Abstract
The poor efficacy of the in vivo anti-tumor immune response has been partially attributed to ineffective T-cell responses mounted against the tumor. Fas-FasL-dependent activation-induced cell death (AICD) of T cells is believed to be a major contributor to compromised anti-tumor immunity. The molecular mechanisms of AICD are well-investigated, yet the possibility of regulating AICD for cancer therapy remains to be explored. In this study, we show that histone deacetylase inhibitors (HDACIs) can inhibit apoptosis of CD4+ T cells within the tumor, thereby enhancing anti-tumor immune responses and suppressing melanoma growth. This inhibitory effect is specific for AICD through suppressing NFAT1-regulated FasL expression on activated CD4+ T cells. In gld/gld mice with mutation in FasL, the beneficial effect of HDACIs on AICD of infiltrating CD4+ T cells is not seen, confirming the critical role of FasL regulation in the anti-tumor effect of HDACIs. Importantly, we found that the co-administration of HDACIs and anti-CTLA4 could further enhance the infiltration of CD4+ T cells and achieve a synergistic therapeutic effect on tumor. Therefore, our study demonstrates that the modulation of AICD of tumor-infiltrating CD4+ T cells using HDACIs can enhance anti-tumor immune responses, uncovering a novel mechanism underlying the anti-tumor effect of HDACIs.
Introduction
Tumors are composed of many different cell types, among which immune cells are claimed to play a critical role in controlling tumor growth.1 During tumor development, immune cells, especially tumor-infiltrating T lymphocytes (TILs), secrete an array of cytokines that can kill tumor cells directly.2 Owing to the important role of immune system in eliminating potential tumor cells, immunotherapy is considered as a very promising strategy for treating tumors. For instance, the adoptive transfer of TILs has been shown to dramatically enhance tumor rejection in some settings.3, 4 Furthermore, antibodies against cytotoxic T-lymphocyte antigen 4 (CTLA4), programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) have been shown to be very effective in treating cancers, a result of enhanced anti-tumor immunity by TILs.5, 6, 7
However, tumor cells are not always successfully eliminated by immune responses. One mechanism is that even as T cells continually migrate into tumor sites, they often undergo apoptosis prior to being able to carry out their anti-tumor functions.8 Among the mechanisms underlying T-cell apoptosis, activation-induced cell death (AICD) is very important as a normal control mechanism for immune response. AICD was first described in 1989 and is considered critical for regulating T-cell viability and immune homeostasis.9 We have shown that activated CD4+ T cells undergo AICD upon re-stimulation. Re-stimulation rapidly induces FasL (CD95L) expression, and FasL-Fas interaction triggers the caspase cascade, leading to T-cell apoptosis.9, 10 Importantly, the impairment of FasL-Fas pathway in humans affects lymphocyte apoptosis and leads to the autoimmune lymphoproliferative syndrome, which is characterized by the accumulation of activated lymphocytes and autoimmune disease.11 Owing to this important role of FasL-mediated AICD in controlling immune response, the possibility of regulating AICD for improved cancer immunotherapy requires further exploration.
Histone deacetylase inhibitors (HDACIs) are small molecules that inhibit the activity of histone deacetylases (HDACs). In recent years, HDACIs have entered the clinic as anti-tumor drugs. Vorinostat, a synthetic compound that is structurally similar to the first-described natural HDACI, trichostatin A (TSA), was the first FDA-approved HDAC inhibitor for the treatment of relapsed and refractory cutaneous T-cell lymphoma. Many other HDACIs are currently in clinical trials, either as mono-therapies or in combination with conventional chemotherapy.12, 13, 14 Still, the mechanisms underlying their therapeutic effects remain elusive.15 Interestingly, substantial evidence has shown that HDACIs can induce apoptosis in a variety of cell types through different mechanisms.16, 17 The role of HDACIs in AICD is unclear, however, and whether this role contributes to their potential utility in tumor therapy remains to be determined.
In this study, we employed TSA, and found that it significantly suppressed the growth of B16F0 melanoma through inhibiting apoptosis of activated CD4+ T lymphocytes within tumor. Furthermore, this effect of TSA was exerted through specifically downregulating FasL expression on infiltrating CD4+ T cells, which resulted in enhanced anti-tumor immune response. This role of FasL was further evidenced by the fact that TSA provided no benefit in the treatment of tumor-bearing gld/gld mice. Importantly, we found that TSA and CTLA4 antibody acted synergistically to greatly enhance CD4+ T-cell infiltration, and together may offer better tumor therapeutic effects than either agent alone. Our findings reveal a novel mechanism underlying the anti-tumor effect of HDACIs, which is inhibiting AICD of tumor-infiltrating CD4+ T lymphocytes.
Results
TSA inhibits tumor growth by promoting the survival of infiltrating CD4+ T cells
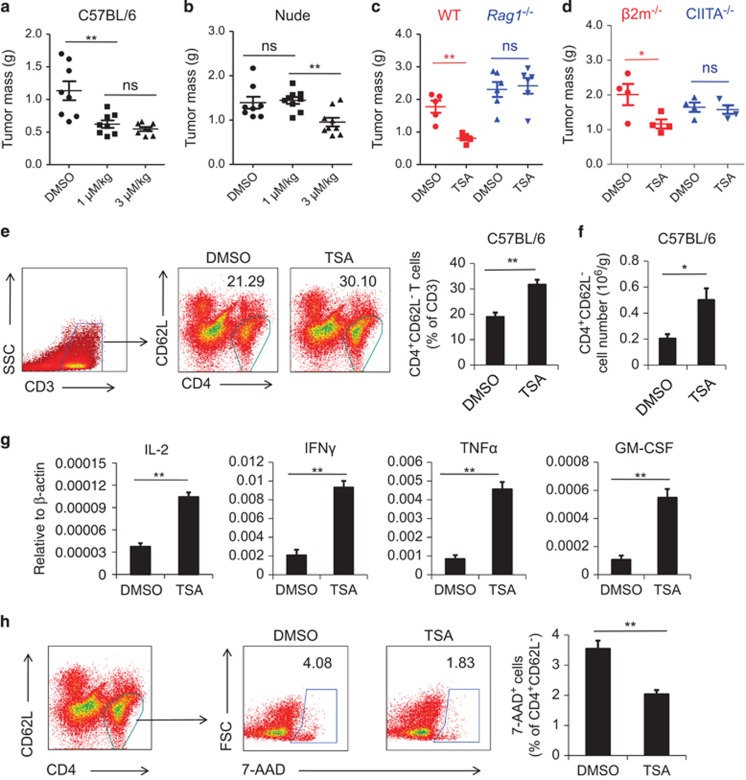
As tumor-infiltrating lymphocytes (TILs) are essential in eliminating tumor cells, we wondered whether HDACIs exert their therapeutic effect through TILs. We tested this possibility in the B16F0 melanoma model established on both C57BL/6 mice and nude mice. To determine the optimal concentration of TSA, two doses of TSA were administrated; 1 μM/kg (referred to as ‘low dose') and 3 μM/kg, the concentration widely used for experimental tumor treatment (referred to as ‘high dose').18 We found that in C57BL/6 mice, regardless of concentration, TSA administration resulted in significantly smaller tumors compared with vehicle alone (Figure 1a). Surprisingly, when similar studies were conducted in nude mice, which lack a thymus and therefore have no T cells, the therapeutic effect of low-dose TSA was completely absent whereas the effect of high-dose TSA remained (Figure 1b). This suggested that the anti-tumor effect of low-dose TSA was exerted through the immune system, most likely through T cells; whereas that of high-dose TSA was independent of T-cell immunity, possibly by directly inducing tumor cell death as previously reported.18 To test these possibilities, we first employed Rag1−/−mice, which have no T lymphocytes. As expected, there was no anti-tumor effect at low-dose TSA (Figure 1c). Next, we stimulated B16F0 cells with different concentrations of TSA in vitro and found as high as 200 nM TSA could indeed induce tumor cell death (Supplementary Figure 1). As lower TSA concentrations should induce fewer side effects and be less toxic, we adopted 1 μM/kg TSA for the subsequent experiments.
