Abstract
The present study examined the physiological impact of a school based sprint interval training (SIT) intervention in replacement of standard physical education (SPE) class on cardio-respiratory fitness (CRF) and glucose homeostasis during the semester following summer vacation. Participants (n=49) were randomly allocated to either intervention (SIT; n=26, aged 16.9 ± 0.3 yrs) or control group who underwent standard physical education (SPE; n=23, aged 16.8 ± 0.6 yrs). CRF (VO2max) and glucose homeostasis were obtained prior-to and following 7 weeks of SIT exercise. Significant group x time interaction was observed for CRF (P < 0.01) with non-significant trends for fasting insulin (P= 0.08), and HOMA-IR (P=0.06). CRF decreased (P < 0.01) in SPE such that POST intervention CRF was significantly lower (P< 0.05) in SPE. Fasting plasma glucose (P < 0.01), insulin (P< 0.01) and HOMA-IR (P< 0.01) increased significantly amongst SPE. The main finding of the present study is that 7-weeks of SIT exercise is an effective method of maintaining (but not improving) CRF and fasting insulin homeostasis amongst school-going adolescents. SIT exercise demonstrates potential as a time efficient physiological adjunct to standard PE class in order to maintain CRF during the school term.
Keywords: adolescents, sprint interval training (SIT), cardio-respiratory fitness (CRF)
INTRODUCTION
Physical inactivity, low cardiorespiratory fitness (CRF) [1] and sedentary behaviour, particularly sitting hours [2] are primary causal risk factors in the pathogenesis of metabolic disorders amongst children and adolescents. In contrast to the school environment, adolescents are known to self-select higher levels of physical activity throughout a summer vacation [3]. During childhood and adolescence, the classroom environment lends itself to seasonal epochs of reduced physical activity with corresponding increases in time spent sitting. However, there is a current lack of objectively determined evidence to support of this tenet.
School based physical activity in the UK, is largely achieved through the medium of standard physical education (SPE) classes. However, there is evidence that pupils spend less than 50% of SPE lessons achieving even moderately-intense levels of physical exertion with the majority of available class-time spent with students being physical inactive [4]. Time constraints have been proposed as a latent obstacle that inhibits adequate physical activity in school based PE class [5]. Although daily physical activity guidelines promote that 60 minutes of moderate to vigorous physical activity should be achieved each day [6], approximately 61% of British adolescents fail to meet these recommendations [7] and the number of younger school aged children (2-15 years) in receipt of inadequate daily physical activity in Scotland has increased ~10% between 2003 and 2012 [8]. Again, parents and older children have cited time constraints as a barrier to achieving adequate physical activity levels [9, 10].
First described by Åstrand and colleagues in 1960 [11], sprint interval training (SIT) is enjoying a recent re-emergence in the scientific literature as an effective and time-efficient means of achieving improving CRF and health outcomes amongst a variety of populations, healthy or otherwise. SIT involves short bursts of high intensity exercise interspersed with low-intensity aerobic recovery intervals. SIT has been demonstrated effectiveness as time efficient method of improving insulin sensitivity and physical fitness amongst adolescents [12, 13]. SIT-induced Improvements in CRF and insulin sensitivity have been found in studies ranging from 2-12 weeks, in healthy adults [14, 15] and in healthy and obese juveniles [16–18]. However, there is a lack of documented evidence relating to the effects of SIT on CRF and glucose and insulin homeostasis in adolescent during the school term.
Therefore, the main aim of this study was to examine the physiological effects of a school based SIT intervention, during the first school semester, on CRF and glucose homeostasis compared with a matched and counterbalanced control group undertaking standard physical education (SPE) class. We hypothesised that a school based physiological intervention of sprint interval training (SIT) would favourably impact CRF compared to a control group undertaking standard physical education classes (SPE). We further hypothesised that this SIT intervention would favourably impact plasma glucose and insulin homeostasis compared with a SPE control.
MATERIALS AND METHODS
Following ethical approval from the University of the West of Scotland Institutional Ethics Committee, apparently healthy adolescent participants (n=49) provided written informed consent to participate in the study. Participants were recruited from a cohort taking physical education classes at the same school, who were screened by physical activity readiness questionnaire (PAR-Q) and deemed to be absent of health related disease. The study employed a quasi-experimental randomly allocated controlled and counterbalanced design where the intervention arm received a 7 week sprint interval training programme (SIT; n=26, aged 16.9 ± 0.3 yrs) while the control arm (SPE; n=23, aged 16.8 ± 0.6 yrs) undertook SPE classes comprising 3 x 1 hr classes per week for 7 consecutive weeks. Homogeneity between groups was achieved via pre-intervention counterbalancing, and confirmed by an absence of difference in CRF, body composition, maturation status and time spent being physically active, between groups.
