Abstract
Recent reports suggest that hypovitaminosis D in athletes is as common as in the general population. This study was devised to examine vitamin D status and determinants of deficiency in athletes living in a sunny country (Tunisia). One hundred and fifty national elite athletes, training outdoors (n = 83) or indoors (n = 67), were enrolled from January to February 2012. Plasma 25-hydroxyvitamin D was measured by radioimmunoassay. Concentrations were between 50 and 75 nmol · l-1 in 21.3% of participants, between 25 and 50 nmol · l-1 in 55.3% of participants and <25 nmol · l-1 in 14.7% of participants. The concentrations were significantly lower in indoor athletes than outdoor athletes (36.2±19.0 nmol · l-1 vs. 49.1±19.2 nmol · l-1; p < 0.001). In multivariate analysis, vitamin D deficiency (25-hydroxyvitamin D <50 nmol · l-1) was associated with indoor sports [multi-adjusted odds ratio (95% confidence interval), 5.03 (1.64-15.4); p = 0.005], female gender [3.72 (1.44-9.65); p = 0.007] and age < 18 years [2.40 (1.01-5.85); p = 0.05]. Athletes living in sun-rich environments are exposed to a high risk of vitamin D inadequacy. Given the importance of vitamin D in health and athletic ability, targeting sufficient levels of plasma 25-hydroxyvitamin D in athletes is well justified.
Keywords: cholecalciferol, hypovitaminosis D, sun exposure, vitamin D inadequacy
INTRODUCTION
Vitamin D plays an important role in optimizing health and preventing disease [1]. Emerging evidence suggests that hypovitaminosis D is widespread worldwide, affecting different age groups and demographics [2–4]. Due to its key role in the musculoskeletal system, immune function and inflammatory response, vitamin D levels may directly affect an athlete's overall health and athletic ability [5–9]. During recent years, some studies have examined vitamin D status in athletes. The available data show that vitamin D inadequacy ranges from 16% to 100%, varying according to latitude, season, gender, type of sport and socio-cultural and ethnic factors [10–16]. Vitamin D is mainly produced endogenously following incidental skin exposure to ultraviolet B irradiation with little derived from dietary sources [1, 17]. In this regard, elite Tunisian athletes, who live in an environment with a high potential for sun exposure and who benefit from sustained nutritional support, are expected to achieve adequate vitamin D status. However, this condition could be influenced by factors such as season, duration/timing of exposure to sunlight, skin colour, amount/type of clothing, sun block usage, setting of training, body composition, diet and supplement use. The present study aimed to examine vitamin D status among young elite Tunisian athletes during the winter season and to identify groups with increased risk of vitamin D deficiency (VDD). Particular attention was paid to the role of sun exposure in the development of vitamin D status.
MATERIALS AND METHODS
Study design and participants. This cross-sectional study included 150 athletes, 57 females and 93 males aged 15 to 26 years. Athletes were enrolled from the Elite Sport Centres of Kairouan (latitude, 33.2° N) and Tunis (latitude, 36.5° N) (Tunisia) from January to February 2012. All participants were identified as being non-smokers and free from illness or current injury. Athletes who admitted having taken vitamin D supplements, fish oils or multivitamins during the past year were excluded. Athletes were divided into two groups according to the mode of training (indoors/outdoors) as a marker for sun exposure. Outdoor sports were athletics (n = 83), while the indoor sports were judo (n = 31), karate (n = 21), boxing (n = 6) and fencing (n = 9). Information on demographic characteristics, lifestyle factors, athletic activity, dietary intake and supplements or medication use was collected. Athletes underwent a brief clinical examination including blood pressure measure. Weight and height were measured with athletes barefooted and lightly clothed, and body mass index (BMI) was calculated. Biceps, triceps, sub-scapular and supra-iliac skin folds thickness were measured using skin fold callipers by experienced staff. Body density was calculated using the sum of the four skin folds, and fat mass relative to total body weight was calculated using the Siri formula [18]. Athletes’ skin colour was classified by the study staff as fair, brown or dark. Dietary intake data were collected by means of a nutritionist-administered three-day diet history method. Energy and nutrient intakes were calculated using a computer program (Nutri Pro 7 software, CERDEN, Brussels, Belgium).