Figure 1.
TSA inhibits B16F0 melanoma growth in vivo through promoting the survival of CD4+ T lymphocytes. (a, b) B16F0 cells (4 × 105) were injected intramuscularly into the left thigh of C57BL/6 mice or nude mice (n=8 each). From day 6, 1 μM/kg or 3 μM/kg TSA was injected i.p. every 2 days, with dimethyl sulfoxide (DMSO) used as a control. On day 12, mice were killed and the resultant tumors were excised and weighed. (c) DMSO and 1 μM/kg TSA were administrated as before on C57BL/6 mice (n=5) or Rag1−/− mice (n=6), and tumors were excised and weighed after 12 days. (d) B16 melanoma was established as in (a) on β2m−/− mice or CIITA−/−mice (n=4 each). TSA (1 μM/kg) was administrated as in (a) and resultant tumors were weighed. (e)Tumors from DMSO group and 1 μM/kg TSA-treated group were mechanically minced for the isolation of infiltrating lymphocytes. Cells were counted and stained for flow cytometry to determine the percentage of CD4+CD62L− lymphocytes. (f) Absolute number of CD4+CD62L− cells per gram tumor was calculated. (g) CD4+CD62L− cells were isolated from infiltrating lymphocytes by isolation kit. The expression of IFNγ, TNFα, IL-2 and GM-CSF by isolated cells were examined by real-time PCR. (h) TILs isolated and identified as CD4+CD62L− cells were also stained for 7-AAD. The extent of apoptosis by activated CD4+ T cells was determined by the percentage of 7-AAD+, as shown. Statistical analysis was performed using the Student's t-test. *P<0.05, **P<0.01; ns, not significant.
To identify which subset of T cells is essential for the anti-tumor effect of TSA, we employed CIITA−/− mice that have no CD4+ T cells, and β2m−/− mice that lack CD8+ T cells. Interestingly, without CD4+ T cells, low-dose TSA had no effect; in the absence of CD8+ T cells, however, TSA did exert significant therapeutic effect (Figure 1d). Therefore, CD4+ T cells are indispensable for the anti-tumor effect of TSA.
Owing to the important role of CD4+ T cells, we next explored the infiltration of activated CD4+ T lymphocytes within the tumor microenvironment in wild-type mice. Interestingly, TSA administration resulted in significant increases in the fraction of CD4+ CD62L− activated T cells in tumor tissue (Figure 1e). Besides, histological analysis also showed elevated infiltration of T cells in tumor (Supplementary Figure 2). Most of these cells were also positive for CD44, which is an indicative marker for effector memory T cells (Supplementary Figure 3). More importantly, the absolute number of these cells per gram of tumor also increased (Figure 1f). We further isolated CD4+ CD62L− cells from tumor and tested the expression of cytokines associated with T cell activation, including interleukin-2 (IL-2), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and granulocyte-macrophage colony stimulating factor (GM-CSF). We found that TSA could significantly increase the expression of these cytokines (Figure 1g). Elevated serum levels of these cytokines were also detected (Supplementary Figure 4). Taken together, TSA administration results in increased CD4+ T cells within tumor tissue, which leads to enhanced anti-tumor immune responses and constrained tumor growth.
To further explore the reasons responsible for the increased activated CD4+ T cells, we examined the apoptotic status of these cells within tumor. Interestingly, we found that the TSA treatment dramatically reduced CD4+ T cell death, as revealed by their permeability to 7-AAD (Figure 1h). Therefore, TSA administration increased the number of activated CD4+ T cells within the tumor possibly through inhibiting apoptosis.
HDAC inhibition promotes the survival of T-cell hybridoma by blocking AICD
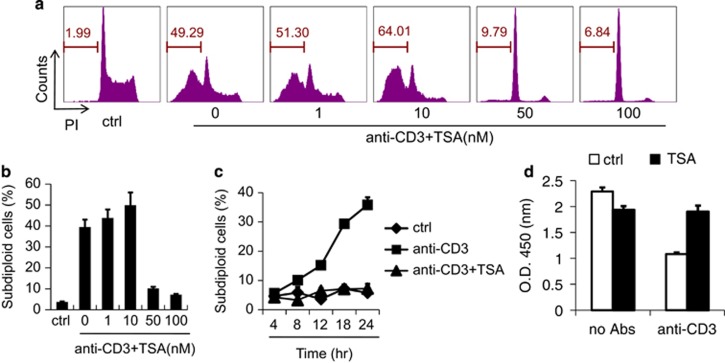
As we demonstrated, TILs undergo decreased apoptosis in response to TSA treatment. As AICD is one of the major mechanisms underlying the apoptosis of TILs, we hypothesize that this is through the inhibition of AICD happening in tumor microenvironment. To this end, we examined the effect of HDAC inhibition on AICD in vitro. We employed A1.1 cells, a T-cell hybridoma that is well established for the investigation of AICD in T lymphocytes.19 AICD of A1.1 cells was induced by TCR ligation using immobilized anti-CD3. The addition of TSA at doses of 50–100 nM resulted in a striking reduction in AICD (Figures 2a and b), a sharp contrast to previous reports of the pro-apoptotic role of TSA.16 TSA alone showed no effect on the survival of A1.1 cells (Supplementary Figure 5). Furthermore, the inhibitory effect of TSA was apparent soon after re-activation (Figure 2c). To corroborate these findings, we employed another HDACI, sodium butyrate (NaBu), and observed similar results (Supplementary Figure 6). To confirm that cell death was indeed reduced by TSA treatment, we measured cell viability using CCK-8 and found a nearly complete reversal of cell death by TSA (Figure 2d). Therefore, inhibition of HDAC activity using HDACIs can protect T cells from AICD and thereby greatly promote their survival.
Figure 2.
Inhibition of HDAC protects T cells from AICD and promotes their survival. (a) A1.1 T cell hybridoma cells were cultured in the presence or absence of the indicated concentrations of TSA in 24-well plates that had been previously coated with anti-CD3 (10 μg/ml) to induce AICD. After 24 h, cells were harvested and DNA content was revealed by staining with propidium iodide and analyzed by flow cytometry to determine the proportion of subdiploid cells (representative experiment). (b) The statistical results of three separate experiments described in a. (c) The percentage of apoptotic cells at various time points after culture as in a. (d) After 20 h of culture as in a in the presence of 100 nM TSA, A1.1 cell viability was evaluated by cellular dehydrogenase activity using CCK-8. Values represent mean ±s.e.m. of three separate experiments performed in duplicate wells, for this and all other figures, unless otherwise indicated.
