Abstract
This prospective blinded randomized study aimed to determine whether the timing of morphine and phenylbutazone administration affects the breathing response to skin incision, recovery quality, behavior, and cardiorespiratory variables in horses undergoing fetlock arthroscopy. Ten Standardbred horses were premedicated with acepromazine (0.04 mg kg−1 IM) and romifidine (0.04 mg kg−1 IV). Anesthesia was induced with diazepam (0.05 mg kg−1) and ketamine (2.2 mg kg−1) IV at T0. Horses in group PRE (n = 5) received morphine (0.1 mg kg−1) and phenylbutazone (2.2 mg kg−1) IV after induction and an equivalent amount of saline after surgery. Horses in group POST (n = 5) received the inversed treatment. Anesthesia was maintained with isoflurane 2% in 100% oxygen. Hypotension (mean arterial pressure <60 mmHg) was treated with dobutamine. All horses breathed spontaneously. Dobutamine requirements, respiratory rate (fR), heart rate (HR), mean arterial blood pressure, end-tidal CO2, inspired (i) and expired (e) tidal and minute volume (VT and ), inspiratory time (IT), and the inspiratory gas flow (VTi/IT) were measured every 5 min. Data were averaged during four 15 min periods before (P1 and P2) and after the incision (P3 and P4). Serial blood–gas analyses were also performed. Recoveries were unassisted, video recorded, and scored by three anesthetists blinded to the treatment. The postoperative behavior of the horses (25 demeanors), HR, and fR were recorded at three time points before induction (T0–24 h, T0–12 h, and T0–2 h) and six time points after recovery (TR) (TR + 2, 4, 6, 12, 24, 48 h). Data were compared between groups using a Wilcoxon test and within groups using a Friedman test or a Kruskal–Wallis signed-rank test when applicable. Tidal volumes (VTe and VTi) were higher in PRE than in POST during all the considered periods but the difference between groups was only significant during P2 (VTe in mL kg−1 in PRE: 13 [9, 15], in POST: 9 [8, 9], p = 0.01). None of the other variables were significantly different between and within groups. Under our experimental conditions, skin incision did not affect respiratory variables. Administration of pre- versus postoperative phenylbutazone and morphine did not influence recovery quality, HR, fR, or animal behavior.
Keywords: horses, noxious stimulus, breathing response, analgesia, opioids
Introduction
Surgery performed in horses undergoing general anesthesia induces nociception which may affect the quality of recovery (1), the most critical phase of equine anesthesia (2). Early treatment of nociception with appropriate multimodal analgesia is therefore mandatory to improve the outcome of equine surgeries. Arthroscopy, in particular, has been used as a pain model in horses due to the fact that it can induce a moderate degree of postoperative pain requiring an optimal pain management (3, 4).
The identification of markers of nociception during the maintenance of anesthesia is important because it could lead to an early treatment of nociceptive stimulation.
In anesthetized human patients, some studies demonstrated that noxious stimuli affect respiratory variables (5–8). The ventilatory response to skin incision during anesthesia is an increase in mean inspiratory flow rate, defined as the amount of air the patient inspires (VTi) by the time the inspiration lasts (IT): VTi/IT. This is the result of an increase in tidal volume (VT) without changes in respiratory frequency. This was observed over the first breaths following this stimulus and it rapidly reverted to pre-incision values in patients anesthetized with halothane (5), enflurane (7), or with a propofol–alfentanil infusion (8). These studies suggest that VTi/IT and VT can be used as markers of nociception.
In veterinary medicine, we did not find similar studies which point to a possible relationship between nociception and respiratory drive under anesthesia. To the authors’ knowledge, the breathing response to skin incision has not been studied in anesthetized horses.
Non-steroidal anti-inflammatory drugs (phenylbutazone) and opioids (morphine) are commonly used as part of the analgesic protocol in equine patients undergoing arthroscopy. Opioids may have a variable influence on respiration (9). When associated to alpha-2 agonists, morphine produced respiratory depression (fall in PaO2 and rise in PaCO2) (10), whereas, when given as an intravenous (IV) bolus during general anesthesia, it did not affect VT, fR, or PaCO2, suggesting a lack of significant centrally induced respiratory depression (11). As opioids may influence the breathing response to noxious stimuli, their inclusion in the anesthetic protocol may question its efficiency as a marker of nociception.
In contrast, phenylbutazone does not seem to have an effect on respiration. This drug did not affect cardiorespiratory variables in healthy standing horses that received a combined administration of phenylbutazone and romifidine in comparison to romifidine alone (12).
The timing of analgesic administration may also affect the breathing response to skin incision. In addition, due to the subsequent surgery, a more severe postoperative pain may occur. The effects of preemptive analgesia on postoperative pain, behaviors, and particularly on recovery quality in horses, remain to be studied (13). Phenylbutazone, when given to horses undergoing arthroscopy preoperatively, seemed to have a postoperative analgesic benefit but altered the recovery score (including the number of attempts to stand, ataxia, and excitement as criteria) in comparison to the placebo (14). Although controversial, several studies demonstrated that morphine administered as preoperative treatment improved the quality of recovery (15, 16).
Nevertheless, the effects of postoperative in comparison to preoperative administration of morphine and phenylbutazone on horses’ recovery quality and postoperative behavior have not been studied yet.
The objective of this study was to determine if skin incision induces a modification of respiratory variables: inspiratory flow rate, tidal volume, and respiratory frequency in anesthetized horses undergoing bilateral fetlock arthroscopy. We also investigated if the preoperative (compared to postoperative) administration of morphine and phenylbutazone affect this response, recovery quality, postoperative behavior, and cardiorespiratory variables.
We tested the hypotheses that the breathing response to skin incision would be an increase in tidal volume and inspiratory flow rate without changes in respiratory frequency. We also hypothesized that the horses receiving morphine and phenylbutazone before surgery would have better recovery qualities and would show less postoperative behavioral changes in favor of pain than those receiving these analgesics after surgery.
Materials and Methods
An abbreviation list is provided in Datasheet S1 in Supplementary Material.
This trial was a part of another study of the Equine Department and was performed in accordance with the EUROGUIDE on the accommodation and care of animals used for experimental and other scientific purposes published by the Royal Society of Medicine Press Limited (London, UK). The experimental procedure was approved by the Animal Ethics Committee of VetAgro-Sup (veterinary campus of Lyon), France (RECH-ETIC-P003-E01).
Animals
Ten healthy (American Society of Anesthesiologists physical status score, ASA I) Standardbred horses (seven geldings and three mares), age (6.57 ± 1.27 years), weight (527 kg ± 49 kg) dedicated to research were included in the study.