Written informed consent was obtained from all the adult participants and the parents of adolescent players after verbal and written explanation of the experimental design and potential risks of the study. The study was conducted according to the Declaration of Helsinki, and the protocol was fully approved by the Ethics Committee of Rabta Hospital before commencement of the assessments.
Biochemical analyses
Blood samples were collected following an overnight fast. Plasma 25-hydroxyvitamin D (25-OHD) was measured by a manual competitive radioimmunoassay method using a commercial kit (DiaSorin, Stillwater, Minnesota). The method used 125I-labelled 25-OHD, an antibody to 25-OHD and a second antibody for precipitation. This assay method allows the combined measurement of both 25-OHD3 and 25-OHD2 with a sensitivity of 3 nmol · l-1 and the imprecision less than 10%. Following international guidelines [7, 19] vitamin D inadequacy, deficiency and severe deficiency were defined by plasma 25-OHD concentrations < 75, < 50 and < 25 nmol · l-1, respectively.
Statistical analysis
Statistical analysis of the data was conducted using SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA). The Gaussian distribution of continuous variables was verified. Descriptive statistics were performed and values are shown as mean (standard deviation) for continuous variables and as percent for categorical variables. Comparisons between groups were performed with Student's t-test for independent samples for continuous variables, and χ2 tests for categorical variables. Mantel-Haenszel odds ratios with 95% confidence intervals were calculated as estimates of relative risk of VDD for several potential risk factors. A backward binary logistic regression model was used to investigate risk factors for VDD, whilst adjusting for possible confounding factors. The adjustment was performed for gender, age (<18 years/ ≥ 18 years), mode of training (outdoors/indoors), skin colour (fair/brown/dark), and dichotomous variables for fat mass and vitamin D and calcium dietary intake, defined as the respective continuous variables split at the median. The fit of logistic models was satisfactory (Hosmer and Lemeshow, χ2, 6.777; p = 0.561). P values < 0.05 were considered statistically significant.
RESULTS
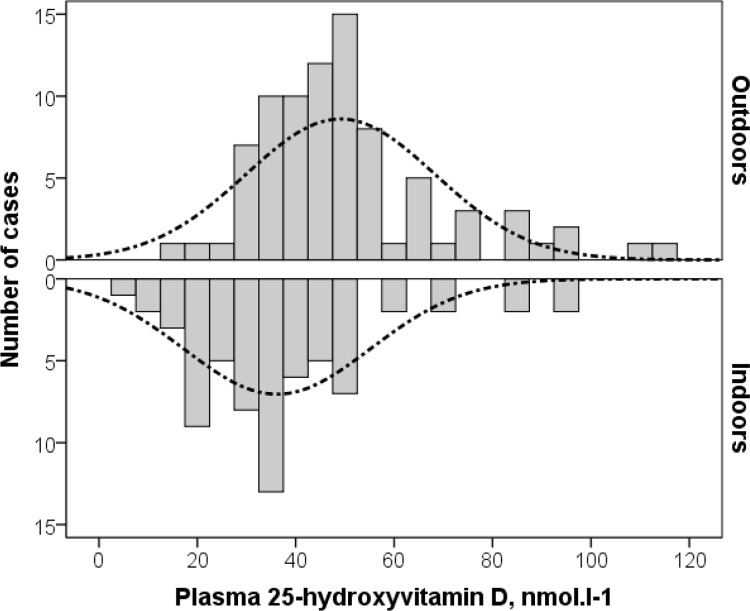
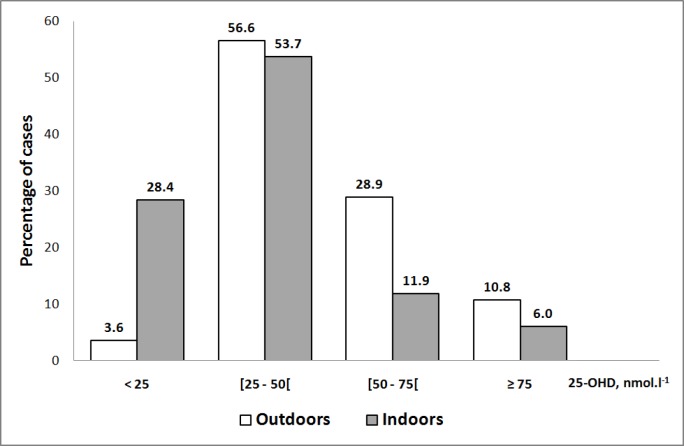
Table 1 shows the main clinical, nutritional and biological characteristics according to the mode of training (indoors/outdoors). Athletes exercising mainly indoors showed higher BMI, fat mass and dietary intake, but lower plasma creatinine concentrations than athletes training outdoors. Plasma 25-OHD varied from 5 to 115 nmol · l-1 with an average of 43.3 nmol · l-1 and was significantly lower in athletes participating in indoor