HDACIs suppress TCR-initiated FasL expression in T-cell hybridoma
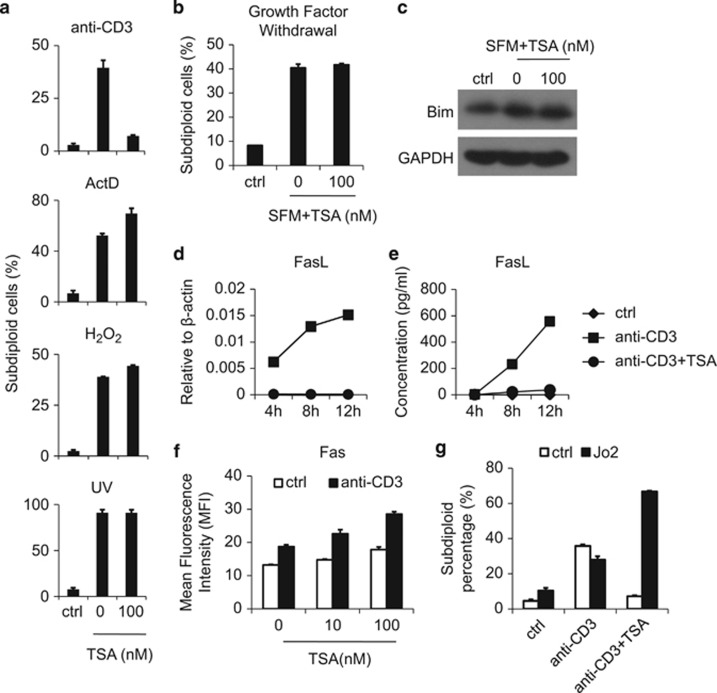
TILs may be susceptible to other types of apoptosis besides AICD; therefore, we next examined the effect of TSA on apoptosis induced by other stimuli. A1.1 cells were exposed to actinomycin D (ActD), hydrogen peroxide (H2O2) or UV irradiation, which are all reported to induce tumor cell death, each through a different mechanism.20, 21 Interestingly, following these cellular insults, TSA treatment did not reduce the proportion of apoptotic cells in A1.1 cell cultures (Figure 3a). Moreover, TSA could not inhibit A1.1 cell death induced by serum deprivation. Thus, TSA could not block growth factor withdrawal-induced T-cell death, one of the mechanisms underlying T-cell death within the tumor microenvironment22, 23 (Figure 3b). Also, levels of Bim, the molecule mediating growth factor withdrawal-induced T-cell death, were unaffected in the presence of TSA (Figure 3c). Therefore, the inhibitory effect of TSA is specific to AICD.
Figure 3.
TSA specifically suppresses FasL expression. (a) A1.1 cells were treated with immobilized anti-CD3 (10 μg/ml), actinomycin D (ActD, 5 μg/ml), hydrogen peroxide (H2O2, 80 mM) or UV irradiation (80 J/m2) in the presence or absence of 100 nM TSA. After 24 h, the apoptotic rate was determined by the proportion of subdiploid cells. (b, c) A1.1 cells were cultured in complete medium or serum-free medium (SFM) with or without TSA (100 nM) treatment. After 48 h, cells were collected for either apoptosis measurement according to subdiploid DNA content or the expression of Bim protein by western blot. (d, e) AICD was induced on A1.1 cells with or without 100 nM TSA, and cells and supernatant were collected at the indicated time points for assay of FasL protein and mRNA by ELISA and real-time PCR, respectively. (f) A1.1 cells were treated with indicated doses of TSA with or without anti-CD3 for 24 h, and cellular Fas expression was analyzed by immunofluorescence staining and flow cytometry. (g) A1.1 cells were treated with anti-CD3 with or without TSA (100 nM) and soluble FasL (Jo2, 8 μg/ml) for 24 h, and apoptosis was determined according to subdiploid DNA content.
It has been shown in A1.1 cells that TCR re-stimulation induces FasL expression, leading to Fas ligation and resultant apoptosis.10, 24 To this end, we next examined the effects of TSA on FasL expression. We found that TSA completely blocked FasL upregulation at both mRNA and protein levels (Figures 3b and c). Similarly, NaBu was also found to inhibit FasL expression (Supplementary Figure 7). When CD90 (Thy-1) antibody was used to activate A1.1 cells, we again found that TSA completely inhibited subsequent FasL expression and apoptosis (Supplementary Figure 8), providing further evidence that HDACIs exert their anti-apoptotic effect by preventing FasL upregulation. In contrast, Fas expression was not suppressed by HDACIs; instead, HDACIs enhanced Fas in a dose-dependent manner (Figure 3d). To further verify the role of FasL in the inhibitory effect of TSA on AICD, we next employed the FasL mimic Jo2. As expected, TSA did not inhibit Jo2-induced cell death (Figure 3e), indicating that the Fas response is unimpeded by TSA; rather, it is the lack of FasL that prevents AICD. Interestingly, we found that in the presence of Jo2, a Fas-activating antibody, TSA dramatically increased the apoptotic rate of A1.1 cells, a finding which may be explained by the elevated level of Fas expression observed following TSA treatment. These results demonstrate that HDACIs inhibit FasL expression by activated A1.1cells, which, despite the increased Fas expression, leads to complete inhibition of AICD.
TSA protects primary CD4+ T cells from AICD by inhibiting FasL expression
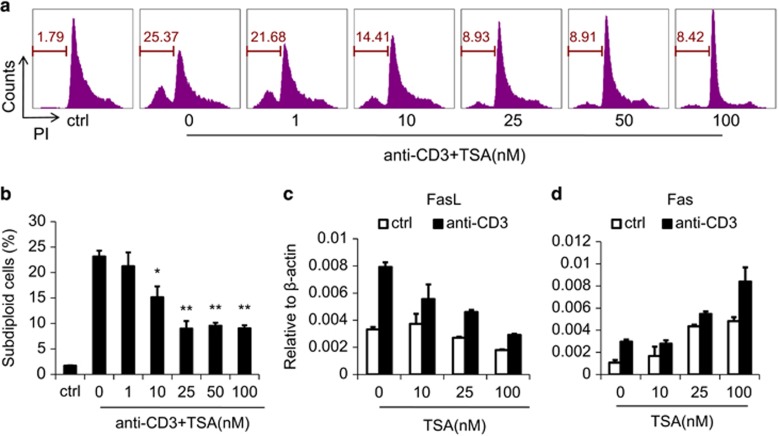
We have shown above that HDAC inhibitors can dramatically suppress AICD of A1.1 cells by inhibiting the expression of FasL. To extend this finding to physiologically more relevant primary CD4+ T cells, we generated CD4+ T cell blasts. We found that the AICD of CD4+ T-cell blasts was also suppressed by TSA (Figures 4a and b), whereas TSA alone showed no effect (Supplementary Figure 9), similar to the T-cell hybridoma results. Significant inhibition occurred at a dose as low as 10 nM and complete inhibition required only 25 nM TSA. Furthermore, during AICD, FasL and Fas were regulated by TSA much as they were in A1.1 cells (Figures 4c and d). Thus, TSA similarly inhibits AICD in both primary CD4+ T cells and A1.1 hybridoma cells through the regulation of FasL expression.