Participant body mass (kg) was recorded to nearest 0.1kg using a calibrated digital scale (Seca 880 digital scale, Ltd. Birmingham UK). Stature (cm) was measured using a stadiometer (Seca Ltd. Birmingham UK). Body Mass Index (BMI) was calculated as body-mass · height-2 (kg · m-2).
Daily nutritional intake was recorded using a previously validated self-reported seven-day food diary and a food frequency questionnaire [19] and analysed using nutritional analysis software (Nutri-check, Health Options Ltd, Cirencester, Gloucester, UK). Participants were instructed to maintain comparable diets in the days leading to physiological assessment and normal physical activity patterns throughout the study. A self-reported Physical Activity Questionnaire for Adolescents (PAQ-A) assessed participant physical activity behaviour during the prior seven days including; number of days spent engaged in sporting activity, physical activity during the school day and physical activity out-with the school day [20]. Self-reported pubertal development scale was used to classify participants sexual maturation solely by pubic hair development [21], which was graded on a scale of 1 to 5 according to Tanner criteria. (Table 1)
TABLE 1.
Developmental characteristics of Sprint Interval Training (SIT) and structured PE (SPE) groups prior to (PRE) 7-week SIT training intervention (mean ± SD where appropriate).
| SIT Group (n= 20) | SPE Group (n= 23) | ||
|---|---|---|---|
| PRE | PRE | ||
| Age | 16.9 ± 0.4 | 16.8 ± 0.5 | |
| Gender boys/girls | 13 / 7 | 18 / 5 | |
| Tanner stage (n/%): | |||
| Pubic hair growth: | 3 | 8 / 40 | 1 / 4 |
| 4 | 6 / 30 | 11 / 48 | |
| 5 | 6 / 30 | 11 / 48 | |
Blood was sampled following an overnight fast, between the hours of 8-10am, from an antecubital forearm vein, using standard venepuncture method. Sampling occurred between 63 and 84 hours following the last exercise session. Blood was collected into heparinised vacutainers and immediately centrifuged at 4,000rpm for 10 minutes at 10°C. Blood plasma was extracted and stored in 1.5ml aliquots at -80°C until subsequent analysis. Plasma glucose and insulin samples were analysed in duplicate with the average being used as the criterion measurement. Glucose was determined using enzymatic standard methods (Randox, Antrim, UK) using a Campspec M107 spectrophotometer (Camspec, Leeds, UK). The intra- and inter assay coefficients of variation (CV's) were 2.4% and 3.5% respectively. Insulin was determined using immunoassay kits (ALPCO, Salem, NH) and a Camspec M107 spectrophotometer (Camspec, Leeds, UK). The intra- and inter assay CV's were 2.6% and 4.0% respectively Homeostatic model of assessment for insulin resistance (HOMA-IR) was calculated as=[(fasting insulin x fasting blood glucose)/22.5] [22]. The homeostatic model of assessment is a reliable and valid method to determine insulin action amongst obese adolescents [23].
Participants CRF was assessed using a previously validated [24] 20m multi-stage fitness test (20 MSFT), employing standard procedures [25]. Maximal oxygen uptake (VO2max) was estimated using the equation outlined by Barnett [26]. Simultaneous measurement of heart rate were evaluated using continuous heart rate telemetry (Hosand, TM200, Hosand Technology, Verbania, Italy) which served to confirm maximal effort and further establish the relative weekly SIT intensity amongst the intervention arm.
All measurements, including the SIT intervention were performed in the same school gymnasium, during time normally allocated to PE classes. Participants presented at SIT intervention wearing standard PE clothing and indoor sports shoes and received a demonstration of SIT manoeuvres. A standardised 5 min warm-up and 5 min of stretching exercise was performed prior to SIT sessions commencing. Each SIT session involved 30 s of running sprints between cones set 20 m apart, interspersed with 30 s of passive recovery. Four sets (4 x) 30 s sprints performed during each session for the first two weeks (6 sessions), 5 x 30 s sprints were performed in each session during weeks three and four (sessions 7-12); 6 x 30 s sprints were performed in each session during weeks 5-7 (sessions 13-21) in accordance to previous similar investigations [27]. Participants were required to complete a minimum of 80% of sessions (17 of 21 sessions) to be included in the final data analysis [28, 29]. The SIT intervention consisted of 21 SIT sessions executed over 7 consecutive weeks consisting of 3 sessions per week equating to a combined total of 61 sprint interval training (effort) minutes. The SPE group performed 3 weekly sessions of 60 mins of standard PE conforming to the national education curriculum for 7 consecutive weeks. Both SPE and SIT groups were instructed to maintain their normal daily physical activity habits.
Sample power was estimated using the procedures of Park and Schutz [30] for ANOVA designs that incorporate a repeated factor. For a medium effect size in the intervention groups of d=0.5, power of 0.8 (suggesting an 80% probability of achieving significance at the P ≤ 0.05 level), indicated that a sample group size of 17 participants were required per group. Effect sizes > 0.5 indicate clinically relevant change [31]. Assuming a typical accession rate of up to 30% for exercise studies in our laboratory, it was deemed appropriate to recruit the entire cohort of 49 students.
