Abstract
The flea beetle Aphthona whitfieldi Bryant (Coleoptera: Chrysomelidae) is the main pest of the bioenergy crop Jatropha curcas L. (Euphorbiaceae) in Burkina Faso and several other West African countries. Adults severely defoliate plants, resulting in seedling mortality, poor growth, and low yields. To study the population dynamics of the pest in the Sissili Province of Burkina Faso, 12 sites were monitored weekly during a year and 31 sites were inspected for damage at the peak period of insect abundance. The effect of cropping systems (hedge, intercropping, and monoculture) and surrounding vegetation on population densities of A. whitfieldi was assessed. Beetles were rarely found in the dry season and peaked in the second half of the rainy season. The cropping system did not significantly influence the abundance and attack level. In contrast, the close vicinity of fallow lands seems to increase damage levels. Many aspects of the biology and ecology of A. whitfieldi remain to be investigated before sustainable control methods can be developed. However, this study already allows us to propose recommendations for further research on management.
Keywords: bioenergy crop, intercropping, Chrysomelidae
Physic nut, Jatropha curcas L. (Euphorbiaceae), is a multipurpose shrub originating from Central America. It was introduced in West Africa during the colonial era, mainly for its use as living fence to protect fields from grazing. It is also traditionally used for soap production, medicinal purposes, fertilizer, pesticide, etc. (Heller 1996). More recently, it has been promoted and planted worldwide as bioenergy crop, its seeds containing oil that is suitable for biodiesel production (Achten et al. 2008). In Burkina Faso, the extensive use of J. curcas for biodiesel started in the 2000s, when thousands of hectares were planted (Derra et al. 2013). To minimize negative effects on food security, it is mainly promoted for planting as living fences or in wide intercropping systems, as supplement to current farming systems (Derra et al. 2013, Jongschaap et al. 2013).
Pests and diseases are listed as one of the factors that limit the utilization of J. curcas in Africa and elsewhere (Jongschaap et al. 2013, Muys et al. 2013, Minengu et al. 2014). In contradiction of the general belief that J. curcas was resistant to pests because of its toxicity, a large number of insects and other pests have been identified that can severely reduce growth and yield (Anitha and Varaprasad 2012, Minengu et al. 2014, Lama et al. 2015, for review). Several studies have identified flea beetles of the genus Aphthona (Coleoptera: Chrysomelidae, Alticinae) as major pests of J. curcas in various regions in Africa (Gagnaux 2009, Nielsen 2009, Rouamba 2011, Anitha and Varaprasad 2012, Minengu et al. 2015c). They are particularly damaging to young plants, which can die from continuous defoliation. Even when the plant is not killed, growth and fructification are severely hampered (Gagnaux 2009, Rouamba 2011, Anitha and Varaprasad 2012, Minengu et al. 2015c). Until recently, the taxonomy of the genus Aphthona in Africa was particularly confusing. In their revision of the Aphthona cookei species group in Sub-Saharan Africa, Biondi et al. (2013) examined many African specimens, including those reared from J. curcas during this study and stated that Aphthona whitfieldi Bryant was the species damaging J. curcas in West Africa. The same species is also cited by Igogo et al. (2011) as damaging J. curcas in Kenya, but Biondi et al. (2013) identified other specimens reared from J. curcas in Kenya as A. cookei (Gerstaecker). In Southern Africa, Aphthona dilutipes Jacobi and an undescribed species were reared from J. curcas by Gagnaux (2009). Interestingly, Aphthona spp. seem to be absent or of minor importance on J. curcas in dryer regions such as Niger (Habou et al. 2014), Sudan (Abdalla and Al Atif 2013), and Senegal (Terren et al. 2012).