Horses were randomly allocated to groups PRE (preoperative analgesia) and POST (postoperative analgesia) by simple randomization, using sequentially numbered, opaque, and sealed envelopes. Group PRE (n = 5) received morphine (Morphine Clorhydrate Aguettant, Aguettant Laboratory, France) 0.1 mg kg−1 and phenylbutazone (Phenylarthrite, Vétoquinol, France) 2.2 mg kg−1 IV immediately after induction of anesthesia (T0) and group POST (n = 5) received morphine and phenylbutazone at the end of the surgical procedure (last skin suture). The same volume of saline was administered in group POST at induction and in group PRE at recovery. The anesthetist was blinded to the treatment.
Study Design
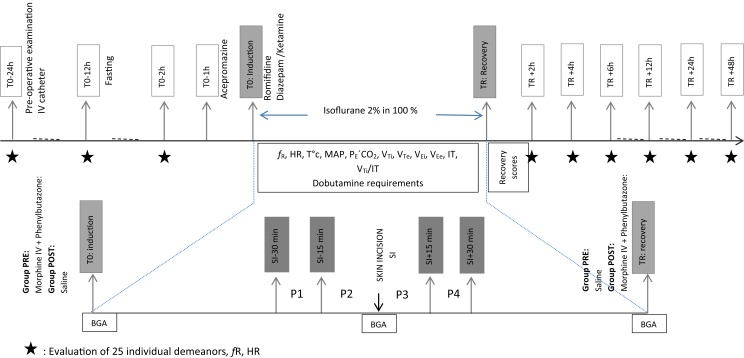
The day before surgery, a 14-G catheter (Angiocath, Becton Dickinson, UT, USA) was inserted percutaneously into the right jugular vein. All the animals were fasted 12 h before anesthesia (Figure 1).
Figure 1.
Study design.
Acepromazine (Calmivet, Vetoquinol, France) 0.04 mg kg−1 was administered intramuscularly (IM) 1 h before induction of general anesthesia. In the induction box, all the horses were sedated with romifidine (Sedivet Boehringer Ingelheim, France) 0.04 mg kg−1 intravenously and anesthesia was induced with diazepam (Valium Roche, France) 0.05 mg kg−1 IV followed by ketamine (Imalgène1000, Merial, France) 2.2 mg kg−1 IV. A silicone endotracheal tube (outer diameter 26 mm) was introduced into the trachea and the cuff was inflated with a manometer pump to reach a pressure of 95 cmH2O to ensure a sealed airway. Horses were transferred to the operating room, placed in dorsal recumbency, and instrumented for monitoring. Ringer’s Lactate (Ringer Lactate Aguettant, Aguettant Laboratories, France) was administered at 10 mL kg−1 h−1.
Anesthesia was maintained with a 2% inspired fraction of isoflurane (FI′ISO) set on the vaporizer (FI′ISO: PRE = 2.0% [1.3, 2.4], POST = 1.9% [1.2, 2.2]; expired fraction of isoflurane, FE′ISO: PRE = 1.6% [1.3, 2], POST = 1.5% [1.2, 1.7]) (Isoflo, Abbot Laboratory, UK) in 100% O2. The horses were allowed to breathe spontaneously. The endpoints of the study were toxic signs of hypercapnia (mainly arrhythmias, hypoxemia) or a PaCO2 > 100 mmHg. In this case, horses would have been mechanically ventilated and withdrawn from the study.
Bilateral fetlock arthroscopy was performed by the same surgeon in all horses and anesthesia time was recorded.
Cardiorespiratory Variables
Heart rate (HR, from the electrocardiogram), fR, and mean arterial blood pressure (MAP) from an arterial 20-G catheter (Insyte-W, Becton Dickinson, UT, USA) placed into the facial artery, body core temperature [T (°C)], peripheral arterial hemoglobin oxygen saturation (by pulse oximetry), end-tidal carbon dioxide tension (PE′CO2), inspired (i) and expired (e) VT and minute volume , and inspiratory time (IT) were recorded every 5 min with a previously calibrated monitor (Datex S/5, Datex-Ohmeda, GE Healthcare, Helsinki, Finland) and a flow sensor (Horse-Lite, Morpheus engineering, Wenum Wiesel, Netherlands). Monitored VT and values were multiplied by six following the manufacturer’s recommendations (17). The pressure transducer was zeroed against the atmospheric pressure and leveled at the height of the scapulohumeral joint. The inspiratory gas flow rate (VTi/IT) was calculated.
Dobutamine Requirements
Dobutamine (Dobutamine Panpharma 250 mg, Panpharma, France) IV was administered with a syringe driver (Syramed μSP6000, Arcomed, Switzerland) at a starting dose rate of 2 μg kg−1 min−1 when MAP decreased below 60 mmHg. Every 5 min, the dobutamine rate of infusion was increased by 2 μg kg−1 min−1 if the target MAP was not achieved, or stopped at any moment, once MAP reached 60 mmHg. The total amount of dobutamine per period was calculated for each horse and then averaged for each group.
Rescue Doses of Thiopental
The depth of anesthesia was considered adequate when palpebral and corneal reflexes were present, the eye was central, with no presence of nystagmus. Light planes of anesthesia (in case of nystagmus or movement) were treated with thiopental (Nesdonal, Merial, France) 0.5 mg kg−1 IV. The number of boli was recorded.
Blood–Gas Analysis
Three blood–gas analyses were performed (VetStat Idexx, France) to measure the pH and the arterial oxygen and carbon dioxide tensions (PaO2 and PaCO2, respectively). Analyses were performed once when the horse was instrumented, once when skin incision was performed, and at the end of the procedure (TR).
Recovery Scores and Characteristics
Once the surgery was finished, the horses were transferred to the recovery box (TR) and were allowed to recover without assistance. Oxygen was provided by insufflation to all horses through a nasal tube (15 L min−1). Recoveries were video recorded and assessed by three experienced anesthetists who were unaware of the treatment.