Data were analysed using SPSS version 18.0. Q-Q plots were employed to confirm normal distribution of data. Independent t-tests were employed to confirm homogeneity between the groups for age (years), aerobic fitness (ml · kg-1 · min-1) and body mass (kg-1). Pearson's Chi-square analysis was used to configure independence of maturation status between the SIT and the SPE group. A three-way repeated measure of analysis of variance (ANOVA) was employed to compare PRE and POST intervention data (gender x group x time). Gender did not exhibit any gender by time (gender x time) interaction. Consequently, training effects were compared using a group x time ANOVA. Bonferroni-corrected paired-sample t-tests were used to compare means from PRE to POST within the SIT and SPE groups. Alpha value of P≤ 0.05 was used to indicate statistical significance. Data is presented as mean ± standard deviation (mean ± SD).
RESULTS
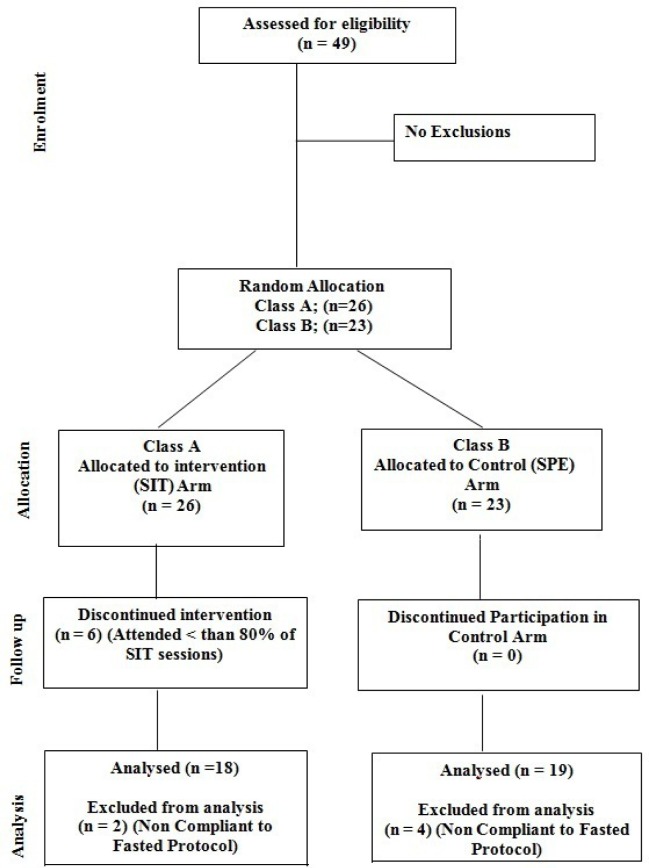
Overall secession rate in the intervention arm was 12.3% with 6 SIT participants in failing to achieve the required 80% (17/21) of sessions required to be included in the final data analysis with complete adherence in the SPE group. Reasons for secession are outlined in Figure 1. Forty-three participants (SIT n=20, SPE n=23) completed the study with 37 participants meeting the criteria for blood sampling analysis (SIT n=18; SPE n=19) (Figure 1). Frequency analysis (χ2) revealed a lack of difference between groups in classifications of maturation status, BMI, waist circumference and physical activity levels. Total body mass (kg) and body mass index (kg · m-2) remained unchanged during the study (Table 2).
FIG. 1.
Flow diagram depicting participant recruitment, allocation and progress through the study.
TABLE 2.
Daily average nutritional intake, weekly physical activity questionnaire (PAQ-A) score, body composition and shuttle-run performance for sprint interval training group (SIT) compared with control group receiving standard physical education (SPE) PRE and POST sprint interval training programme.
| SIT | SPE | |||
|---|---|---|---|---|
| PRE | POST | PRE | POST | |
| Daily Nutritional Intake: | ||||
| Energy intake (Kcal) | 1574 ± 297 | 1635 ± 385 | 1667 ± 486 | 1704 ± 439 |
| Dietary Fat (%) | 37 ± 4.4 | 35 ± 6.1 | 34 ± 5.2 | 32 ± 8.6 |
| Saturated Fat (%) | 13 ± 9 | 11 ± 12 | 16 ± 8 | 14 ± 10 |
| Carbohydrate (%) | 37 ± 9.2 | 38 ± 10.1 | 36 ± 12.4 | 37 ± 13.2 |
| Protein (%) | 13 ± 7.3 | 16 ± 5.1 | 14 ± 5.4 | 17 ± 7.2 |
| PAQ-A score | 2.4 ± 0.5 | 3.8 ± 0.4 Ψ | 2.30 ± 0.51 | 2.1 ± 0.6* |
| Physiological Measures: | ||||
| Body Mass (Kg) | 64.2 ± 7.82 | 63.5 ± 7.7 | 66.0 ± 10.4 | 67.0 ± 10.4 |
| BMI (kg·m-2) | 22.2 ± 2.3 | 21.6 ± 2.5 | 22.3 ± 3.2 | 22.5 ± 3.0 |
| Shuttle Run Performance | 82 ± 11 | 92 ± 12 | 80 ± 13 | 67 ±10*Ψ |
Note: Data presented as group mean ± standard deviation (SD).