sports (figure 1). The concentrations were between 50 and 75 nmol · l-1 in 21.3% of all athletes, between 25 and 50 nmol · l-1 in 55.3% and less than 25 nmol · l-1 in 14.7%. Only 8.7% of tested athletes had the recommended plasma vitamin D status (25-OHD > 75 nmol · l-1). Indoor trained athletes exhibited significantly higher prevalence of severe vitamin D deficiency (28.4% vs. 3.6%; p < 0.001) as well as VDD (82.1% vs. 60.2%; p = 0.004) than outdoor trained athletes (figure 2). Univariate analysis suggested that VDD was more frequent in females, athletes training indoors, those less than eighteen years old and those with estimated percentage fat mass over 15%. VDD was associated neither with BMI or skin colour, nor with dietary intake of vitamin D or calcium (Table 2). Multivariate analysis showed that indoor sports [multi-adjusted odds ratio (95% confidence interval), 5.03 (1.64-15.4); p = 0.005], female gender [3.72 (1.44-9.65); p = 0.007] and age less than 18 years [2.47 (1.01-6.24); p = 0.05] are independent risk factors for VDD in these athletes.
TABLE 1.
Main characteristics of athletes according to the mode of training (indoors/outdoors)
| Outdoor sports (n = 83) |
Indoor sports (n = 67) |
P | |
|---|---|---|---|
| Sex-ratio (males/females) | 45/38 | 43/24 | 0.218 |
| Age, years | 17.7 ± 1.37 | 18.3 ± 2.61 | 0.08 |
| Systolic blood pressure, mm Hg | 113 ± 10.1 | 112 ± 10.4 | 0.505 |
| Diastolic blood pressure, mm Hg | 69.6 ± 8.71 | 68.0 ± 9.81 | 0.329 |
| Body mass index, Kg·m-2 | 20.8 ± 2.44 | 23.3 ± 4.33 | < 0.001 |
| Fat mass,% Males | 12.4 ± 4.18 | 15.7 ± 4.10 | < 0.001 |
| Females | 20.0 ± 4.44 | 22.5 ± 4.48 | 0.012 |
| Duration of sport training, years | 4.57 ± 1.87 | 7.37 ± 3.59 | < 0.001 |
| Training volume per week, hours | 12.9 ± 1.70 | 14.4 ± 3.31 | 0.001 |
| Total energy intake, calories·day-1 | 2677 ± 847 | 3060 ± 831 | 0.007 |
| Protein dietary intake, g·day-1 | 92.8 ± 34.1 | 108 ± 33.5 | 0.006 |
| Fat dietary intake, g·day-1 | 104 ± 45.5 | 117 ± 36.9 | 0.048 |
| Carbohydrate dietary intake, g·day-1 | 343 ± 105 | 392 ± 113 | 0.008 |
| Calcium dietary intake, mg·day-1 | 1114 ± 430 | 1312 ± 684 | 0.036 |
| Phosphorus dietary intake, mg·day-1 | 1450 ± 530 | 1712 ± 611 | 0.007 |
| Vitamin D dietary intake, µg·day-1 | 9.67 ± 4.78 | 10.9 ± 7.38 | 0.267 |
| Total cholesterol, mmol·l-1 | 3.89 ± 0.68 | 3.79 ± 0.56 | 0.342 |
| HDL cholesterol, mmol·l-1 | 1.24 ± 0.26 | 1.20 ± 0.22 | 0.282 |
| Triglycerides, mmol·l-1 | 0.76 ± 0.29 | 0.77 ± 0.35 | 0.847 |
| Total protein, mg·dl-1 | 735 ± 32.0 | 727 ± 62.1 | 0.337 |
| Creatinine, µmol·l-1 | 646 ± 77.8 | 602 ± 124 | 0.008 |
| Calcium, mmol·l-1 | 2.38 ± 0.15 | 2.35 ± 0.14 | 0.227 |
| Phosphorus, mmol·l-1 | 1.10 ± 0.10 | 1.12 ± 0.14 | 0.239 |
| Magnesium, mmol·l-1 | 0.91 ± 0.09 | 0.92 ± 0.07 | 0.486 |
FIG. 1.
