Abstract
The relationship between oxidative stress and some exercise components of resistance exercise (e.g. intensity, exercise volume) has not been clearly defined. Additionally, the oxidative stress markers may respond differently in various conditions. This study aims to determine the effects of progressive intensity of resistance exercise (RE) on oxidative stress and antioxidants in trained and untrained men, and also to investigate the possible threshold intensity required to evoke oxidative stress. RE trained (N=8) and untrained (N=8) men performed the leg extension RE at progressive intensities standardized for total volume: 1x17 reps at 50% of one-repetition maximum (1RM); 1x14 reps at 60% of 1RM; 1x12 reps at 70% of 1RM; 2x5 reps at 80% of 1RM; and 3x3 reps at 90% of 1RM. Blood samples were drawn before (PRE) and immediately after each intensity, and after 30 minutes, 60 minutes and 24 hours following the RE. Lipid-hydroperoxide (LHP) significantly increased during the test and then decreased during the recovery in both groups (p<0.05); the POST-24 h LHP level was lower than PRE-LHP. Protein carbonyl (PCO) and superoxide dismutase (SOD) significantly increased (p<0.05); however, 8-hydroxy-2’-deoxyguanosine (8-OHdG) and glutathione (GSH) were not affected by the RE (p > 0.05). The results indicated that there was no significant training status x intensity interaction for examined variables (p > 0.05). Standardized volume of RE increased oxidative stress responses. Our study suggests that lower intensity (50%) is enough to increase LHP, whereas higher intensity (more than 80%) is required to evoke protein oxidation.
Keywords: free radicals, weight training, progressive intensity, training status, oxidative stress
INTRODUCTION
Oxidative stress is a biological phenomenon marked by an imbalance between reactive free radicals (FR) and the antioxidant defence system [1]. Many studies have reported that acute exercise increases FR production, intensifies oxidative stress, and causes cellular damage [2]. Conversely, it has been shown that aerobic or anaerobic (resistance) exercises performed regularly decrease oxidative stress and attenuate lipid peroxidation levels while significantly increasing antioxidant enzyme activity [3–5]. This may be explained by a well-adapted antioxidant defence system in physically active people [6]. Moreover, most studies on oxidative stress and antioxidant responses to exercise have been performed using prolonged endurance exercise protocols [7, 8]. In this respect, the physiological load of intermittent exercise, such as resistance exercise (RE), differs from a continuous steady-state exercise.
RE training or strength training is the preferred form of exercise for increasing an individual's physical fitness [9]. Intensity, volume, and frequency are basic components of RE training. The intensity in RE training is often quantified as a function of the maximum weight that can be lifted once (one-repetition maximum, 1RM), and the number of repetitions is limited for a given intensity. It has been reported that regular RE training decreases oxidative stress in some patient groups (e.g. Parkinson's, rheumatoid arthritis, and obesity) [10–12]. Additionally RE has been suggested as an alternative to improve the physical fitness status of individuals, especially when it is considered that long-term performance of aerobic exercises is generally unsuccessful under conditions such as obesity [12]. Therefore, RE grows in importance every day.
Conflicting results associated with oxidative stress and some exercise components of RE (e.g. intensity, exercise volume) influenced the design of this study. Additionally, it has been shown that oxidative stress markers may respond differently in various conditions [1]. Understanding how oxidative stress and the antioxidant defence system respond to the exercise intensity during RE in trained and untrained men may therefore provide new information on the physiological effects of RE training. Given the above information, the purposes of this study were 1) to examine time-dependent effects of progressive exercise intensity during RE on oxidative stress biomarkers and antioxidants, 2) to determine effects of training status (RE trained and untrained groups) on examined variables, and 3) to investigate a possible threshold intensity required to evoke oxidative stress, assessed by different biomarkers; for this reason, while the exercise intensity was increasing, the set numbers and repetition count were designed to expose equal session work (exercise volume).
MATERIALS AND METHODS
Participants
RE trained (RET group, n=8) and untrained males (UT group, n=8) participated in this study. Inclusion criteria were: non-tobacco user, free of any antioxidant supplements for at least six weeks before the start of this study [13], maximal oxygen consumption (VO2max) less than 60 ml · kg-1 · min-1 [14], no RE experience for UT group, and minimum of one year continuous whole body RE experience for RET group, best classified as recreationally trained [14, 15]. Major exclusion criteria were current use of any medicine (ergogenic or dietary aid), and following a reduced caloric intake diet that could alter the metabolism [15]. Participants completed a health history questionnaire before enrolment to ensure that there were no existing health risks and to quantify each subject's physical activity status [13, 15]. Written informed consent was obtained from each participant. All participants were completely informed about the study. The study was conducted according to the Declaration of Helsinki and approved by the Ethic Committee of Eskişehir Osmangazi University.
Anthropometric data collection and evaluation of VO2max
Participants’ heights were measured using a stadiometer (Holtain, UK) and participants’ weights, BMI (kg · m-2) and percent body fat (%) were estimated using a bioelectrical impedance analyser (Tanita MC-180-MA, Japan) (Table 1) [16]. After collecting the anthropometric data, each participant underwent a graded treadmill exercise test – using the Bruce protocol – to determine VO2max [3, 14]. Oxygen consumption, carbon dioxide production and heart rate were measured continuously using the computerized, breath-by-breath analysing system Master-Screen-CPX (Care Fusion, Germany).
TABLE 1.