– Significant between group difference (P< 0.05) ;
– Significant within group difference (P < 0.01).
PAQ-A did not differ between groups at PRE but was significantly different at POST (P <0.05, ES = 0.73). PAQ-A remained unchanged amongst the SPE group but increased from PRE to POST in the SIT group (P< 0.01) (Table 1.). Analysis of 7-day food diaries did not reveal any differences in daily energy intake (Kcal), percentage intake of macronutrients between groups did not change during the intervention (Table 2).
Maximum heart rate (HRmax) during 20m MSFT were consummate with maximal exertion in both groups both PRE and POST intervention. (SIT; 206±8 and 203±11 bpm, SPE; 208±9 and 200±10 bpm, respectively). Average weekly heart rate during SIT was 179 ±1.9 bpm, equating to an average weekly HRmax of 87% during the SIT intervention.
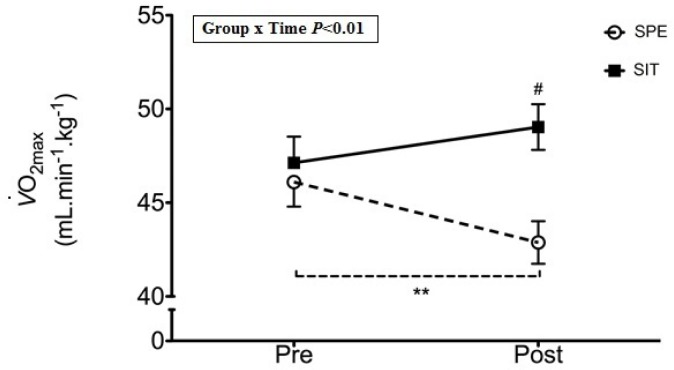
A significant group x time interaction was observed for CRF (P< 0.01, ES=0.93). CRF was equal between SIT and SPE at PRE (47.1 ± 6.3 ml·kg-1·min-1 and 46.1 ± 7.3 ml·kg-1·min-1, respectively), CRF was significantly higher in SPE at POST intervention (49.1 ± 6.2 ml·kg-1·min-1 and 42.9 ± 7.1 ml·kg-1·min-1; P< 0.01), which was achieved through a non-significant trend towards an increase in SIT (P=0.06) with a concomitant significant decrease from PRE to POST in the SPE (P< 0.01) (Figure 2).
FIG. 2.
Aerobic fitness PRE to POST intervention.
Note: ** – Significant difference PRE to POST (P < 0.01).
# – Significant difference between groups at POST (P < 0.05).
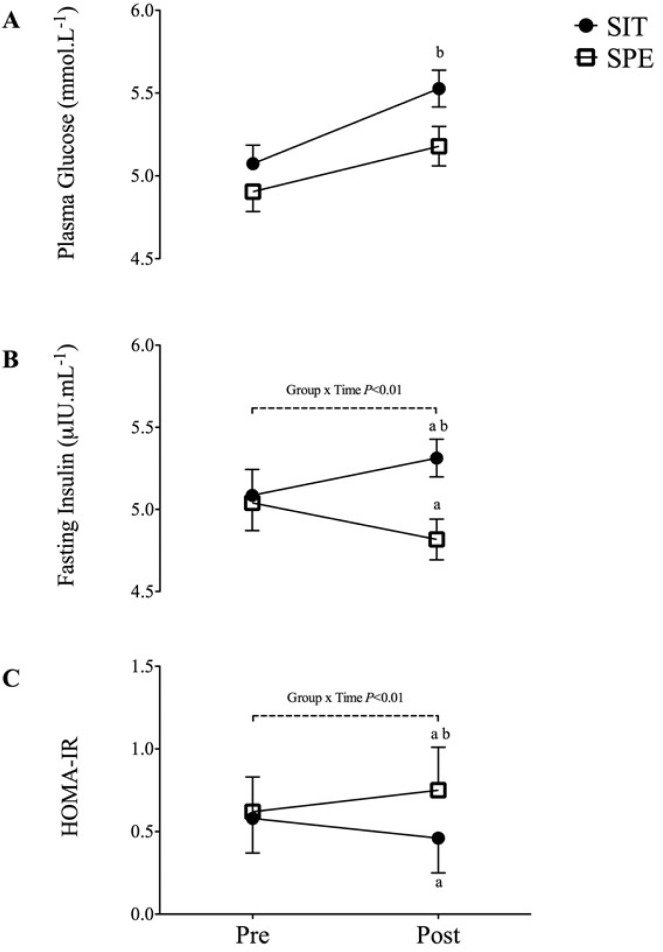
Fasted plasma glucose levels were similar between groups SPE (4.91 ± 0.12 mmol · mL-1 and 5.07 ± 0.11 mmol · mL-1, respectively) PRE intervention with a small but statistically significant difference between groups POST (5.16 ± 0.65 mmol · mL-1 and 5.50 ± 0.42 mmol · mL-1, respectively (P< 0.05, ES=0.32). Plasma glucose increased significantly from PRE to POST intervention in SPE (P< 0.01) whilst remaining unchanged in SIT (Figure 3A).