Surprisingly, very little is known of the biology and ecology of the African Aphthona spp. Adults damage the plants by feeding on leaves during the rainy season, but there is no information available in the literature on the immature stages’ biology. Larvae and pupae have been found in the soil around the trunk of J. curcas (A.S., unpublished data), suggesting that eggs are laid in the soil and larvae feed on J. curcas roots. The seasonal occurrence of an unidentified Aphthona sp. has been monitored in the tropical humid climate of the region of Kinshasa, Democratic Republic of the Congo (DRC), by Minengu et al. (2015c), who showed that the beetle mainly occurs in the rainy seasons. Other basic information on the phenology, population dynamics, host range, habitat preferences, etc. is lacking, which is severely impeding the development of sustainable control methods. Insecticides are efficient in controlling the pest (Cassimo et al. 2011, Minengu et al. 2015c), but their extensive use is neither desirable nor affordable for small scale farmers in a context of intercropping. Plant extracts from Lantana camara L. and Tephrosia vogelii (Hook) have been tested as repellent in Kenya, with promising results (Igogo et al. 2011). Anitha and Varapasad (2012) also mentioned the use of trap plants around J. curcas fields, and other cultural practices such as early planting and deep ploughing have been suggested by Gagnaux (2009), but these methods still have to be tested and validated.
In this article, we describe observations on the population dynamics of A. whitfieldi in Burkina Faso. We also test the effect of cropping systems and surrounding vegetation on population densities of A. whitfieldi in the hope that this information will help developing cultural practices to mitigate the effect of the beetle on J. curcas.
Materials and Methods
Region of Investigations
The study was carried out in the Sissili Province, southern Burkina Faso. All sites were situated in the communes of Léo, Boura, and Biéha, within a radius of 30 km from the city of Léo (11.10° N, 02.10° W). The climate is characterized by a unimodal rainfall pattern, with the rainy season starting in May and ending in October. Mean annual precipitation varies between 900 and 1,200 mm. The work was carried out at farmers’ fields and hedges of J. curcas, planted between 2009 and 2012.
Population Dynamics of A. whitfieldi
To study the population dynamics of A. whitfieldi over a year’s time, 12 sites planted in 2009 were selected in the communes of Léo and Biéha. In each commune, two monocultures of J. curcas, two fields where J. curcas was intercropped with various other crops, and two J. curcas hedges were monitored from 10–12 September 2012 to 2–4 September 2013 in Léo and Biéha, respectively. In the intercropping systems, planting distances for J. curcas was 2 by 2 by 8 m, i.e., two rows of J. curcas were planted at 2 m distance and at 8 m from two other rows. Other crops were usually planted at 1.3 m distance from J. curcas stems. Intercropped plants included soybean, maize, groundnut, okra or pepper, and the species varied among fields and seasons. Every week, five trees were selected haphazardly in each plantation and the number of A. whitfieldi found on each tree was counted. Tree size varied within and between plantations because trees were growing rapidly and were pruned by the farmers. However, there was no evidence of significant differences in tree size among plantation systems throughout the year of observation.
Environmental Factors Influencing the Abundance of A. whitfieldi
The monitoring system for assessing the population dynamics in the 12 plantations described earlier was also used to compare abundance of A. whitfieldi in monocultures, intercropped fields, and hedges. However, because of the low number of plantations involved in this study, another study to assess environmental factors influencing the abundance of A. whitfieldi was carried out from 19 September to 1 October 2012. At that time, A. whitfieldi was present at various levels of infestation in all plantations of the region. Thirty-one plantations were chosen haphazardly in the communes of Léo, Boura, and Biéha and surveyed for A. whitfieldi: 10 hedges, 13 monoculture fields of J. curcas, and 8 fields where J. curcas was intercropped with other plants. All fields were first planted in 2009 or 2010, but in many cases, young seedlings were planted later to replace dead trees.
In each plantation, 20 trees of at least 2-yr old (i.e., planted in 2009 or 2010) were randomly selected. For each tree, damage by A. whitfieldi was assessed only in the lower part of the tree (50 cm from the soil) to minimize the effect of tree size, taking into account that trees had different heights and damage is usually higher in the lower part of the crown. Damage was assessed by evaluating, on 10 leaves, the average surface of leaves with damage by A. whitfieldi, i.e., the surface where photosynthesis cannot be performed, whether by direct feeding or drying out of the leaf due to the insect pest. We did not measure the exact surface affected by the insect for each leaf. Instead, we scored the average attack as follows: <1% (average 0.5%) of leaf surface damaged; 1–5% (average 3%); 5–20% (average 12.5%); 20–50% (average 30%); 50–90% (average 70%); and >90% (average 95%). To score the general level of infestation of a plantation, we averaged all the average percentages of infestation of the 20 trees. At each plantation, we also assessed the proximity with fallow lands, i.e., we noted whether fields and hedges were directly adjacent to fallow lands or only to cultivated lands (i.e., where all assessed trees were at least 100 m from the next fallow). For fields, we also noted whether the field itself was left as fallow or weeded. When the field was not weeded, we considered it as a fallow.