Comparative distribution of plasma 25-hydroxyvitamin D in indoor and outdoor athletes
FIG. 2.
Prevalence of vitamin D status classes according to the mode of training (indoors/outdoors)
TABLE 2.
Plasma vitamin D and multi-adjusted odds ratio for vitamin D deficiency according to various potential determinants of vitamin D status
| N | 25hydroxyvitamin D | Vitamin D deficiency a | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD), nmol.l1 | P | % | ORb (95% CI) | P | |||
| Training | Outdoor | 83 | 49.1 (19.2) | <0.001 | 60.2 | - | 0.005 |
| Indoor | 67 | 36.2 (19.0) | 82.1 | 5.03 (1.64-15.4) | |||
| Gender | Male | 93 | 46.5 (18.6) | 0.013 | 62.4 | - | 0.007 |
| Female | 57 | 38.2 (21.6) | 82.5 | 3.72 (1.44-9.65) | |||
| Age | ≥ 18 years | 81 | 46.2 (22.9) | 0.045 | 63.3 | - | 0.050 |
| < 18 years | 69 | 39.6 (16.2) | 80.6 | 2.47 (1.01-6.24) | |||
| Skin colour | Fair | 75 | 45.4 (21.1) | 0.261 | 68.4 | - | |
| Brown | 71 | 41.8 (19.3) | 70.0 | 1.20 (0.48-2.99) | 0.696 | ||
| Dark | 4 | 31.2 (10.3) | 100 | unfeasible* | - | ||
| Fat mass | < 15% | 75 | 47.8 (20.1) | 0.006 | 62.7 | - | 0.685 |
| ≥ 15% | 75 | 38.8 (19.3) | 77.3 | 1.26 (0.41-3.92) | |||
| Vitamin D intake | < 9,30 µg/day | 75 | 44.2 (20.7) | 0.977 | 70.2 | - | 0.840 |
| ≥ 9,30 µg/day | 75 | 44.1 (16.6) | 67.9 | 0.91 (0.36-2.30) | |||
| Calcium intake | < 1190 mg/day | 75 | 44.1 (19.0) | 0.416 | 69.4 | - | 0.592 |
| ≥ 1190 mg/day | 75 | 41.4 (20.4) | 71.8 | 1.29 (0.513.22) | |||
Note: N, Number; SD, standard deviation; OR, odd-ratio; 95% CI, 95% confidence interval; a, 25-OH vitamin D < 50 nmol.l-1; b, multi-adjusted OR: adjustment on gender, age group (<18 years/ ≥ 18 years), mode of training (outdoors/indoors), skin colour (fair/brown/dark) and dichotomous variables for fat mass and vitamin D and calcium dietary intake defined as the respective continuous variables split at the median);
OR for vitamin D deficiency cannot be calculated as all four of the dark skinned athletes were classified as vitamin D deficiency.
DISCUSSION
The present study conducted among young Tunisian athletes from various sports disciplines demonstrated an extremely high prevalence (over 90%) of vitamin D inadequacy. This finding is surprising for young, healthy athletes living at a latitude where endogenous vitamin D synthesis is possible throughout the year. However, it fits well with the high prevalence found in athletes living in similar latitudes [11–13, 20]. Most previous studies were carried out in athletes from the developed world and few studies were made in athletes living in the Middle East. The prevalence of vitamin D inadequacy is often high in athletes, but varies according to combined factors including gender, season, setting of training, and ethnic and cultural factors. Overall, vitamin D insufficiency/deficiency is common when the blood is drawn in the cold season and when the athlete is female, training indoors, has dark pigmentation and is short of sun exposure. Table 3 presents the currently available data on vitamin D status in athletic populations worldwide.
TABLE 3.