Descriptive data of trained (RET) and untrained (UN) participants
| RET group (n=8) |
UN group (n=8) |
|
|---|---|---|
| Anthropometric data | ||
| Age (years) | 25.50 ± 4.72 | 29.00 ± 5.87 |
| Height (cm) | 178.5 ± 3.09 | 176.5 ± 5.76 |
| Weight (kg) | 79.46 ± 9.72 | 86.31 ± 12.33 |
| Percent Body Fat (%) | 16.27 ± 6.24* | 23.75 ± 5.38 |
| Body Mass Index (kg·m-2) | 24.91 ± 2.56 | 27.72 ± 3.58 |
| Exercise performance | ||
| VO2max (ml·kg-1·min-1) | 46.52 ± 8.61* | 36.23 ± 4.18 |
| 1 RM for leg extension (kg) | 137.4 ± 19.9* | 110.5 ± 17.7 |
| Relative strength [1RM (kg) / weight (kg)] | 1.742 ± 0.27** | 1.293 ± 0.22 |
| RE experience (years) | 5.313 ± 3.40 | -- |
| RE experience (day·week-1) | 3.625 ± 1.18 | -- |
| Resting SBP (mmHg) | 111.2 ± 12.2 | 112.5 ± 11.0 |
| Resting DBP (mmHg) | 70.00 ± 5.97 | 73.75 ± 5.17 |
Note: Data are presented as mean ± SD. VO2max: maximal oxygen consumption; 1RM: 1 repetition maximal of leg extension resistance exercise; RET: resistance exercise trained; UN: untrained; SBP: systolic blood pressure; DBP: diastolic blood pressure.
p<0.05
p<0.01 significantly different from UN group's value.
Determination of one-repetition maximum (1RM) and familiarization
Three to five days following the VO2max evaluation, the 1RM strength for leg extension RE was determined according to the Brzycki formula [17]: “predicted 1RM=weight lifted · [1.0278-(0.0278 x the number of repetitions performed)]-1”. The 1RM can be determined by attempting the 1RM test with heavy weights (near to maximum), which may cause an inordinate amount of stress on the muscles, bones, and connective tissues and may cause muscle soreness or even more serious injury [17, 18]. However, the 1RM lift is a highly specialized skill that requires a great deal of technique [17], and it is not recommended for novice lifters without any previous experience, such as our untrained participants. Instead, submaximal weights with multiple repetitions were used to predict the 1RM using the Brzycki formula [17]. The leg extension was chosen because it activates large muscle groups during the movement, uses a similar muscle mass as the squat exercise [13, 19, 20], which was used in previous studies, and produces results that are easy to compare.
All participants were instructed to refrain from strenuous physical tasks during the 24 hours prior to 1RM testing. Participants sat upright and performed the leg extension exercise following the cadence of an audible metronome set to 45 beats per minute while keeping their hands crossed in front of their chest to avoid the supporting their upper body with their arms. Following assessment of 1RM, participants rested and the test procedure was explained in detail. The participants were familiarized with the first two steps of the test [16, 19].
Resistance exercise test
Three to seven days following the familiarization, the leg extension exercise was performed in progressive intensities standardized for total volume: 1x17 reps at 50% of 1RM; 1x14 reps at 60% of 1RM; 1x12 reps at 70% of 1RM; 2x5 reps at 80% of 1RM; 3x3 reps at 90% of 1RM with 5 minutes of rest between intensities and 90-120 seconds of rest between sets. Participants were instructed to avoid strenuous exercise for the 48 hours prior to the test [14, 20] and for the 24 hours following the test, during the recovery time. All testing was completed in the morning (09.00–12.00 am) after 8-10 hours of overnight fasting [19]. After 10-15 minutes of rest in a sitting position, systolic and diastolic blood pressures were read using a sphygmomanometer (Erka Perfect Aneroid, Germany). The participant then sat upright on leg extension equipment and placed one of his hands parallel to the ground on the armrest (modified for the experiment), with the other arm crossed in front of his chest to avoid using the arms to support the upper body. Stabilizing straps were placed around the thorax and the thighs. At that time, a 20-gauge cannula was placed in a superficial forearm vein to obtain a PRE test blood sample [13]. Following the warm-up with light loads, the test started. After completion of the test, participants sat for 60 minutes to obtain POST 30 minutes and POST 60 minutes blood samples.
Dietary analysis
All participants were instructed to maintain their normal dietary habits during the investigation and to complete a diet recall for three days before the test and for 24 hours following the test. The diet recalls were used to quantify the average intake of calories, protein, carbohydrate, fat, vitamin C, vitamin A, and vitamin E [14, 19], which were analysed using a computerized dietary assessment program (BEBİS, version 6.1, Turkey).
Blood collection
With the participants in an upright, seated position, blood samples were obtained before (PRE) and immediately after (within one minute) each intensity. In addition, POST 30 minutes, POST 60 minutes and POST 24 hour blood samples were obtained during the rest period. All blood samples (except POST 24 hour) were drawn using a 20-gauge cannula inserted into a superficial forearm vein, which was removed 60 minutes after the test. Eight ml of blood collected without the use of an anticoagulant were centrifuged at 2000g for 20 minutes at 4°C and 8 ml of blood collected into lithium-heparin tubes were centrifuged at 1000g for 15 minutes at 4°C. Serum and plasma samples were separated, aliquoted into tubes, and stored at -80°C until analysis. To perform GSH analyses, serum was deproteinized with the addition of 0.1% metaphosphoric acid (1:1 vol/vol), incubated for five minutes at room temperature and re-centrifuged at 2500g for three minutes. The supernatants were collected carefully and stored at -20°C until total GSH analysis.