Figure 4.
TSA suppresses AICD of CD4+ T-cell blasts by inhibiting FasL expression. (a) CD4+ T-cell blasts (2.5 × 105), generated as described, were cultured with the indicated concentrations of TSA in anti-CD3 pre-coated 24-well plates for 12 h and apoptosis revealed by subdiploid DNA content. (b) Result of three separate experiments, as in a. (c, d) CD4+ T-cell blasts were cultured as in a, harvested and prepared for quantitation of FasL and Fas by real-time PCR. Statistical analysis was performed using Student's t-test. *P<0.05, **P<0.01.
TSA inhibits FasL expression by regulating NFAT1 and Egr2
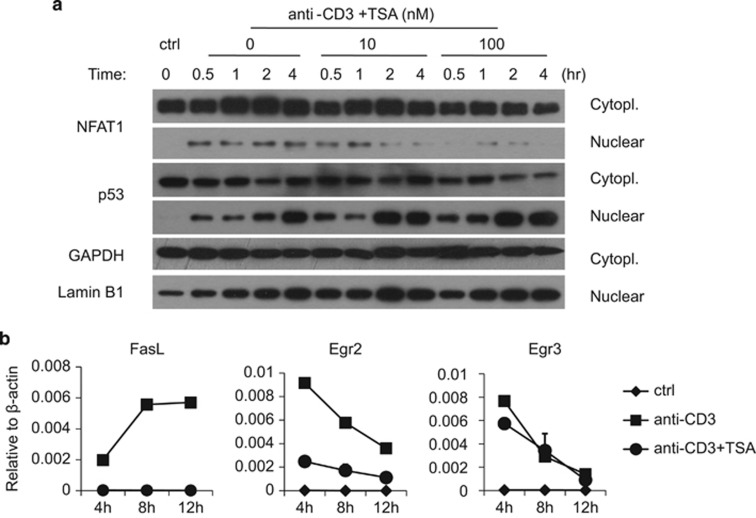
It is well known that TCR activation triggers two separate pathways, calcium/nuclear factor of activated T cells (NFAT) signaling and PKC signaling.25 It has been proposed that NFAT1 (NFATp, NFATc2) and PKC are both required for the expression of FasL, whereas Fas expression needs only PKC.26, 27, 28 As TSA specifically inhibits FasL but not Fas, we examined the activity of the key molecule, NFAT1. Cytoplasmic and nuclear exacts were prepared from anti-CD3 re-activated A1.1 cells to examine the distribution of NFAT1. We found that with high concentrations of TSA, nucleus-associated NFAT1 was decreased, but not p53 or p65, which both regulate Fas expression during AICD29, 30 (Figure 5a, Supplementary Figure 10). These findings indicate that the inhibitory effect of TSA on FasL expression is closely related to the specific suppression of NFAT1 activity.
Figure 5.
TSA inhibits FasL expression in an NFAT1- and Egr2-dependent manner. (a) A1.1 cells were treated with immobilized anti-CD3 and graded concentrations of TSA. At indicated time points, cytoplasmic (cytopl.) and nuclear fractions were extracted and the expression of NFAT1, p53, GAPDH and lamin B1 were analyzed by western blot. A representative of three experiments is shown. (b) A1.1 cells were incubated with or without anti-CD3 re-activation in the presence or absence of 100 nM TSA. Cells were harvested at indicated time points and the expression of FasL, Egr2 and Egr3 mRNA was analyzed by real-time PCR.
It has been reported that NFAT1 translocation induces expression of early growth factor 2 and 3 (Egr2/3), which would directly bind to the FasL promoter and launch FasL expression.28 Accordingly, to verify the effect of TSA on NFAT1 translocation, we next examined Egr2/3 expression. We found that TSA inhibited the expression of NFAT-induced Egr2, but not Egr3, indicating that Egr2 rather than Egr3 is the critical regulator of FasL in A1.1 cells (Figure 5b). Taken together, these results demonstrate that TSA exerts its effect on transcription factors that are known to regulate FasL expression.
TSA inhibits apoptosis of CD4+ T cells in tumors by suppressing FasL expression
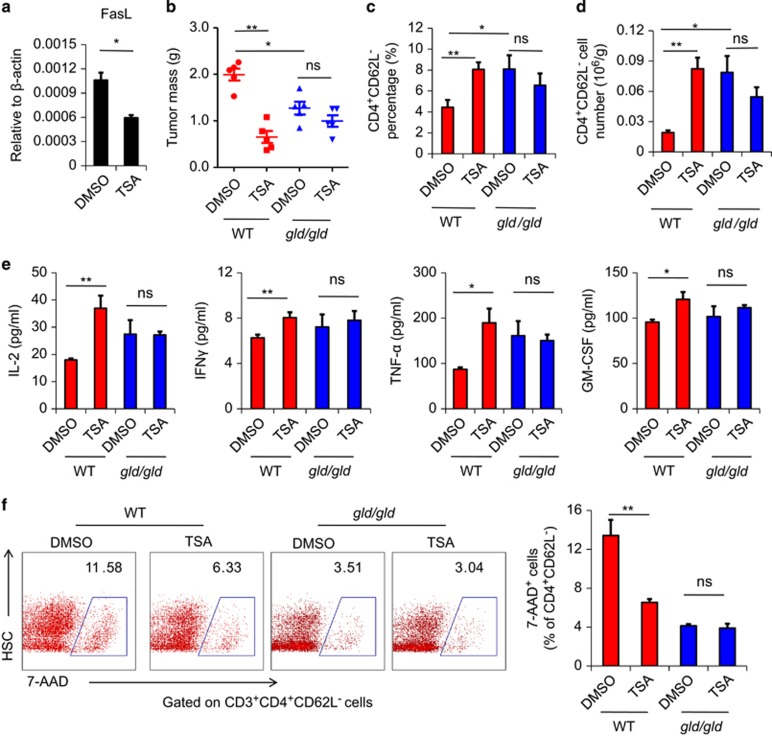
We have demonstrated in vitro that the anti-apoptotic effects of HDACIs result from inhibition of FasL expression. To determine whether this effect is responsible for decreased apoptosis of CD4+ T cells following TSA administration in vivo, we specifically examined FasL expression by tumor-infiltrating CD4+ T cells, and found that TSA treatment dramatically reduced the expression of FasL on these cells (Figure 6a). This shows that TSA indeed inhibits the FasL-mediated AICD of tumor-infiltrating CD4+ T cells in vivo. To further verify that this phenomenon was responsible for the therapeutic effect of TSA, we again utilized the B16F0 melanoma model and compared the effects of TSA on resultant tumor mass in both wild-type and gld/gld mice, in which FasL is mutated. We found that the therapeutic effect of TSA that was apparent in the wild-type mice and was completely absent in gld/gld mice (Figure 6b). Similarly, in gld/gld mice, TSA did not increase the number or percentage of CD4+ T cells in the tumor, or the serum level of T-cell cytokines (Figures 6c and e). Furthermore, the AICD of CD4+ T cells was unaffected by TSA in gld/gld mice (Figure 6f). Thus, the inhibition of FasL-mediated AICD by TSA is crucial for its ability to boost the numbers of infiltrating T lymphocytes. This conclusion was further supported by the finding that the lack of FasL in gld/gld mice also led to greater numbers of tumor-infiltrating T cells and smaller tumors, as compared with wild-type mice.