Reported vitamin D status in athletic populations worldwide
| First author, year [reference] | Country, latitude | Number (M/F); Age, years (Indoors/Outdoors) | Month/Season | Plasma 25-hydroxyvitamin D, nmol·l-1 | |||
|---|---|---|---|---|---|---|---|
| Mean | < 75 | < 50 | < 25 | ||||
| Lehtonen-Veromaa, 1999 [10] | Finland, 60° N | 131 F; 9-15 y (66/65) | Winter | 33.9 | - | 67.7% a | 13.4% b |
| Magee, 2013 [23] | Ireland, 51-56° N | 75 M/9 F; 22-30 y (17/67) | November/March | 48.4 M | 55% | - | - |
| Wilson, 2012 [25] | UK, 53° N | 36 M; 25 ± 5.0 y (0/36) | Winter | 36.5* | - | 77.8% | 47.2% |
| Morton, 2012 [14] | UK, 53°N | 20 M; 26 ± 4 y (0/20) | December | 51.0 | - | 65% | - |
| August | 104 | - | 0% | - | |||
| Close, 2013 [15] | UK, 53° N | 61 M; 15-30 y (0/61) | Winter | 41.0* | - | 62% | 34.4% c |
| Close, 2013 [22] | UK, 53°N | 30 M; 21 ± 2 y (0/30) | Winter | 52.0 | 57% | 20% | - |
| Wolman, 2013 [24] | UK, 53° N | 6 M/13 F; 26 ± 8.8 y (19/0) | Winter | 37.3 | 100% | - | 26.3% |
| Summer | 59.8 | 84.2% | 10.5% | ||||
| Bescos-Garcia, 2011 [20] | Spain, 41° N | 21 M; 25 ± 4.3 y (21/0) | August to May | 47.8 | 90.5% | 57% | 9.5% |
| Halliday, 2011 [21] | USA, 41° N | 41 M/F; > 18 y (12/29) | Winter | 76.2 | 60.6% | 3.0% | - |
| Spring | 105 | 16.0% | 4.0% | - | |||
| Present study | Tunisia, 33-36° N | 89 M/57 F; 15-26 y (83/67) | Winter | 43.3 | 91,8% | 73% | 15% |
| Lovell, 2008 [11] | Australia, 35° S | 18 F; 10-17 y (18/0) | Winter (May) | 56.0 | 83.3% | 33.3% | 5.6% c |
| Constantini, 2010 [12] | Israel, 31° N | 52 M/46 F; 10-30 y (77/21) | Winter/Summer | 63.2 | 73.5% | 25.5% | 6.1% a |
| Willis, 2012 [8] | USA, 30° N | 19 M/F; 19-45 y (0/19) | Not stated | 96.2* | 42% | 10.5% | - |
| Hamilton, 2010 [13] | Qatar, 25° N | 93 M; 13-45 y (0/93) | Summer | - | 100% | 91.4% | 58.1% |
| Hamilton, 2014 [16] | Qatar, 25° N | 274 M; 24.4 ± 8.3 (0/274) | Summer | 51.8 | 84% | 55.5% | 12% |
As expected, our study showed that vitamin D insufficiency/deficiency is noticeably more frequent among athletes training mainly indoors than those involved in outdoor sports. A study conducted on 98 young athletes also living in a sunny country (Israel, 31.8°N), showed a prevalence of vitamin D inadequacy of 80% in indoor training athletes compared to 48% in outdoor training athletes [12]. The present study revealed that vitamin D inadequacy is also very common in outdoor athletes. This finding is somewhat surprising in athletes training outdoors for 10 to 15 hours weekly in latitudes where vitamin D endogenous synthesis is possible almost all the year. However, it may be understandable for many reasons. Firstly, blood samples were collected during winter when the sky is partly cloudy and the athletes wear extensive clothing due to the cool environmental temperatures. It has been clearly demonstrated that plasma 25-OHD concentrations vary noticeably according to the seasons, with a nadir during winter in the Northern hemisphere [24, 26, 27]. Secondly, training sessions were held in the early mornings and late afternoons when the sun's rays are less efficient for vitamin D synthesis [27]. Thirdly, while not properly assessed, it is our observation that these athletes, like most people living in hot and sunny environments, consciously avoid exposure to sunlight during the midday hours when vitamin D synthesis is most effective [1]. Finally, inactivation of pre-vitamin D and vitamin D following excessive exposure to intense sunlight during the summer [17, 28] could also contribute to hypovitaminosis D during the winter. In a group of 324 Qatar-based footballers (25°N), Hamilton et al. [16] observed that footballers from Africa, the Gulf Countries, Persia and other Middle Eastern countries had significantly lower 25-OHD levels than European/ American and Asian players. They speculate that this predominantly reflects culturally specific sun exposure practices, compliance with supplementation practices and the dark skin colour of African and Middle Eastern players.