Biochemical analysis
Blood lactate (LA) was analysed based on the enzymatic-amperometric measuring method and was administered with the aid of the technical device Biosen C-line (EKF Diagnostic GmbH, Germany). The FOX2 (ferrous oxidation with xylenol orange, version 2) method was used to study lipid peroxidation (LHP) in plasma [21]. The recipe for measuring LHP is as follows: 100 μM xylenol orange, 250 μM ammonium ferrous sulfate, 90% methanol (HPLC grade), 4 mM butylated hydroxytoluene, 25 mM H2SO4. 50 μl of the sample was added to 950 μL of FOX2 reagents, vortexed, and incubated at room temperature for 30 minutes. The absorbance was read at 560 nm after removing all flocculated material by centrifugation. The signal was read against a H2O2 standard curve (0-100 μmol: r2=0.98). The intra-assay coefficient of variation (CV) for LHP was 4.99% and all samples were studied on the same day.
Plasma protein carbonyls (PCO) were measured spectrophotometrically by using the Reznick and Packer method [22]. PCO groups react with 2,4-dinitrophenylhydrazine (DNPH) to generate chromophoric dinitrophenylhydrazones. DNPH was dissolved in HCl, and after the DNPH reaction, proteins were precipitated with an equal volume of 20% (w/v) trichloroacetic acid, and were washed three times with 4 ml of an ethanol/ethyl acetate mixture (1:1). Washings were achieved by mechanical disruption of pellets in the washing solution using a small spatula and re-centrifugation. Finally, the precipitates were dissolved in 6M guanidine–HCl solution and the absorbance was read at 360 nm, using the molar extinction coefficient of DNPH, ε=2.2 · 104 (M) l · mol-1 · cm-1. Protein contents were determined by the Biuret method [23] using a BSA standard curve (1-10 mg · ml-1; r2=0.99). The intra-assay CV for PCO was 4.33%. Serum 8-hydroxy-2’-deoxyguanosine (8-OHdG) was analysed using an enzyme-linked immunosorbent assay kit (ADI-EKS-350; Enzo Life Science, Germany; intra-assay CV: <10%). Serum total glutathione (GSH) and superoxide dismutase (SOD) activity were determined using commercial kits (Cayman Chemical, Ann Arbor, MI, USA; 703002 and 706002 respectively). Using a GSSG standard curve (0–8.0 μM GSSG equivalents 0–16.0 μM total GSH; r2=0.99; intra-assay CV: <2%), the total GSH concentration was calculated. The SOD assay measures all three types of SOD (Cu/Zn-, Mn-, and Fe-SOD). Using a bovine erythrocyte SOD (Cu/Zn) standard curve (0–0.25 U · ml-1; r2=0.99; intra-assay CV: 3.2%), the serum SOD activity was calculated.
Statistical analysis
All the data were tested for normal distribution with the Kolmogorov-Smirnov test. Independent sample t-tests were performed to determine possible group differences for the following variables: age, height, weight, percent body fat, BMI, VO2max, 1RM, relative strength (1RM/weight), dietary variables, and PRE test values of LA, LHP, PCO, 8-OHdG, total GSH, and SOD.
The effects of progressive intensity of RE on examined variables were analysed using mixed design (a mixture of between group and repeated measures variables) analysis of variance (ANOVA). In the event of a significant F-ratio (p<0.05), the Bonferroni and LSD (least significant difference) methods were applied to determine pairwise differences. A sample size of eight participants for both groups provided >96% statistical power (1-β) at an α level of 0.05 (2-tailed). Data are presented as mean ± SEM, except for descriptive data (Table 1) and dietary intake data (Table 2), which are reported as mean ± SD. All statistical analyses were performed using the 15.0 version of SPSS (Statistical Package for Social Sciences, SPSS Inc.) for Windows, and p<0.05 was accepted as statistically significant.
TABLE 2.
Dietary intakes assessed during the 3 days before the test and during the 24 hours after the test
| RET group (n=8) |
UN group (n=8) |
|
|---|---|---|
| Calories (kcal·day-1) | 2549.7 ± 407.5 | 2775.3 ± 325.9 |
| Carbohydrate (g) | 298.31 ± 47.31 | 343.66 ± 42.93 |
| Fat (g) | 103.59 ± 24.49 | 109.24 ± 18.48 |
| Protein (g) | 95.21 ± 23.98 | 95.41 ± 18.35 |
| Vitamin A (μg) | 1196.48 ± 481.03 | 1185.65 ± 332.14 |
| Vitamin C (mg) | 94.84 ± 40.18 | 87.71 ± 29.91 |
| Vitamin E (mg) | 20.41 ± 6.82 | 17.34 ± 2.03 |
Note: RET – resistance-exercise trained, UN – untrained. Vitamin A values provide retinol equivalents. Data are presented as mean ± SD. No significant differences were observed between groups.
RESULTS
The RET and the UN groups’ participants were not different in terms of age, height, weight, and BMI (p > 0.05), but they were different as regards percentage of body fat (p<0.05; Table 1). The VO2max, 1RM, and relative strength were significantly greater in the RET group compared to the UN group (p<0.05 and p<0.01, Table 1). No significant differences were observed for dietary variables between the two groups (Table 2). Additionally, PRE test values of LA, LHP, PCO, 8-OHdG, total GSH, and SOD were not significantly different between groups (p > 0.05).