Figure 6.
The anti-tumor effect of TSA occurs through inhibition of the FasL-mediated AICD of activated CD4+ T cells. (a) TILs isolated and identified as in Figure 1 were examined for the expression of FasL by real-time PCR. (b) B16F0 cells (4 × 105) were injected intramuscularly in the left thigh of C57BL/6 mice (n=5) and gld/gld mice (n=5). From day 6, 1 μM/kg TSA was injected i.p. every 2 days, with dimethyl sulfoxide as a control. On day 14, mice were killed, and tumors were excised and weighed. (c) Tumors were then minced for the isolation of infiltrated lymphocytes. Cells were counted and stained by CD4 and CD62L for flow cytometry to detect the percentage of CD4+CD62L− lymphocytes. (d) The absolute numbers of CD4+CD62L− cells were calculated. (e) The serum levels of cytokines were detected by Bio-plex. (f) The percentage of 7-AAD+ cells in infiltrating CD4+ T cells were analyzed by flow cytometry. Statistical analysis was performed using the Student's t-test. *P<0.05, **P<0.01; ns, not significant.
To test whether this phenomenon can be generalized to other tumor types, we employed EL4 lymphoma model and obtained results similar to those with melanoma: EL4 lymphoma growth was suppressed by TSA administration, and infiltration by CD4+ T lymphocytes was enhanced in wild-type mice, but not in gld/gld mice (Supplementary Figure 11).
The combined administration of TSA and CTLA4 antibody achieves better anti-tumor effect
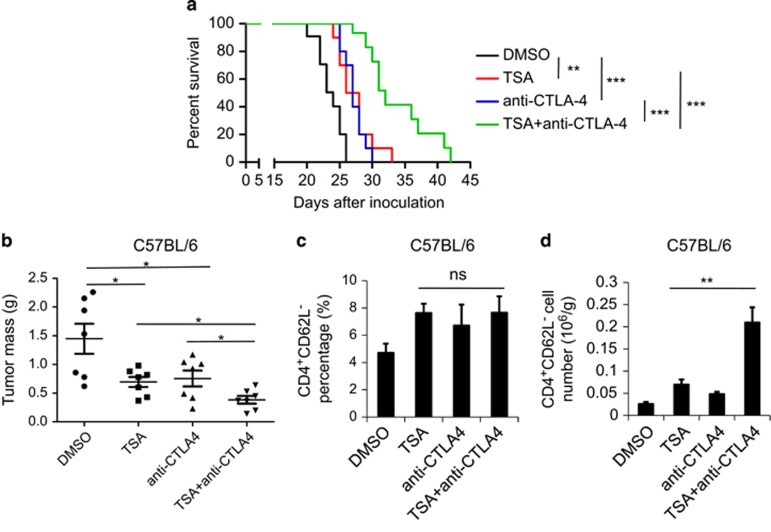
It has been reported that HDACIs have a much stronger therapeutic effect when combined with other anti-tumor agents.31 CTLA4 antibody has been shown to be effective in the treatment of several types of cancers, especially melanoma, owing to its ability to enhance the T-lymphocyte response.32 Therefore, we reasoned that combining TSA and anti-CTLA4 might have a synergistic therapeutic effect. To test this, anti-CTLA4 was co-administered with TSA in the B16 melanoma model. As expected, the combination of these two agents prolonged the survival of tumor-bearing mice to a much greater extent than did either alone (Figure 7a). Co-administration of TSA and anti-CTLA4 also showed a stronger anti-tumor effect, limiting melanoma growth more than TSA or anti-CTLA4 alone (Figure 7b). Furthermore, although the percentage of activated CD4+ CD62L− T lymphocytes in the tumors was no greater with the combined treatment, the absolute number of these cells was strikingly increased compared with either treatment alone (Figure 7c and d). Taken together, these results demonstrate that TSA and CTLA4 antibody can act synergistically to increase the number of activated CD4+ T lymphocytes, compared with either alone, resulting in a more potent therapeutic effect on melanoma growth. This finding has great potential to guide the clinical application of HDACIs in the treatment of cancer.
Figure 7.
Co-administration of TSA and anti-CTLA4 antibodies could further enhance the therapeutic effect of TSA alone. (a) Survival of mice challenged with 4 × 105 B16F0 cells and treated every other day from day 6 with the indicated agents i.p. Mice with tumor size >1000 mm3 or severe locomotion difficulties were killed. Each curve represents one independent experiment with 10 mice per group. Statistical significance was determined by Log-Rank test, **P<0.01, ***P<0.001. (b) B16 melanoma was established on C57BL/6 mice as described before. From day 6, 1 μM/kg TSA or 200 μg anti-CTLA4 antibodies or both was i.p. injected every 2 days (n=7 each). On day 14, mice were killed, and tumors were excised and weighed. (c) Tumors were minced and infiltrated lymphocytes were isolated. The number of these cells were counted and then stained by CD4 and CD62L for the detection of the percentage of CD4+CD62L− lymphocytes. (d) Total numbers of CD4+CD62L− lymphocytes per gram tumor were then calculated. Statistical analysis was performed using the Student's t-test. *P<0.05, **P<0.01; ns, not significant.
Discussion
For the past two decades, a large number of HDAC inhibitors have been purified from natural sources, and others have been synthesized.12 In recent years, accumulating evidence has demonstrated that HDACIs have promising therapeutic potential for the treatment of various tumors.12, 13, 14 At least two such agents (Vorinostat and Romidepsin) have been approved by the FDA, and many others are under clinical trial.33 Still, the mechanisms underlying the therapeutic effects of HDACIs remain elusive.
There is substantial evidence that tumor cells can induce Fas-FasL-mediated apoptosis of TILs as a way to escape immune surveillance.2, 8 In fact, the restoration of TIL function within tumor by inhibition of apoptosis has been adopted as a therapeutic strategy and found to dramatically improve tumor rejection.3, 34 Therefore, the apoptotic status of TILs is closely related to tumor growth and might be associated with the anti-tumor effect of HDACIs. TILs are susceptible to several types of apoptosis, among which AICD is the major mechanism regulating the viability of activated T cells, which are the main effectors in anti-tumor immune responses. Additionally, AICD is also mainly mediated through the Fas-FasL pathway.9, 10, 24 For these reasons, we focused on the effect of HDACIs on the AICD of activated T lymphocytes in tumors.