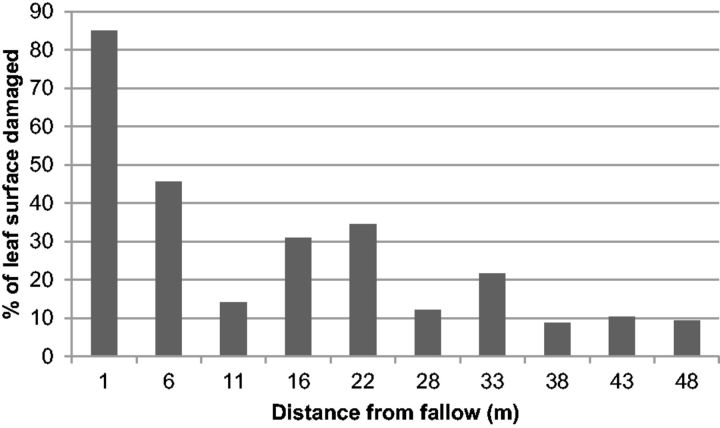
To further assess the influence of the distance to fallow lands on damage, in September 2012 we chose a field with 3-mo-old plants, weeded and intercropped with soybean, adjacent to fallow land at only one side, the three other sides being occupied by various weeded crops. We assessed the level of infestation, using the same scale, of five randomly selected trees at 1, 6, 11, 16, 22, 28, 33, 38, 43, and 48 m from the fallow land.
Statistical Analyses
To compare the abundance of A. whitfieldi between the three cropping systems at the 12 sites where beetle populations were monitored during 1 yr, we used a linear mixed effects model with cropping system as fixed factor, village as random factor, and the sum of beetle number per field as dependent variable. To analyze the influence of cropping systems and environmental factors on the level of infestation by A. whitfieldi at the 31 plantations monitored in September 2012, generalized linear models were used to fit a gamma regression to infestation scores. Fixed factors used were J. curcas as hedge versus fields (all data); monoculture versus intercropping (fields only); and J. curcas plants adjacent to fallows in or outside the fields (all data and fields only). In the 3-month-old J. curcas field adjacent to fallow lands, the relationship between the level of infestation by the beetle and the distance to the nearest fallow was analyzed using a Pearson correlation. All statistics were carried out using SPSS (IBM Corp. 2013).
Results
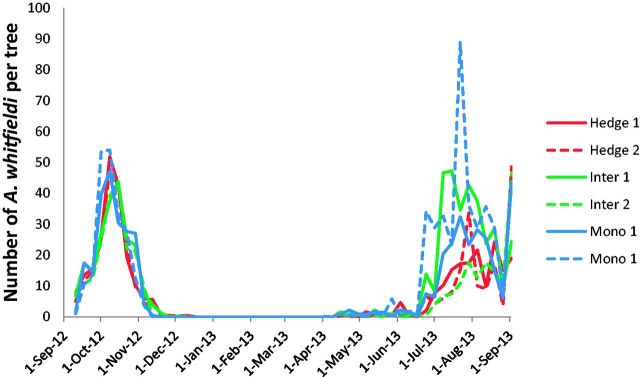
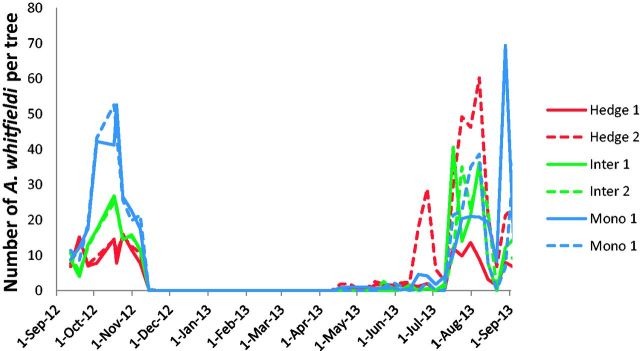
The yearly abundance of A. whitfieldi at the 12 plantations is shown in Figs. 1 and 2. The beetle is absent on J. curcas during most of the dry season. It appears before the main rains in April, but it becomes abundant only when the rainy season is well established, i.e., in late June and July. Populations crash in November, a few weeks after the termination of the rain. There was no significant difference in abundance between the different cropping systems (F = 11.451, P = 0.080, df = 2,6).