Acute effects of resistance exercise
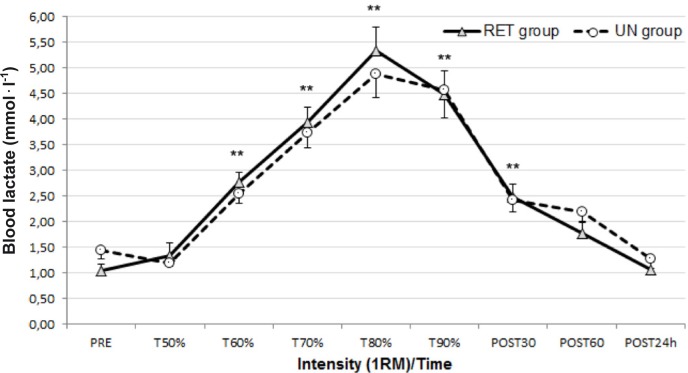
Blood LA concentration increased significantly and gradually in both groups (p<0.001; 1-β= 1.00) (Figure 1), in parallel with the rise of exercise intensity. This rise in blood LA was transient and returned to the pre-test value in the recovery period.
FIG. 1.
Blood lactate (LA) responses to resistance exercise before (PRE) and after each intensity and in recovery period (mean±SEM)
Note: RET – resistance-exercise trained, UN – untrained. **p<0.01 significantly different from PRE, T50% and POST 24 hours.
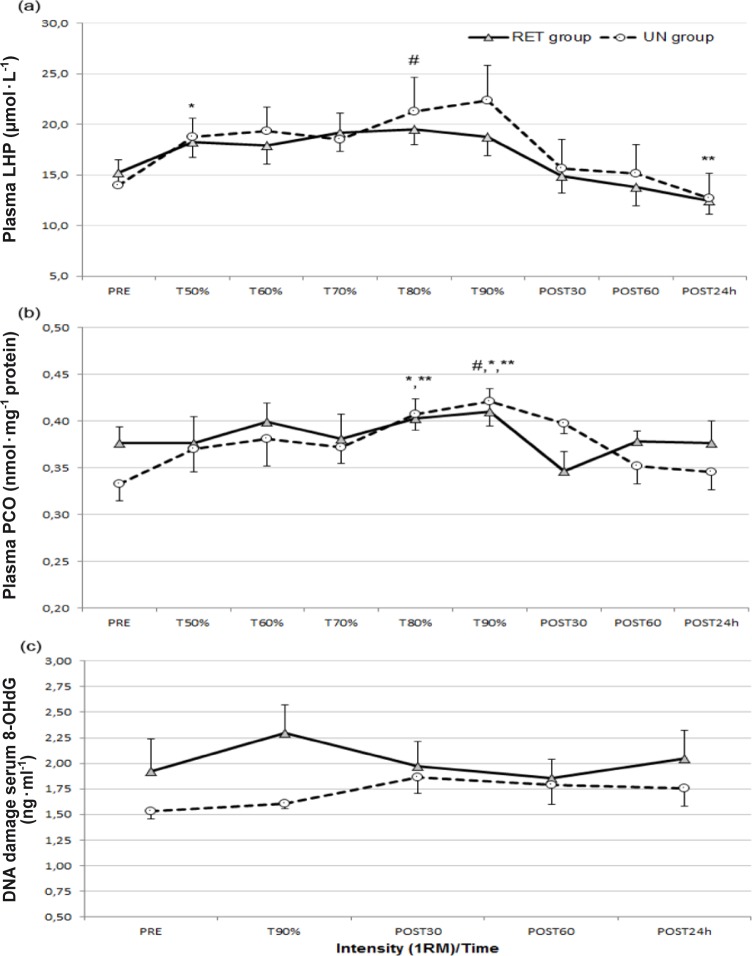
LHP, a product of lipid peroxidation, was used as an indirect marker for oxidative stress. The results indicated that plasma LHP significantly increased during the test and decreased in the recovery period in both groups (p<0.001; 1-β=0.99) (Figure 2a). As a result of the pairwise comparison, plasma T50% LHP values significantly increased in both groups compared to PRE LHP values (p<0.05). Mean LHP values increased from 15.25±2.90 (μmol · L-1) to 18.29±4.28 (μmol · L-1) in the RET group and increased from 13.99±7.05 (μmol · L-1) to 18.73±5.33 (μmol · L-1) in the UN group. The peak LHP concentration occurred at 80% of 1RM in the RET group and at 90% of 1RM in the UN group. The POST 24 hour LHP value was the lowest value measured during the experiment and it was significantly decreased in both groups compared to T50%, T70%, T80%, and T90% measurement times. In the recovery period (POST 30 min, POST 60 min and POST 24 h), the mean LHP value decreased from 14.89±4.66 (μmol · L-1) to 13.82±5.18 (μmol · L-1), and 12.47±3.84 (μmol · L-1) in the RET group whereas it decreased from 15.63±8.24 (μmol · L-1) to 15.19±8.10 (μmol · L-1) and 12.71±6.91 (μmol · L-1) in the UN group.
FIG. 2.
Oxidative stress biomarkers’ responses to progressive resistance exercise intensity (mean±SEM).
Note: RET – resistance-exercise trained, UN – untrained. (a): Lipid hydroperoxides (LHP) before (PRE) and after each intensity and in the recovery period; *p<0.05 significantly different from PRE value, **p<0.01 significantly different from T50%, T70%, T80%, and T90% values, #p<0.05 significantly different from POST 60 minute value. (b): Protein carbonyl (PCO) concentrations before (PRE) and after each intensity and in recovery period; *p<0.05 significantly different from POST 30 minute and POST 60 minute values, #p<0.05 significantly different from T50%, T60%, **p<0.01 significantly different from PRE, T70%, and POST 24 hour values. (c): DNA damage (8-OHdG) before (PRE) and after each intensity and in recovery period. No significant differences were observed.