In our studies, we have shown that HDACIs can inhibit the apoptosis of activated T cells. In recent years, however, there has arisen substantial evidence suggesting that HDACIs can themselves induce T-cell death.35, 36 These reports are inconsistent with our findings. We noticed, however, that the infiltrating cells responsible for attacking tumors are mostly activated functional T cells, and little has been reported about the effect of HDACIs on activated T cells. To our knowledge, our current study is the first to report that HDACIs have an anti-apoptotic effect on activated T lymphocytes. Furthermore, our studies suggested that the anti-apoptotic effects of HDACIs on the activated T cells were observed at lower concentrations of the drug, whereas we also observed direct induction of tumor cell apoptosis at higher levels. It is possible that both the activation state of the CD4+ T cells, as well as the dose of HDACI used may determine whether HDACIs exert pro- or anti-apoptotic effects in T cells.
We have demonstrated the anti-apoptotic effect of HDACIs on tumor-infiltrating CD4+ T cells resulted from inhibition FasL expression following re-activation. The Fas-FasL interaction is known to mediate the AICD of Th1 cells, whereas the major regulator of AICD of Th2 cells is granzyme B.37, 38 We found that granzyme B expression on TILs was not changed by TSA treatment like FasL (data not shown), suggesting that TSA may preferentially suppress AICD in Th1 CD4+ cells. Interestingly, increased numbers of activated Th1 cells are reported to be associated with prolonged disease-free survival in various tumor models and human cancers; whereas Th2 cells are associated with cancer progression and metastasis.39, 40, 41 Therefore, it is possible that the anti-tumor effect of HDACIs may be due to inhibition of FasL-mediated apoptosis of Th1 cells, but this hypothesis remains to be further tested.
HDACIs inhibit the activity of multiple HDACs. Among the 11 classical HDACs, nearly half of them have been reported to regulate cell death. Inhibition of either HDAC1 or HDAC2 was reported to be associated with increased apoptosis in human cancer cells.42 Also, an HDAC8-specific inhibitor was shown to selectively induce apoptosis in T-cell-derived lymphoma and leukemic cells, but not in solid cancer cell lines.42 On the other hand, Bolger et al.43 claimed that inhibition of HDAC4 could suppress cell death. Especially, HDAC7 was reported to be exported to the cytoplasm of T cells after TCR activation and mutation in HDAC7 inhibits its nucleocytoplasmic shuttling and suppresses TCR-induced apoptosis.44 Owing to the strong similarity between HDAC7 and FasL in both expression pattern and functional effects on T-cell apoptosis, we considered that HDAC7 may be a candidate FasL regulator. In fact, we examined the expression of HDAC7 in A1.1 cells during AICD induction and found that it was upregulated by anti-CD3 ligation and suppressed by HDACI treatment, much as was FasL expression, further indicating a possible relationship between HDAC7 and FasL (data not shown). However, the specific HDACs responsible for the regulation of FasL remain obscure and need to be identified.
In our study, we have shown that the inhibitory effect of TSA on FasL expression is related to decreased NFAT activity. Such inhibition can result in the suppression of AICD of T cells. As a well-known NFAT inhibitor, cyclosporin A (CsA) can suppress T-cell activation45 and FasL expression during AICD.9 CsA has been widely used as an immunosuppressant for the treatment of rheumatoid arthritis or inflammation induced by organ transplantation.46, 47 Moreover, its application can promote tumor growth by suppressing the overall immune surveillance by T cells.48 The differences between TSA- and CsA-mediated effects on tumors most likely can be attributed to their different regulatory effects on T cells. Unlike CsA, when TSA inhibits the progression of AICD, it shows no inhibitory effect on the proliferation of T cells by the same concentration (data not shown). Our results suggest that this regulation is the potential mechanism mediating tumor inhibition by TSA. Compared with TSA, in addition to its well-known suppression on T cells, CsA is also known to regulate cancer progression via promoting transforming growth factor beta (TGFβ), through a mechanism independent of host immunity.49 Therefore, the mechanism underlying the different effects of CsA and TSA on tumor growth needs further exploration.
Our studies have proven that FasL inhibition results in increased numbers of tumor-infiltrating T cells, which is the basis of the anti-tumor effect of TSA. FasL expression by tumor cells is also one of the mechanisms underlying the death of tumor-infiltrating lymphocytes.8 However, we found that the expression of FasL on B16F0 cells is very low and TSA shows no inhibitory effect on its expression (data not shown). In 2002, it was reported that the main anti-tumor mechanism of TSA was Fas ligation-induced tumor cell death.18 If that were the case, however, then tumor mass in our gld/gld mice would be expected to be even greater than that in wild-type mice, as gld/gld mice should have less tumor cell death. Instead, our studies show that tumor growth was suppressed in gld/gld mice compared with wild-type mice, a result that we attribute to the elevated numbers of infiltrating T cells. Of note, Maecker et al.18 inoculated with MEF cells rather than melanoma cells to induce murine tumors, and it might be possible that TSA exerts its anti-tumor effect through different mechanisms in different types of tumors. Furthermore, the concentration of TSA Maecker et al. used was around 3 μM/kg, whereas in our experiment, we employed much less concentration to 1 μM/kg. We have already shown that high-dose TSA could indeed induce tumor cell death, which is consistent with their results. Overall, we believe the anti-tumor effect of HDACIs is due, at least in part, to enhanced T lymphocyte survival within tumor. Nevertheless, the details of the mechanism need to be further investigated.
In conclusion, the results presented here support the following scenario: HDAC inhibitors suppress the apoptosis of activated T lymphocytes by inhibiting their expression of FasL, thus enhancing their survival. This leads to increased numbers of activated T cells within tumor, thereby enhancing anti-tumor immunity and constraining tumor growth. Thus, we have proposed a new mechanism by which HDACIs exert their therapeutic effects in cancer. With further study and a more complete understanding of how HDACIs regulate T lymphocyte survival, the use of HDACIs can be exploited for broader and more effective cancer therapy.
Materials and methods
Animals
C57BL/6 and nude mice were purchased from the Shanghai Laboratory Animal Center of Chinese Academy of Sciences, Shanghai, China, and maintained under specific pathogen-free conditions. Rag1−/− mice, gld/gld mice, CIITA−/− mice and β2m−/− mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in the Vivarium of Shanghai Jiao Tong University School of Medicine. All procedures were approved by the Institutional Animal Care and Use Committee of the Institute of Health Sciences, Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences. Animals were matched for age and gender in each experiment.
Antibodies and reagents
TSA, sodium butyrate, propidium iodide, actinomycin D and dimethyl sulfoxide were from Sigma-Aldrich (St Louis, MO, USA). Mouse CD90 (Thy-1) functional grade purified antibody, recombinant mouse IL-2, anti-mouse CD3 FITC, anti-mouse CD4 PerCP-Cyanine 5.5, anti-mouse CD4 PE, anti-mouse CD62L APC, anti-7-AAD PerCP-Cyanine5.5 and anti-mouse CD95 (Fas) PE were from eBiosciences (La Jolla, CA, USA). Anti-CD95 (Jo2) was from BD Pharmingen (San Diego, CA, USA). Mouse CD3 and CD28 polyclonal antibodies were from Abcam (Cambridge, MA, USA). Anti-CTLA4 was generated as described previously.50 Hydrogen peroxide (30%) was from Sinopharm Chemical Reagent (Shanghai, China). SCIENTZ 03-II UV crosslinker was from Scientz Biotechnology (Ningbo, China).