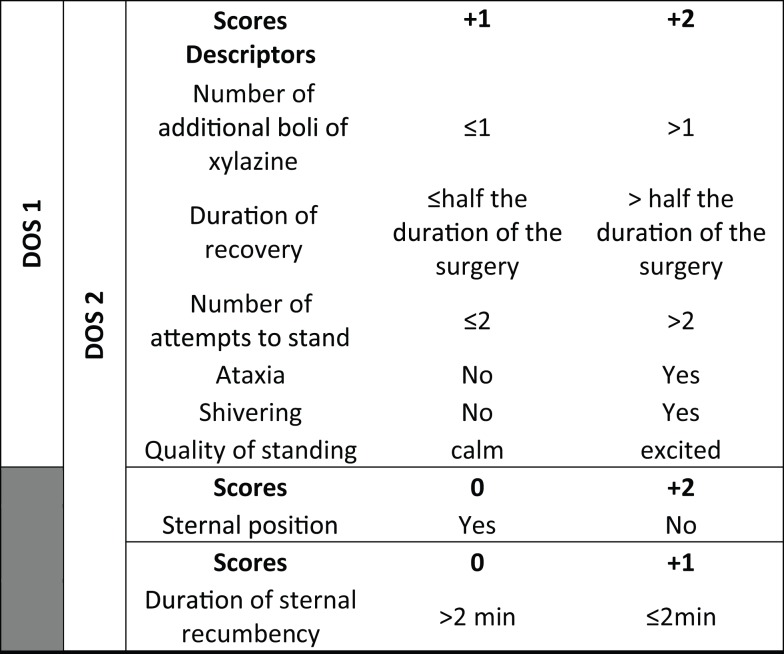
Recoveries were assessed with three scoring systems: a visual analog scale (RVAS: 0 cm: worst possible recovery and 10 cm: best possible recovery), a dichotomous subjective scale (DSS: good versus bad recovery), and a dichotomous objective score (DOS) (18) (Figure 2). Dichotomous objective descriptors (DODs) were used for objective scoring of recovery. DOSs (DOS1 and DOS2) were created compiling all the DODs (in DOS2) or rejecting the DODs “sternal position” and “duration of sternal position” (in DOS1). Descriptive objective scores for DOS 1 and DOS2 were calculated adding the scores mentioned in the table. For DOS2, the minimal possible score is 6 and the maximal possible score is 14. For DOS1, the minimal possible score is 6 and maximal possible score is 12. Contrary to the VAS, high DOS scores show bad recoveries and low DOS scores reveal good recoveries.
Figure 2.
Dichotomous objective scores.
In addition to the recovery scores, the following recovery characteristics were analyzed: the time from placement in the recovery box to the time the horse was standing (RT), the time until the horse first moved (MOV), the time from the horses’ first movement until it was standing (MovUp), the number of tries (Tries), and the agitation index (AI), which is the ratio of tries and the time from the first movement to the horse standing. Xylazine boluses (0.1 mg kg−1) were prepared in advance in case the horse became dangerously agitated.
Pre- and Postoperative Behavior
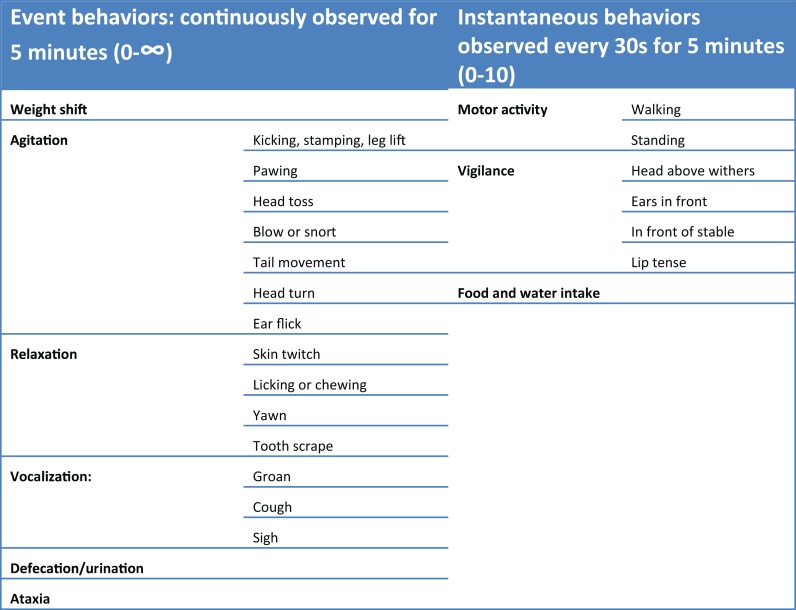
Preanesthetic behaviors (baseline) were assessed at three time points, corresponding to 24, 12, and 2 h before anesthesia induction (T0–24 h, T0–12 h, and T0–2 h, respectively) by an observer blinded to the treatment from outside the box. After recovery from general anesthesia, all the horses returned to their boxes and 2 h were allowed for their acclimatization. Postoperative behaviors were assessed at 2, 4, 6, 12, 24, and 48 h after recovery (TR + 2 h, TR + 4 h, TR + 6 h, TR + 12 h, TR + 24 h, and TR + 48 h, respectively). Twenty-five individual demeanors were assessed separately. We have classified them into nine categories to facilitate the presentation of the results (Figure 3). Event behaviors were continuously observed for 5 min, the possible values obtained were between 0 and the infinity. Instantaneous behaviors were observed every 30 s for 5 min, which allowed a maximum score of 10 per descriptor.
Figure 3.
Pre and post-operative behavior evaluation.
Additionally to the behavior assessment, HR and fR were measured at each time point.
Statistical Analysis
Statistical analysis was performed with R Statistical software [R Core Team (19)]. Due to the small samples size, non-parametric tests were used.
Data Recorded During Surgery
Data were averaged within four periods: from 30 to 15 min before skin incision (P1), from 15 min to time of skin incision (P2), from skin incision to 15 min after incision (P3), and from 15 to 30 min after skin incision (P4).
A Wilcoxon rank-sum test was used to compare data between groups at the same period. A Wilcoxon signed-rank test was used to compare data at different periods within groups. Differences were considered significant if p was <0.05 unless a Bonferroni correction was applied (when more than two comparisons were performed) (p < 0.01). Data are presented as median [minimum, maximum].
Data Recorded During Recovery and the Postoperative Period
Treatment and time interactions were assessed using a Friedman test. A Wilcoxon signed-rank test or sum test was then used for comparison within or between groups.
Data are presented as median [minimum, maximum].
Results
Anesthesia Time and Rescue Doses of Thiopental
The anesthesia time was not significantly different between groups and was 115 [66, 120] min in PRE and 107 [52, 131] min in POST. Mean time for incision was not significantly different between groups and was 61 [54, 65] min in PRE and 57 [52, 71] min in POST.
Only two horses presented some nystagmus in group POST and required thiopental. The total number of boluses was two and four, respectively and they were administered after induction of general anesthesia, while the horses were being transported to the surgical table.
Cardiorespiratory Variables
Respiratory rate, , IT, and VTi/IT did not differ within or between groups (Table 1).