The results indicated that plasma PCO significantly increased during the test in both groups (p<0.01; 1-β=0.96) (Figure 2b). The peak plasma PCO concentration occurred at 90% of 1RM in both groups. The rise of plasma PCO was transient and approached the pre-test value in the recovery period. As a result of the pairwise comparison the T90% PCO value differed significantly from all measurements (p<0.05 and p<0.01) except the T80% value. In addition, the T80% PCO value was significantly different from all measurements (p<0.05 and p<0.01) except T50%, T60%, and T90% values.
Statistical analysis of data indicated no significant main effect of progressive intensity for 8-OHdG, an indirect marker of DNA damage. The 8-OHdG level increased in both groups, but these increases were not significant (p > 0.05) (Figure 2c). The peak serum 8-OHdG concentration occurred at 90% of 1RM in the RET group (from 1.92±0.88 ng · ml-1 to 2.30±0.77 ng · ml-1) and at the POST 30 minutes measurement time in the UN group (from 1.53±0.20 ng · ml-1 to 1.86±0.45 ng · ml-1).
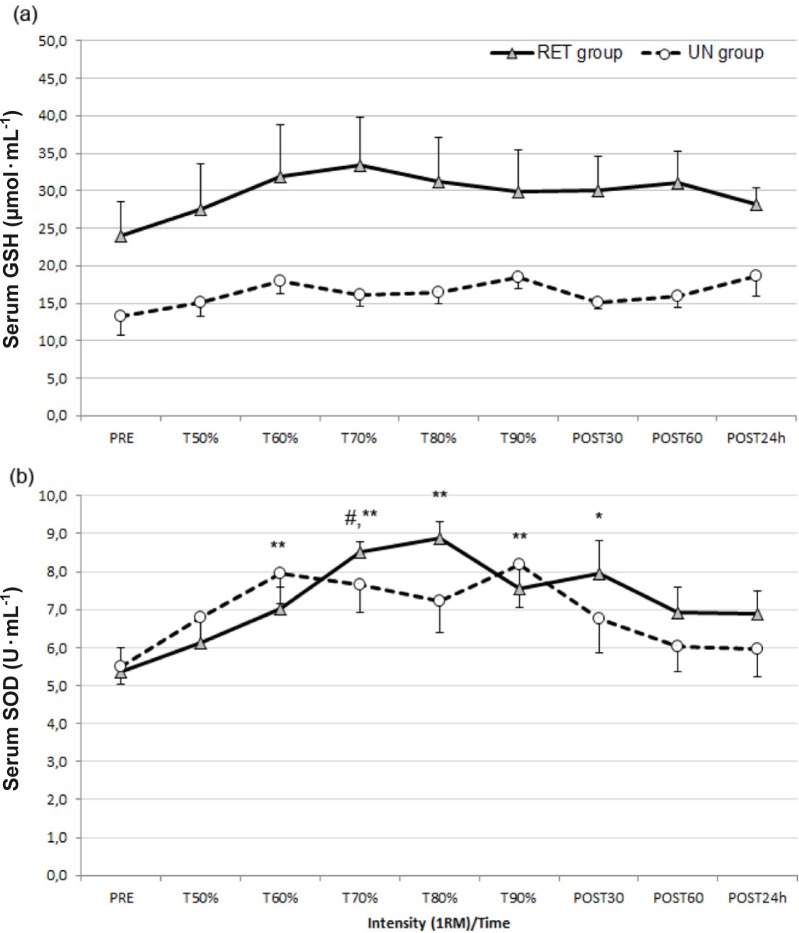
The results revealed no significant main effect of progressive intensity for total GSH (p > 0.05). Moreover, total GSH levels were higher in the RET group at all measurement points (Figure 3a). However, SOD significantly increased during the test (p<0.001; 1-β=0.99). The T60%, T70%, T80% T90%, and POST 30 minute SOD values were significantly higher than PRE SOD values (p<0.05 and p<0.01; Figure 3b). The T60% SOD value significantly increased from the PRE value of 5.36±1.8 U · ml-1 to 7.01±1.6 U · ml-1 in the RET group and from the PRE value of 5.49±1.2 U · ml-1 to 7.96±2.3 U · ml-1 in the UN group. The peak serum SOD concentration occurred at 80% of 1RM in the RET group (8.89±1.26 U · ml-1) and at 90% of 1RM in the UN group (8.20±3.30 U · ml-1).
FIG. 3.
Antioxidant responses to progressive resistance exercise intensity (mean±SEM).
Note: RET – resistance-exercise trained, UN – untrained. (a): Serum total glutathione (GSH) before (PRE) and after each intensity and in the recovery period. No significant differences were observed. (b): Serum superoxide dismutase (SOD) before (PRE) and after each intensity and in recovery period; *p<0.05 and **p<0.01 significantly different from PRE value, #p<0.05 significantly different from T50% value.
Group (training status) x intensity (time) interactions
One of the purposes of this study was to determine whether RE training performed regularly (training status) affects the time-dependent alterations of oxidative stress and antioxidants. Statistical analysis of data indicated that there were no significant group (training status) x intensity (time) interactions for LA, LHP, PCO, 8-OHdG, total GSH, and SOD concentrations. However, the interaction of training status x time for SOD approached statistical significance (p=0.069; 1-β=0.64).
DISCUSSION
To our knowledge, the effects of progressive resistance exercise intensity on oxidative stress biomarkers and antioxidants in resistance-trained and untrained men have not yet been studied. Likewise, minimum intensity required to evoke oxidative stress has not been defined. The results of the present study indicate that performing the leg extension RE one set at an intensity of 50% of 1RM was enough to evoke lipid peroxidation. Alternatively, higher intensity (≥80% of 1RM) was required to enhance protein oxidation, assessed by PCO. During the test, DNA damage (8-OHdG) and total GSH levels increased in both groups but these increases were not significant. Furthermore, the results showed that regular RE training increases the antioxidants such as GSH, which defends the system against oxidative damage. However, there were no significant group differences or group (training status) x intensity (time) interaction for oxidative stress biomarkers.