Cells
A1.1 T cell hybridomas were maintained as previously described.19 CD4+ T cell blasts were generated as follows. Naïve splenocytes (1 × 106 cells/ml) were cultured on plastic-bound anti-CD3 and soluble anti-CD28 for 48 h. These cells were harvested, and CD4+ T cells were isolated by mouse CD4+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany), and then cultured with IL-2 (200 U/ml, R&D, Minneapolis, MN, USA) alone for 48 h. All cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin and 50 mM β-ME (all from Invitrogen, Carlsbad, CA, USA). B16F0 mouse melanoma cells and EL4 lymphoma cells were cultured in DMEM medium supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin.
AICD induction
A1.1 T cell hybridoma cells or CD4+ T cell blasts (1 × 106 cells/ml) were activated on plastic-bound anti-CD3 in 12-well plates for various times. Cell viability following AICD induction was determined by culture with the prescribed reagent in the Cell Counting Kit-8 (Beyotime Biotechnology, Shanghai, China) for 2 h following the manufacturer's instructions, and the optical density at 450 nm was measured for the supernatant.
Flow cytometric analysis
Cells were suspended at a concentration of 2 × 106 cells/ml (in phosphate-buffered saline with 2% FBS) and 200 μl of suspension was incubated with propidium iodide staining buffer (0.1 mg/ml propidium iodide, 0.1% saponin in phosphate-buffered saline) or fluorescently labeled antibodies for 30 min. Antibody-stained cells were washed twice with phosphate-buffered saline. Fluorescence intensity was measured by flow cytometry (BD FACSCalibur, BD Bioscience, Shanghai, China).
Real-time PCR
Total RNA was isolated using RNAprep pure Cell/Bacteria Kit (Tiangen biotech, Beijing, China). First-strand cDNA synthesis was performed using PrimeScript RT Master Mix (TaKaRa Biotech, Dalian, China). The levels of mRNA of genes of interest were measured by real-time PCR (7900 HT by Applied Biosystems, Foster City, CA, USA) using SYBR Green Master Mix (TaKaRa Biotech). Total amount of mRNA was normalized to endogenous β-actin mRNA. Sequences of PCR primer pairs were as follows: mouse FasL, forward 5′-TCCGTGAGTTCACCAACCAAA-3′ and reverse 5′-GGGGGTTCCCTGTTAAATGGG-3′ mouse Egr2, forward 5′-GCCAAGGCCGTAGACAAAATC-3′ and reverse 5′-CCACTCCGTTCATCTGGTCA-3′ mouse Egr3, forward 5′-CCGGTGACCATGAGCAGTTT-3′ and reverse 5′-TAATGGGCTACCGAGTCGCT-3′ mouse IL-2, forward 5′-GTGCTCCTTGTCAACAGCG-3′ and reverse 5′-GGGGAGTTTCAGGTTCCTGTA-3′ mouse GM-CSF, forward 5′-GGCCTTGGAAGCATGTAGAGG-3′ and reverse 5′-GGAGAACTCGTTAGAGACGACTT-3′ mouse IFNγ, forward 5′-ATGAACGCTACACACTGCATC-3′ and reverse 5′-CCATCCTTTTGCCAGTTCCTC-3′ mouse TNFα, forward 5′-GACGTGGAACTGGCAGAAGAG-3′ and reverse 5′-TTGGTGGTTTGTGAGTGTGAG-3′ mouse β-actin, forward 5′-CCACGAGCGGTTCCGATG-3′ and reverse 5′-GCCACAGGATTCCATACCCA-3′
Mouse tumor model
For the melanoma model, each mouse was injected with 0.4 × 106 B16F0 in 100 ml phosphate-buffered saline intramuscularly on the left thigh on day 1. In the TSA dosing experiment, 1 μM/kg or 3 μM/kg TSA was administrated intraperitoneally to C57BL/6 or nude mice every other day starting on day 6, with dimethyl sulfoxide serving as a vehicle control. In other experiments, melanoma was established as described and 1 μM/kg TSA was administrated with or without 200 μg/ml anti-CTLA4 antibodies. Mice were observed daily and killed when tumor began to significantly affect mobility or >1000 mm3. Cytokine levels in serum were determined by multiplexed bead immunoassay using the Luminex Technology (Bio-Plex, Bio-Rad Laboratories, Hercules, CA, USA). The melanoma tumors were excised and weighed and mechanically minced, then separated by discontinuous density gradient centrifugation. Briefly, cells were pelleted, resuspended in 70% Percoll (GE healthcare, Piscataway, NJ, USA), overlaid with 30% Percoll, and then centrifuged at 2000 r.p.m. for 20 min at room temperature. Lymphocytes were aspirated from the interface and washed twice with medium. CD4+CD62L− lymphocytes were isolated by mouse CD4+CD62L− T cell isolation kit (Miltenyi) according to the manufacturer's instructions. Four micrometer paraffin sections were prepared and stained with indicated antibody according to the protocol provided by the manufacturer. For the EL4 lymphoma model, the cells and TSA were injected as in the B16 model. The resultant tumors were excised and weighed, and digested by type I collagenase (0.5 mg/ml) at 37 °C for 2 h, and lymphocytes were then isolated by Percoll gradient.
Western blotting analysis
For the detection of Bim and P-65 protein, nuclear or cytoplasmic proteins were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the manufacturer's instructions. Samples were heated in sodium dodecyl sulfate sample buffer at 95 °C for 10 min, separated on a polyacrylamide gel, and separated proteins were electroblotted onto polyvinylidene difluoride membranes. Specific proteins were revealed by mouse and rabbit antibodies against NFAT1, p53, laminB or GAPDH (Cell Signaling Technology, Danvers, MA, USA) by overnight incubation at 4 °C, followed by chemiluminescent detection according to the manufacturer's instructions.
Statistical analysis
Data are presented as mean±s.e.m. Statistical significance was assessed using unpaired two-tailed Student's t-test, *P<0.05, **P<0.01, or Log-Rank test in survival experiment: **P<0.01, ***P<0.001.
Acknowledgments
This work was supported by grants from the Scientific Innovation Project of the Chinese Academy of Science (XDA 01040107 and XDA 01040110), the Programs of National Natural Science of China (81330046), the Ministry of Science and Technology of China (2015CB964400), the External Cooperation Program of BIC, Chinese Academy of Sciences (GJHZ201307), Shanghai Municipal Key Projects of Basic Research (12JC1409200), Shanghai Rising-Star Program (14QA1404200). The Child Health Institute of New Jersey is supported by a grant from the Robert Wood Johnson Foundation (grant number 67038).