Table 1.
Cardiorespiratory variables, arterial blood–gas analysis, and dobutamine requirements in 10 isoflurane-anesthetized horses undergoing bilateral fetlock arthroscopy.
| Variable | PRE | POST | |
|---|---|---|---|
| fR (breaths min−1) | P1 | 3 [1, 11] | 6 [1, 9] |
| P2 | 3 [2, 8] | 5 [1, 7] | |
| P3 | 4 [3, 7] | 4 [3, 7] | |
| P4 | 4 [2, 5] | 4 [2, 5] | |
| VTi (mL kg−1) | P1 | 14 [10, 15] | 9 [8, 14] |
| P2 | 14 [11, 15] | 11 [9, 13]a | |
| P3 | 16 [9, 20] | 11 [9, 13] | |
| P4 | 16 [10, 21] | 12 [9, 13] | |
| VTe (mL kg−1) | P1 | 14 [8, 24] | 8 [8, 14] |
| P2 | 13 [9, 15] | 9 [8, 9]a | |
| P3 | 14 [8, 19] | 10 [8, 11] | |
| P4 | 15 [10, 21] | 12 [8, 13] | |
| IT (s) | P1 | 3 [1, 4] | 2 [1, 3] |
| P2 | 3 [1, 5] | 3 [2, 4] | |
| P3 | 3 [2, 6] | 3 [2, 8] | |
| P4 | 3 [2, 6] | 4 [2, 6] | |
| (mL kg−1 min−1) | P1 | 51 [24, 10] | 62 [14 76] |
| P2 | 64 [31, 85] | 61 [60, 66] | |
| P3 | 61 [39, 69] | 55 [22, 68] | |
| P4 | 48 [47, 86] | 33 [12, 48] | |
| (mL kg−1 min−1) | P1 | 46 [24, 90] | 54 [14, 73] |
| P2 | 59 [30, 70] | 49 [40, 58] | |
| P3 | 59 [37, 64] | 53 [21, 55] | |
| P4 | 46 [44, 89] | 32 [13, 47] | |
| VTi/IT (mL kg−1 s−1) | P1 | 3 [1, 3] | 5 [1, 5] |
| P2 | 4 [2, 6] | 5 [5, 5] | |
| P3 | 4 [2, 8] | 5 [2, 5] | |
| P4 | 6 [2, 8] | 3 [1, 4] | |
| PE′CO2 (mmHg) | P1 | 41 [31, 48] | 40 [33, 50] |
| P2 | 43 [38, 54] | 45 [40, 46] | |
| P3 | 45 [37, 56] | 44 [38, 50] | |
| P4 | 47 [39, 49] | 40 [37, 53] | |
| HR (beats min−1) | P1 | 41 [31, 48] | 40 [33, 50] |
| P2 | 43 [38, 54] | 45 [40, 46] | |
| P3 | 45 [37, 46] | 44 [38, 50] | |
| P4 | 47 [39, 49] | 40 [37, 43] | |
| MAP (mmHg) | P1 | 54 [50, 60] | 61 [51, 62] |
| P2 | 56 [53, 59] | 61 [57, 77] | |
| P3 | 64 [53, 58] | 69 [59, 88] | |
| P4 | 73 [62, 76] | 63 [62, 85] | |
| Dobutamine requirements by period (μg kg−1) | P1 | 18 [9, 36] | 15 [6, 33] |
| P2 | 18 [15, 27] | 15 [0, 24] | |
| P3 | 9 [0, 21] | 3 [0, 3] | |
| P4 | 0 [0, 6] | 6 [0, 15] | |
| T (°C) | P1 | 35.0 [34.8, 36.6] | 35.4 [34.7, 36.2] |
| P2 | 34.9 [34.4, 36.3] | 35.1 [34.6, 36.2] | |
| P3 | 34.4 [33.9, 36.1] | 34.8 [34.4, 35.8] | |
| P4 | 34.1 [33.5, 36.0] | 34.7 [34.2, 35.4] | |
| pH | G1 | 7.35 [7.27, 7.37] | 7.33 [7.31, 7.35] |
| G2 | 7.28 [7.22, 7.36] | 7.28 [7.20, 7.33] | |
| G3 | 7.29 [7.32, 7.23] | 7.29 [7.19, 7.30] | |
| PaCO2 (mmHg) | G1 | 56 [48, 76] | 56 [55, 66] |
| G2 | 73 [61, 75] | 66 [60, 83] | |
| G3 | 75 [69, 85] | 71 [60, 91] | |
| PaO2 (mmHg) | G1 | 322 [74, 399] | 356 [284, 442] |
| G2 | 186 [171, 287] | 254 [135, 391] | |
| G3 | 108 [72, 148] | 125 [74, 285] | |
Horses received morphine and phenylbutazone before skin incision (PRE, n = 5) or at the end of the procedure (POST, n = 5). Data are reported as median [min, max]. fR, respiratory frequency; VTi, inspired tidal volume; VTe, expired tidal volume; , inspired minute volume; , expired minute volume; VTi/IT, inspiratory flow rate; PECO2, expired pressure of carbon dioxide; HR, heart rate; MAP, mean arterial blood pressure; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; P1, from 30 to 15 min before skin incision; P2, from 15 min to time of skin incision; P3, from skin incision to 15 min after incision; P4 from 15 to 30 min after skin incision. Arterial gas analysis periods: G1, once when the horse was instrumented, G2, once when skin incision was performed, and G3, at the end of the procedure. Data are reported as median [min, max].
aIndicates statistically significant differences between groups (p < 0.05).