It has been shown that the lipid peroxidation (assessed by malondialdehyde – MDA) significantly increased following circuit RE training [24] and after performing the squat exercise at intensity of 60% and 90% 1RM [13] in RE-trained men. Another study equalizing the total work of the exercise showed that both strength (90% 1RM) and hypertrophy (75% 1RM) RE intensified oxidative stress responses in trained men [1]. In contrast to these studies, it has been observed that there was no alteration in the plasma MDA level following the squat exercise performed at 70% of 1RM in well-trained males whose 1RM was more than 1.5 times their body weight (6-7 sets [25]; 5-12 reps per set performed for 30 min [20]; 1x15 reps. [19]).
MDA is the most commonly used marker to determine lipid peroxidation in the exercise and oxidative stress literature. However, it is criticized in many ways because all lipid peroxidation products do not form MDA [15, 26, 27]. Additionally, it has been emphasized that MDA is removed from plasma following highly intensive exercise – in the recovery period – and therefore it is not a suitable indicator of oxidative stress for such exercises [28]. Due to these limitations specified for MDA, in the present study, LHP was used as an indicator of lipid peroxidation. Our data demonstrated that LHP significantly increased following the RE performed at 50% of 1RM in both groups when compared to the PRE LHP level (Figure 2a). Hudson et al. [1] identified that, although the exercise volume was equal, the LHP level did not change following the squat exercise performed at 90% of 1RM, but the LHP level increased significantly following the squat exercise performed at 75% of 1RM. Similarly, in the present study, the total work was equal for each load. As a result, performing one set of the leg extension exercise at 50% of 1RM was sufficient to intensify oxidative stress responses. In previous studies, moderate intensity (60%, 70-75% of 1RM) was commonly used. In the present study, the test protocol started with 50% of 1RM and compared it with classical training practice (low load – high total work, high load – low total work), while the exercise volume was kept quite low. Moreover, untrained individuals were included and the higher set numbers were not used. However, the plasma LHP level exceeded its initial value; the LHP level increased by 20% in the RET group and 33% in the UN group. Our data are in agreement with studies that found an increase in lipid peroxidation after acute RE [1, 13].
The study results indicated that squat exercise performed at 70% of 1RM did not affect the MDA level, but it increased the PCO level significantly (74% and 1.8 times the initial value) [19, 20]. Similarly, it has been determined that the squat exercise performed at 90% of 1RM [1] and the biceps curl and calf extension exercises performed at 70% of 1RM [15] significantly increased the PCO level in trained men. It has been reported that after acute eccentric exercise bouts and circuit resistance exercise training, the protein carbonyls significantly increased [29, 30]. Conversely, it has been reported that the PCO level did not change significantly in well-trained individuals (1RM ≥1.5 times the body weight) following the squat exercise performed at 70% of 1RM [25]. These conflicting results have been explained by a low exercise load (total 40±2 reps) and the high training status of individuals [25]. The findings of the present study indicated that the PCO level significantly increased following the leg extension performed at 80% and 90% of 1RM. The PCO level increased by 7.2% in the RET group and 22.5% in the UN group after a performance at 80% of 1RM, and it increased by 9.3% in the RET group and 26.4% in the UN group after a performance at 90% of 1RM (compared to the PRE level) (Figure 2b). Even though long rest periods were given between intensities, the elevation of PCO may be due to the cumulative exercising load. The significant increases determined at 80% and 90% of 1RM actually reflect the effects of prior practices (intensities) and the work done. In addition, the increased PCO level – as a result of the 40-minute exercise test – returned to its initial level after 24 hours by decreasing during the recovery period.
No significant alteration was recorded for 8-OHdG values following the squat exercise performed at two different occasions [19, 20]. In fact, the 8-OHdG level increased by 21% following the squat exercise [19] and it remained high even 24 hours after the RE [20]. However, these increases were not statistically significant. In contrast to the other markers examined in the current study, the change in the 8-OHdG level was examined before the test, after the test, and during the recovery period. Because of the short practice time of the test protocol (1 set x 17 reps etc.), the total effect at the end of the RE test was analysed. The results indicated that the 8-OHdG level increased by 19.5% and 5% in the RET and UN groups, respectively. Higher exercise volume, because of the higher 1RM, may explain the greater DNA damage in the RE-trained males. However, these differences were not statistically significant.
It has been reported that the GSH level did not increase significantly after circuit training (4%) but it did increase significantly after traditional interval training (14%) [16]. Our data indicated that RE performed in a single set (17 reps) increased the total GSH level. However, this increase was not statistically significant. First, the test protocol may not have been strenuous enough. Although blood LA values increased proportionally to the exercise load, they were very low when compared to the findings of Deminice et al. [16] (9.3 mM and 9.6 mM vs. 5.33 mM and 4.87 mM in the RET and UN groups respectively). Second, as previously mentioned, because of the untrained individuals, the classical training practices that cause high total work were not used.
The total GSH concentrations were observed to be higher in the RET group. The present study results showed that there were no statistically significant differences between the two groups at the beginning. However, the p value of 0.061 for GSH was very close to the value of 0.05 which was accepted as statistically significant. Antioxidant capacity can be modulated by diet; however, the diet analysis proved that vitamin A, vitamin C, and vitamin E consumption was similar for both groups. Hence, it has been accepted that the higher GSH level was not due to the diet but due to the training. It has been demonstrated that long-term RE training increases GSH and SOD levels while decreasing lipid peroxidation and DNA damage (8-OHdG) [4, 5, 31]. In the current study, the VO2max, 1RM, and relative strength values that give information about participants’ training status were significantly higher in the RET group.
