ibarra JL. Sympathoadrenal mechanisms in hemodynamic responses to gastric distension in cats. Am J Physiol-Heart C. 1982;243:H748–H753. doi: 10.1152/ajpheart.1982.243.5.H748. [DOI] [PubMed] [Google Scholar]
- 27.Edwards D, Wetzel K, Wyner D. Intercollegiate soccer: Saliva cortisol and testosterone are elevated during competition, and testosterone is related to status and social connectedness with teammates. Physiol Behav. 2006;87:135–147. doi: 10.1016/j.physbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Noakes TD, Wilson G, Gray DA, Lambert MI, Dennis SC. Peak rates of diuresis in healthy humans during oral fluid overload. S Afr Med J. 2001;91(10):852–857. [PubMed] [Google Scholar]