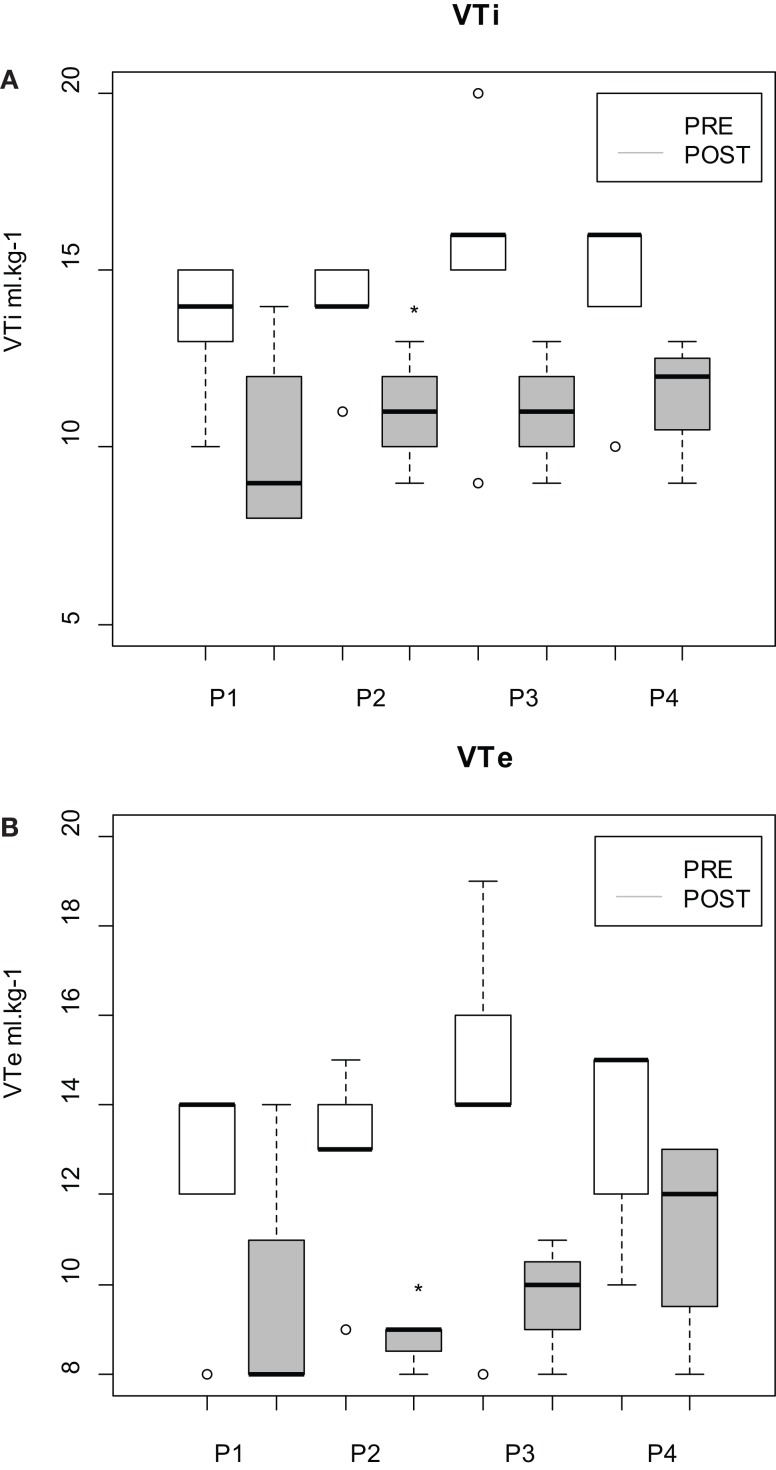
During all the periods, VTe and VTi were higher in PRE than in POST. Nevertheless, the difference was significant only during P2 (p = 0.01) (Figure 4).
Figure 4.
(A) Inspired (i) and (B) expired (e) tidal volume (VT) measured in isoflurane-anesthetized horses undergoing fetlock arthroscopy. Horses received morphine and phenylbutazone before skin incision (PRE) or at the end of the procedure (POST). Four periods of time were defined: P1, from 30 to 15 min before skin incision; P2, from −15 min to skin incision; P3, from skin incision to 15 min after incision; and P4, from 15 to 30 min after skin incision. The boxplots represent the first and third quartile (Q1 and Q3, respectively) and the median. The lower dispersion bar represents, in the distribution, the value which is immediately superior to the adjacent value [Q1 − 1.5(Q3 − Q1)]. The higher dispersion bar represents, in the distribution, the value which is immediately inferior to the adjacent value [Q1 + 1.5(Q3 − Q1)]. *Indicates statistically significant differences between groups (p < 0.05).
Heart rate and MAP did not differ within or between groups.
Dobutamine Requirements
There was a tendency, in horses allocated to group POST, to receive less dobutamine from P2 to P3. However, this difference was not significant when the Bonferroni correction was applied (p = 0.03) (Table 1).
Blood–Gas Analysis
Arterial pH, PaO2, and PaCO2 did not differ within or between groups (Table 1).
Recovery Scores and Characteristics
No significant differences between groups were found in any of the recovery scores or characteristics. No horse received xylazine (Table 2).
Table 2.
Recovery scores and characteristics in 10 isoflurane-anesthetized horses undergoing bilateral fetlock arthroscopy.
| PRE | POST | |
|---|---|---|
| RVAS | 3.8 [3.7–9.2] | 4.4 [3.1–8.5] |
| DSS | 2 [1–2] | 2 [1–2] |
| DOS 1 | 9 [8–10] | 9 [8–10] |
| DOS 2 | 10 [8–11] | 9 [8–10] |
| RT (min) | 17 [4–52] | 16 [5–22] |
| Mov (min) | 4 [1–24] | 6 [1–13] |
| MovUp (min) | 12 [3–2] | 9 [3–10] |
| Tries | 3 [1–6] | 7 [2–10] |
| AI | 0.3 [0.04–2] | 0.9 [0.22–2.3] |
Horses received morphine and phenylbutazone before skin incision (PRE, n = 5) or at the end of the procedure (POST, n = 5). A visual analog scale (RVAS), a dichotomous subjective scale (DSS), a dichotomous objective score (DOS), the time from placement in the recovery box to the time the horse was standing (RT), the time until the horse first moved (MOV), the time from the horse’s first movement until it was standing (MovUp), the number of tries (Tries), and the agitation index (AI) were calculated. Data are reported as median [min, max].
Pre- and Postoperative Behavior
Concerning the behavior assessment, horses in PRE spent significantly more time walking at T0 + 2 h than in POST (p = 0.02).
No other significant difference was found in any of the nine categories. No significant differences were identified in HR or fR between the groups.
These results are detailed in Datasheet S2 in Supplementary Material.
Discussion
In this study, skin incision did not modify respiratory variables in horses undergoing fetlock arthroscopy under general anesthesia in dorsal recumbency. Horses that received morphine and phenylbutazone preoperatively had a higher VT but the same fR than horses that received the same treatment postoperatively. The quality of recovery was the same whether morphine and phenylbutazone were administered before or after the surgery. Horses also showed few postoperative behavioral differences between groups.
A needle prick is considered to be a high-intensity, noxious stimulus, leading to an adaptive and protective reaction, due to a brief and localized signal of potential tissue damage (20). Therefore, we expected all the more that skin incision evokes somatic nociception. Surgical incision has been used as a nociceptive stimulus in former (5, 7, 8) as well as in more recent (21–23) studies in man. In animals, we found only one clinical study using skin incision preceding castration as a model of nociception (24).