Studies that compared trained and untrained subjects found that the MDA level did not change [14, 32], or it first decreased and then increased [33]. In concordance with these results [14, 32], our data showed that training status has no effect on any of the examined variables (LHP, PCO, 8-OHdG, and total GSH), and only the interaction of training status x intensity for the SOD level approached statistical significance (p=0.069).
Parker et al. [34] demonstrated that increasing exercise intensity resulted in a significantly greater biological antioxidant capacity at 70%, 85%, and 100% of VO2max compared to resting levels in untrained males. As compared to the current study, first, resistance exercise was used instead of aerobic exercise, second, both trained (experienced in resistance exercise training) and untrained males were included, and third, the exercise volume (the weight lifted and the work done) was kept equal at each step. To our knowledge, this is the only study that has examined the responses of FR and antioxidant capacity to the progressive intensity of RE.
CONCLUSIONS
Our data indicated that plasma LHP, PCO, and serum SOD concentrations significantly increased during the RE. On the other hand, there was no statistically significant difference between groups, and there were no statistically significant group (training status) x intensity (time) interactions for examined variables. Acute RE did not significantly increase the DNA damage, but working with higher exercise volume (lifting heavier weights) caused greater DNA damage as in the RE-trained males. Moreover, although the GSH values were not altered significantly during the test, it was observed that the GSH level was higher in trained men. Exercises performed at very low intensity (30%-40% of 1RM) may fail to develop adaptation and up-regulation in the antioxidant defence system. Therefore, when planning the RE training, different intensities are suggested for use in practice. Different oxidative stress biomarkers are affected by different exercise intensities so that instead of classical RE training planning (training for a week with a given exercise intensity and then increasing or decreasing the intensity), it seems more beneficial to use a combination of different intensities in a training unit.
Acknowledgements
This study was supported by Anadolu University Research Fund (1001S42) and was presented at 17th annual Congress of the European College of Sport Science, Bruges, Belgium, 2012. The authors would like to thank all the participants for their voluntary participation and to Professor of Financing Dr. Güven Sevil for his valuable help.
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Hudson MB, Hosick PA, McCaulley GO, Schrieber L, Wrieder J, McAnulty SR, Triplett NT, McBride JM, Quindry JC. The effects of resistance exercise on humoral markers of oxidative stress. Med Sci Sports Exerc. 2008;40(3):542–548. doi: 10.1249/MSS.0b013e31815daf89. [DOI] [PubMed] [Google Scholar]
- 2.Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7(1):34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Fatouros IG, Jamurtas AZ, Villiotou V, Pouliopoulou S, Fotinakis P, Taxildaris K, Deliconstantinos G. Oxidative stress responses in older men during endurance training and detraining. Med Sci Sports Exerc. 2004;36(12):2065–2072. doi: 10.1249/01.mss.0000147632.17450.ff. [DOI] [PubMed] [Google Scholar]
- 4.Parise G, Brose AN, Tarnopolsky MA. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp Gerontol. 2005;40(3):173–180. doi: 10.1016/j.exger.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Vincent KR, Vincent HK, Braith RW, Lennon SL, Lowenthal DT. Resistance exercise training attenuates exercise-induced lipid peroxidation in the elderly. Eur J Appl Physiol. 2002;87(4-5):416–423. doi: 10.1007/s00421-002-0640-2. [DOI] [PubMed] [Google Scholar]
- 6.Brites FD, Evelson PA, Christiansen MG, Nicol MF, Bası´lico MJ, Wikinski RW, Llesuy SF. Soccer players under regular training show oxidative stress but an improve plasma antioxidant status. Clin Sci (Lond) 1999;96(4):381–385. [PubMed] [Google Scholar]
- 7.Aguilo A, Tauler P, Fuentespina E, Tur JA, Cordova A, Pons A. Antioxidant response to oxidative stress induced by exhaustive exercise. Physiol Behav. 2005;84(1):1–7. doi: 10.1016/j.physbeh.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Tauler P, Aguilo A, Guix P, Jimenez F, Villa G, Tur JA, Cordova A, Pons A. Pre-exercise antioxidant enzyme activities determine the antioxidant enzyme erythrocyte response to exercise. J Sports Sci. 2005;23(1):5–13. doi: 10.1080/02640410410001716724. [DOI] [PubMed] [Google Scholar]
- 9.Fleck SJ, Kraemer WJ. Champaign, IL: Human Kinetics; 1997. Designing Resistance Training Programs. [Google Scholar]
- 10.Bloomer RJ, Schilling BK, Karlage RE, Ledoux MS, Pfeiffer RF, Callegari J. Effect of resistance training on blood oxidative stress in Parkinson disease. Med Sci Sports Exerc. 2008;40(8):1385–1389. doi: 10.1249/MSS.0b013e31816f1550. [DOI] [PubMed] [Google Scholar]