Skin colour is thought to be a key factor that determines vitamin D status [4]. Dark-skinned individuals require greater UV doses than light-skinned ones for comparable vitamin D biosynthesis [17]. In the present study, we did not observe a significant trend for darker skin to be associated with VDD. The lack of significance may be due to the rough estimation of skin colour in this study. Also, other sun exposure related factors (i.e. duration/timing of sun exposure, amount/type of clothing and sun block use) could interfere with the relationship. Hence, the impact of skin colour on 25-OHD concentration in these athletes requires further evaluation.
Obesity and fat mass excess are considered to be risk factors for hypovitaminosis D [29, 30]. Low plasma 25-OHD levels in obese subjects are likely due to a sequestration/lack of mobilization of this fat-soluble vitamin by inflated adipose tissue [31]. In our study, VDD was associated with a higher fat mass, but the relationship lost its significance in multivariate analysis. This disparity probably resulted from the confounding effect of gender and mode of training rather than from excess body fat per se. While none of our athletes would be considered obese, fat mass was obviously greater in females than males and in our indoor compared with outdoor trained subjects. On the other hand, too little fat mass may also result in low vitamin D status. In normal conditions, an excess of pre-vitamin D synthesized during the summer is stored in adipose tissue to be released into the circulation during the winter [17]. Due to regular and intense physical activity, most athletes have little fat mass and consequently have limited vitamin D storage capacity [32].
Low dietary intake is an established risk factor for vitamin D inadequacy. Hypovitaminosis D can be found despite seemingly sufficient sun exposure [13, 33, 34], suggesting that a concomitant proper dietary intake is required. Dietary analyses revealed that over 80% of Tunisian athletes ingested a lower food-related vitamin D intake than the Institute of Medicine Recommended Dietary Allowance of 600 IU [35]. No athlete reached the daily intake of 1500–2000 IU/day recommended by the Endocrine Society [19] despite having the benefit of sustained nutritional support. Our lack of association between estimated vitamin D intake from foods and plasma 25-OHD concentrations is not surprising given that most circulating 25-OHD originates from endogenous synthesis rather than diet [17, 27]. Foods naturally rich in or enriched with vitamin D (e.g. oily fish, fortified dairy products and juices) are costly and not often consumed by Tunisian athletes. Consequently, a lack of vitamin D in the diet may well be an additional factor that increases the risk of vitamin D inadequacy in these athletes.
The present study showed that the risk of VDD was about twice as great in athletes aged less than 18 years compared with older athletes. Hamilton et al. [13] also found that athletes with VDD were younger than the group with higher 25-OHD concentrations. Lower vitamin D status in younger athletes is likely related to an accelerated growth rate with a higher vitamin D demand throughout puberty [36, 37]. Hypovitaminosis D was more frequent in our female athletes, which agrees with other studies in this area [12, 33, 38]. These data could be explained by a greater fat mass and a widespread use of sun block in females compared with male subjects. Differences in the hormonal milieu between genders may also affect transport proteins and enzymes involved in vitamin D metabolism.
The present study has some limitations. Firstly, factors such as sun exposure duration and timing, precise skin colour, clothing amount and type, and sun block application frequency and type were not properly recorded. Secondly, seasonal variations were not assessed due to the cross-sectional design of the study. The collected data may be only relevant for the winter season, and VDD might not turn out to be as common throughout the year in this group of athletes. However, we provide novel data demonstrating a high rate of vitamin D inadequacy in elite Tunisian athletes during winter. The risk of hypovitaminosis D is even greater for younger athletes, female athletes, and those training indoors.
CONCLUSIONS
Tunisian athletes are exposed to a high risk of hypovitaminosis D despite living in a sun-rich environment. Even if sunlight determines the main source of vitamin D in the body, these athletes may well not receive adequate sunlight exposure due to cultural and ethnic factors. Given the accumulating evidence implicating vitamin D in maintaining health and optimal functioning of skeletal muscle, it is important that young athletes have appropriate circulating 25-OHD concentrations. Routine monitoring of athletes would include the assessment of plasma 25-OHD during the winter season. Management of hypovitaminosis D should include education regarding safe and efficient sunlight exposure, intake of vitamin D-rich/fortified foods and direct vitamin D supplementation when required. Further research is warranted to determine the optimal combinations of sun exposure, dietary intake and supplements required to achieve an adequate year round vitamin D status in athletes living in sun-rich environments.