However, in our study, this stimulus did not seem to produce nociception. MAP seems to be the most sensitive and reliable indicator of nociception in isoflurane-anesthetized horses (24). In the present study, skin incision did not affect this variable (even in group POST) and might, therefore, be considered as an insufficient nociceptive stimulus.
Heart rate and MAP were mainly higher than 40 bpm and lower than 70 mmHg, respectively, which could have reflected deep plane of anesthesia that, in turn, may have interfered with the recognition of nociception. However, this is unlikely because a palpebral reflex was maintained during the whole procedure confirming appropriate or even light stage of anesthesia.
The clinical properties of the drugs used for premedication and induction of general anesthesia may also have altered the nociceptive effects of skin incision. Nevertheless, premedication with alpha-2 agonist is mandatory before induction of anesthesia in horses. All alpha-2 agonists present analgesic properties, and therefore, it would have been difficult to draw up a protocol without analgesic drugs. The analgesic effects of romifidine after IV injection may last 120 min (25), and in our study, the mean time for incision from induction was 60 min (26, 27). Ketamine half-life is 40–60 min. It is, therefore, possible that romifidine and ketamine had an effect on the response to skin incision. However, in the study by Haga and Dolvik (24), alpha-2 agonist (detomidine) and ketamine did not conceal the nociceptive effect of surgery when administered to horses for premedication and induction of anesthesia respectively. Furthermore, the response to skin incision was not suppressed in men that had not received any premedication but a constant rate infusion (CRI) of opioid throughout the procedure (8).
A study in deeply anesthetized rabbits showed that low- and high-frequency tooth pulp stimulation (tooth afferent fibers almost exclusively provides nociceptive information) evoked respiratory response (increased and fR) only when it was associated with muscular contractions of the digastric muscle (28). This suggests that noxious stimulus could only affect respiratory variables when ergoreceptors are stimulated by muscular contraction. In our study, incision was performed at the fetlock level and did not involve muscular fibers or muscular contraction. Abdominal incision during colic surgery, for example, could be a more accurate nociceptive stimulus.
An increase in VT and VTi/IT in group POST was expected following skin incision. Previous studies in human patients showed that noxious stimulus affects the drive (represented by VTi/IT) and the timing of respiration (inspiratory and expiratory times) (7, 8). The mean inspiratory flow rate indicates the rate of increase in central inspiratory neural activity and is, therefore, a good indicator of central neural output. The duration of the phases of the respiratory cycle (IT) reflects the central control and the pulmonary and somatic afferent influences. In anesthetized human patients, some studies demonstrated that noxious stimulus induces an increase in respiratory drive and a decrease (6) or no change (7) in respiratory timing. These aspects of the control of breathing may, on one hand, be affected by opioids that may reduce the drive and increase expiration time (29) and, on the other hand, by the noxious stimulus that may reduce these effects (8). However, it is not possible to comment on how skin incision affected the control of breathing in the present study as skin incision did not seem to produce nociception.
Nevertheless, it is interesting to note that VTe and VTi were overall higher in PRE than in POST. Among the analgesic drugs used in PRE, phenylbutazone does not seem to affect cardiorespiratory variables (12), but morphine may stimulate pulmonary ventilation via central nervous excitation (30). Nevertheless, it is unlikely to occur under anesthesia. In man, opioids are rather known to induce respiratory depression (31). Morphine decreased minute volume by 10.3% in patients suffering from acute postoperative pain (32). However, we found little information in the literature about the effects of morphine on respiratory volumes in anesthetized animals. Nolan et al. (11) found that anesthetized horses, receiving an IV bolus of 0.05 mg kg−1 morphine, had significantly higher compared with controls. Nevertheless, VT and PaCO2 were not significantly different between groups and higher resulted from lower fR in the saline group. In addition, in the morphine group, , VT, and PaCO2 were not significantly different before and after the injection of the drug. Although breathing volumes were not measured, other authors studied the effect of a bolus of morphine on respiratory variables. Arterial carbon dioxide tension was not modified by a bolus of morphine (0.25 mg kg−1) administered to horses anesthetized with isoflurane in oxygen (33). In the same study, a higher dose of morphine (2 mg kg−1) induced respiratory depression objectified by increased PaCO2. Mircica et al. (34) also reports minimal respiratory effects of low clinical doses of morphine (0.1–0.17 mg kg−1) in the halothane-anesthetized horse.
In our study, it remains unclear why horses treated with morphine and phenylbutazone at induction had higher VT than horses that received the drugs postoperatively unless a greater inspired concentration of isoflurane was administered in order to maintain surgical depth of anesthesia in group POST. Isoflurane has indeed been shown to produce respiratory depression (35). This is unlikely because FI′ISO and FE′ISO were not significantly different in both groups. In addition, respiratory frequency did not differ between groups. This is in agreement with other studies (15, 34, 36) that demonstrated that morphine has no effect on fR and arterial blood–gas analysis.
With regard to recovery quality and characteristics, although better recoveries were expected in PRE than in POST, significant differences were not observed between groups.
Preemptive analgesia refers to evidence that analgesic preoperative treatment is more effective than the same treatment administered after incision or surgery (37). Clark (13) reviewed both the human and veterinary literature on this subject. The author concluded that the evidence pertaining to preemptive analgesia is equivocal and not convincing in human literature. In veterinary medicine, only one study in cats (38) met the criteria (same pre- and postintervention) of this concept. The effects of preemptive analgesia on the quality of recovery from anesthesia in horses are not known. Nevertheless, some authors compared the postoperative effect of some analgesics given preoperatively with a placebo. Although there is no strong evidence in the literature that phenylbutazone can adversely affect recoveries, one study in horses undergoing arthroscopy showed that horses premedicated with phenylbutazone had slightly worse subjective recovery scores than controls with saline (4). The effects of morphine on the quality of recovery in equine anesthesia are also controversial, although there is stronger opinion to support its beneficial effects when used at low doses. Steffey et al. (33) considered that recovery from anesthesia after administration of high dose of morphine (2 mg kg−1) was dangerous, but horses recovered reasonably well after administration of a lower “clinical” dose (0.25 mg kg−1). Horses undergoing elective surgical procedure and receiving a bolus of morphine before induction of anesthesia and a CRI of morphine during anesthesia showed fewer attempts to attain sternal recumbency and standing [Clark et al. (16)] than horses that received the same volume of saline. Love et al. (15) also found better recovery subjective scores in horses treated with a bolus of morphine (0.1–0.2 mg kg−1) after induction than in controls. This study failed to show the beneficial effects of the preoperative analgesic treatment on the quality of recovery. The analgesic effects of the anesthetic molecules can have concealed the difference between both groups.