Glossary
- HDAC
histone deacetylase
- HDACIs
histone deacetylase inhibitors
- AICD
activation-induced cell death
- TILs
tumor-infiltrating T lymphocytes
- TSA
trichostatin A
- Nabu
sodium butyrate
- ActD
actinomycin D
- H2O2
hydrogen peroxide
- NFAT
nuclear factor of activated T cells
- Egr
early growth factor
- IFN-γ
interferon-γ
- TNF-α
tumor necrosis factor-α
- IL-2
interleukin-2
- GM-CSF
granulocyte-macrophage colony stimulating factor
- CTLA-4
cytotoxic T-lymphocyte antigen 4
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
Supplementary Material
References
- 1Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 2Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest 2006; 86: 231–245. [DOI] [PubMed] [Google Scholar]
- 3Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol 2004; 5: 141–149. [DOI] [PubMed] [Google Scholar]
- 4Boni A, Muranski P, Cassard L, Wrzesinski C, Paulos CM, Palmer DC et al. Adoptive transfer of allogeneic tumor-specific T cells mediates effective regression of large tumors across major histocompatibility barriers. Blood 2008; 112: 4746–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science 1996; 274: 1363–1366. [DOI] [PubMed] [Google Scholar]
- 9Shi YF, Sahai BM, Green DR. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature 1989; 339: 625–626. [DOI] [PubMed] [Google Scholar]
- 10Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 1995; 373: 444–448. [DOI] [PubMed] [Google Scholar]
- 11Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 1995; 81: 935–946. [DOI] [PubMed] [Google Scholar]
- 12Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006; 6: 38–51. [DOI] [PubMed] [Google Scholar]
- 13Geng L, Cuneo KC, Fu A, Tu T, Atadja PW, Hallahan DE. Histone deacetylase (HDAC) inhibitor LBH589 increases duration of gamma-H2AX foci and confines HDAC4 to the cytoplasm in irradiated non-small cell lung cancer. Cancer Res 2006; 66: 11298–11304. [DOI] [PubMed] [Google Scholar]
- 14Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res 2000; 60: 5165–5170. [PubMed] [Google Scholar]
- 15Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 2007; 26: 5541–5552. [DOI] [PubMed] [Google Scholar]
- 16Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA 2004; 101: 18030–18035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Marks PA, Jiang X. Histone deacetylase inhibitors in programmed cell death and cancer therapy. Cell Cycle 2005; 4: 549–551. [DOI] [PubMed] [Google Scholar]
- 18Maecker HL, Yun Z, Maecker HT, Giaccia AJ. Epigenetic changes in tumor Fas levels determine immune escape and response to therapy. Cancer Cell 2002; 2: 139–148. [DOI] [PubMed] [Google Scholar]
- 19Brunner T, Yoo NJ, LaFace D, Ware CF, Green DR. Activation-induced cell death in murine T cell hybridomas. Differential regulation of Fas (CD95) versus Fas ligand expression by cyclosporin A and FK506. Int Immunol 1996; 8: 1017–1026. [DOI] [PubMed] [Google Scholar]
- 20Vaux DL. Toward an understanding of the molecular mechanisms of physiological cell death. Proc Natl Acad Sci USA 1993; 90: 786–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Jacobson MD. Reactive oxygen species and programmed cell death. Trends Biochem Sci 1996; 21: 83–86. [PubMed] [Google Scholar]
- 22Colotta F, Polentarutti N, Sironi M, Mantovani A. Expression and involvement of c-fos and c-jun protooncogenes in programmed cell death induced by growth factor deprivation in lymphoid cell lines. J Biol Chem 1992; 267: 18278–18283. [PubMed] [Google Scholar]
- 23Jaattela M. Escaping cell death: survival proteins in cancer. Exp Cell Res 1999; 248: 30–43. [DOI] [PubMed] [Google Scholar]
- 24Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev 2003; 193: 70–81. [DOI] [PubMed] [Google Scholar]
- 25Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol 1996; 14: 259–274. [DOI] [PubMed] [Google Scholar]
- 26Wang R, Zhang L, Yin D, Mufson RA, Shi Y. Protein kinase C regulates Fas (CD95/APO-1) expression. J Immunol 1998; 161: 2201–2207. [PubMed] [Google Scholar]
- 27Manicassamy S, Sun Z. The critical role of protein kinase C-theta in Fas/Fas ligand-mediated apoptosis. J Immunol 2007; 178: 312–319. [DOI] [PubMed] [Google Scholar]
- 28Rengarajan J, Mittelstadt PR, Mages HW, Gerth AJ, Kroczek RA, Ashwell JD et al. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity 2000; 12: 293–300. [DOI] [PubMed] [Google Scholar]
- 29Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M et al. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med 1998; 188: 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Liu F, Bardhan K, Yang D, Thangaraju M, Ganapathy V, Waller JL et al. NF-kappaB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J Biol Chem 2012; 287: 25530–25540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 2006; 5: 769–784. [DOI] [PubMed] [Google Scholar]
- 32Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol 2006; 18: 206–213. [DOI] [PubMed] [Google Scholar]
- 33West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 2014; 124: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 2003; 3: 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Moreira JM, Scheipers P, Sorensen P. The histone deacetylase inhibitor Trichostatin A modulates CD4+ T cell responses. BMC Cancer 2003; 3: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Dangond F, Gullans SR. Differential expression of human histone deacetylase mRNAs in response to immune cell apoptosis induction by trichostatin A and butyrate. Biochem Biophys Res Commun 1998; 247: 833–837. [DOI] [PubMed] [Google Scholar]
- 37Devadas S, Das J, Liu C, Zhang L, Roberts AI, Pan Z et al. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity 2006; 25: 237–247. [DOI] [PubMed] [Google Scholar]
- 38Xu G, Shi Y. Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res 2007; 17: 759–771. [DOI] [PubMed] [Google Scholar]
- 39Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 40Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007; 117: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313: 1960–1964. [DOI] [PubMed] [Google Scholar]
- 42Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: What are the cancer relevant targets? Cancer Lett 2009; 277: 8–21. [DOI] [PubMed] [Google Scholar]
- 43Bolger TA, Yao TP. Intracellular trafficking of histone deacetylase 4 regulates neuronal cell death. J Neurosci 2005; 25: 9544–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Dequiedt F, Kasler H, Fischle W, Kiermer V, Weinstein M, Herndier BG et al. HDAC7, a thymus-specific class II histone deacetylase, regulates Nur77 transcription and TCR-mediated apoptosis. Immunity 2003; 18: 687–698. [DOI] [PubMed] [Google Scholar]
- 45Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 1997; 15: 707–747. [DOI] [PubMed] [Google Scholar]
- 46Merion RM, White DJ, Thiru S, Evans DB, Calne RY. Cyclosporine: five years' experience in cadaveric renal transplantation. N Engl J Med 1984; 310: 148–154. [DOI] [PubMed] [Google Scholar]
- 47Kahan BD. Cyclosporine. N Engl J Med 1989; 321: 1725–1738. [DOI] [PubMed] [Google Scholar]
- 48Tanaka T, Takahara S, Hatori M, Suzuki K, Wang J, Ichimaru N et al. A novel immunosuppressive drug, FTY720, prevents the cancer progression induced by cyclosporine. Cancer Lett 2002; 181: 165–171. [DOI] [PubMed] [Google Scholar]
- 49Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature 1999; 397: 530–534. [DOI] [PubMed] [Google Scholar]
- 50Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995; 182: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.