Fig. 1.
Average number of A. whitfieldi adults per tree in the six fields in Léo: two hedges, two intercropped fields, and two monocultures, between Sept. 2012 and Sept. 2013.
Fig. 2.
Average number of A. whitfieldi adults per tree it the six fields in Biéha: two hedges, two fields intercropped fields, and two monocultures, between Sept. 2012 and Sept. 2013.
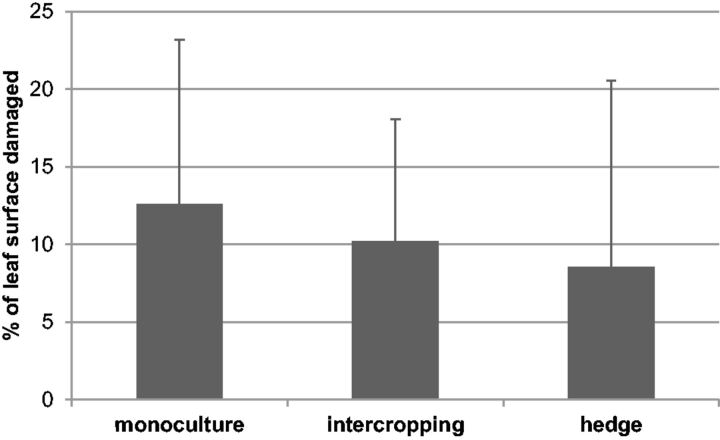
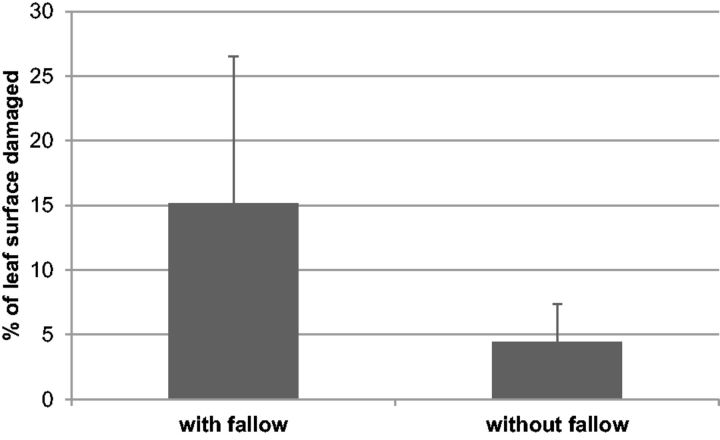
Beetles were found at all 31 field sites surveyed in September 2012, with mean percentages of leaf surface damage varying from 0.5 to 39.5%. Single trees with 100% defoliation were not uncommon. The type of cropping system did not significantly affect damage levels (hedges vs. fields, P = 0.463, Wald χ2 = 0.538; intercropped field vs. monoculture, P = 0.573, Wald χ2 = 0.318, df = 1) (Fig. 3). However, the presence or absence of fallow land within or besides the plantation was important, i.e., A. whitfieldi was significantly more abundant on plants that were in the vicinity of fallows or when the field itself was not weeded (P = 0.001, Wald χ2 = 11.97 with all data; P = 0.027, Wald χ2 = 4.92 with fields only, df = 1) (Fig. 4).
Fig. 3.
Percentage of leaf surface of J. curcas damaged by A. whitfieldi in 13 monoculture fields, 8 fields where J. curcas was intercropped with other plants, and 10 living hedges. Vertical bars indicate SD.
Fig. 4.
Percentage of leaf surface of J. curcas damaged by A. whitfieldi in plantations in or adjacent to fallow lands compared with plantations where no fallow land occurred at <100 m from the assessed trees. Vertical bars indicate SD.