Postoperative behaviors and cardiorespiratory variables did not differ between groups. Some studies demonstrated more box walking or signs of discomfort and agitation in horses that received a morphine CRI during general anesthesia compared to dexmedetomidine CRI (39). As mentioned above, in the present study, skin incision followed by arthroscopy may be considered as an insufficient nociceptive stimulus. Arthroscopy was used as a model of postoperative pain behavior in several clinical studies (3, 4). In our study, horses had healthy joints and were pain free at presentation. Consequently, they may have experienced a lower degree of pain than horses presented with damaged joints.
The performance of this study is not free of other limitations. All horses received the antinociceptive treatment (whether pre- or postoperatively), which may have masked the effect of surgical stimulation on postoperative behaviors. Although unethical, a third group with no analgesia at all would have been interesting to show any effect of central sensitization caused by inflammatory injury (40).
The lack of effect of the surgical stimulus on cardiorespiratory variables may also result from the insufficient sample size. We were not able to control the sample size because, in order to respect the three Rs of animal experimentation, we chose to benefit from another clinical study realized on horses in our faculty. A post hoc sample size was calculated on the basis of VT, showing that 19 horses would have been necessary in each group to get a 90% chance to detect, with a risk of 5%, an average difference of VT of 1 L between the two groups, considering a common SD of 1.3 L.
Conclusion
With the protocol used in this study and the number of horses included, it is difficult to conclude if tidal volume and respiratory frequency can be used as markers of nociception in horses because skin incision did not induce nociception in this study. For further experimental studies, higher sample size, anesthetic drugs without analgesic properties or more efficient nociceptive stimulus, and pain markers should be used. Nevertheless, this pilot study delivers important information for prospective future trials.
Author Contributions
Participated in research design: CCR, ICCB, and KP. Conducted experiments: CCR, ICCB, IG, and KP. Performed data analysis: CCR, ICCB, IG, and KP. Wrote or contributed to the writing of the manuscript: CCR, ICCB, and KP. Revised the manuscript: CCR, ICCB, IG, KP, and SG.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank Ms. Samantha Gallagher for assessing the manuscript’s English language.
Funding
We wish to thank VetAgro Sup for providing the funding of this study.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fvets.2015.00058
References
- 1.Valverde A, Rickey E, Sinclair M, Rioja E, Hathaway A, Cruz A. Comparison of cardiovascular function and quality of recovery in isoflurane-anaesthetized horses administered a constant rate infusion (CRI) of lidocaine or lidocaine and medetomidine during elective surgery. Equine Vet J (2010) 42:192–9. 10.1111/j.2042-3306.2010.00027.x [DOI] [PubMed] [Google Scholar]
- 2.Johnston G, Eastment J, Wood J, Taylor P. The confidential enquiry into perioperative equine facilities (CEPEF): mortality results of phases 1 and 2. Vet Anaesth Analg (2002) 29:159–70. 10.1046/j.1467-2995.2002.00106.x [DOI] [PubMed] [Google Scholar]
- 3.Price J, Catriona S, Welsh E, Waran N. Preliminary evaluation of a behaviour-based system for assessment of post-operative pain in horses following arthroscopic surgery. Vet Anaesth Analg (2003) 30:124–37. 10.1046/j.1467-2995.2003.00132_15.x [DOI] [PubMed] [Google Scholar]
- 4.Raekallio M, Taylor P, Blomfield M. A comparison of methods for evaluation of pain and distress after orthopaedic surgery in horses. J Vet Anaesth (1997) 24:17–20. 10.1111/j.1467-2995.1997.tb00150.x [DOI] [Google Scholar]
- 5.Cullen D, Eger E, Stevens W, Smith N, Cromwell T, Cullen B, et al. Clinical signs of anesthesia. Anesthesiology (1972) 36:21–36. 10.1097/00000542-197201000-00005 [DOI] [PubMed] [Google Scholar]
- 6.Eger E, Dolan W, Stevens W, Miller R, Way W. Surgical stimulation antagonizes the respiratory depression produced by forane. Anesthesiology (1972) 36:544–9. 10.1097/00000542-197206000-00005 [DOI] [PubMed] [Google Scholar]
- 7.Sutherland R, Drummond G. Effects of surgical skin incision on respiration in patients anaesthetized with enflurane. Br J Anaesth (1996) 76:777–9. 10.1093/bja/76.6.777 [DOI] [PubMed] [Google Scholar]
- 8.Dockery M, Drummond G. Respiratory response to skin incision during anaesthesia with infusions of propofol and alfentanil. Br J Anaesth (2002) 88:649–52. 10.1093/bja/88.5.649 [DOI] [PubMed] [Google Scholar]
- 9.Clutton R. Opioid analgesia in horses. Vet Clin North Am Equine Pract (2010) 26:493–514. 10.1016/j.cveq.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 10.Clarke K, Paton B. Combined use of detomidine with opiates in the horse. Equine Vet J (1998) 20:331–4. 10.1111/j.2042-3306.1988.tb01540.x [DOI] [PubMed] [Google Scholar]
- 11.Nolan A, Chambers J, Hale G. The cardiorespiratory effects of morphine and butorphanol in horses anaesthetized under clinical conditions. J Vet Anaesth (1991) 18:19–24. 10.1111/j.1467-2995.1991.tb00007.x [DOI] [Google Scholar]
- 12.Kruger K, Stegmann G, Becker P. Preliminary investigation of concurrent administration of phenylbutazone and romifidine in healthy horses. Vet Anaesth Analg (2011) 38:505–9. 10.1111/j.1467-2995.2011.00642.x [DOI] [PubMed] [Google Scholar]
- 13.Clark L. Editorial: pre-emptive or preventive analgesia-lessons from the human literature? Vet Anaesth Analg (2014) 41:109–12. 10.1111/vaa.12119 [DOI] [PubMed] [Google Scholar]