FIG. 3.
Fasting blood glucose levels PRE to POST intervention. 3B- Fasting insulin levels PRE to POST intervention. 3C- HOMA-IR PRE to POST intervention.
a – Significant difference PRE to POST within group (P < 0.05).
b – Significant difference between groups at POST (P < 0.05).
A non-significant trend towards a group x time interaction was observed for fasting insulin (P=0.08, ES=0.58) (Figure 3B). Fasting plasma insulin levels did not differ between the SIT and SPE at PRE (5.04 ± 0.17 µIU · mL-1 and 5.09 ± 0.16 µIU · mL-1, respectively). Plasma insulin differed significantly between groups POST intervention (SIT: 4.81 ± 0.40 µIU · mL-1 and SPE: 5.31 ± 0.12 µIU · mL-1, respectively (P < 0.01), which was an achieved trend towards a decrease from PRE to POST in SIT (P=0.08) with concomitant significant increase from PRE to POST in SPE (P=0.01) (Figure 3B).
A non-significant trend towards a group x time interaction was observed for HOMA-IR (P=0.06, ES=0.57). No significant difference was observed for HOMA-IR between the SIT and SPE at PRE (1.11 ± 0.05 and 1.11 ± 0.04, respectively). However, groups were significantly different at POST (1.12 ± 0.04 and 1.31 ± 0.04 respectively; P < 0.01), with a non-significant trend towards a decrease in HOMA-IR (P= 0.06) from PRE to POST in SIT while HOMA-IR increased significantly in SPE (P< 0.01) from PRE to POST (Figure 3B).
DISCUSSION
The main findings of the present study were that a school based programme of SIT allowed the maintenance of CRF with a corresponding decrease evident amongst the control participants receiving SPE. Similarly, whilst remaining within healthy limits, insulin centred glucose homeostasis became less favourable in SPE compared with those in receipt of the SIT intervention. To our knowledge, this is the first study to examine the impact of a school-based SIT on CRF and insulin sensitivity in adolescents, with a further novel finding relating to the decrease in CRF experienced by the SPE control group during the semester following summer vacation.
Comparable studies of running SIT in similarly aged participants have demonstrated increases in CRF amongst apparently healthy [13, 17, 32] and obese adolescents [12, 18, 33]. Conversely, CRF was maintained but not significantly improved amongst the SIT intervention group in the present study. The present findings relating to effects of SIT on CRF are in contrast with previous SIT studies in adolescents that usually report significant improvements in CRF following SIT interventions [12, 13, 16, 17, 32, 33]. The relatively high initial CRF levels exhibited by the SIT and SPE cohorts upon enrolment to the study may provide some explanation for the lack of improvement following the SIT intervention and offer similar explanation for the decrease in CRF amongst the SPE control group. It may be that the margin for available improvement in CRF was compromised amongst a group that were above the 90th percentile for age related CRF and more importantly, that the SPE group also being above the 90th percentile experienced a significant reduction in CRF. Furthermore, the delta change in completed 20m MSFT shuttles from PRE to POST amongst the current cohort is similar to those reported in studies where changes in aerobic capacity reached significance [13, 17]. There was also a strong trend towards an increase in CRF amongst the SIT group (P=0.06) coupled with large effect size (ES =0.93) in the present study, suggesting that a longer intervention, or a higher intensity may have produced significant improvements as demonstrated by similar SIT investigations [12, 18, 34], each of whom report significant improvement in CRF following 12 weeks of SIT in obese adolescents.
An increased PAQ-A score amongst the SIT group following the intervention, in the absence of any change to SPE, with a concomitant large effect size (d=0.73) is an unexpected and novel finding. This suggests that a programme of school based SIT may encourage participants to self-select higher levels of overall physical activity outwith the school environment. As the PAQ-A relies on self-reported data and does not account for changes in the relative intensities of physical activity, we regard our findings as preliminary until further study can replicate these data using objectively measures of physical activity and intensities thereof (ie. accelerometery). Compared with CRF related investigations, the effects of SIT on measures of insulin and glucose homeostasis have been less well-defined, particularly amongst apparently healthy adolescent cohorts [13, 17]. In contrast to beneficial changes demonstrated amongst obese and overweight adolescents [12, 18, 33, 34], which indicates that the capacity for SIT related improvements in insulin cantered metabolic homeostasis is more evident amongst adolescents with an existing disorder of known association to metabolic disturbances such as overweight and obesity. Despite this, the impact of SIT amongst adult cohorts whether healthy [35, 36] or overweight/obese [37] demonstrate improved insulin sensitivity in those receiving the SIT intervention.