- 11.Rall LC, Roubenoff R, Meydani SN, Han SN, Meydani M. Urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG) as a marker of oxidative stress in rheumatoid arthritis and aging: Effect of progressive resistance training. J Nutr Biochem. 2000;11(11-12):581–584. doi: 10.1016/s0955-2863(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 12.Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity. 2006;14(11):1921–1930. doi: 10.1038/oby.2006.224. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman JR, Im J, Kang J, Maresh CM, Kraemer WJ, French D, Nioka S, Kime R, Rundell KW, Ratamess NA, Faigenbaum AD, Chance B. Comparison of low- and high-intensity resistance exercise on lipid peroxidation: Role of muscle oxygenation. J Strength Cond Res. 2007;21(1):118–122. doi: 10.1519/00124278-200702000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Dixon CB, Robertson RJ, Goss FL, Timmer JM, Nagle EF, Evans RW. The effect of acute resistance exercise on serum malondialdehyde in resistance-trained and untrained collegiate men. J Strength Cond Res. 2006;20(3):693–698. doi: 10.1519/R-15854.1. [DOI] [PubMed] [Google Scholar]
- 15.Goldfarb AH, Garten RS, Chee PD, Cho C, Reeves GV, Hollander DB, Thomas C, Aboudehen KS, Francois M, Kraemer RR. Resistance exercise effects on blood glutathione status and plasma protein carbonyls: influence of partial vascular occlusion. Eur J Appl Physiol. 2008;104(5):813–819. doi: 10.1007/s00421-008-0836-1. [DOI] [PubMed] [Google Scholar]
- 16.Deminice R, Sicchieri T, Mialich MS, Milani F, Ovidio PP, Jordao AA. Oxidative stress biomarker responses to an acute session of hypertrophy-resistance traditional interval training and circuit training. J Strength Cond Res. 2011;25(3):798–804. doi: 10.1519/JSC.0b013e3181c7bac6. [DOI] [PubMed] [Google Scholar]
- 17.Brzycki M. Strength testing - predicting a one-rep max from reps–to-fatigue. J Phys Educ Recreation Dance. 1993;64:88–90. [Google Scholar]
- 18.Niewiadomski W, Laskowska D, Gąsiorowska A, Cybulski G, Strasz A, Langfort J. Determination and prediction of one repetition maximum (1RM): Safety considerations. J Hum Kinetics. 2008;19:109–120. [Google Scholar]
- 19.Bloomer RJ, Fry AC, Falvo MJ, Moore CA. Protein carbonyls are acutely elevated following single set anaerobic exercise in resistance trained men. J Sci Med Sport. 2007;10(6):411–417. doi: 10.1016/j.jsams.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Bloomer RJ, Goldfarb AH, Wideman L, McKenzie MJ, Consitt LA. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J Strength Cond Res. 2005;19(2):276–285. doi: 10.1519/14823.1. [DOI] [PubMed] [Google Scholar]
- 21.Strength testing - predicting a one-rep max from reps–to-fatigue. Wolff SP. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. [Google Scholar]
- 22.Strength testing - predicting a one-rep max from reps–to-fatigue. Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 23.Gornall AG, Bardawill CJ, David MN. Determination of serum proteins by means of the Biüret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 24.McBride JM, Kraemer WJ, Triplett-McBride T, Sebastianelli W. Effect of resistance exercise on free radical production. Med Sci Sports Exerc. 1998;30(1):67–72. doi: 10.1097/00005768-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Bloomer RJ, Falvo MJ, Fry AC, Schilling BK, Smith WA, Moore CA. Oxidative stress response in trained men following repeated squats or sprints. Med Sci Sports Exerc. 2006;38(8):1436–1442. doi: 10.1249/01.mss.0000227408.91474.77. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez MC, Rosenfeld J, Tarnopolsky MA. Plasma malondialdehyde increases transiently after ischemic forearm exercise. Med Sci Sports Exerc. 2003;35(11):1859–1865. doi: 10.1249/01.MSS.0000093609.75937.70. [DOI] [PubMed] [Google Scholar]
- 27.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1-2):41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 28.Groussard C, Rannou-Bekono F, Machefer G, Chevanne M, Vincent S, Sergent O, Cillard J, Gratas-Delamarche A. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur J Appl Physiol. 2003;89(1):14–20. doi: 10.1007/s00421-002-0767-1. [DOI] [PubMed] [Google Scholar]
- 29.Cardoso AM, Bagatini MD, Roth MA, Martins CC, Rezer JF, Mello FF, Lopes LF, Morsch VM, Schetinger MR. Acute effects of resistance exercise and intermittent intense aerobic exercise on blood cell count and oxidative stress in trained middle-aged women. Braz J Med Biol Res. 2012;45(12):1172–1182. doi: 10.1590/S0100-879X2012007500166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margaritelis NV, Kyparos A, Paschalis V, Theodorou AA, Panayiotou G, Zafeiridis A, Dipla K, Nikolaidis MG, Vrabas IS. Reductive stress after exercise: The issue of redox individuality. Redox Biol. 2014;2:520–528. doi: 10.1016/j.redox.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Çakır-Atabek H, Demir S, Pınarbaşılı RD, Gündüz N. Effects of different resistance training intensity on indices of oxidative stress. J Strength Cond Res. 2010;24(9):2491–2497. doi: 10.1519/JSC.0b013e3181ddb111. [DOI] [PubMed] [Google Scholar]
- 32.Ramel A, Wagner KH, Elmadfa I. Plasma antioxidants and lipid oxidation after submaximal resistance exercise in men. Eur J Nutr. 2004;43(1):2–6. doi: 10.1007/s00394-004-0432-z. [DOI] [PubMed] [Google Scholar]
- 33.Viitala PE, Newhouse IJ, LaVoie N, Gottardo C. The effects of antioxidant vitamin supplementation on resistance exercise induced lipid peroxidation in trained and untrained participants. Lipids Health Dis. 2004;3:14. doi: 10.1186/1476-511X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker L, McGuckin T, Leicht A. Influence of exercise intensity on endogenous oxidative stress and antioxidant capacity. J Sci Med Sport. 2012;15:S13–S14. [Google Scholar]