- 14.Raekallio M, Taylor P, Bennett R. Preliminary investigations of pain and analgesia assessment in horses administered phenylbutazone or placebo after arthroscopic surgery. Vet Surg (1997) 26:150–5. 10.1111/j.1532-950X.1997.tb01478.x [DOI] [PubMed] [Google Scholar]
- 15.Love E, Lane J, Murison P. Morphine administration in horses anesthetized for upper respiratory tract surgery. Vet Anaesth Analg (2006) 33:179–88. 10.1111/j.1467-2995.2005.00247.x [DOI] [PubMed] [Google Scholar]
- 16.Clark L, Clutton R, Blissitt K, Chase-Topping M. The effects of morphine on the recovery of horses from halothane anaesthesia. Vet Anaesth Analg (2008) 35:22–9. 10.1111/j.1467-2995.2007.00350.x [DOI] [PubMed] [Google Scholar]
- 17.Moens Y, Gootjes P, Ionita J, Heinonen E, Schatzmann U. In vitro validation of a Pitot-based flow meter for the measurement of respiratory volume and flow in large animal anaesthesia. Vet Anaesth Analg (2009) 36:209–19. 10.1111/j.1467-2995.2009.00449.x [DOI] [PubMed] [Google Scholar]
- 18.Portier K, Séna A, Senior M, Clutton R. A study of the correlation between objective and subjective indices of recovery quality after inhalation anaesthesia in equids. Vet Anaesth Analg (2010) 37:329–36. 10.1111/j.1467-2995.2010.00542.x [DOI] [PubMed] [Google Scholar]
- 19.R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; (2013). Available from: http://www.R-project.org/ [Google Scholar]
- 20.Muir W. Pain: mechanisms and management in horses. Vet Clin North Am Equine Pract (2010) 26:467–80. 10.1016/j.cveq.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 21.Seitsonen E, Korhonen I, van Gils M, Huiku M, Lötjönen J, Korttila K, et al. EEG spectral entropy, heart rate, photoplethysmography and motor responses to skin incision during sevoflurane anaesthesia. Acta Anaesthesiol Scand (2005) 49:384–92. 10.1111/j.1399-6576.2005.00654.x [DOI] [PubMed] [Google Scholar]
- 22.Rantanen M, Yli-Hankala A, van Gils M, Yppärilä-Wolters H, Takala P, Huiku M, et al. Novel multiparameter approach for measurement of nociception at skin incision during general anaesthesia. Br J Anaesth (2006) 96:367–76. 10.1093/bja/ael005 [DOI] [PubMed] [Google Scholar]
- 23.Takeyama M, Matsunaga A, Kakihana Y, Masuda M, Kuniyoshi T, Kanmura Y. Impact of skin incision on the pleth variability index. J Clin Monit Comput (2011) 25:215–21. 10.1007/s10877-011-9298-9 [DOI] [PubMed] [Google Scholar]
- 24.Haga H, Dolvik N. Electroencephalographic and cardiovascular variables as nociceptive indicators in isoflurane-anaesthetized horses. Vet Anaesth Analg (2005) 32:128–35. 10.1111/j.1467-2995.2005.00194.x [DOI] [PubMed] [Google Scholar]
- 25.Rohrbach H, Korpivaara T. Comparison of the effects of the alpha-2 agonists detomidine, romifidine and xylazine on nociceptive withdrawal reflex and temporal summation in horses. Vet Anaesth Analg (2009) 36:384–95. 10.1111/j.1467-2995.2009.00466.x [DOI] [PubMed] [Google Scholar]
- 26.Kaka J, Klavano P, Hayton W. Pharmacokinetics of ketamine in the horse. Am J Vet Res (1979) 40:978–81. [PubMed] [Google Scholar]
- 27.Waterman A, Robertson S, Lane J. Pharmacokinetics of intravenously administered ketamine in horse. Res Vet Sci (1987) 42:162–6. [PubMed] [Google Scholar]
- 28.Raimondi G, Legramante J, Iellamo F, Frisardi G, Cassarino S, Peruzzi G. Noxious stimuli do not determine reflex cardiorespiratory effects in anesthetized rabbits. J Appl Physiol (1996) 81:2421–7. [DOI] [PubMed] [Google Scholar]
- 29.Martin W. Pharmacology of opioids. Pharmacol Rev (1983) 35:283–323. [PubMed] [Google Scholar]
- 30.Muir W. Drugs used to produce standing chemical restraint in horses. Vet Clin North Am Large Anim Pract (1981) 3:17–44. [DOI] [PubMed] [Google Scholar]
- 31.Eckenhoff J, Oech S. The effects of narcotics and antagonists upon respiration and circulation in man. A review. Clin Pharmacol Ther (1960) 1:483–524. [PubMed] [Google Scholar]
- 32.Nikoda V, Petrov R, Sandrikov V, Chizhov A. [Effects of opioid analgesics on pulmonary ventilation and metabolism in patients with acute postoperative pain]. Anesteziol Reanimatol (1994) 4:28–9. [PubMed] [Google Scholar]
- 33.Steffey E, Eisele J, Baggot J. Interactions of morphine and isoflurane in horses. Am J Vet Res (2003) 64:166–75. 10.2460/ajvr.2003.64.166 [DOI] [PubMed] [Google Scholar]
- 34.Mircica E, Clutton R, Kyles K. Problems associated with perioperative morphine in horses: a retrospective case analysis. Vet Anaesth Analg (2003) 30:147–55. 10.1046/j.1467-2995.2003.00092.x [DOI] [PubMed] [Google Scholar]
- 35.Steffey E, Howland DJ. Comparison of circulatory and respiratory effects of isoflurane and halothane anesthesia in horses. Am J Vet Res (1980) 41:821–5. [PubMed] [Google Scholar]
- 36.Clark L, Clutton R, Blissitt K, Chase-Topping M. Effects of perioperative morphine administration during halothane anaesthesia in horses. Vet Anaesth Analg (2005) 32:10–5. 10.1111/j.1467-2995.2004.00174.x [DOI] [PubMed] [Google Scholar]
- 37.Katz J, McCartney C. Current status of preemptive analgesia. Curr Opin Anaesthesiol (2002) 15:435–41. 10.1097/00001503-200208000-00005 [DOI] [PubMed] [Google Scholar]
- 38.Ingwersen W, Fox R, Cunningham G, Winhall MC. Efficacy and safety of 3 versus 5 days of meloxicam as an analgesic for feline onychectomy and sterilization. Can Vet J (2012) 53:257–64. [PMC free article] [PubMed] [Google Scholar]
- 39.Gozalo-Marcilla M, Steblaj B, Schauvliege S, Duchateau L, Gasthuys F. Comparison of the influence of two different constant-rate infusions (dexmedetomidine versus morphine) on anaesthetic requirements, cardiopulmonary function and recovery quality in isoflurane anaesthetized horses. Res Vet Sci (2013) 95:1186–94. 10.1016/j.rvsc.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 40.Kissin I. Pre-emptive analgesia. Anesthesiology (2000) 93:1138–43. 10.1097/00000542-200010000-00040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.