High levels of fasting blood glucose and insulin are well-established risk factors for the development of metabolic disease [38]. While SIT remained unaffected in the present study, SPE experienced a significant increase in fasting blood glucose during the course of the intervention and levels of fasting insulin increased amongst SPE such that groups became significantly different at POST measurement and reflected by a substantial effect size (d=0.57). Although the magnitudes of glucose and insulin changes were small, in concordance with the decrease in CRF amongst SPE, have little clinical significance; it does signal the initiation of a process that may potentiate adverse metabolic health outcomes. As such, when compared to the studies that report improvements in metabolic status amongst obese adolescents with existing obesity [34], it stands to reason that SIT is likely to be more effective amongst adolescents with impaired fasting glucose or existing insulin resistance.
Changes in homeostasis model (HOMA), based on plasma levels of fasting glucose and insulin has been widely applied for quantifying insulin resistance. In contrast to previous work in obese adolescent females [18], the present study did not result in any changes in HOMA-IR following SIT exercise. However, there was a significant increase in HOMA-IR amongst SPE, which is largely accounted for by concomitant increases in fasting insulin concentrations. Consequently it is likely that the decline in CRF observed in SPE is responsible for the adverse changes in insulin and HOMA-IR as has previously demonstrated in adult cohorts [39–42] and can be supported, in part, by the substantial effect size (ES=0.58) in the present study. Although the absolute increase remained within normal limits for HOMA-IR, it does support the potential for decreases in CRF to instigate the pathogenesis towards insulin resistance in normally healthy cohorts.
Although the long-term effects are unclear given the high initial CRF levels of both cohorts, the present study identifies a reduction in CRF during the first semester following summer vacation. This provides the first evidence for a detrimental impact of the school environment amongst normally physically active adolescents and identifies the initiation of a process that, if left unchecked, may progress to a state of increased cardiometabolic risk. Given that poor levels of CRF during adolescence are associated with the development of future cardio metabolic disease risk [3], decrements in CRF as a consequence of the school environment should become more widely known, particularly if future studies can confirm the present finding amongst adolescents with moderate and low CRF levels upon study enrolment.
SIT interventions within the school environment require limited resources and have been effectively and safely used within adult [15] and adolescent populations [13, 17] and indeed games such as the Tunisian “raqassa” that have an inherent high intensity component, can offer practical solutions for comparable cardiovascular stimulation [43] in a PE setting. Moreover, it has been suggested that some participants report SIT to be a more enjoyable method of exercise than prolonged continuous exercise in adults [44] and adolescents [34]. The finding of the present study supports the contention that SIT routines may be a physiologically effective, teacher-friendly and time-efficient exercise modality as an adjunct to or incorporation into school PE routines.
There are some important limitations to the present study. Firstly there were a lack of objective determinants of daily physical activity to further scrutinise the quantity and intensity of physical activity during and outwith the school the school environment. Furthermore, the present cohort, were in the 95th percentile for age related fitness in their age group which limits the generalizability of some findings. However, differences exhibited in a fit group are likely to be more pronounced in more heterogeneous physically inactive, cohorts with existing cardiometabolic risk as demonstrated in studies of obese adolescents. Further to this, the use of food diaries to assess energy intake is known to result in under-reporting of calorie intake. In addition, the lack of dietary intake data for the (Pre-study) vacation period might account for some of the changes to blood glucose homeostasis and should be accounted for in future similar studies. Finally, the authors propose that future similar study should extend this work by incorporating objective measurements of physical activity to confirm our finding of increased self-selected physical activity in the SIT intervention group.
CONCLUSIONS
The present study identifies the first semester of school, following summer vacation, to be associated with a decrease in maximal CRF and altered glucose and insulin homeostasis. Our findings indicate that a 7 week running SIT intervention is beneficial for maintaining aerobic fitness and insulin homeostasis during the first term of school following a summer vacation. SIT exercise routines demonstrate potential as a time efficient physiological adjunct to standard PE class in order to maintain CRF during the school term.
Acknowledgements
The authors would like to acknowledge the participants for their involvement in this study.