The importance of the proximity of fallows was confirmed by the separate observation in a plantation of 3-month-old J. curcas plants beside a fallow land. The level of attack by A. whitfieldi was significantly negatively correlated with the distance to the fallow (R = −0.590; P < 0.001). Trees at 1 m of the fallow were defoliation at 85%, whereas those situated at more than 10 m of the fallow were defoliated at <40% and those at 38 m or more were defoliated at <10% (Fig. 5). In the first 6 m from the fallow, 50% of the young trees were totally defoliated.
Fig. 5.
Percentage of leaf surface of J. curcas damaged by A. whitfieldi in a plantation intercropped with soybean, adjacent to a fallow, according to the distance from the fallow.
Discussion
Our long-term observations showed that population densities of A. whitfieldi and their damage on J. curcas were particularly high in the second half of the rainy season. Minengu et al. (2015c) made similar observations with an undetermined Aphthona sp. in DRC. Possibly, most beetles die during the dry season. This could also explain why Aphthona spp. seem to be absent or less prevalent on J. curcas in dry areas (Terren et al. 2012, Abdalla and Al Atif 2013, Habou et al. 2014). Furthermore, J. curcas loses a large part of its leaves during the dry season, which does not favor the continuous presence of defoliating insects such as Aphthona spp. Damage was particularly serious on young plants. It was common to observe newly planted seedlings totally defoliated and even killed by A. whitfieldi (A. S. unpublished observations).
This study failed to show a significant difference in damage level in the different cropping systems, i.e., on hedges, monoculture plantations, or intercropping. Thus, the common observation that crops and trees are less vulnerable to pests in intercropping systems and mixed stands than in monocultures (e.g., Andow et al. 1991, Jactel and Brockerhoff 2007) was not verified for A. whitfieldi on J. curcas. Nevertheless, in this preliminary study, we considered all intercropping systems together because we had no control on the associated crops, which varied not only between fields but also within the same field between seasons and years. It cannot be ruled out that a specific intercropping system could be found that would be able to lower pest populations, e.g., by repelling beetle or by attracting or hosting natural enemies. For example, in the region of Kinshasa, DRC, Minengu et al. (2015b) observed a decrease of the damage by Aphtona sp. when J. curcas was intercropped with maize and the legume plant Stylosanthes guianensis (Aublet). Furthermore, there is a general agreement that, in Africa, hedges and wide intercropping J. curcas systems should be favored over monocultures for economic, risk management, and food security reasons (e.g., Jongschaap et al. 2013, Minengu et al. 2015a).
Interestingly, A. whitfieldi seemed to be favored by the direct vicinity of fallow lands (wild grasses), including when J. curcas is planted in nonweeded land. Since we did not find A. whitfieldi on wild plants despite extensive surveys around J. curcas fields (A. S. unpublished observations), it is likely that the lower abundance of the pest in a clean environment is not due to the absence of wild hosts. Instead, it may rather be due to less favorable conditions for the immature stages (eggs, larvae, and pupae) in the soil. Indeed, weeding or ploughing the soil may affect immature stages, either directly or indirectly, by causing unfavorable abiotic conditions. Unfortunately, for the moment, little is known about the biology and ecology of A. whitfieldi, in particular during the immature stages. For example, it is still unclear in which stage the insect spends the dry season and how it survives the drought, how many generations it may have during the rainy season, what are its alternative host plants, etc. As other members of the Alticinae subfamily, eggs are most probably laid on the ground and the larvae feed on roots. Several European Aphthona spp. used for the biological control of invasive Euphorbiaceae in North America have been well studied (Jan 1998, Hodur et al. 2006, Larson et al. 2008). But these are temperate species, univoltine with an obligatory diapause in the soil the larval stage, broken by winter cold. It is likely that Afro-tropical species have a very different bio-ecology that remains to be investigated before sustainable control can be developed.