Non-standard abbreviations
25-OHD, 25-hydroxyvitamin D; VDD, vitamin D deficiency
Acknowledgments
This work was financed by the Ministry of Higher Education and Scientific Research of Tunisia (UR/05/08-08 funds). The authors thank the athletes who volunteered to participate in the study and their coaches for their valuable help.
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, Saluja B, Ganie MA, Singh S. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr. 2005;82:477–482. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- 3.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, Ragi-Eis S, Chandler J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–254. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 4.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared to 2000–2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannell JJ, Hollis BW, Sorenson MB, Taft TN, Anderson JJ. Athletic performance and vitamin D. Med Sci Sports Exerc. 2009;41:1102–1110. doi: 10.1249/MSS.0b013e3181930c2b. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports. 2010;20:182–190. doi: 10.1111/j.1600-0838.2009.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7:337–345. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 8.Willis K, Smith D, Broughton K, Larson-Meyer DE. Vitamin D status and biomarkers of inflammation in runners. Open Access J Sports Med. 2012;3:35–42. doi: 10.2147/OAJSM.S31022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyon MA, Koutedakis Y, Wolman R, Nevill AM, Allen N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J Sci Med Sport. 2014;17:8–12. doi: 10.1016/j.jsams.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Lehtonen-Veromaa M, Möttönen T, Irjala K, Kärkkäinen M, Lamberg-Allardt C, Hakola P, Viikari J. Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur J Clin Nutr. 1999;53:746–751. doi: 10.1038/sj.ejcn.1600844. [DOI] [PubMed] [Google Scholar]
- 11.Lovell G. Vitamin D status of females in an elite gymnastics program. Clin J Sport Med. 2008;18:159–161. doi: 10.1097/JSM.0b013e3181650eee. [DOI] [PubMed] [Google Scholar]
- 12.Constantini NW, Arieli R, Chodick G, Dubnov-Raz G. High prevalence of Vitamin D insufficiency in athletes and dancers. Clin J Sport Med. 2010;20:368–371. doi: 10.1097/JSM.0b013e3181f207f2. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton B, Grantham J, Racinais S, Chalabi H. Vitamin D deficiency is endemic in Middle Eastern sportsmen. Public Health Nutr. 2010;13:1528–1534. doi: 10.1017/S136898000999320X. [DOI] [PubMed] [Google Scholar]
- 14.Morton JP, Iqbal Z, Drust B, Burgess D, Close GL, Brukner PD. Seasonal variation in Vitamin D status in professional soccer players of the English Premier League. Appl Physiol Nutr Metab. 2012;37:1–5. doi: 10.1139/h2012-037. [DOI] [PubMed] [Google Scholar]
- 15.Close GL, Russell J, Cobley JN, Owens DJ, Wilson G, Gregson W, Fraser WD, Morton JP. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. J Sports Sci. 2013;31:344–353. doi: 10.1080/02640414.2012.733822. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton B, Whiteley R, Farooq A, Chalabi H. Vitamin D concentration in 342 professional football players and association with lower limb isokinetic function. J Sci Med Sport. 2014;17:139–143. doi: 10.1016/j.jsams.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 18.Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4:239–280. doi: 10.1016/b978-1-4832-3110-5.50011-x. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 20.Bescos Garcia R, Rodriguez Guisado FA. Low levels of vitamin D in professional basketball players after wintertime: relationship with dietary intake of vitamin D and calcium. Nutr Hosp. 2011;26:945–951. doi: 10.1590/S0212-16112011000500004. [DOI] [PubMed] [Google Scholar]
- 21.Halliday TM, Peterson NJ, Thomas JJ, Kleppinger K, Hollis BW, Larson-Meyer DE. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med Sci Sports Exerc. 2011;43:335–343. doi: 10.1249/MSS.0b013e3181eb9d4d. [DOI] [PubMed] [Google Scholar]