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Shaibi GQ, Michaliszyn SB, Fritschi C, Quinn L, Faulkner MS. Type 2 diabetes in youth: a phenotype of poor cardiorespiratory fitness and low physical activity. Int J Pediatr Obes. 2009;4(4):332–7. doi: 10.3109/17477160902923341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriska A, Delahanty L, Edelstein S, Amodei N, Chadwick J, Copeland K, et al. Sedentary behavior and physical activity in youth with recent onset of type 2 diabetes. Pediatrics. 2013;131(3):850–6. doi: 10.1542/peds.2012-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanningham-Foster L, Foster RC, McCrady SK, Manohar CU, Jensen TB, Mitre NG, et al. Changing the school environment to increase physical activity in children. Obesity (Silver Spring) 2008;16(8):1849–53. doi: 10.1038/oby.2008.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLure SA, Summerbell CD, Reilly JJ. Objectively measured habitual physical activity in a highly obesogenic environment. Child Care Health Dev. 2009;35(3):369–75. doi: 10.1111/j.1365-2214.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 5.Boyle SE, Jones GL, Walters SJ. Physical activity among adolescents and barriers to delivering physical education in Cornwall and Lancashire, UK: a qualitative study of heads of PE and heads of schools. BMC public health. 2008;8:273. doi: 10.1186/1471-2458-8-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organisation. World Health Statistics 2013; Geneva: World Health Organisation; 2013. [Google Scholar]
- 7.The Health and Social Care Information Centre. Statistics on obesity, physical activity and diet: England, 2011; England: The Health and Social Care Information Centre; 2012. [Google Scholar]
- 8.Scottish Government. Scottish Health Survey 2012; Edinburgh: Scottish Government; 2013. [Google Scholar]
- 9.Dwyer JJ, Allison KR, Barrera M, Hansen B, Goldenberg E, Boutilier MA. Teachers’ perspective on barriers to implementing physical activity curriculum guidelines for school children in Toronto. CJPH. 2003;94(6) doi: 10.1007/BF03405083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allison KR, Dwyer JJ, Makin S. Perceived barriers to physical activity among high school students. Preventive Medicine. 1999;28(6):608–15. doi: 10.1006/pmed.1999.0489. [DOI] [PubMed] [Google Scholar]
- 11.Åstrand I, Åstrand PO, Christensen EH, Hedman R. Intermittent muscular work. Acta physiologica Scandinavica. 1960;48:448–53. doi: 10.1111/j.1748-1716.1960.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 12.Corte de Araujo AC, Roschel H, Picanco AR, do Prado DM, Villares SM, de Sa Pinto AL, et al. Similar health benefits of endurance and high-intensity interval training in obese children. PloS one. 2012;7(8):42747. doi: 10.1371/journal.pone.0042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchan DS, Ollis S, Young JD, Thomas NE, Cooaper SM, Tong TK, et al. The effects of time and intensity of exercise on novel and established markers of CVD in adolescent youth. Am J Hum Biol. 2011;23(4):517–26. doi: 10.1002/ajhb.21166. [DOI] [PubMed] [Google Scholar]
- 14.Babraj JA, Vollaard NB, Keast C, Guppy FM, Cottrell G, Timmons JA. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord. 2009;9:3. doi: 10.1186/1472-6823-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandvei M, Jeppesen PB, Stoen L, Litleskare S, Johansen E, Stensrud T, et al. Sprint interval running increases insulin sensitivity in young healthy subjects. Arch Physiol Biochem. 2012;118(3):139–47. doi: 10.3109/13813455.2012.677454. [DOI] [PubMed] [Google Scholar]
- 16.Barker AR, Day J, Smith A, Bond B, Williams CA. The influence of 2 weeks of low-volume high-intensity interval training on health outcomes in adolescent boys. J Sports Sci. 2014;32(8):757–65. doi: 10.1080/02640414.2013.853132. [DOI] [PubMed] [Google Scholar]
- 17.Buchan DS, Ollis S, Young JD, Cooper SM, Shield JP, Baker JS. High intensity interval running enhances measures of physical fitness but not metabolic measures of cardiovascular disease risk in healthy adolescents. BMC public health. 2013;13:498. doi: 10.1186/1471-2458-13-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racil G, Ben Ounis O, Hammouda O, Kallel A, Zouhal H, Chamari K, et al. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol. 2013;113(10):2531–40. doi: 10.1007/s00421-013-2689-5. [DOI] [PubMed] [Google Scholar]
- 19.Food Standards Agency. McCance and Widdowson's the Composition of Foods; Cambridge UK: Royal Society of Chemistry; 2002. [Google Scholar]
- 20.Kowalski K, Crocker P, Faulkner R. Validation of the physical activity questionnaire for older children. Pediatr Exerc Sci. 1997;9:174–86. [Google Scholar]
- 21.Tanner J. Growth at adolescence. Oxford: Blackwell; 1962. [Google Scholar]
- 22.Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005;54(2):333–9. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- 23.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):500–3. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz JR, Silva G, Oliveira N, Ribeiro JC, Oliveira JF, Mota J. Criterion-related validity of the 20-m shuttle run test in youths aged 13-19 years. J Sports Sci. 2009;27(9):899–906. doi: 10.1080/02640410902902835. [DOI] [PubMed] [Google Scholar]