Aphthona spp. can be easily controlled by chemical insecticides but their use is neither economically nor environmentally sustainable in Africa (Cassimo et al. 2011, Minengu et al. 2015c). Based on preliminary observations described in this article and elsewhere, several recommendations can already be suggested for the control of A. whitfieldi and probably other Aphthona spp. in other regions in Africa. These recommendations will have to be tested through proper field experiments before being implemented. First, early plant establishment is critical, since the flea beetles are most damaging on young seedlings (Rouamba 2011, Minengu et al. 2015a,c) and, as shown by this study, mainly occurs in high numbers in the second half of the rainy season. Thus, every effort should be made to favor the fast growth of newly planted seedlings in the first part of the rainy season, by planting early and by planting as intercropping with fertilized crops, e.g., maize. Second, although the reasons are still unclear, we also showed that A. whitfieldi is more abundant in badly maintained (i.e., not weeded) fields and in the close vicinity of fallows. Therefore, such situations should be avoided, in particular for young seedlings. Furthermore, attention should be paid to seedlings planted right beside older J. curcas plants, even if these do not appear much affected. Established plants always sustain a variable level of flea beetles in the lower part of their crown (A. S. unpublished observations) and will act as a source of beetles for young, vulnerable seedlings.
Acknowledgments
We thank Prof. Maurizio Biondi (University of L'Aquila, Italy) for his support in the identification of the Aphthona species and Dr. Dirk Babendreier (CABI Switzerland) for his advices on statistics. This study was supported by ADECIA, the Agence Française de Développement (AFD) through a support to the Fonds Français pour l’Environnement (FFEM), Fondation Fasobiocarburant, and the Swiss Agency for Development and Cooperation (SDC) through the ERA-ARD project JATROPHABILITY.
References Cited
- Abdalla I., Al Atif M. A. 2013. Arthropods community associated with physic nut (J. curcas L.) in dry lands (a case study in Sudan), pp. 661–671. In Efe R., Atalay I., Cüreba I. (eds.), Proceedings, 3rd International Geography Symposium-GEOMED 2013, 10–13 June 2013, Kemer, Antalya, Turkey. [Google Scholar]
- Achten W., Verchot L., Franken Y., Mathijs E., Singh V., Aerts R., Muys B. 2008. Jatropha bio-diesel production and use. Biomass Bioenergy 32: 1063–1084. [Google Scholar]
- Andow D. A. 1991. Vegetational diversity and arthropod population response. Annu. Rev. Entomol. 36: 561–568. [Google Scholar]
- Anitha K., Varaprasad K. S. 2012. Jatropha pests and diseases, an overview, pp. 175–218. In Carels N., Sujatha M., Bahadur B. (eds.), Jatropha, challenges for a new energy crop, Springer, New York. [Google Scholar]
- Biondi M., Urbani F., D’Alessandro P. 2013. Revision of the Aphthona cookei species group in Sub-Saharan Africa: pests of Jatropha curcas L. in biodiesel plantations (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Entomologia 1: e7. [Google Scholar]
- Cassimo A. C., João E.C.B., Coelho J. P., Santos L. 2011. Evaluation of insecticide doses for the control of jatropha leaf beetle and jatropha leaf miner in Mozambique, pp. 187–190. In Proceedings, 10th African Crop Science Conference, 10–13 October 2011, Maputo, Mozambique. [Google Scholar]
- Derra A. N., Yélémou B., Sanon K. B., Hilou A., Millogo/Rasolodimby J., Hien V. 2013. Management patterns of Jatropha curcas: impact on the microbial and the mycorrhizial biomasses in different phyto-geographic zones of Burkina Faso. Adv. Appl. Sci. Res. 4: 256–267. [Google Scholar]
- Gagnaux P. C. 2009. Entomofauna associada a` cultura da Jatrofa (Jatropha curcas L.) em Moçambique, 79 pp. Projecto fianal. Universidade Eduardo Mondlane, Faculdade De Agronomia E Engenharia Florestal, Maputo, Mozanbique. [Google Scholar]