- 22.Close GL, Leckey J, Patterson M, Bradley W, Owens DJ, Fraser WD, Morton JP. The effects of vitamin D3 supplementation on serum total 25-OHD concentration and physical performance: a randomised dose-response study. Br J Sports Med. 2013;47:692–696. doi: 10.1136/bjsports-2012-091735. [DOI] [PubMed] [Google Scholar]
- 23.Magee PJ, Pourshahidi LK, Wallace JMW, Cleary J, Conway J, Harney E, Madigan SM. Vitamin D Status and Supplementation in Elite Irish Athletes. Int J Sport Nutr Exerc Metab. 2013;23:441–448. doi: 10.1123/ijsnem.23.5.441. [DOI] [PubMed] [Google Scholar]
- 24.Wolman R, Wyon MA, Koutedakis Y, Nevill AM, Eastell R, Allen N. Vitamin D status in professional ballet dancers: winter vs. summer. J Sci Med Sport. 2013;16:388–391. doi: 10.1016/j.jsams.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Wilson G, Fraser WD, Sharma A, Eubank M, Drust B, Morton JP, Close GL. Markers of bone health, renal function, liver function, anthropometry and perception of mood: a comparison between Flat and National Hunt Jockeys. Int J Sports Med. 2013;34:453–459. doi: 10.1055/s-0032-1321898. [DOI] [PubMed] [Google Scholar]
- 26.Hill TR, Cotter AA, Mitchell S, Boreham CA, Dubitzky W, Murray L, Strain JJ, Flynn A, Robson PJ, Wallace JM, Kiely M, Cashman KD. Vitamin D status and its determinants in adolescents from the Northern Ireland Young Hearts 2000 cohort. Br J Nutr. 2008;99:1061–1067. doi: 10.1017/S0007114507842826. [DOI] [PubMed] [Google Scholar]
- 27.Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev. 2008;13:6–20. [PubMed] [Google Scholar]
- 28.Lenders CM, Feldman HA, Von Scheven E, Merewood A, Sweeney C, Wilson DM, Lee PD, Abrams SH, Gitelman SE, Wertz MS, Klish WJ, Taylor GA, Chen TC, Holick MF, Elizabeth Glaser Pediatric Research Network Obesity Study Group Relation of body fat indexes to vitamin D status and deficiency among obese adolescents. Am J Clin Nutr. 2009;90:459–467. doi: 10.3945/ajcn.2008.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGill AT, Stewart JM, Lithander FE, Strik CM, Poppitt SD. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr J. 2008;7:4. doi: 10.1186/1475-2891-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 2012;36:387–396. doi: 10.1038/ijo.2011.119. [DOI] [PubMed] [Google Scholar]
- 31.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 32.Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, Kohn N, Martinello S, Berkowitz R, Holick MF. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meddeb N, Sahli H, Chahed M, Abdelmoula J, Feki M, Salah H, Frini S, Kaabachi N, Belkahia Ch, Mbazaa R, Zouari B, Sellami S. Vitamin D deficiency in Tunisia. Osteoporos Int. 2005;16:180–183. doi: 10.1007/s00198-004-1658-6. [DOI] [PubMed] [Google Scholar]
- 34.Goswami R, Kochupillai N, Gupta N, Goswami D, Singh N, Dudha A. Presence of 25(OH) D deficiency in a rural North Indian village despite abundant sunshine. J Assoc Physicians India. 2008;56:755–757. [PubMed] [Google Scholar]
- 35.Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14:938–939. doi: 10.1017/S1368980011000565. [DOI] [PubMed] [Google Scholar]
- 36.Koenig J, Elmadfa I. Status of calcium and vitamin D of different population groups in Austria. Int J Vitam Nutr Res. 2000;70:214–220. doi: 10.1024/0300-9831.70.5.214. [DOI] [PubMed] [Google Scholar]
- 37.González-Gross M, Valtueña J, Breidenassel C, Moreno LA, Ferrari M, Kersting M, De Henauw S, Gottrand F, Azzini E, Widhalm K, Kafatos A, Manios Y, Stehle P, HELENA Study Group Vitamin D status among adolescents in Europe: the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br J Nutr. 2012;107:755–764. doi: 10.1017/S0007114511003527. [DOI] [PubMed] [Google Scholar]
- 38.Hill TR, McCarthy D, Jakobsen J, Lamberg-Allardt C, Kiely M, Cashman KD. Seasonal changes in vitamin D status and bone turnover in healthy Irish postmenopausal women. Int J Vitam Nutr Res. 2007;77:320–325. doi: 10.1024/0300-9831.77.5.320. [DOI] [PubMed] [Google Scholar]