- 25.Leger LA, Mercier D, Gadoury C, Lambert J. The multistage 20 metre shuttle run test for aerobic fitness. J Sports Sci. 1988;6(2):93–101. doi: 10.1080/02640418808729800. [DOI] [PubMed] [Google Scholar]
- 26.Barnett A, Chan LYS, Bruce IC. A preliminary study of the 20-m multistage shuttle run as a predictor of a peak VO2 in Hong Kong Chinese students. Pediatr Exerc Sci. 1993;5:42–50. [Google Scholar]
- 27.Buchan DS, Ollis S, Thomas NE, Baker JS. The influence of a high intensity physical activity intervention on a selection of health related outcomes: an ecological approach. BMC public health. 2010;10:8. doi: 10.1186/1471-2458-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rognmo O, Hetland E, Helgerud J, Hoff J, Slordahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11(3):216–22. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- 29.Molmen-Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;19(2):151–60. doi: 10.1177/1741826711400512. [DOI] [PubMed] [Google Scholar]
- 30.Park I, Schutz RW. An introduction to latent growth models: analysis of repeated measures physical performance data. Res Q Exerc Sport. 2005;76(2):176–92. doi: 10.1080/02701367.2005.10599279. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. A power primer. Psychological bulletin. 1992;112(1):155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 32.Baquet G, Gamelin FX, Mucci P, Thevenet D, Van Praagh E, Berthoin S. Continuous vs. interval aerobic training in 8- to 11-year-old children. J Strength Cond Res. 2010;24(5):1381–8. doi: 10.1519/JSC.0b013e3181d1575a. [DOI] [PubMed] [Google Scholar]
- 33.Koubaa A, Trabelsi H, Masmoudi L, Elloumi M, Sahnoun Z, Zeghal KM, et al. The effects of intermittent and continuous training on body composition, cardiorespiratory fitness and lipid profile in obese adolescents. OOSR J Pharm Biol Sci. 2013;3(2):31–7. [Google Scholar]
- 34.Tjonna AE, Stolen TO, Bye A, Volden M, Slordahl SA, Odegard R, et al. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin Sci (Lond) 2009;116(4):317–26. doi: 10.1042/CS20080249. [DOI] [PubMed] [Google Scholar]
- 35.Babraj J, Vollaard N, Keast C, Guppy F, Cottrell G, Timmons J. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC endocrine disorders. 2009;9(1):3. doi: 10.1186/1472-6823-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards JC, Johnson TK, Kuzma JN, Lonac MC, Schweder MM, Voyles WF, et al. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to beta-adrenergic stimulation. J Physiol. 2010;588(Pt 15):2961–72. doi: 10.1113/jphysiol.2010.189886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whyte LJ, Ferguson C, Wilson J, Scott RA, Gill JM. Effects of single bout of very high-intensity exercise on metabolic health biomarkers in overweight/obese sedentary men. Metabolism. 2013;62(2):212–9. doi: 10.1016/j.metabol.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Artz E, Freemark M. The pathogenesis of insulin resistance in children: metabolic complications and the roles of diet, exercise and pharmacotherapy in the prevention of type 2 diabetes. Pediatr Endocrinol Rev. 2004;1(3):296–309. [PubMed] [Google Scholar]
- 39.Moliner-Urdiales D, Ortega FB, Vicente-Rodriguez G, Rey-Lopez JP, Gracia-Marco L, Widhalm K, et al. Association of physical activity with muscular strength and fat-free mass in adolescents: the HELENA study. Eur J Appl Physiol. 2010;109(6):1119–27. doi: 10.1007/s00421-010-1457-z. [DOI] [PubMed] [Google Scholar]
- 40.Ortega FB, Ruiz JR, Hurtig-Wennlof A, Vicente-Rodriguez G, Rizzo NS, Castillo MJ, et al. Cardiovascular fitness modifies the associations between physical activity and abdominal adiposity in children and adolescents: the European Youth Heart Study. Br J Sports Med. 2010;44(4):256–62. doi: 10.1136/bjsm.2008.046391. [DOI] [PubMed] [Google Scholar]
- 41.Rizzo NS, Ruiz JR, Hurtig-Wennlof A, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: the European youth heart study. J Pediatr. 2007;150(4):388–94. doi: 10.1016/j.jpeds.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 42.Ortega A, Ruiz JR, Hurtig-Wennlof A, Sjostrom M. Physically Active Adolescents Are More Likely to Have a Healthier Cardiovascular Fitness Level Independently of Their Adiposity Status. The European Youth Heart Study. Rev Esp Cardiol. 2008;61(2):123–9. [PubMed] [Google Scholar]
- 43.Jebali T, Moalla W, Elloumi M, Padulo J, Baquet G, Chamari K. The relevant use of the traditional tunisian game „raqassa” for cardiovascular stimulation in schoolchildren. Biol Sport. 2013;30(3):219–25. doi: 10.5604/20831862.1059304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartlett JD, Close GL, MacLaren DP, Gregson W, Drust B, Morton JP. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J Sports Sci. 2011;29(6):547–53. doi: 10.1080/02640414.2010.545427. [DOI] [PubMed] [Google Scholar]