- Habou Z. A., Adam T., Haubruge E., Mergeai G., Verheggen F. J. 2014. Insects associated with Jatropha curcas Linn. (Euphorbiaceae) in West Niger. J. Insect Sci. 14: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. 1996. Physic nut, Jatropha curcas L. Promoting the conservation and use of underutilized and neglected crops, 88 pp. Institute of Plant Genetic and Crop Plant Research, Gatersleben, Germany, and International Plant Genetic Resource Institute, Rome, Italy. [Google Scholar]
- Hodur N. M., Leistritz F. L., Bangsund D. A. 2006. Biological control of leafy spurge: utilization and implementation. Rangeland Ecol. Manage. 59: 445–452. [Google Scholar]
- IBM Corp. 2013. IBM SPSS statistics for windows, version 22.0. IBM Corp., Armonk, NY. [Google Scholar]
- Igogo J. M., Ogendo J. O., Kariuki S. T., Otaye D. O. 2011. Insecticidal, antifeedant and repellent effects of Tephrosia vogelii Hook. and Lantana camara L. aqueous crude extracts against golden flea beetle, Aphthona whitfieldi Bryant in Jatropha, Jatropha curcas L. Biopest. Int. 7: 93–103. [Google Scholar]
- Jactel H., Brockerhoff E. G. 2007. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 10: 835–848. [DOI] [PubMed] [Google Scholar]
- Jan J. J. 1998. Biology of Aphthona nigriscutis (Coleoptera: Chrysomelidae) in the laboratory. Ann. Entomol. Soc. Am. 90: 433–437. [Google Scholar]
- Jongschaap R.E.E., Kenis M., Ellison C., Rouamba M., Freyer B. 2013. Jatropha growth and oilseed production in Africa. Jatropha Facts Series, Issue 1, ERA-ARD. [Google Scholar]
- Lama A. D., Vuorisalo T., Niemelä P. 2015. Global patterns of arthropod herbivory on an invasive plant, the physic nut (Jatropha curcas L.). J. Appl. Entomol. 139: 1–10. [Google Scholar]
- Larson D. L., Grace J. B., Larson J. L. 2008. Long-term dynamics of leafy spurge (Euphorbia esula) and its biocontrol agent, flea beetles in the genus Aphthona. Biol. Control 47: 250–256. [Google Scholar]
- Minengu J.D.D., Mobambo P., Mergeai G. 2014. Influence de l’environnement et des pratiques culturales sur la productivité de Jatropha curcas L. en Afrique subsaharienne (synthèse bibliographique). Biotechnol. Agric. Soc. Environ. 18: 290–300. [Google Scholar]
- Minengu J.D.D., Mobambo P., Mergeai G. 2015a. Analysis of the technical/economic performance of four cropping systems involving Jatropha curcas L. in the Kinshasa region (Democratic Republic of the Congo). Tropicultura 33: 67–76. [Google Scholar]
- Minengu J.D.D., Mobambo P., Mergeai G. 2015b. Study of the production possibilities of Jatropha curcas L. in permanent cover of Stylosanthes guianensis (Aublet) Swartz in association with maize (Zea mays L.) and soybean (Glycine max (L.) Merr.) under the conditions of the Batéké Plateau in Kinshasa. Tropicultura 33 (in press). [Google Scholar]
- Minengu J.D.D., Verheggen F., Mergeai G. 2015c. Dynamic and impact of major insect pests of Jatropha curcas L. in two cropping systems with contrasting characteristics in the province of Kinshasa (DRC). Tropicultura 33 (in press). [Google Scholar]
- Muys B., Norgrove L., Alamirew T., Birech R., Chirinian E., Delelegn Y., Ehrensperger A., Ellison C. A., Feto A., Freyer B., et al. 2014. Integrating mitigation and adaptation into development: the case of Jatropha curcas in Sub-Saharan Africa. GCB Bioenergy 6: 169–171. [Google Scholar]
- Nielsen F. 2009. Jatropha curcas oil production for local development in Mozambique. Afr. Crop Sci. Conf. Proc. 9: 71–75. [Google Scholar]
- Rouamba M. 2011. Inventaire des insectes ravageurs et des maladies fongiques du pourghère (Jatropha curcas) au Burkina Faso. Diploma thesis, Institut du Développement Rural, Bobo-Dioulasso, Burkina Faso. [Google Scholar]
- Terren M., Mignon J., Declerck C., Jijakli H., Savery S., Jacquet de Haveskercke P., Winandy S., Mergeai G. 2012. Principal disease and insect pests of Jatropha curcas L. in the lower valley of the Senegal River. Tropicultura 30: 222–229. [Google Scholar]